Endocrine Disruption: Structural Interactions of Androgen Receptor against Di(2-ethylhexyl) Phthalate and Its Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Preparation
2.2. Ligand Preparation
2.3. Induced Fit Docking
2.4. Binding Affinity Calculations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate exposure and long-term epigenomic consequences: A review. Front. Genet. 2020, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Draft Scope of the Risk Evaluation for Di-ethylhexyl Phthalate (1,2-Benzenedicarboxylic acid, 1,2-bis(2-ethylhexyl) ester). The United States Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention (OCSPP), Office of Pollution Prevention and Toxics (OPPT). EPA Document# EPA-740-D-20-017. 2020; pp. 1–113. Available online: https://www.epa.gov/sites/production/files/2020-04/documents/casrn_117-81-7-diethylhexyl_phthalate_draft_scope_4-15-2020.pdf (accessed on 7 December 2020).
- Yuan, X.Q.; Du, Y.Y.; Liu, C.; Guo, N.; Teng, X.M.; Hua, X.; Yao, Y.C.; Deng, Y.L.; Zeng, Q.; Deng, T.R.; et al. Phthalate metabolites and biomarkers of oxidative stress in the follicular fluid of women undergoing in vitro fertilization. Sci. Total Environ. 2020, 738, 139834. [Google Scholar] [CrossRef] [PubMed]
- Plastics Insight. Global Plasticizers Market. 2016. Available online: https://www.plasticsinsight.com/global-plasticisers-market/ (accessed on 7 December 2020).
- Ceresana. Global demand for plasticizers continues to rise. Add. Polym. 2017, 10, 10–11. [Google Scholar]
- Guo, Y.; Kannan, K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef]
- Balalian, A.A.; Whyatt, R.M.; Liu, X.; Insel, B.J.; Rauh, V.A.; Herbstman, J.; Factor-Litvak, P. Prenatal and childhood exposure to phthalates and motor skills at age 11 years. Environ. Res. 2020, 171, 416–427. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency; Office of Chemical Safety and Pollution Prevention. Proposed Designation of di-ethylhexyl Phthalate (DEHP) (1,2-Benzene-dicarboxylic acid, 1,2-bis (2-ethylhexyl) Ester) (CASRN 117-81-7) as a High-Priority Substance for Risk Evaluation. 2019; pp. 1–59. Available online: https://www.epa.gov/sites/production/files/2019-08/documents/di-ethylhexyl_phthalate_117-81-7_proposeddesignation_082219.pdf (accessed on 7 December 2020).
- International Agency for Research on Cancer. Di(2-ethylhexyl)phthalate. In Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 101. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2013; pp. 149–284. Available online: https://publications.iarc.fr/125 (accessed on 7 December 2020).
- European Communities. Commission Directive 2001/59/EC of 6 August 2001 adapting to technical progress for the 28th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. Off. J. Eur. Communities 2001, L225, 1–333. [Google Scholar]
- Japan Society for Occupational Health. Occupational exposure limit. Sangyo Eiseigaku Zasshi 2015, 57, 270–274. (In Japanese) [Google Scholar]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical review on the presence of phthalates in food and evidence of their biological impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, F.; Seiler, F.; Rapp, D.; Eckert, E.; Müller, J.; Metz, C.; Bals, R.; Drexler, H.; Lepper, P.M.; Göen, T. Exposure of patients to di(2-ethylhexy)phthalate (DEHP) and its metabolite MEHP during extracorporeal membrane oxygenation (ECMO) therapy. PLoS ONE 2020, 15, e0224931. [Google Scholar] [CrossRef]
- Apel, P.; Kortenkamp, A.; Koch, H.M.; Vogel, N.; Rüther, M.; Kasper-Sonnenberg, M.; Conrad, A.; Brüning, T.; Kolossa-Gehring, M. Time course of phthalate cumulative risks to male developmental health over a 27-year period: Biomonitoring samples of the German Environmental Specimen Bank. Environ. Int. 2020, 137, 105467. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Bolt, H.M.; Angerer, J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch. Toxicol. 2004, 78, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Lindh, C.H.; Jönsson, B.A.; Giwercman, A. Prenatal phthalate exposure and reproductive function in young men. Environ. Res. 2015, 138, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Bolt, H.M.; Preuss, R.; Angerer, J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol. 2005, 79, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Strømmen, K.; Lyche, J.L.; Blakstad, E.W.; Moltu, S.J.; Veierød, M.B.; Almaas, A.N.; Sakhi, A.K.; Thomsen, C.; Nakstad, B.; Brække, K.; et al. Increased levels of phthalates in very low birth weight infants with septicemia and bronchopulmonary dysplasia. Environ. Int. 2016, 89–90, 228–234. [Google Scholar] [CrossRef]
- Stroustrup, A.; Bragg, J.B.; Busgang, S.A.; Andra, S.S.; Curtin, P.; Spear, E.A.; Just, A.C.; Arora, M.; Gennings, C. Sources of clinically significant neonatal intensive care unit phthalate exposure. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 137–148. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, L.X.; Gu, Y.P.; Wang, J.Y.; Zhang, Y.H.; Song, W.M. Levels of environmental endocrine disruptors in umbilical cord blood and maternal blood of low-birth-weight infants. Zhonghua Yu Fang Yi Xue Za Zhi 2008, 42, 177–180. [Google Scholar]
- Huang, P.C.; Kuo, P.L.; Chou, Y.Y.; Lin, S.J.; Lee, C.C. Association between prenatal exposure to phthalates and the health of newborns. Environ. Int. 2009, 35, 14–20. [Google Scholar] [CrossRef]
- Krotz, S.P.; Carson, S.A.; Tomey, C.; Buster, J.E. Phthalates and bisphenol do not accumulate in human follicular fluid. J. Assist. Reprod. Genet. 2012, 29, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, J.; Hanberg, A.; Berglund, M.; Skerfving, S.; Remberger, M.; Calafat, A.M.; Filipsson, A.F.; Jansson, B.; Johansson, N.; Appelgren, M.; et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 2008, 116, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Kavlock, R.; Boeckelheide, K.; Chapin, R.; Cunningham, M.; Faustman, E.; Foster, P.; Golub, M.; Henderson, R.; Hinberg, I.; Little, R.; et al. NTP Center for the Evaluation of Risks to Human Reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl)phthalate. Reprod. Toxicol. 2002, 16, 529–653. [Google Scholar] [CrossRef]
- Regnier, J.; Bowden, C.; Lhuguenot, J. Effects on rat embryonic development in vitro of di-(2-ethylhexyl) phthalate (DEHP) and its metabolites. Toxicologist 2004, 78, 38–39. [Google Scholar]
- Swan, S.H.; Sathyanarayana, S.; Barrett, E.S.; Janssen, S.; Liu, F.; Nguyen, R.H.; Redmon, J.B.; TIDES Study Team. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod. 2015, 30, 963–972. [Google Scholar] [CrossRef]
- Huang, L.P.; Lee, C.C.; Fan, J.P.; Kuo, P.H.; Shih, T.S.; Hsu, P.C. Urinary metabolites of di(2-ethylhexyl) phthalate relation to sperm motility, reactive oxygen species generation, and apoptosis in polyvinyl chloride workers. Int. Arch. Occup. Environ. Health 2014, 87, 635–646. [Google Scholar] [CrossRef]
- Demirel, A.; Çoban, A.; Yıldırım, S.; Doğan, C.; Sancı, R.; İnce, Z. Hidden toxicity in neonatal intensive care units: Phthalate exposure in very low birth weight infants. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 298–304. [Google Scholar] [CrossRef]
- Gore, A.C.; Crews, D.; Doan, L.L.; La Merrill, M.; Patisaul, H.; Zota, A. Introduction to Endocrine Disrupting Chemicals (EDCs)—A Guide for Public Interest Organizations and Policy Makers. Endocrine Society Reports and White Papers. 2014, pp. 1–76. Available online: https://www.endocrine.org/-/media/endosociety/files/advocacy-and-outreach/important-documents/introduction-to-endocrine-disrupting-chemicals.pdf (accessed on 7 December 2020).
- Sarath Josh, M.K.; Pradeep, S.; Vijayalekshmy Amma, K.S.; Sudha Devi, R.; Balachandran, S.; Sreejith, M.N.; Benjamin, S. Human ketosteroid receptors interact with hazardous phthalate plasticizers and their metabolites: An in silico study. J. Appl. Toxicol. 2016, 36, 836–843. [Google Scholar] [CrossRef]
- Engel, A.; Buhrke, T.; Imber, F.; Jessel, S.; Seidel, A.; Völkel, W.; Lampen, A. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERalpha, ERbeta, and AR. Toxicol. Lett. 2017, 277, 54–63. [Google Scholar] [CrossRef]
- Kambia, N.K.; Séverin, I.; Farce, A.; Moreau, E.; Dahbi, L.; Duval, C.; Dine, T.; Sautou, V.; Chagnon, M.C. In vitro and in silico hormonal activity studies of di-(2-ethylhexyl)terephthalate, a di-(2-ethylhexyl)phthalate substitute used in medical devices, and its metabolites. J. Appl. Toxicol. 2019, 39, 1043–1056. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.G.; Kim, S.H.; Kim, Y.J. The effects of mono-(2-ethylhexyl) phthalate (MEHP) on human estrogen receptor (hER) and androgen receptor (hAR) by YES/YAS in vitro assay. Molecules 2019, 24, 1558. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.A.; Turki, R.F.; Abuzenadah, A.M.; Damanhouri, G.A.; Beg, M.A. Endocrine disruption: Computational perspectives on human sex hormone-binding globulin and phthalate plasticizers. PLoS ONE 2016, 11, e0151444. [Google Scholar] [CrossRef] [PubMed]
- Nabuurs, S.B.; Wagener, M.; de Vlieg, J. A flexible approach to induced fit docking. J. Med. Chem. 2007, 50, 6507–6518. [Google Scholar] [CrossRef] [PubMed]
- Araki, N.; Ohno, K.; Nakai, M.; Takeyoshi, M.; Lida, M. Screening for androgen receptor activities in 253 industrial chemicals by in vitro reporter gene assays using AR-EcoScreen cells. Toxicol. In Vitro 2005, 19, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Krüger, T.; Long, M.; Bonefeld-Jørgensen, E.C. Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology 2008, 246, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Shen, O.; Du, G.; Sun, H.; Wu, W.; Jiang, Y.; Song, L.; Wang, X. Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol. Lett. 2009, 191, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.S.; Anand-Ivell, R.; Nørgaard-Pedersen, B.; Jönsson, B.A.G.; Bonde, J.P.; Hougaard, D.M.; Cohen, A.; Lindh, C.H.; Ivell, R.; Toft, G. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology 2015, 26, 91–99. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Ligocka, D.; Radwan, P.; Bochenek, M.; Jakubowski, L.; Hanke, W. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod. Toxicol. 2013, 42, 232–241. [Google Scholar] [CrossRef]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 12–236. [Google Scholar] [CrossRef]
- Huen, K.; Calafat, A.M.; Bradman, A.; Yousefi, P.; Eskenazi, B.; Holland, N. Maternal phthalate exposure during pregnancy is associated with DNA methylation of LINE-1 and Alu repetitive elements in Mexican-American children. Environ. Res. 2016, 148, 55–62. [Google Scholar] [CrossRef]
- Lien, Y.J.; Ku, H.Y.; Su, P.H.; Chen, S.J.; Chen, H.Y.; Liao, P.C.; Chen, W.J.; Wang, S.L. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan maternal and infant cohort study. Environ. Health Perspect. 2015, 123, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cao, Y.; Shen, Q.; Zhao, Y.; Zhang, Z.; Zhang, Y. Association between urinary phthalates and pubertal timing in Chinese adolescents. J. Epidemiol. 2015, 25, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
- Araki, A.; Mitsui, T.; Goudarzi, H.; Nakajima, T.; Miyashita, C.; Itoh, S.; Sasaki, S.; Cho, K.; Moriya, K.; Sinohara, N.; et al. Prenatal di(2-ethylhexyl) phthalate exposure and disruption of adrenal androgens and glucocorticoids levels in cord blood: The Hokkaido Study. Sci. Total Environ. 2017, 581–582, 297–304. [Google Scholar] [CrossRef]
- Araki, A.; Mitsui, T.; Miyashita, C.; Nakajima, T.; Naito, H.; Ito, S.; Sasaki, S.; Cho, K.; Ikeno, T.; Nonomura, K.; et al. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: The Hokkaido Study on Environment and Children’s Health. PLoS ONE 2014, 9, e109039. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, W.; Weng, S.; Pan, T.; Hu, X.; Wang, F.; Xia, T.; Chen, H.; Luo, T. Effects of the environmental endocrine disruptors di-2-ethylhexyl phthalate and mono-2-ethylhexyl phthalate on human sperm function invitro. Reprod. Fertil. Dev. 2020, 32, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.M.; Roudot, A.C.; Gallissot, F.; Sabate, J.P. Prenatal developmental toxicity studies on di-n-heptyl and di-n-octyl phthalates in Sprague-Dawley rats. Reprod. Toxicol. 2011, 32, 268–276. [Google Scholar] [CrossRef]
- Zarean, M.; Keikha, M.; Poursafa, P.; Khalighinejad, P.; Amin, M.; Kelishadi, R. A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ. Sci. Pollut. Res. Int. 2016, 23, 24642–24693. [Google Scholar] [CrossRef]
- Kay, V.R.; Bloom, M.S.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 2014, 44, 467–498. [Google Scholar] [CrossRef]
- Lioy, P.J.; Hauser, R.; Gennings, C.; Koch, H.M.; Mirkes, P.E.; Schwetz, B.A.; Kortenkamp, A. Assessment of phthalates/phthalate alternatives in children’s toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 343–353. [Google Scholar] [CrossRef]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. BioMed Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef] [PubMed]

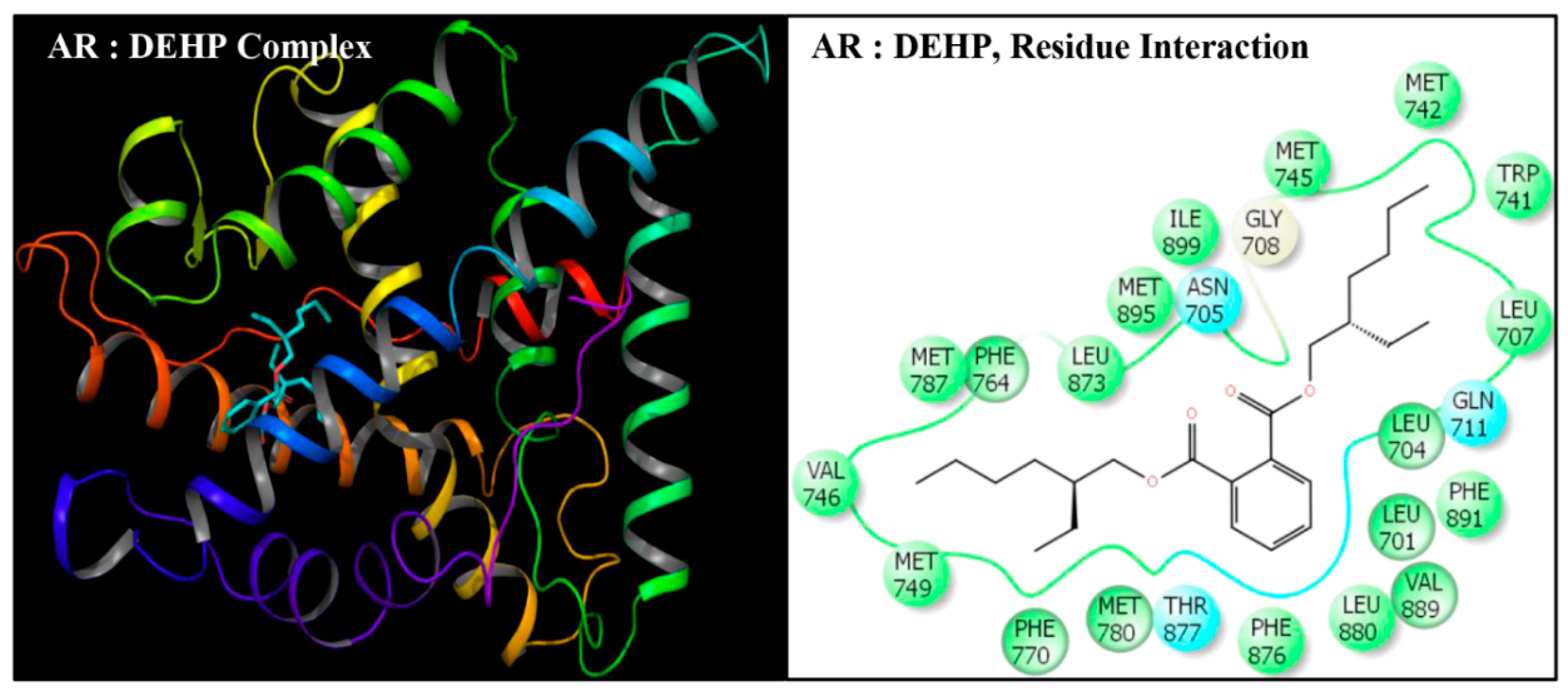
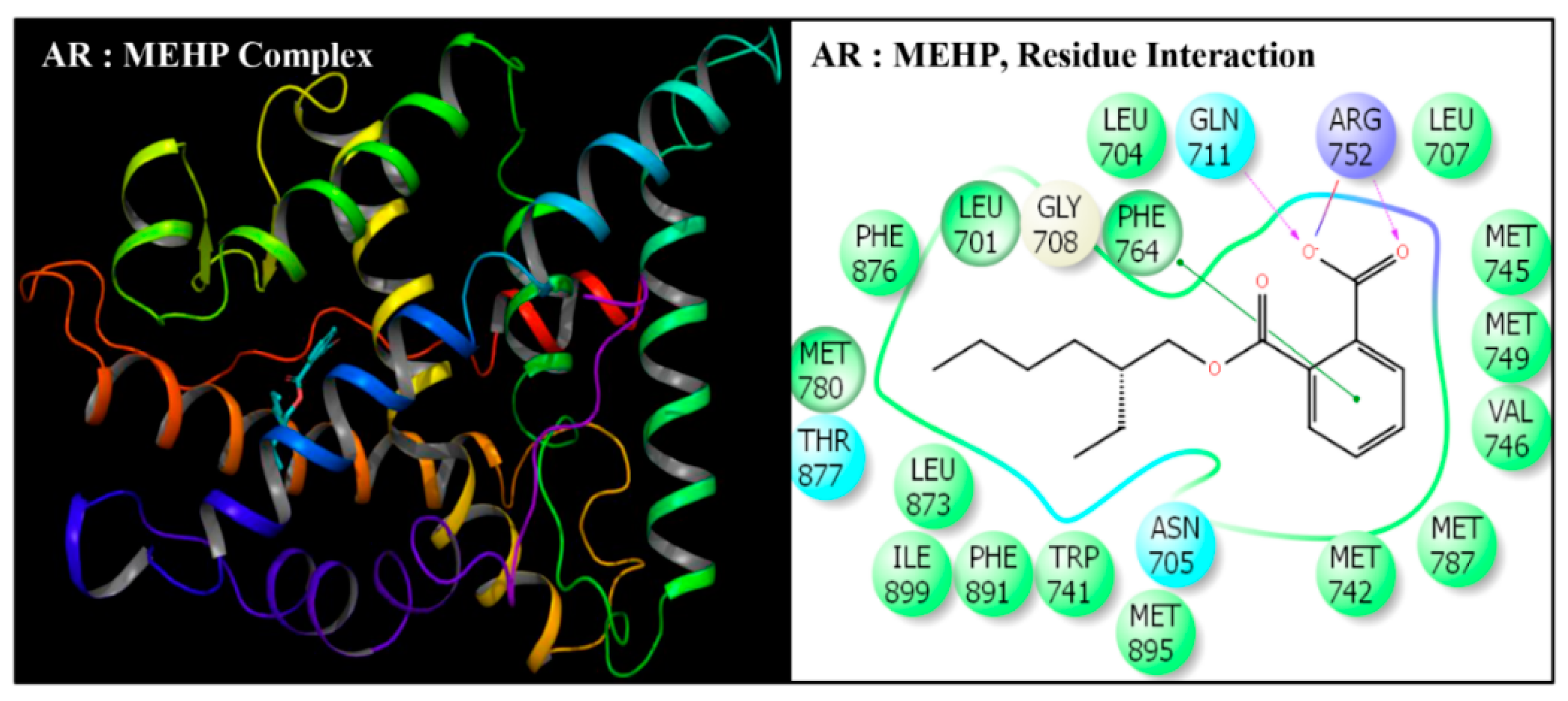
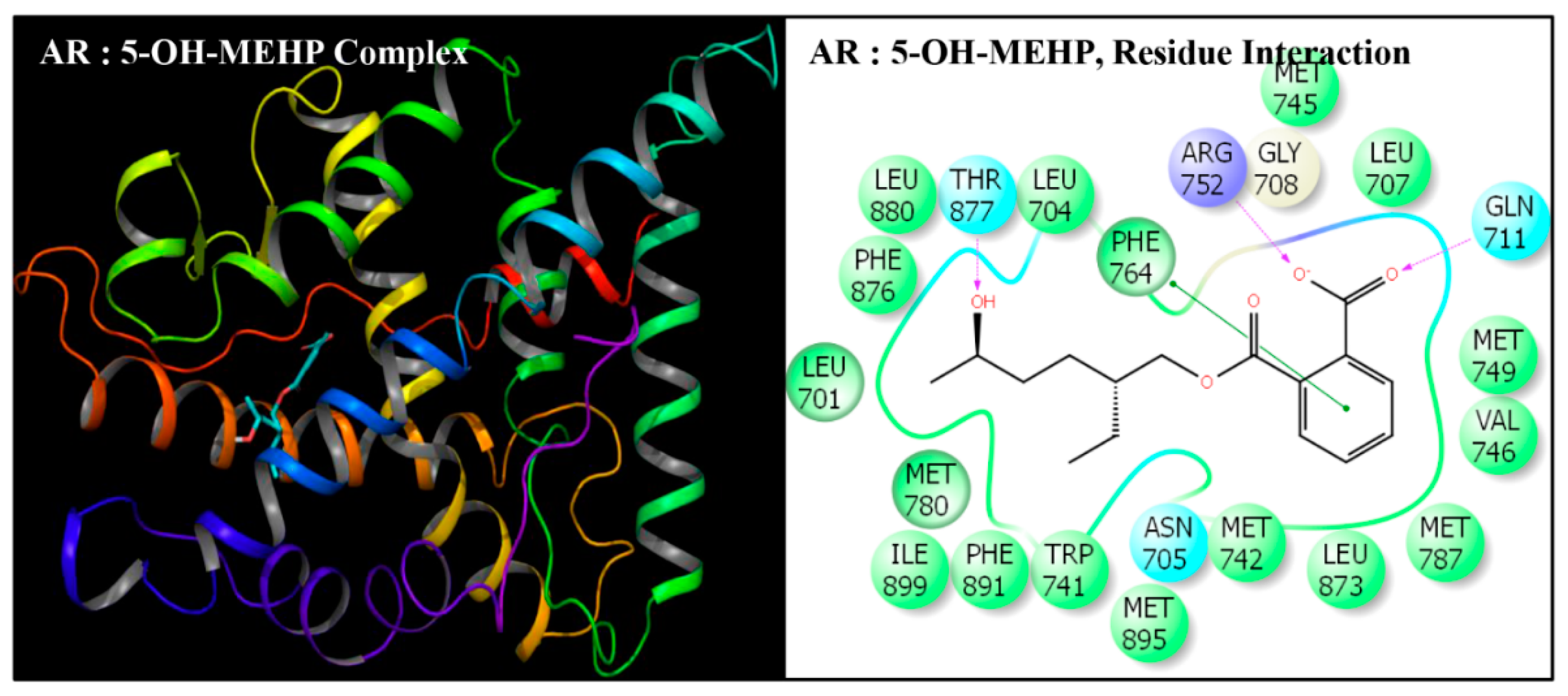
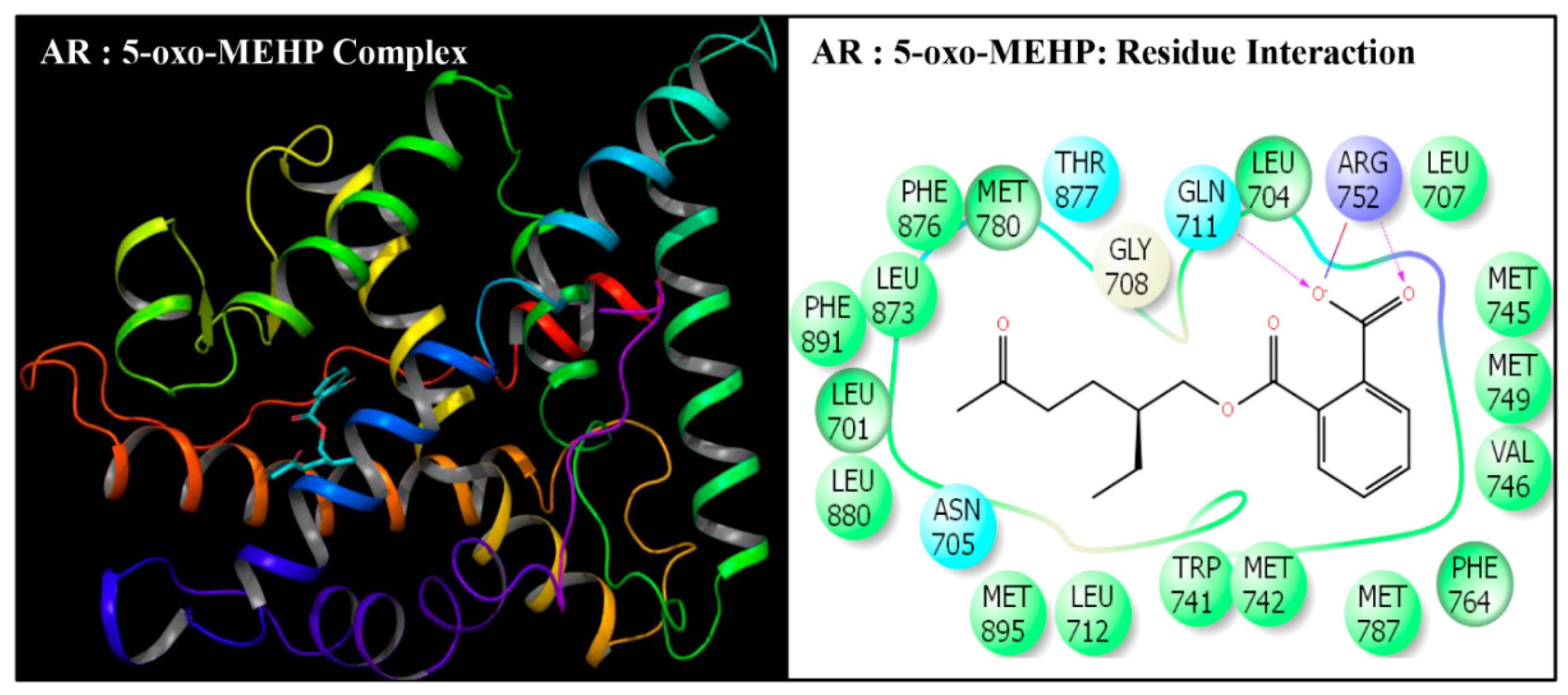
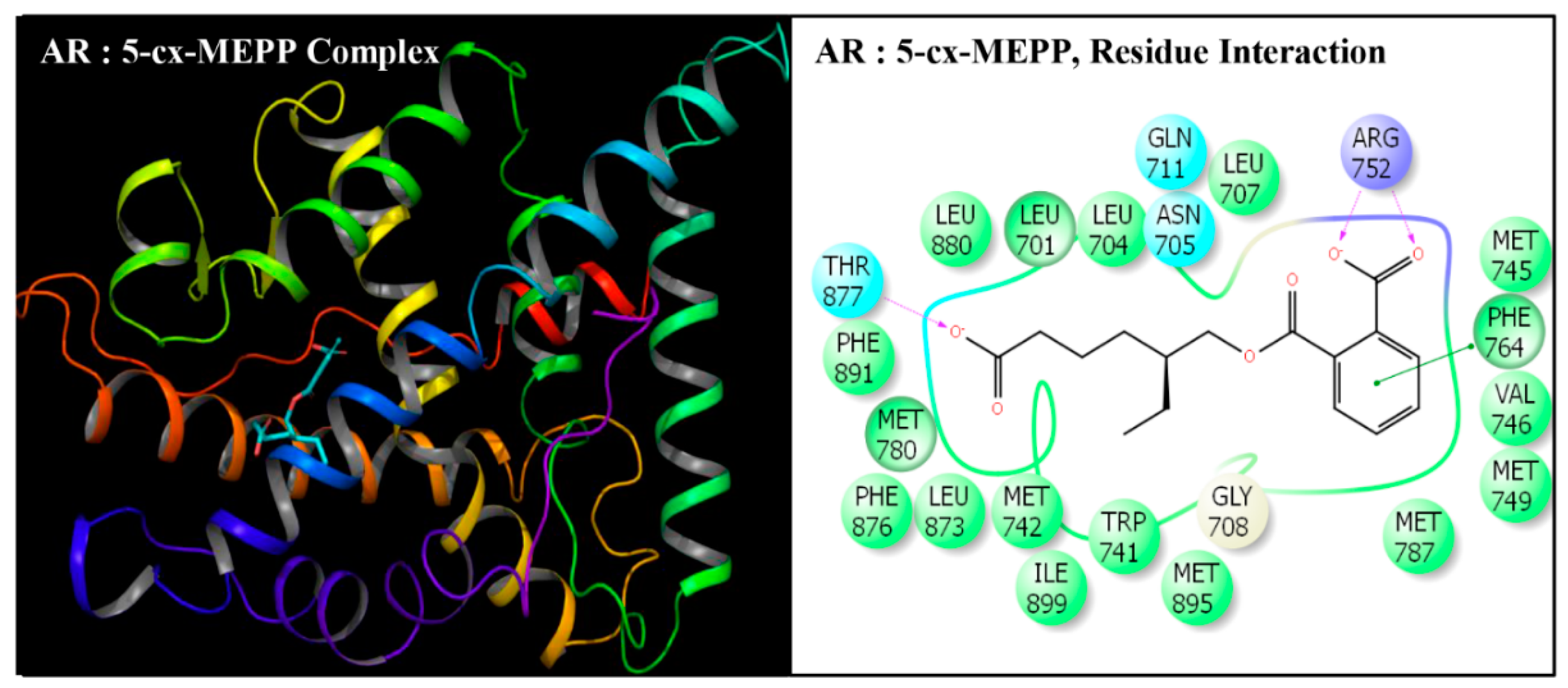
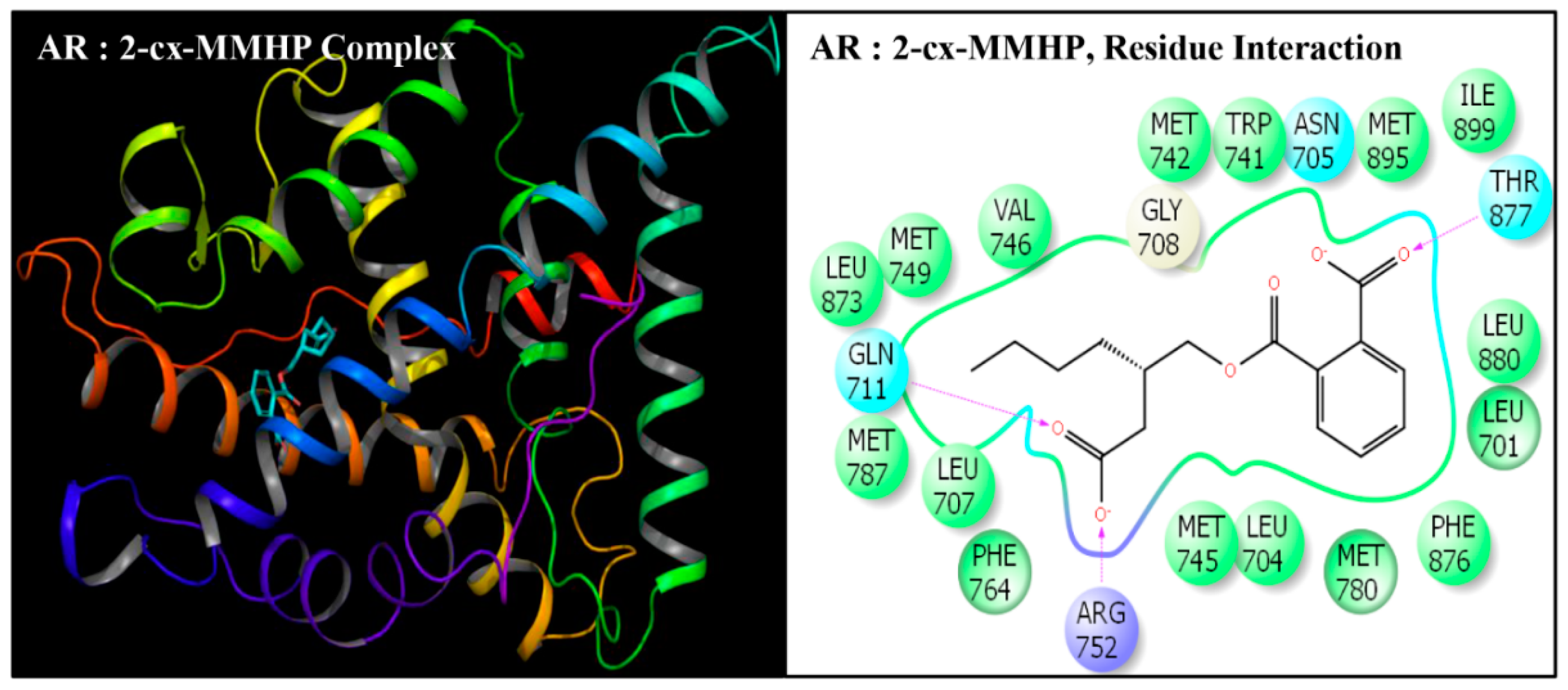
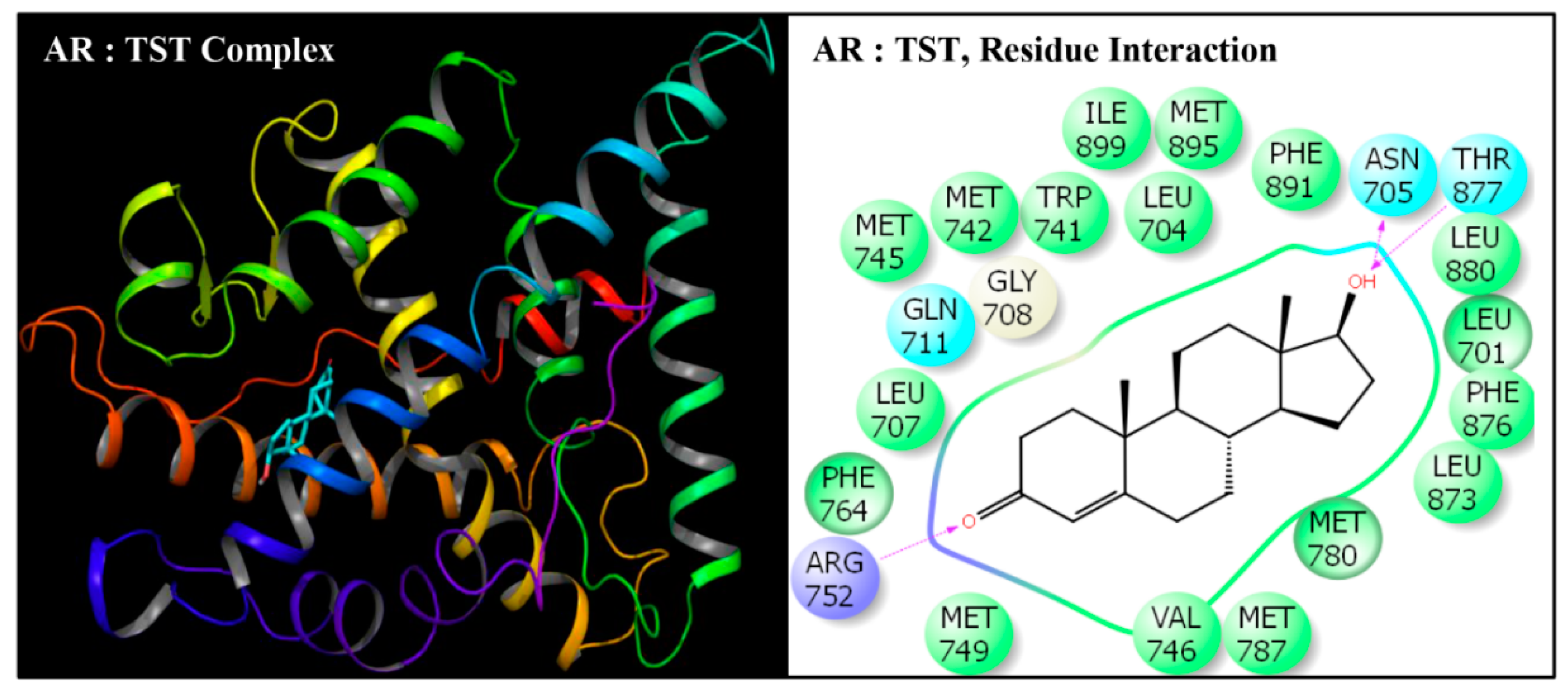
| Serial No. | Name | Abbreviation | PubChem ID | CAS No |
|---|---|---|---|---|
| 1 | Di(2-ethylhexyl)phthalate | DEHP | 8343 | 117-81-7 |
| 2 | Mono-(2-ethylhexyl)phthalate | MEHP | 20393 | 4376-20-9 |
| 3 | Mono-(2-ethyl-5-hydroxyhexyl)phthalate | 5-OH-MEHP | 170295 | 40321-99-1 |
| 4 | Mono-(2-ethyl-5-oxohexyl)phthalate | 5-oxo-MEHP | 119096 | 40321-98-0 |
| 5 | Mono-(2-ethyl-5-carboxypentyl)phthalate | 5-cx-MEPP | 148386 | 40809-41-4 |
| 6 | Mono-[2-(carboxymethyl)hexyl]phthalate | 2-cx-MMHP | 187353 | 82975-93-7 |
| Serial No. | Ligand | Number of Interacting AR Residues | Number of Interacting Residues Common with TST (%) | IFD Score | Docking Score (kcal/mol) | Glide Score (kcal/mol) | MMGB-SA (kcal/mol) |
|---|---|---|---|---|---|---|---|
| 1 | DEHP | 23 | 21 (95%) | −573.86 | −9.17 | −9.17 | −136.12 |
| 2 | MEHP | 21 | 21 (95%) | −573.30 | −9.42 | −9.42 | −109.19 |
| 3 | 5-OH-MEHP | 22 | 22(100%) | −573.33 | −9.65 | −9.65 | −107.50 |
| 4 | 5-oxo-MEHP | 22 | 20 (91%) | −574.45 | −9.73 | −9.73 | −105.80 |
| 5 | 5-cx-MEPP | 21 | 22 (100%) | −575.94 | −11.69 | −11.69 | −92.03 |
| 6 | 2-cx-MMHP | 21 | 21 (95%) | −574.48 | −10.41 | −10.41 | −90.60 |
| 7 | TST | 22 | 22 (100%) | −577.85 | −12.84 | −12.84 | −152.11 |
| Serial No. | Interacting Residue | Serial No. | Interacting Residue |
|---|---|---|---|
| 1 | Leu-701 | 12 | Arg-752 * |
| 2 | Leu-704 | 13 | Phe-764 |
| 3 | Asn-705 | 14 | Met-780 |
| 4 | Leu-707 | 15 | Met-787 |
| 5 | Gly-708 | 16 | Leu-873 |
| 6 | Gln-711 | 17 | Phe-876 |
| 7 | Trp-741 | 18 | Thr-877 |
| 8 | Met-742 | 19 | Leu-880 $ |
| 9 | Met-745 | 20 | Phe-891 & |
| 10 | Val-746 | 21 | Met-895 |
| 11 | Met-749 | 22 | Ile-899 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beg, M.A.; Sheikh, I.A. Endocrine Disruption: Structural Interactions of Androgen Receptor against Di(2-ethylhexyl) Phthalate and Its Metabolites. Toxics 2020, 8, 115. https://doi.org/10.3390/toxics8040115
Beg MA, Sheikh IA. Endocrine Disruption: Structural Interactions of Androgen Receptor against Di(2-ethylhexyl) Phthalate and Its Metabolites. Toxics. 2020; 8(4):115. https://doi.org/10.3390/toxics8040115
Chicago/Turabian StyleBeg, Mohd Amin, and Ishfaq Ahmad Sheikh. 2020. "Endocrine Disruption: Structural Interactions of Androgen Receptor against Di(2-ethylhexyl) Phthalate and Its Metabolites" Toxics 8, no. 4: 115. https://doi.org/10.3390/toxics8040115
APA StyleBeg, M. A., & Sheikh, I. A. (2020). Endocrine Disruption: Structural Interactions of Androgen Receptor against Di(2-ethylhexyl) Phthalate and Its Metabolites. Toxics, 8(4), 115. https://doi.org/10.3390/toxics8040115





