Influence of Glyphosate Formulations on the Behavior of Sulfentrazone in Soil in Mixed Applications
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents for the Laboratory Tests
2.2. Soil Collecting
2.3. Sorption and Desorption Experiment of Sulfentrazone Alone and Mixed with Glyphosate Formulations
2.3.1. Experimental Design
2.3.2. Determination of the Equilibrium Time
2.3.3. Sulfentrazone Sorption
2.3.4. Sulfentrazone Desorption
2.4. Leaching Experiment of Sulfentrazone Isolated and Mixed with Glyphosate Formulations
2.4.1. Experimental Design
2.4.2. Preparation of the Columns
2.4.3. Herbicide Application and Rain Simulation
2.4.4. Method of Extraction for Sulfentrazone
2.4.5. Validation of the Quantification and Extraction Method
2.4.6. Chromatographic Conditions
2.5. Statistical Analysis of the Experiments
3. Results and Discussion
3.1. Adsorption Kinetics of Sulfentrazone Isolated and Mixed with Glyphosate Formulations
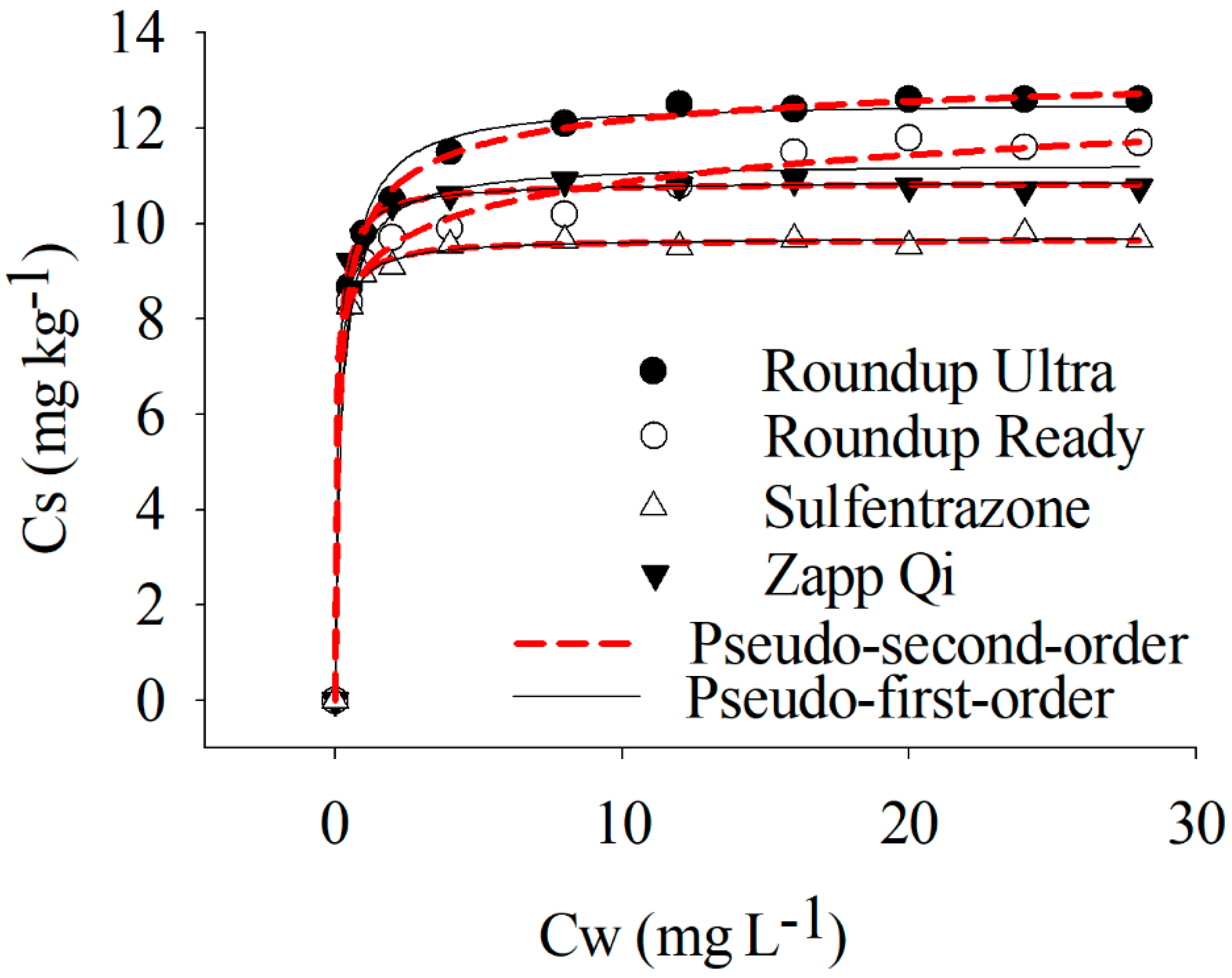
3.2. Sorption and Desorption Isotherms of Sulfentrazone Alone and Mixed with Glyphosate Formulations
3.3. Estimated Leaching of Sulfentrazone Applied Alone and Mixed with Glyphosate Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Law, S.E. Agricultural electrostatic spray application: A review of significant research and development during the 20th century. J. Electrost. 2001, 51, 25–42. [Google Scholar] [CrossRef]
- Silva, A.D.; Ferreira, F.A.; Ferreira, L.R. Herbicidas: Classificação e mecanismo de ação. In Tópicos em Manejo de Plantas Daninhas; Universidade Federal de Viçosa: Viçosa, Brazil, 2013; Volume 2, pp. 58–117. [Google Scholar]
- Silva, T.S.; Souza, M.D.F.; Teófilo, T.M.D.S.; Dos Santos, M.S.; Porto, M.A.F.; Souza, C.M.M.; Dos Santos, J.B.; Silva, D.V. Use of neural networks to estimate the sorption and desorption coefficients of herbicides: A case study of diuron, hexazinone, and sulfometuron-methyl in Brazil. Chemosphere 2019, 236, 124333. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S.-M. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Di Marsico, A.; Scrano, L.; Amato, M.; Gámiz, B.; Real, M.; Cox, L. Mucilage from seeds of chia (Salvia hispanica L.) used as soil conditioner; effects on the sorption-desorption of four herbicides in three different soils. Sci. Total Environ. 2018, 625, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Azcarate, M.P.; Montoya, J.C.; Koskinen, W.C. Sorption, desorption and leaching potential of sulfonylurea herbicides in Argentinean soils. J. Environ. Sci. Health Part B 2015, 50, 229–237. [Google Scholar] [CrossRef]
- Braga, R.R.; Dos Santos, J.B.; Zanuncio, J.C.; Bibiano, C.S.; Ferreira, E.A.; Oliveira, M.C.; Silva, D.V.; Serrão, J.E. Effect of growing Brachiria brizantha on phytoremediation of picloram under different pH environments. Ecol. Eng. 2016, 94, 102–106. [Google Scholar] [CrossRef]
- Faria, A.T.; Souza, M.D.F.; Passos, A.B.R.D.J.; Da Silva, A.A.; Silva, D.V.; Zanuncio, J.C.; Rocha, P.R.R. Tebuthiuron leaching in three Brazilian soils as affected by soil pH. Environ. Earth Sci. 2018, 77, 214. [Google Scholar] [CrossRef]
- Neto, M.D.D.C.; Souza, M.D.F.; Silva, D.V.; Faria, A.T.; Da Silva, A.A.; Pereira, G.A.M.; De Freitas, M.A.M. Leaching of imidazolinones in soils under a clearfield system. Arch. Agron. Soil Sci. 2016, 63, 897–906. [Google Scholar] [CrossRef]
- Gazziero, D.L.P. Mixtures of pesticides in tank, in Brazilian farms. Planta Daninha 2015, 33, 83–92. [Google Scholar] [CrossRef]
- AGROFIT. Sistema de Agrotóxicos Fitossanitários. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 23 November 2017).
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sulfentrazone#section=Top (accessed on 1 October 2018).
- De Carvalho, F.T.; Cavazzana, M.A. Eficácia de herbicidas no manejo de plantas daninhas para o plantio direto de soja. Rev. Bras. Herbic. 2000, 1, 167. [Google Scholar] [CrossRef][Green Version]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária Centro Nacional de Pesquisa de Solos. Manual de Métodos de Análise de Solo, 2th ed.; EMBRAPA: Rio de Janeiro, Brazil, 1997; 212p. [Google Scholar]
- OECD—Organization for Economic Co-Operation and Development. OECD—Guidelines for Testing of Chemicals, Adsorption, 106; OECD: Paris, France, 2000. [Google Scholar]
- Peruchi, L.M.; Fostier, A.H.; Rath, S. Sorption of norfloxacin in soils: Analytical method, kinetics and Freundlich isotherms. Chemosphere 2015, 119, 310–317. [Google Scholar] [CrossRef]
- Costa, A.I.G.; Queiroz, M.E.L.R.; Neves, A.A.; De Assis, R.C.; Dos Soares, C.E.S.; Da Silva, A.A.; D’Antonino, L.; De Oliveira, A.F.; Bellato, C.R. Mobility and persistence of the herbicide fomesafen in soils cultivated with bean plants using SLE/LTP and HPLC/DAD. Environ. Sci. Pollut. Res. 2014, 22, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- European Union. Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed. Document n. SANCO/12495/2011; Supersedes Document n. Sanco/10684/2009; Implemented by 01/01/2012. Available online: https://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_Sanco_2011_12495.pdf (accessed on 12 January 2017).
- Ribani, M.; Bottoli, C.B.G.; Collins, C.H.; Jardim, I.C.L.F.; Melo, L.F.C. Validação em métodos cromatográficos e eletroforéticos. Quím. Nova 2004, 27, 771–780. [Google Scholar] [CrossRef]
- INMETRO. Orientações sobre Validação de Métodos de Ensaios Químicos; DOQ-CGCRE-008; Instituto Nacional de Metrologia, Normalização e Qualidade Industrial: Rio de Janeiro, Brazil, 2003.
- Ohmes, G.A.; Mueller, T.C. Liquid Chromatographic Determination of Sulfentrazone in Soil. J. AOAC Int. 1999, 82, 1214–1216. [Google Scholar] [CrossRef]
- Passos, A.B.R.; Freitas, M.A.M.; Torres, L.G.; Silva, A.A.; Queiroz, M.E.L.; Lima, C.F. Sorption and desorption of sulfentrazone in Brazilian soils. J. Environ. Sci. Health Part B 2013, 48, 646–650. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete sample). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Bartlett, M.S. An Inverse Matrix Adjustment Arising in Discriminant Analysis. Ann. Math. Stat. 1951, 22, 107–111. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Liu, Y.; Yu, J.; Yi, H.; Ye, S.; Deng, R. Sorption, transport and biodegradation—An insight into bioavailability of persistent organic pollutants in soil. Sci. Total Environ. 2018, 610, 1154–1163. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Loewy, R.M. Characterization of soil organic matter by FT-IR spectroscopy and its relationship with chlorpyrifos sorption. J. Environ. Manag. 2017, 196, 316–322. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H.; Sheng, J.; Zhao, Z. Adsorption kinetics of pesticide in soil assessed by optofluidics-based biosensing platform. Chemosphere 2015, 120, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Wu, X.; Gui, W.; Zhu, G. Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. J. Hazard. Mater. 2010, 178, 462–468. [Google Scholar] [CrossRef]
- Mirzaei, A.; Ebadi, A.; Khajavi, P. Kinetic and equilibrium modeling of single and binary adsorption of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) onto nano-perfluorooctyl alumina. Chem. Eng. J. 2013, 231, 550–560. [Google Scholar] [CrossRef]
- Jiang, X.; Ouyang, Z.; Zhang, Z.; Yang, C.; Li, X.; Dang, Z.; Wu, P. Mechanism of glyphosate removal by biochar supported nano-zero-valent iron in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 64–72. [Google Scholar] [CrossRef]
- Mendes, K.F.; Dos Reis, M.R.; Inoue, M.H.; Pimpinato, R.F.; Tornisielo, V.L. Sorption and desorption of mesotrione alone and mixed with S-metolachlor+terbuthylazine in Brazilian soils. Geoderma 2016, 280, 22–28. [Google Scholar] [CrossRef]
- Munira, S.; Farenhorst, A.; Akinremi, W. Phosphate and glyphosate sorption in soils following long-term phosphate applications. Geoderma 2018, 313, 146–153. [Google Scholar] [CrossRef]
- Grey, T.L.; Walker, R.H.; Wehtje, G.R.; Adams, J. Behavior of sulfentrazone in ionic exchange resins, electrophoresis gels, and cation-saturated soils. Weed Sci. 2000, 48, 239–247. [Google Scholar] [CrossRef]
- Alves, P.L.C.A.; Marques, J., Jr.; Ferraudo, A.S. Soil attributes and efficiency of sulfentrazone on control of purple nutsedge (Cyperus rotundus L.). Sci. Agric. 2004, 61, 319–325. [Google Scholar] [CrossRef]
- Gehrke, V.R.; Camargo, E.R.; Avila, L.A. Sulfentrazone: Environmental Dynamics and Selectivity. Planta Daninha 2020, 38. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-83582020000100401&lng=en&nrm=iso (accessed on 20 November 2020). [CrossRef]
- Radian, A.; Fichman, M.; Mishael, Y. Modeling binding of organic pollutants to a clay–polycation adsorbent using quantitative structural–activity relationships (QSARs). Appl. Clay Sci. 2015, 116, 241–247. [Google Scholar] [CrossRef]
- López-Cabeza, R.; Gámiz, B.; Cornejo, J.; Celis, R. Behavior of the enantiomers of the herbicide imazaquin in agricultural soils under different application regimes. Geoderma 2017, 293, 64–72. [Google Scholar] [CrossRef]
- Hall, K.E.; Ray, C.; Ki, S.J.; Spokas, K.; Koskinen, W.C. Pesticide sorption and leaching potential on three Hawaiian soils. J. Environ. Manag. 2015, 159, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lin, J.L.; Jia, L.X.; Liu, Y.; Pan, B.; Yang, Y.; Lin, Y. Effects of two different organic amendments addition to soil on sorption–desorption, leaching, bioavailability of penconazole and the growth of wheat (Triticum aestivum L.). J. Environ. Manage. 2016, 167, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Arroyave, J.M.; Waiman, C.V.; Zanini, G.P.; Avena, M. Effect of humic acid on the adsorption/desorption behavior of glyphosate on goethite. Isotherms and kinetics. Chemosphere 2016, 145, 34–41. [Google Scholar] [CrossRef]
- Philipp, D.M.; Watson, M.A.; Yu, H.S.; Steinbrecher, T.B.; Bochevarov, A.D. Quantum chemical pKa prediction for complex organic molecules. Int. J. Quantum Chem. 2018, 118, e25561. [Google Scholar] [CrossRef]
- Servien, R.; Mamy, L.; Li, Z.; Rossard, V.; Latrille, E.; Bessac, F.; Patureau, D.; Benoit, P. TyPol—A new methodology for organic compounds clustering based on their molecular characteristics and environmental behavior. Chemosphere 2014, 111, 613–622. [Google Scholar] [CrossRef]
- Davey, R.J.; Back, K.R.; Sullivan, R.A. Crystal nucleation from solutions–transition states, rate determining steps and complexity. Faraday Discuss. 2015, 179, 9–26. [Google Scholar] [CrossRef]
- Vasileiadis, M.; Pantelides, C.C.; Adjiman, C.S. Prediction of the crystal structures of axitinib, a polymorphic pharmaceutical molecule. Chem. Eng. Sci. 2015, 121, 60–76. [Google Scholar] [CrossRef]
- Barriuso, E.; Laird, D.A.; Koskinen, W.C.; Dowdy, R.H. Atrazine Desorption from Smectites. Soil Sci. Soc. Am. J. 1994, 58, 1632–1638. [Google Scholar] [CrossRef]
- Khan, M.A.; Brown, C.D. Influence of commercial formulation on leaching of four pesticides through soil. Sci. Total Environ. 2016, 573, 1573–1579. [Google Scholar] [CrossRef]
- Cederlund, H.; Börjesson, E.; Stenström, J. Effects of a wood-based biochar on the leaching of pesticides chlorpyrifos, diuron, glyphosate and MCPA. J. Environ. Manage. 2017, 191, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Brown, C.D. Influence of commercial formulation on the sorption and leaching behaviour of propyzamide in soil. Sci. Total Environ. 2017, 578, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.N.; Locke, M.A. Sulfentrazone sorption, desorption, and mineralization in soils from two tillage systems. Weed Sci. 1998, 46, 494–500. [Google Scholar] [CrossRef]

| Sand (%) | Silt (%) | Clay (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 76 | 7 | 17 | ||||||||
| pH | P | K+ | Ca²+ | Mg²+ | Al3+ | H + Al | SB | CEC (t) | OM | |
| H2O | --------------------------------------- cmolc dm−3 --------------------------------------------------------- | % | ||||||||
| 4.7 | 2.3 | 41 | 2.2 | 0.7 | 0.2 | 5.61 | 3.0 | 3.2 | 2.5 | |
| Kinetic Model | Parameters | |||
|---|---|---|---|---|
| Sulfentrazone | Sulfentrazone + Roundup Ready® | Sulfentrazone + Roundup Ultra® | Sulfentrazone + Zapp Qi® | |
| Pseudo 1st order | ** qe: 9.63 mg | ** qe: 11.67 mg** | *** qe: 12.98 mg | ** qe: 10.86 mg |
| * k1: 4.24 min−1 | *** k1: 2.70 min−1 | ** k1: 4.02 min−1 | ** k1: 4.17 min−1 | |
| R2: 0.99 | R2: 0.99 | R2: 0.97 | R2: 0.99 | |
| Pseudo 2nd order | * qe: 9.22 mg | ** qe: 11.02 mg | ** qe: 12.92 mg | ** qe: 10.33 |
| ** k2: 2.06 g mg−1 min | ** k2: 1.67 g mg−1 min | ** k2: 1.92 g mg−1 min | *** k2: 2.04 g mg−1 min | |
| R2: 0.96 | R2: 0.96 | R2: 0.99 | R2: 0.99 | |
| Herbicides | Parameters | Estimative | Std. Error | a RMSE | Rd |
|---|---|---|---|---|---|
| Sulfentrazone | Kfs | 1.3 *** | ±0.1 | 0.05 | - |
| 1/n | 0.8 *** | ±0.1 | |||
| Sulfentrazone + Roundup Ready® | Kfs | 2.1 * | ±0.4 | 0.06 | 1.65 |
| 1/n | 0.8 ** | ±0.1 | |||
| Sulfentrazone + Roundup Ultra® | Kfs | 2.3 *** | ±0.1 | 0.05 | 1.76 |
| 1/n | 1.0 *** | ±0.0 | |||
| Sulfentrazone + Zapp Qi® | Kfs | 1.9 * | ±0.5 | 0.03 | 1.46 |
| 1/n | 0.9 ** | ±0.1 |
| Property Name | Formulations | ||
|---|---|---|---|
| Roundup Ultra | Roundup Ready | Zapp Qi | |
| Salt | Glyphosate ammonium | Isopropylamine salt | Potassium |
| Molecular weight (g mol−1) | 186.1 | 346.4 | 207.1 |
| Hydrogen bond donor count | 4 | 4 | 3 |
| Hydrogen bond acceptor count | 6 | 6 | 6 |
| Rotatable bond count | 4 | 2 | 4 |
| Parameters | Estimative | Std. Error | a RSME | H | |
|---|---|---|---|---|---|
| Sulfentrazone | Kfd | 65.7 ** | 5.3 | 0.03 | 0.88 |
| 1/n | 0.7 * | 0.1 | |||
| Sulfentrazone + Roundup Ready | Kfd | 125.2 * | 8.7 | 0.06 | 0.88 |
| 1/n | 0.7 * | 0.1 | |||
| Sulfentrazone + Roundup Ultra | Kfd | 733.3 *** | 6.4 | 0.07 | 0.90 |
| 1/n | 0.9 ** | 0.1 | |||
| Sulfentrazone + Zapp Qi | Kfd | 239.8 * | 4.2 | 0.04 | 0.89 |
| 1/n | 0.8 * | 0.1 |
| Depth | Sulfentrazone | Sulfentrazone + Roundup Ready | Sulfentrazone + Roundup Ultra | Sulfentrazone + Zapp Qi |
|---|---|---|---|---|
| mg kg−1 | ||||
| 0–5 | 0.62 ± 0.04 | 1.10 ± 0.04 | 1.25 ± 0.04 | 0.92 ± 0.03 |
| 5–10 | 0.50 ± 0.03 | 0.55 ± 0.02 | 0.25 ± 0.04 | 0.60 ± 0.02 |
| 10–15 | 0.35 ± 0.02 | 0.15 ± 0.02 | - | 0.35 ± 0.02 |
| 15–20 | - | 0.20 ± 0.02 | - | - |
| 20–25 | - | 0.20 ± 0.02 | - | - |
| Total | 1.47 ± 0.03 | 2.20 ± 0.02 | 1.5 ± 0.04 | 1.87 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langaro, A.C.; Souza, M.d.F.; Pereira, G.A.M.; Barros, J.P.A.; Silva, A.A.d.; Silva, D.V.; Passos, A.B.R.d.J.; Mendonça, V. Influence of Glyphosate Formulations on the Behavior of Sulfentrazone in Soil in Mixed Applications. Toxics 2020, 8, 123. https://doi.org/10.3390/toxics8040123
Langaro AC, Souza MdF, Pereira GAM, Barros JPA, Silva AAd, Silva DV, Passos ABRdJ, Mendonça V. Influence of Glyphosate Formulations on the Behavior of Sulfentrazone in Soil in Mixed Applications. Toxics. 2020; 8(4):123. https://doi.org/10.3390/toxics8040123
Chicago/Turabian StyleLangaro, Ana Cláudia, Matheus de Freitas Souza, Gustavo Antônio Mendes Pereira, João Pedro Ambrósio Barros, Antonio Alberto da Silva, Daniel Valadão Silva, Ana Beatriz Rocha de Jesus Passos, and Vander Mendonça. 2020. "Influence of Glyphosate Formulations on the Behavior of Sulfentrazone in Soil in Mixed Applications" Toxics 8, no. 4: 123. https://doi.org/10.3390/toxics8040123
APA StyleLangaro, A. C., Souza, M. d. F., Pereira, G. A. M., Barros, J. P. A., Silva, A. A. d., Silva, D. V., Passos, A. B. R. d. J., & Mendonça, V. (2020). Influence of Glyphosate Formulations on the Behavior of Sulfentrazone in Soil in Mixed Applications. Toxics, 8(4), 123. https://doi.org/10.3390/toxics8040123





