Phytoextraction of Cr(VI)-Contaminated Soil by Phyllostachys pubescens: A Case Study
Abstract
:1. Introduction
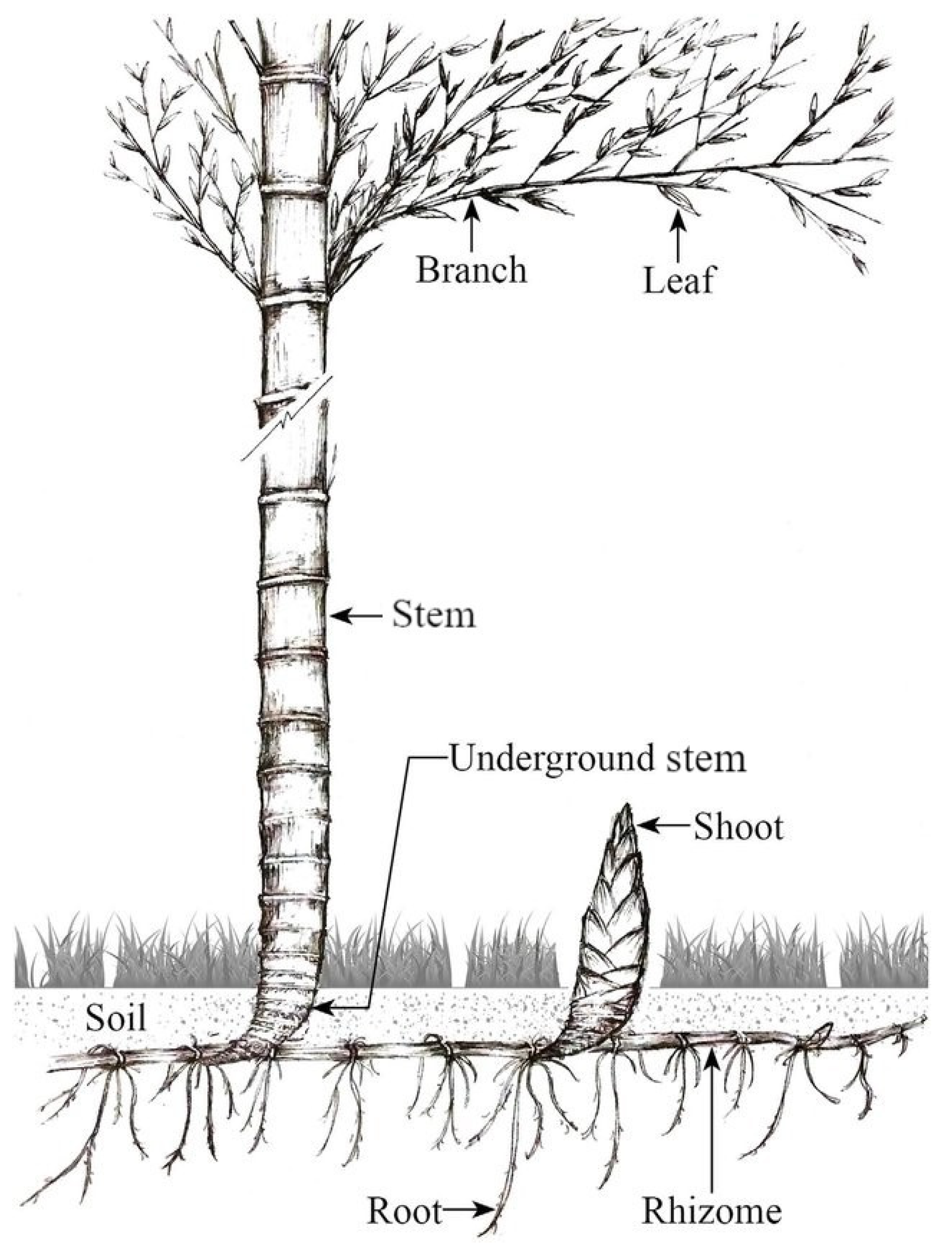
2. Materials and Methods
2.1. Analysis of the Bamboo Growth
2.2. Sample Preparation and Characterization
2.3. Statistical Analysis
3. Results and Discussion
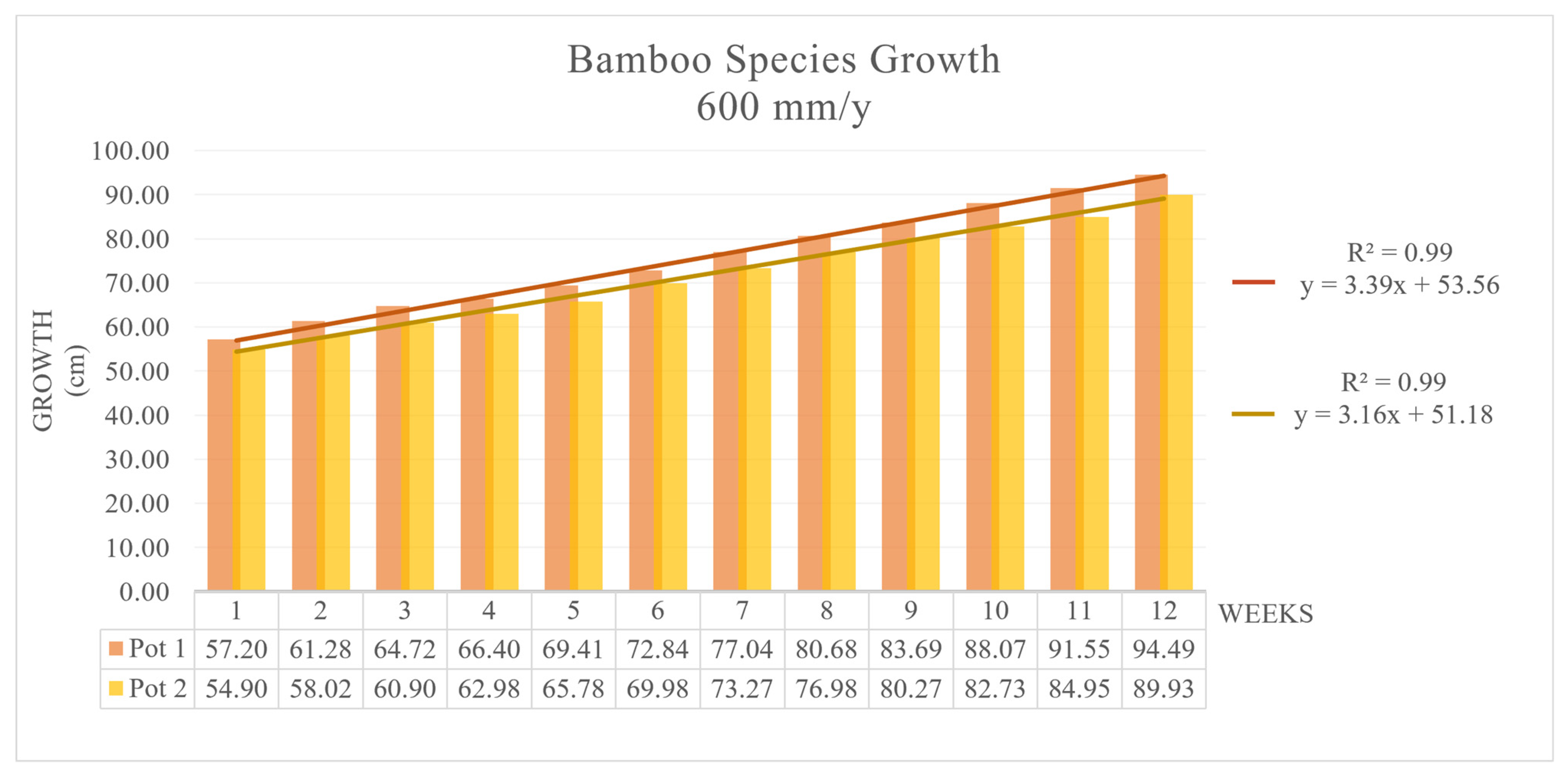
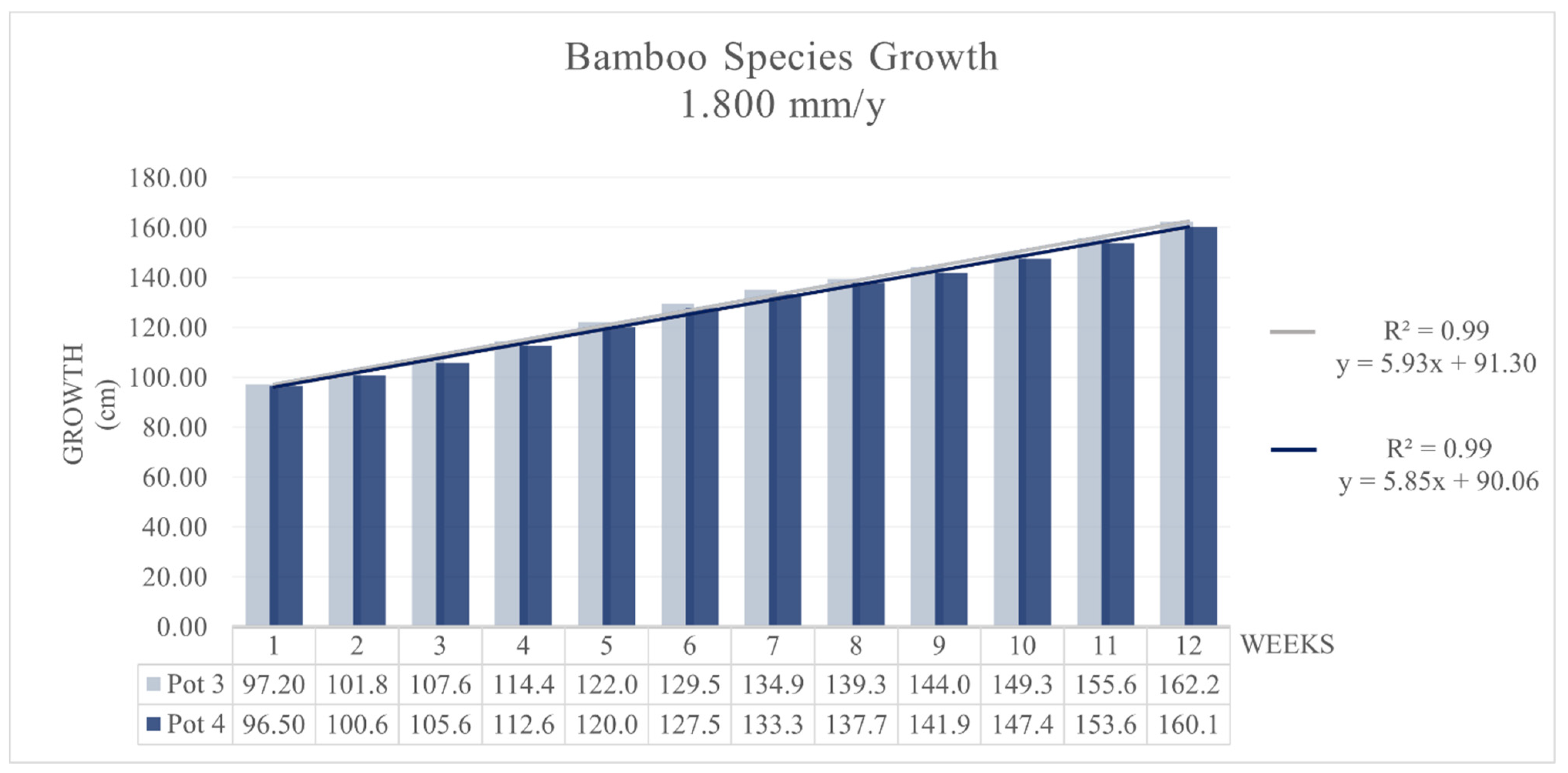
3.1. Growth Rate Test on MB
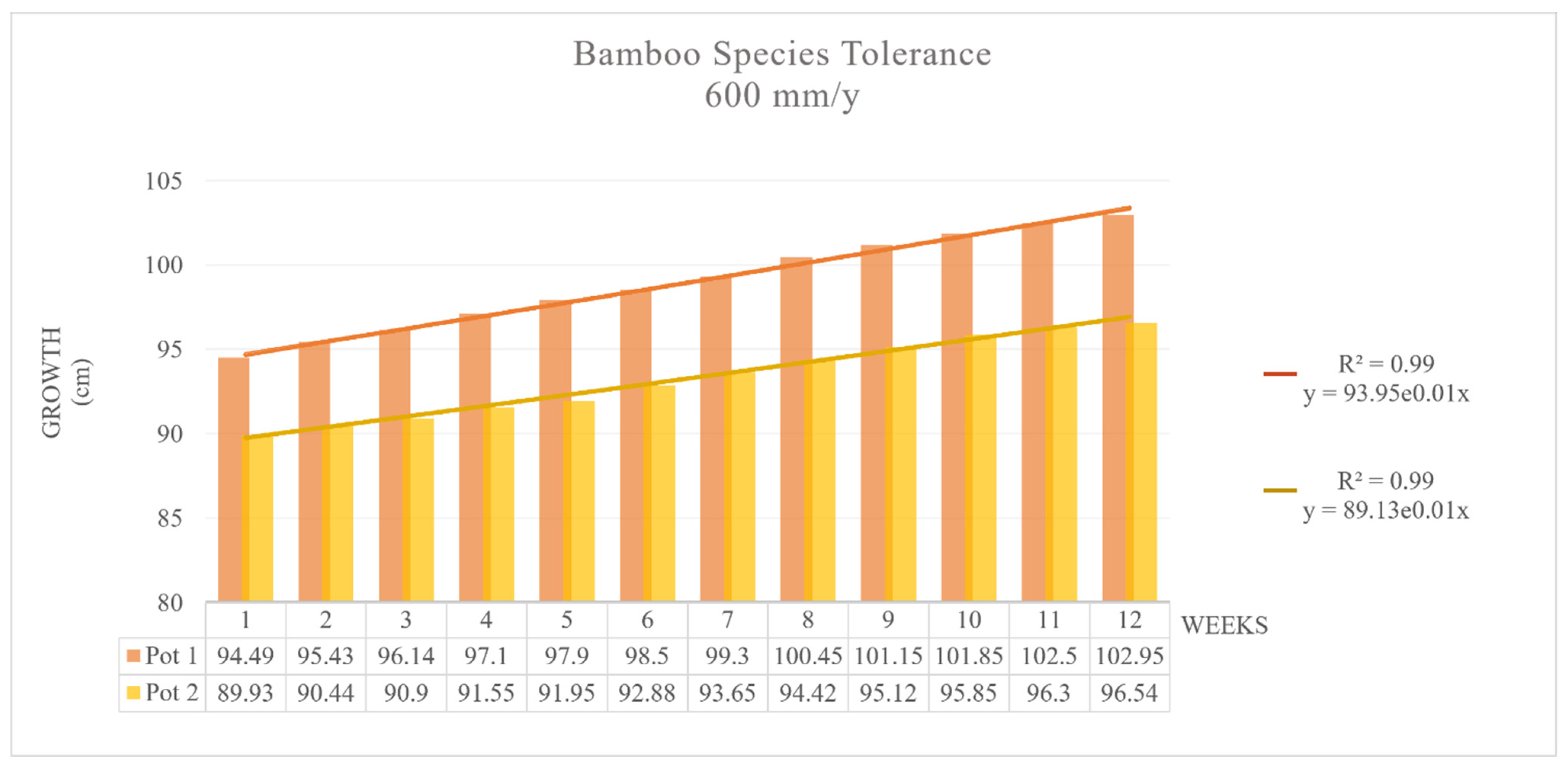
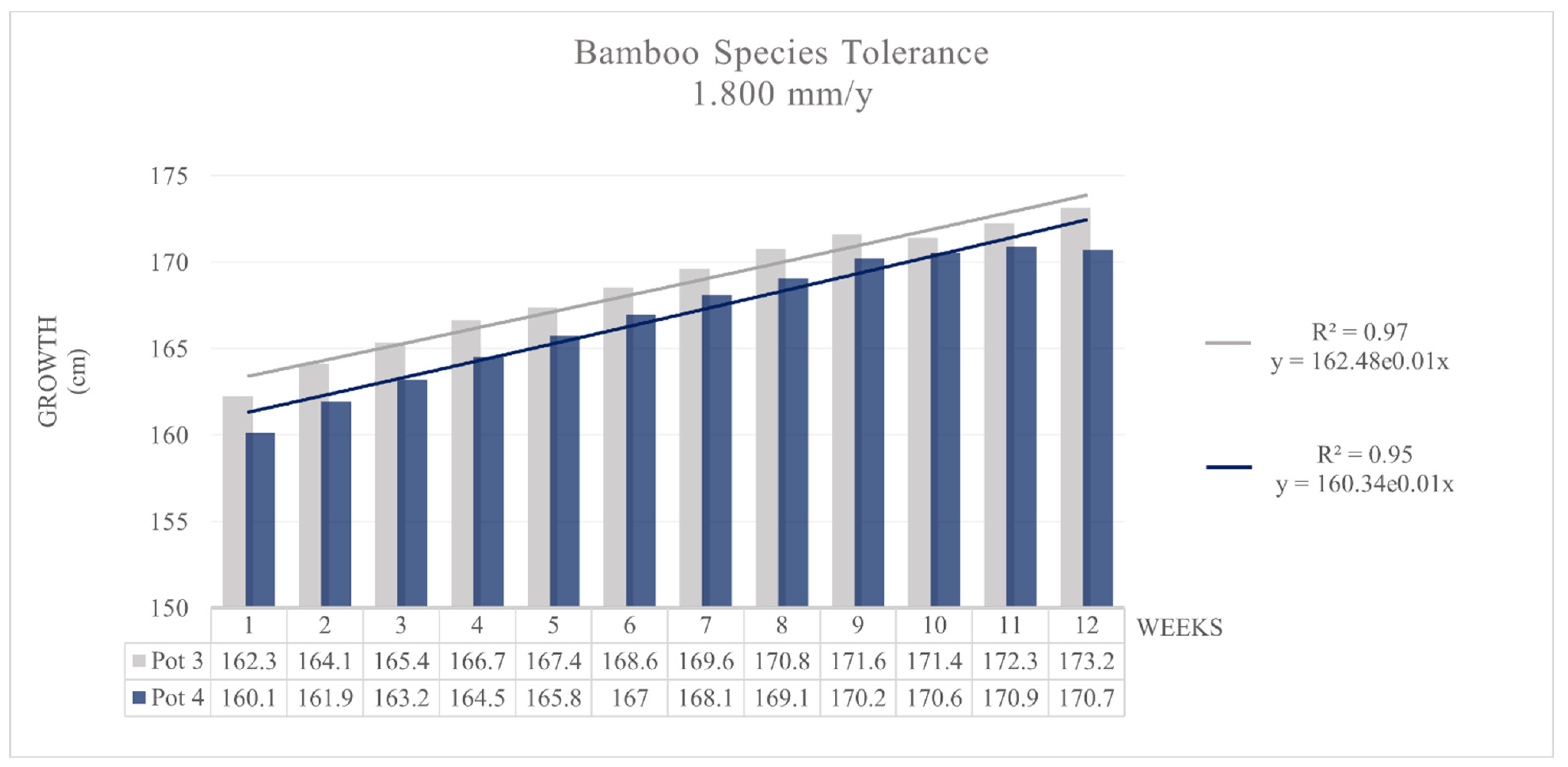
3.2. Contamination and Tolerance Test
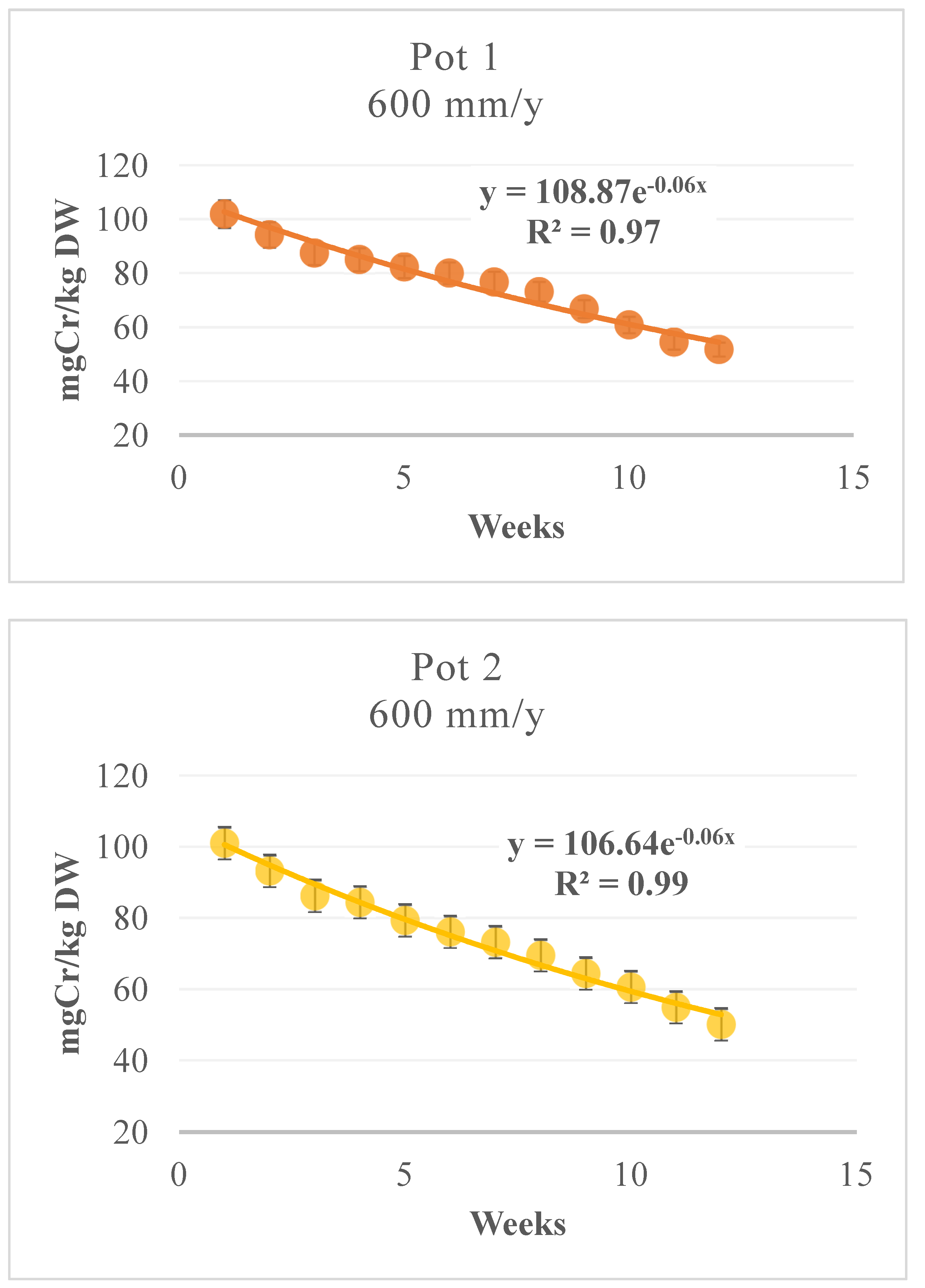
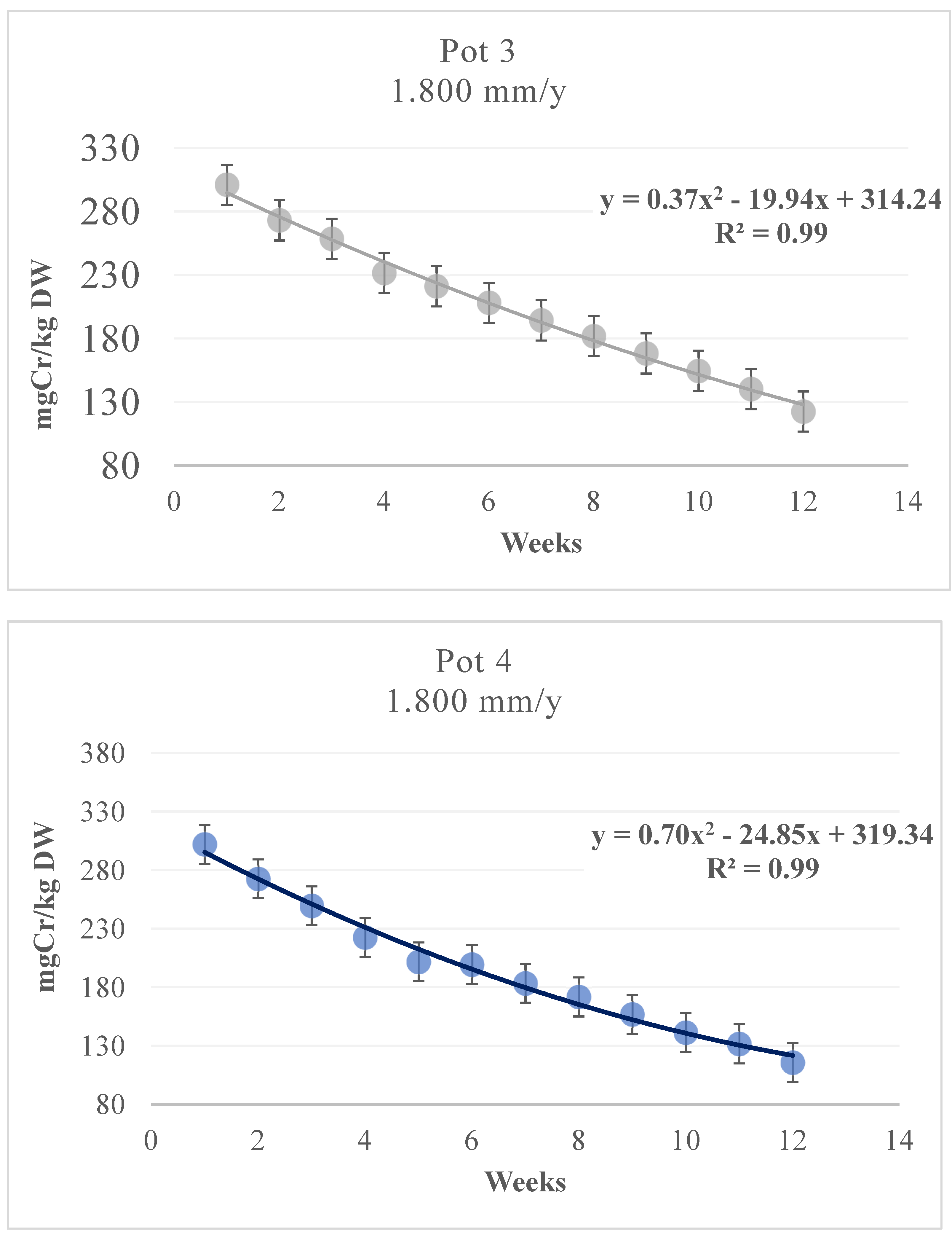
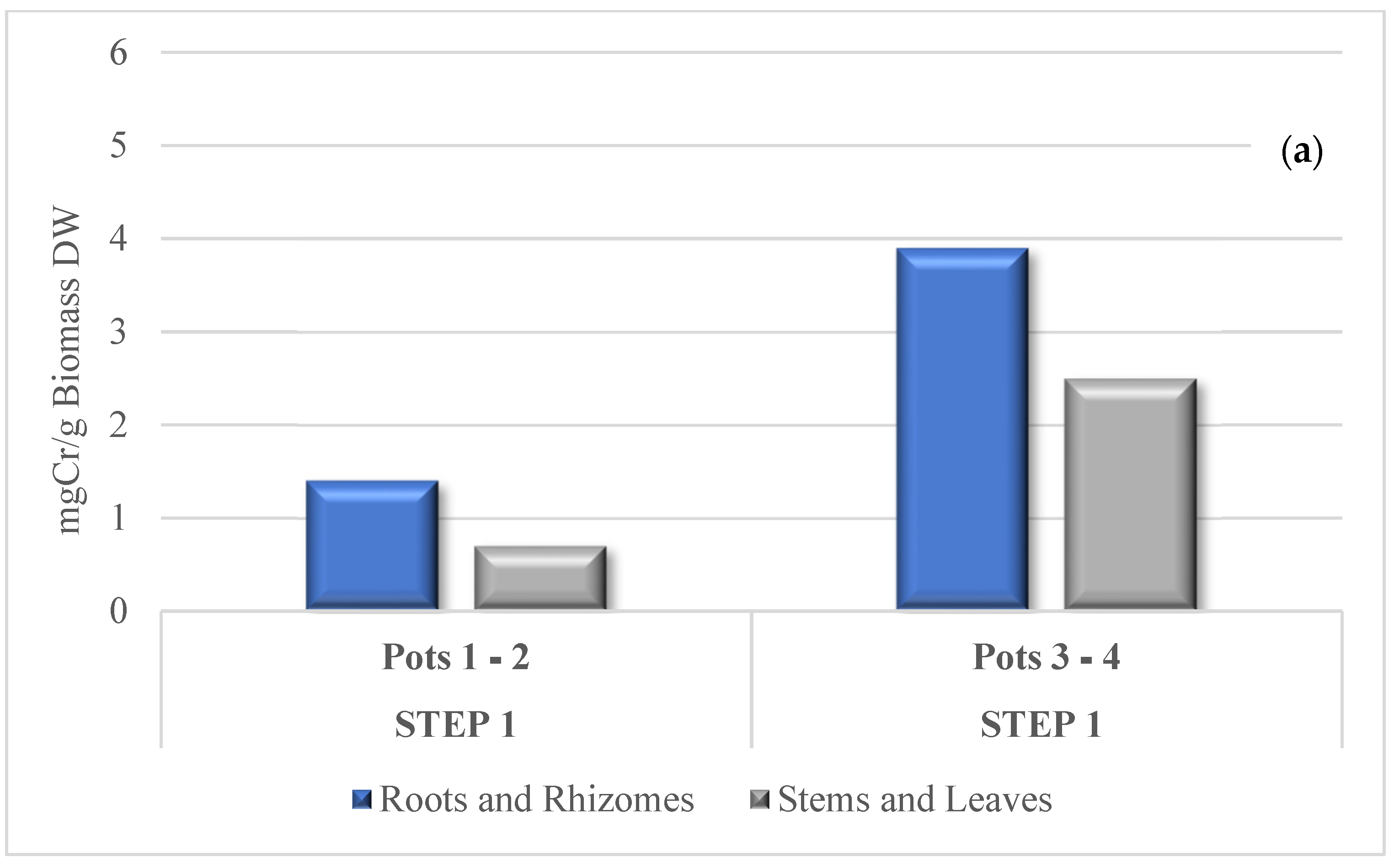
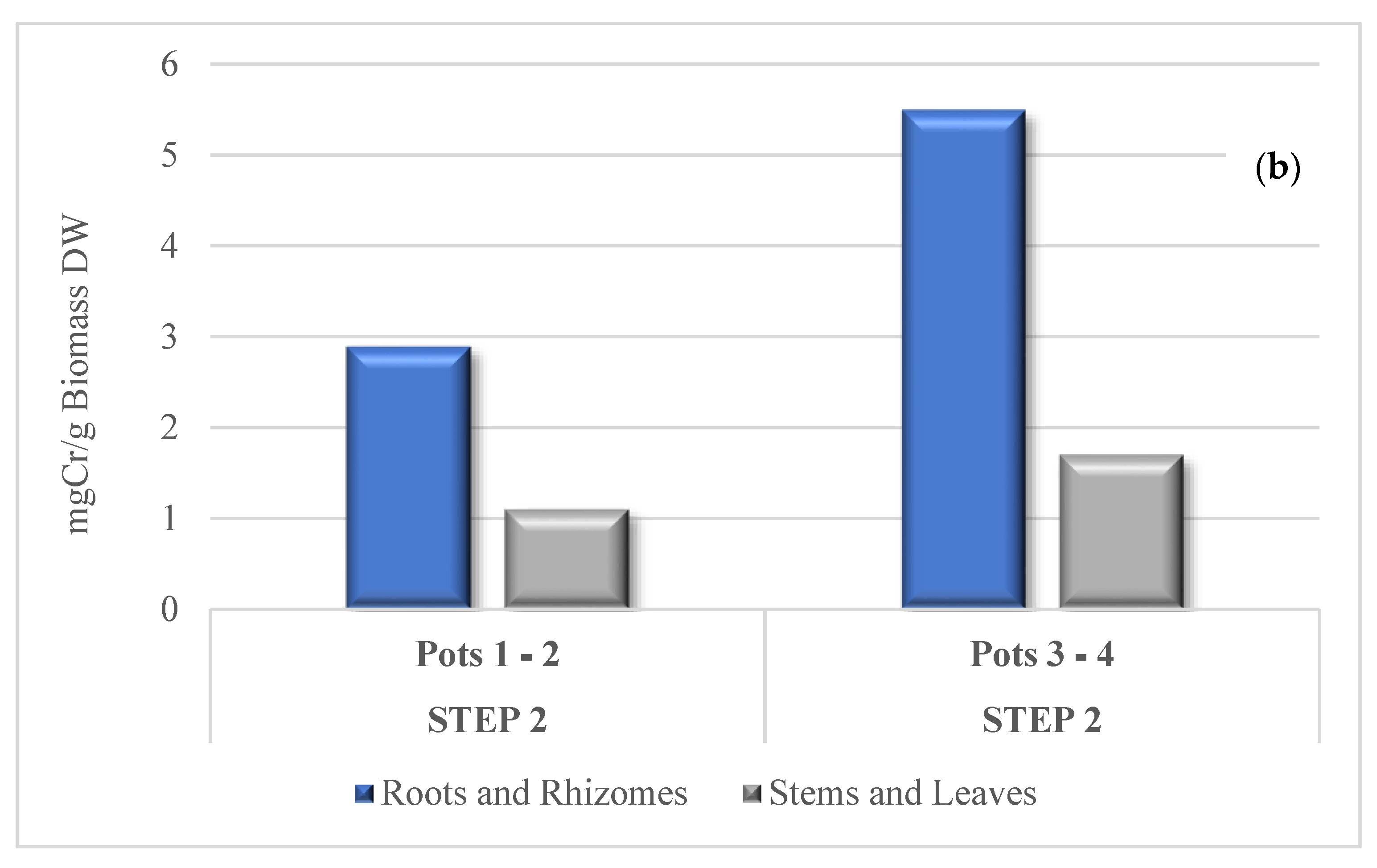
3.3. Cr Phytoextraction from the Soil
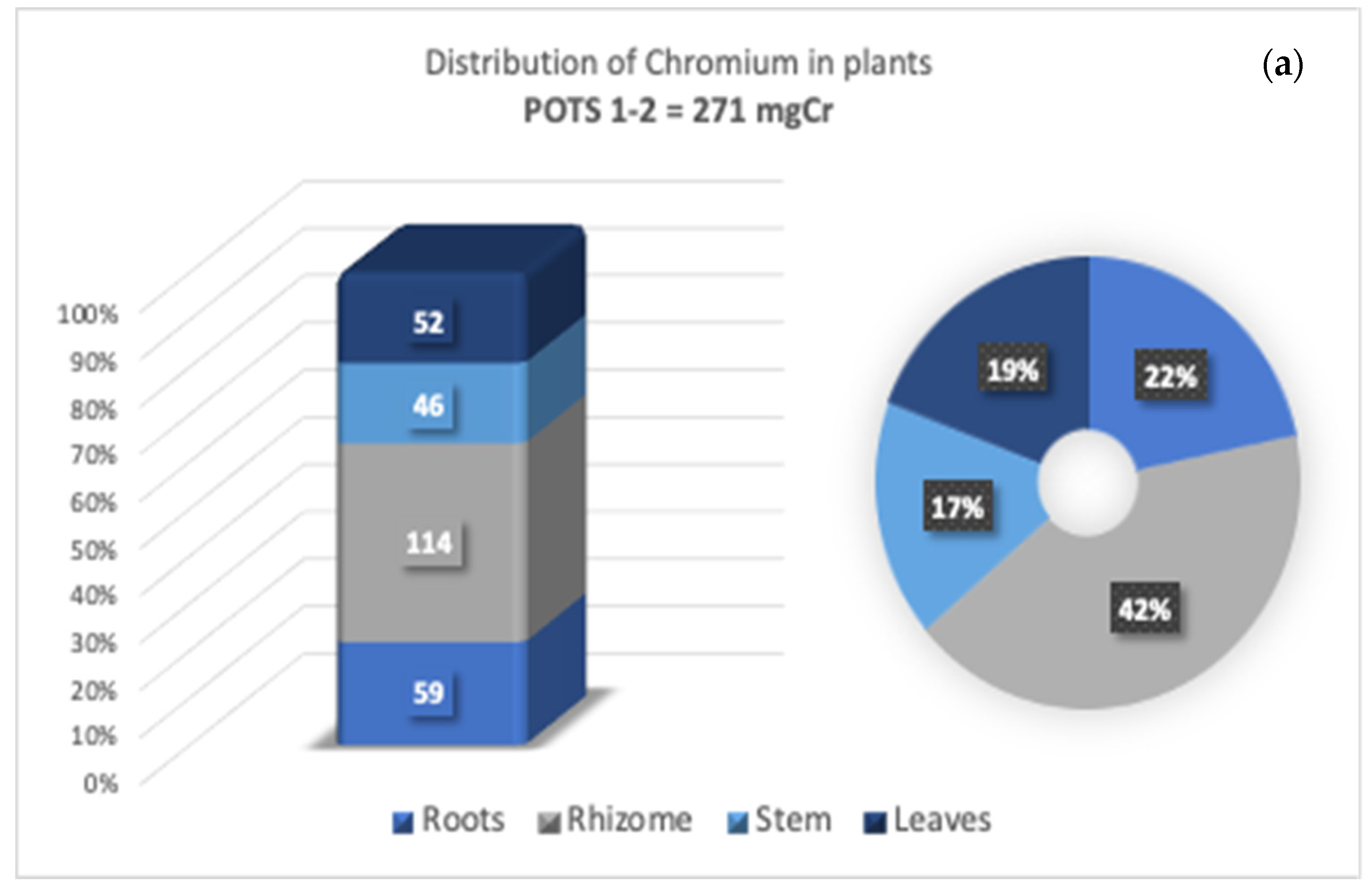
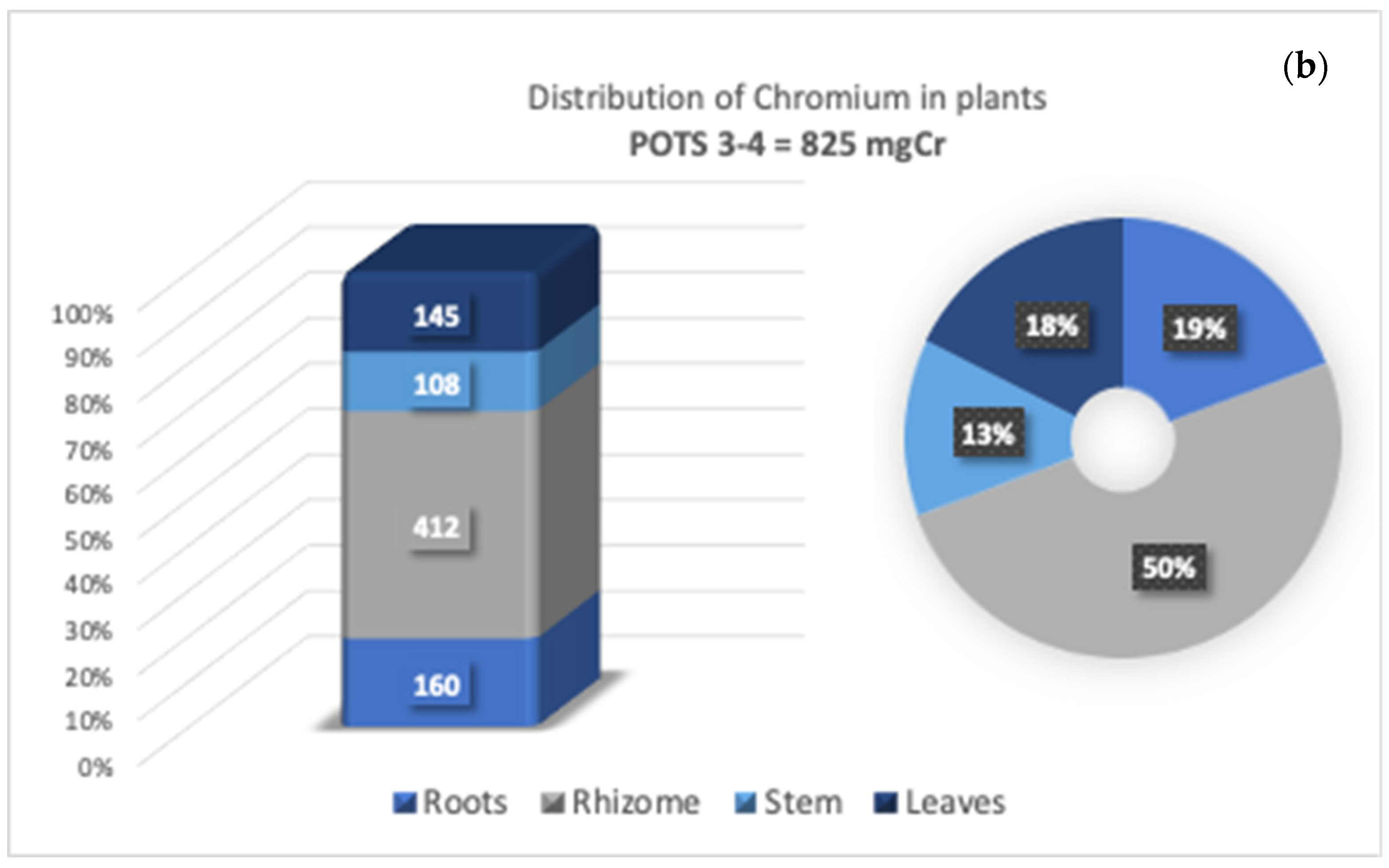
3.4. Cr Distribution in Tissues
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Chandra, R.; Saxena, G.; Kumar, V. Phytoremediation of environmental pollutants: An eco-sustainable green technology to environmental management. In Advances in Biodegradation and Bioremediation of Industrial Waste, 1st ed.; CRC Press LLC: Boca Raton, FL, USA; Taylor and Francis Group LLC: Boca Raton, FL, USA, 2015; pp. 1–30. [Google Scholar]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achmad, R.T.; Budiawan; Auerkari, E.I. Effects of Chromium on Human Body. Annu. Res. Rev. Biol. 2017, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cary, E.E. Chromium in air, soils, and natural waters. In Biological and Environmental Aspects of Chromium; Langard, S., Ed.; Elsevier Biomedical: New York, NY, USA, 1982; pp. 49–63. [Google Scholar]
- Ranieri, E. Chromium and nickel control in full- and small-scale subsuperficial flow constructed wetlands. Soil Sediment Contam. 2012, 21, 802–814. [Google Scholar] [CrossRef]
- Ranieri, E. Hydraulics of sub-superficial flow constructed wetlands in semi arid climate conditions. Water Sci. Technol. 2003, 47, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Das, P.K.; Das, B.P.; Dash, P. Green technology to limit the effects of hexavalent chromium contaminated water bodies on public health and vegetation at industrial sites. J. App. Bio. Biotech. 2021, 9, 28–35. [Google Scholar]
- Ranieri, E.; Tursi, A.; Giuliano, S.; Spagnolo, V.; Ranieri, A.C.; Petrella, A. Phytoextraction from Chromium-Contaminated Soil Using Moso Bamboo in Mediterranean Conditions. Water Air Soil Pollut. 2020, 231, 1–12. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Witkowicz, R.; Tabor, S. Using Jerusalem Artichoke to Extract Heavy Metals from Municipal Sewage Sludge Amended Soil. Pol. J. Environ. Stud. 2018, 27, 513–527. [Google Scholar] [CrossRef]
- Ullah, S.; Mahmood, S.; Ali, R.; Khan, M.R.; Akhtar, K.; Depar, N. Comparing chromium phyto-assessment in Brachiaria mutica and Leptochloa fusca growing on chromium polluted soil. Chemosphere 2021, 269, 128728. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.; Peczalski, R.; Andrieu, J. Optimization of crystalline powders vacuum contact drying with intermittent stirring. Chem. Eng. Res. Des. 2008, 86, 606–611. [Google Scholar] [CrossRef]
- Collin, B.; Doelsch, E.; Keller, C.; Panfili, F.; Meunier, J.D. Effects of silicon and copper on bamboo grown hydroponically. Environ. Sci. Pollut. R. 2013, 20, 6482–6495. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Peng, D.L.; Shafi, M.; Li, S.; Wu, J.S.; Ye, Z.Q.; Wang, Y.; Yan, W.B.; Liu, D. Phytoremediation Potential of Moso Bamboo (Phyllostachys pubescens) for Zinc and Ultrastructure Changes Under Zinc Toxicity. Russ. J. Ecol. 2015, 46, 444–449. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Islam, E.; Chen, J.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Lu, K. Lead accumulation and tolerance of Moso bamboo (Phyllostachys pubescens) seedlings: Applications of phytoremediation. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2015, 16, 123–130. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Wang, S.; Chen, T.; Liu, E.; Liu, C.P. Accessing the nursing behaviour of Moso bamboo (Phyllostachys edilus) on carbohydrates dynamics and photosystems. Sci. Rep. 2020, 10, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukina, A.; Boutin, C.; Rowland, O.; Carpenter, D. Evaluating trivalent chromium toxicity on wild terrestrial and wetland plants. Chemosphere 2016, 162, 355–364. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Kalčíková, G.; Zupančič, M.; Jemec, A.; Gotvajn, A.Ž. The impact of humic acid on chromium phytoextraction by aquatic macrophyte Lemna minor. Chemosphere 2016, 147, 311–317. [Google Scholar] [CrossRef]
- Arshad, M.; Silvestre, J.; Pinelli, E.; Kallerhoff, J.; Kaemmerer, M.; Tarigo, A.; Shahid, M.; Guiresse, M.; Pradere, P.; Dumat, C. A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere 2008, 71, 2187–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, S.; Bhatti, A.A.; Dar, Z.A.; Bhat, S.A. Phytoremediation of Heavy Metals: An Eco-Friendly and Sustainable Approach. Bioremed. Biotechnol. 2020, 215–231. [Google Scholar]
- Anderson, C.; Moreno, F.; Meech, J. A field demonstration of gold phytoextraction technology. Miner. Eng. 2005, 18, 385–392. [Google Scholar] [CrossRef]
- Cho-Ruk, K.; Kurukote, J.; Supprung, P.; Vetayasupo, S. Perennial Plants in the Phytoremediation of Lead-contaminated Soils. Biotechnology 2005, 5, 1–4. [Google Scholar] [CrossRef] [Green Version]
- McGrath, S.P.; Lombi, E.; Gray, C.W.; Caille, N.; Dunham, S.J.; Zhao, F.J. Field evaluation of Cd and Zn phytoextraction potential by the hyperaccumulators Thlaspi caerulescens and Arabidopsis helleri. Environ. Pollut. 2006, 141, 115–125. [Google Scholar] [CrossRef]
- Karimi, N.; Ghaderian, S.M.; Raab, A.; Feldmann, J.; Meharg, A. An arsenic-accumulating, hypertolerant brassica, Isatis capadocica: Rapid report. New Phytol. 2009, 184, 41–47. [Google Scholar] [CrossRef]
- Baker, A.; McGrath, S.; Reeves, R.; Smith, J. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In Phytoremediation of Contaminated Soils; Terry, N., Vangronsveld, J., Banuelos, G., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 85–107. [Google Scholar]
- López-Luna, J.; González-Chávez, M.; Esparza-García, F.; Rodríguez-Vázquez, R. Toxicity assessment of soil amended with tannery sludge, trivalent chromium and hexavalent chromium, using wheat, oat and sorghum plants. J. Hazard. Mater. 2009, 163, 829–834. [Google Scholar] [CrossRef]
- Kalimeris, A.; Ranieri, E.; Founda, D.; Norrant, D. Variability modes of precipitation along a Central Mediterranean area and their relations with ENSO, NAO, and other climatic patterns. Atmos. Res. 2017, 198, 56–80. [Google Scholar] [CrossRef]
- Ranieri, E.; Świetlik, J. DBPS control in European drinking water treatment plants using chlorine dioxide: Two case studies. J. Environ. Eng. Landsc. Manag. 2010, 18, 85–91. [Google Scholar] [CrossRef]
- Van Lienden, C.; Shan, L.; Rao, S.; Ranieri, E.; Young, T.M. Metals Removal from Stormwater by Commercial and Non-Commercial Granular Activated Carbons. Water Environ. Res. 2010, 82, 351–356. [Google Scholar] [CrossRef]
- Petrella, A.; Mascolo, G.; Murgolo, S.; Petruzzelli, V.; Ranieri, E.; Spasiano, D.; Petruzzelli, D. Photocatalytic Oxidation of Organic Micro-Pollutants: Pilot Plant Investigation and Mechanistic Aspects of the Degradation Reaction. Chem. Eng. Commun. 2016, 203, 1298–1307. [Google Scholar] [CrossRef]
- Ciudin, R.; Isarie, C.; Cioca, L.; Petrescu, V.; Nederita, V.; Ranieri, E. Vacuum waste collection system for an historical city centre. Sci. Bull. 2014, 76, 215–222. [Google Scholar]
- Ranieri, E.; Rada, E.C.; Ragazzi, M.; Masi, S.; Montanaro, C. Critical analysis of the integration of residual municipal solid waste incineration and selective collection in two Italian tourist areas. Waste Manag. Res. 2014, 32, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; Gikas, P. Effects of Plants for Reduction and Removal of Hexavalent Chromium from a Contaminated Soil. Water Air Soil Pollut. 2014, 225, 1–9. [Google Scholar] [CrossRef]
- Al-Bataina, B.B.; Young, T.M.; Ranieri, E. Effects of compost age on the release of nutrients. Int. Soil Water Conserv. Res. 2016, 4, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Petrella, A.; Petruzzelli, V.; Ranieri, E.; Catalucci, V.; Petruzzelli, D. Sorption of Pb(II), Cd(II) and Ni(II) from single- and multimetal solutions by recycled waste porous glass. Chem. Eng. Commun. 2016, 203, 940–947. [Google Scholar] [CrossRef]
- Gorgoglione, A.; Gioia, A.; Iacobellis, V.; Piccinni, A.F. A Rationale for Pollutograph Evaluation in Ungauged Areas, Using Daily Rainfall Patterns: Case Studies of the Apulian Region in Southern Italy. Appl. Environ. Soil Sci. 2016, 2016, 9327614. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G.; Ranieri, E.; Torretta, V. Process enhancement for maximization of methane production in codigestion biogas plants. Manag. Environ. Qual. Int. J. 2016, 27, 289–298. [Google Scholar] [CrossRef]
- Ranieri, E.; Moustakas, K.; Barbafieri, M.; Ranieri, A.C.; Herrera-Melián, J.A.; Petrella, A.; Tommasi, F. Phytoextraction technologies for mercury- and chromium-contaminated soil: A review. J. Chem. Technol. Biotechnol. 2020, 95, 317–327. [Google Scholar] [CrossRef]
- Bosire, G.O. Rehabilitation and Phytoremediation of Heavy Metal Polluted Riverine Wetlands Using Bamboo for Phytoextraction in Kibera. Ph.D. Thesis, Kenyatta University, Nairobi, Kenya, 2014. Available online: http://ir-library.ku.ac.ke/handle/123456789/9256 (accessed on 1 November 2021).
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo—An untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere 2020, 246, 125750. [Google Scholar] [CrossRef]
- Zachariah, E.J.; Sabulal, B.; Nair, D.N.K.; Johnson, A.J.; Kumar, C.S.P.; Elavinamannil, Z. Carbon dioxide emission from bamboo culms. Plant Biol. 2016, 18, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, G.; Jiang, P.; Wu, J.; Lin, L. Carbon Accumulation and Carbon Forms in Tissues During the Growth of Young Bamboo (Phyllostachy pubescens). Bot. Rev. 2011, 77, 278–286. [Google Scholar] [CrossRef]
- Zhou, G.; Meng, C.; Jiang, P.; Xu, Q. Review of Carbon Fixation in Bamboo Forests in China. Bot. Rev. 2011, 77, 262–270. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Zhou, G.; Gu, H.; Li, Q.; Zhang, C. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wong, M.; Yang, J.; Ye, Z.; Jiang, P.; Zheng, S. Dynamics of Carbon Accumulation During the Fast Growth Period of Bamboo Plant. Bot. Rev. 2011, 77, 287–295. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Zhou, G.; Jiang, H.; Wang, W.F.; Xiang, W.H. Climate warming-induced upward shift of Moso bamboo population on Tianmu Mountain, China. J. Mt. Sci. 2013, 10, 363–369. [Google Scholar] [CrossRef]
- Were, F.H.; Wafula, G.A.; Wairungu, S. Phytoremediation Using Bamboo to Reduce the Risk of Chromium Exposure from a Contaminated Tannery Site in Kenya. J. Health Pollut. 2017, 7, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Pulford, I.D.; Riddell-Black, D.; Stewart, C. Heavy metal uptake by willow clones from sewage sludge-treated soil: The potential for phytoremediation. Int. J. Phytoremediat. 2002, 4, 59–72. [Google Scholar] [CrossRef]
- Purdy, J.J.; Smart, L.B. Hydroponic screening of shrub willow (Salix spp.) for arsenic tolerance and uptake. Int. J. Phytoremediat. 2008, 10, 515–528. [Google Scholar] [CrossRef]
- Melo, È.E.C.; Nascimento, C.W.A.; Accioly, A.M.; Santos, A.C.Q. Phytoextraction and fractionation of heavy metals in soil after multiple applications of natural chelants. Sci. Agric. 2008, 65, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Shelmerdine, P.A.; Black, C.R.; McGrath, S.P.; Young, S.D. Modelling phytoremediation by the hyperaccumulating fern, Pteris vittata, of soils historically contaminated with arsenic. Environ. Pollut. 2009, 157, 1589–1596. [Google Scholar] [CrossRef]
- Gadd, G.M. Phytoremediation of toxic metals; using plants to clean up the environment. Edited by ilya raskin and burt D ensley, john wiley sons, inc, New York, 2000, 304 pp, price UK£ 58.80, ISBN 0 471 19254 6. J. Chem. Technol. Biotechnol. 2001, 76, 325. [Google Scholar] [CrossRef]
- De Carvalho, T.S.D.A.; de Santana Santos, T.; Pestana, E.M.; Souza, F.N.; Lage, V.M.G.B.; Nunesmaia, B.J.B.; Sena, P.T.S.; Mariano-Neto, E.; da Silva, E.M. Natural humic substances effects on the life history traits of Latonopsis australis SARS (1888) (Cladocera–Crustacea). Chemosphere 2015, 120, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Shehata, S.M.; Badawy, R.K.; Aboulsoud, Y.I.E. Phytoremediation of some heavy metals in contaminated soil. Bull. Natl. Res. Cent. 2019, 43, 189. [Google Scholar] [CrossRef] [Green Version]
- Sajad, M.A.; Khan, M.S.; Bahadur, S.; Naeem, A.; Ali, H.; Batool, F.; Shuaib, M.; Batool, S. Evaluation of chromium phytoremediation potential of some plant species of Dir Lower, Khyber Pakhtunkhwa, Pakistan. Acta Ecol. Sin. 2020, 40, 158–165. [Google Scholar] [CrossRef]
- Gopal, R.; Rizvi, A.H.; Nautiyal, N. Chromium alters iron nutrition and water relations of spinach. J. Plant Nutr. 2009, 32, 1551–1559. [Google Scholar] [CrossRef]
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water rela-tion, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 2007, 68, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Awalla, O. Phytoremediation of sewage sludge in soils contaminated with heavy metals. Glob. J. Environ. Sci. 2013, 12, 13–19. [Google Scholar]
- Shahid, M.; Rafiq, S.S.M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Yen, T.-M. Culm height development, biomass accumulation and carbon storage in an initial growth stage for a fast-growing moso bamboo (Phyllostachy pubescens). Bot. Stud. 2016, 57, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.; Tiwari, M.; Dutta, P.; Singh, P.; Chawda, K.; Kumari, M.; Chakrabarty, D. Chromium Stress in Plants: Toxicity, Tolerance and Phytoremediation. Sustainability 2021, 13, 4629. [Google Scholar] [CrossRef]
- Mohanty, M.; Patra, H.K. Phytoassessment of in situ weed diversity for their chromium distribution pattern and ac-cumulation indices of abundant weeds at South Kaliapani chromite mining area with their phytoremediation prospective. Ecotoxicol. Environ. Saf. 2020, 194, 110399. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranieri, E.; D’Onghia, G.; Ranieri, F.; Petrella, A.; Spagnolo, V.; Ranieri, A.C. Phytoextraction of Cr(VI)-Contaminated Soil by Phyllostachys pubescens: A Case Study. Toxics 2021, 9, 312. https://doi.org/10.3390/toxics9110312
Ranieri E, D’Onghia G, Ranieri F, Petrella A, Spagnolo V, Ranieri AC. Phytoextraction of Cr(VI)-Contaminated Soil by Phyllostachys pubescens: A Case Study. Toxics. 2021; 9(11):312. https://doi.org/10.3390/toxics9110312
Chicago/Turabian StyleRanieri, Ezio, Gianfranco D’Onghia, Francesca Ranieri, Andrea Petrella, Vincenzo Spagnolo, and Ada Cristina Ranieri. 2021. "Phytoextraction of Cr(VI)-Contaminated Soil by Phyllostachys pubescens: A Case Study" Toxics 9, no. 11: 312. https://doi.org/10.3390/toxics9110312
APA StyleRanieri, E., D’Onghia, G., Ranieri, F., Petrella, A., Spagnolo, V., & Ranieri, A. C. (2021). Phytoextraction of Cr(VI)-Contaminated Soil by Phyllostachys pubescens: A Case Study. Toxics, 9(11), 312. https://doi.org/10.3390/toxics9110312







