Influence of Vegetarian Dietary Intervention on Urinary Paraben Concentrations: A Pilot Study with ‘Temple Stay’ Participants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Laboratory Analyses
2.3. Estimation of Daily Intake of Parabens
2.4. Statistical Analyses
3. Results
3.1. Urinary Concentrations of Parabens and Benzophenones
3.2. Estimated Daily Intake of Parabens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Bishop, A.M.; Needham, L.L. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ. Health Perspect. 2010, 118, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Kannan, K. Phthalates and parabens in personal care products from China: Concentrations and human exposure. Arch. Enviton. Contam. Toxicol. 2014, 66, 113–119. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Concentrations and composition profiles of parabens in currency bills and paper products including sanitary wipes. Sci. Total Environ. 2014, 475, 8–15. [Google Scholar] [CrossRef]
- Kirchhof, M.G.; de Gannes, G.C. The health controversies of parabens. Skin Ther. Lett. 2013, 18, 5–7. [Google Scholar]
- Kang, K.-S.; Che, J.-H.; Ryu, D.-Y.; Kim, T.-W.; Li, G.-X.; Lee, Y.-S. Decreased sperm number and motile activity on the F1 offspring maternally exposed to butyl p-hydroxybenzoic acid (butyl paraben). J. Vet. Med. Sci. 2002, 64, 227–235. [Google Scholar] [CrossRef]
- Oishi, S. Effects of propyl paraben on the male reproductive system. Food Chem. Toxicol. 2002, 40, 1807–1813. [Google Scholar] [CrossRef]
- Oishi, S. Effects of butyl paraben on the male reproductive system in mice. Arch. Toxicol. 2002, 76, 423–429. [Google Scholar] [CrossRef]
- Geer, L.A.; Pycke, B.F.; Waxenbaum, J.; Sherer, D.M.; Abulafia, O.; Halden, R.U. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J. Hazard. Mater. 2017, 323, 177–183. [Google Scholar] [CrossRef]
- Smarr, M.M.; Sundaram, R.; Honda, M.; Kannan, K.; Louis, G.M. Urinary Concentrations of Parabens and Other Antimicrobial Chemicals and Their Association with Couples’ Fecundity. Environ. Health Perspect. 2017, 125, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.J.; Ferguson, K.K.; Anzalota Del Toro, L.V.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Environ. Health 2015, 218, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.K.; Angerer, J.; Dierkes, G.; Brüning, T.; Koch, H.M. Metabolism and elimination of methyl, iso-and n-butyl paraben in human urine after single oral dosage. Arch. Toxicol. 2016, 90, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Aylward, L.L.; Hays, S.M.; Zidek, A. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: Implications for exposure assessment and reverse dosimetry. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Nishihama, Y.; Ameda, R.; Yoshinaga, J.; Konishi, S.; Yoneyama, M.; Nakajima, D.; Shiraishi, H.; Imai, H. Inter- and intra-individual variation in urinary concentrations of parabens in male and female Japanese subjects. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2017, 1–6. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Shin, C.; Lee, J.; Kim, S.; Lee, A.; Park, J.; Kho, Y.; Moos, R.K.; Koch, H.M. Urinary parabens and triclosan concentrations and associated exposure characteristics in a Korean population—A comparison between night-time and first-morning urine. Int. J. Hyg. Environ. Health 2018, 221, 632–641. [Google Scholar] [CrossRef]
- Moos, R.K.; Apel, P.; Schroter-Kermani, C.; Kolossa-Gehring, M.; Bruning, T.; Koch, H.M. Daily intake and hazard index of parabens based upon 24 h urine samples of the German Environmental Specimen Bank from 1995 to 2012. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 591–600. [Google Scholar] [CrossRef]
- Moos, R.K.; Koch, H.M.; Angerer, J.; Apel, P.; Schroter-Kermani, C.; Bruning, T.; Kolossa-Gehring, M. Parabens in 24 h urine samples of the German Environmental Specimen Bank from 1995 to 2012. Int. J. Hyg. Environ. Health 2015, 218, 666–674. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Colacino, J.A.; Lewis, R.C.; Meeker, J.D. Personal care product use among adults in NHANES: Associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 326–332. [Google Scholar] [CrossRef]
- Jimenez-Diaz, I.; Artacho-Cordon, F.; Vela-Soria, F.; Belhassen, H.; Arrebola, J.P.; Fernandez, M.F.; Ghali, R.; Hedhili, A.; Olea, N. Urinary levels of bisphenol A, benzophenones and parabens in Tunisian women: A pilot study. Sci. Total Environ. 2016, 562, 81–88. [Google Scholar] [CrossRef]
- Davidson, P.M.; Sofos, J.N.; Branen, A.L. Antimicrobials in Food; CRC press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Liao, C.; Liu, F.; Kannan, K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ. Sci. Technol. 2013, 47, 3918–3925. [Google Scholar] [CrossRef]
- Ma, W.L.; Wang, L.; Guo, Y.; Liu, L.Y.; Qi, H.; Zhu, N.Z.; Gao, C.J.; Li, Y.F.; Kannan, K. Urinary concentrations of parabens in Chinese young adults: Implications for human exposure. Arch. Environ. Contam. Toxicol. 2013, 65, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Morgan, R.; Kannan, K. Parabens in human urine from several Asian countries, Greece, and the United States. Chemosphere 2018, 201, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, S.; Park, J.; Kim, H.J.; Lee, J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.B.; et al. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci. Total Environ. 2013, 461–462, 214–221. [Google Scholar] [CrossRef]
- Kang, H.S.; Kyung, M.S.; Ko, A.; Park, J.H.; Hwang, M.S.; Kwon, J.E.; Suh, J.H.; Lee, H.S.; Moon, G.I.; Hong, J.H.; et al. Urinary concentrations of parabens and their association with demographic factors: A population-based cross-sectional study. Environ. Res. 2016, 146, 245–251. [Google Scholar] [CrossRef]
- Dewalque, L.; Pirard, C.; Charlier, C. Measurement of urinary biomarkers of parabens, benzophenone-3, and phthalates in a Belgian population. Biomed. Res. Int. 2014, 2014, 649314. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jensen, T.K.; Jørgensen, N.; Kyhl, H.B.; Husby, S.; Skakkebæk, N.E.; Main, K.M.; Juul, A.; Andersson, A.-M. Human urinary excretion of non-persistent environmental chemicals: An overview of Danish data collected between 2006 and 2012. Reproduction 2014, 147, 555–565. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jorgensen, N.; Andersson, A.M. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). J. Expo. Sci. Environ. Epidemiol. 2011, 21, 262–271. [Google Scholar] [CrossRef]
- Ji, K.; Lim Kho, Y.; Park, Y.; Choi, K. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: A pilot study with “Temple Stay” participants. Environ. Res. 2010, 110, 375–382. [Google Scholar] [CrossRef]
- Li, A.J.; Kannan, K. Elevated Concentrations of Bisphenols, Benzophenones, and Antimicrobials in Pantyhose Collected from Six Countries. Environ. Sci. Technol. 2018, 52, 10812–10819. [Google Scholar] [CrossRef]
- Rastogi, S.C. UV filters in sunscreen products—A survey. Contact Dermat. 2002, 46, 348–351. [Google Scholar] [CrossRef]
- Ye, X.; Bishop, A.M.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Parabens as urinary biomarkers of exposure in humans. Environ. Health Perspect. 2006, 114, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Advises on the Safety of Paraben Usage in Food; European Food Safety Authority: Parma, Italy, 2014. [Google Scholar]
- Kortenkamp, A.; Faust, M. Combined exposures to anti-androgenic chemicals: Steps towards cumulative risk assessment. Int. J. Androl. 2010, 33, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, J.-Y.; Park, E.-Y.; Won, J.; Hong, K.K.; Moon, G.-I.; Kim, M.-S.; Hong, J.-H. Assessment of estimated daily intakes of preservatives in the Korean population. Korean J. Food Sci. Technol. 2008, 40, 503–509. [Google Scholar]
- Kim, H.-y.; Lee, Y.-J.; Hong, K.-H.; Ha, S.-C.; Ahn, M.-S.; Jo, J.-S.; Kim, K.-S. Intake of food additives in foods by total diet. Korean J. Food Sci. Technol. 1998, 30, 767–774. [Google Scholar]
- The Korea National Health and Nutrition Examination Survey (KNHANES). National Food & Nutrition StatisticsI: Based on 2014 Korea National Health and Nutrition Examination Survey. Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar]
- Asimakopoulos, A.G.; Thomaidis, N.S.; Kannan, K. Widespread occurrence of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters (parabens), benzophenone type-UV filters, triclosan, and triclocarban in human urine from Athens, Greece. Sci. Total Environ. 2014, 470–471, 1243–1249. [Google Scholar] [CrossRef]
- Ishiwata, H.; Nishijima, M.; Fukasawa, Y.; Ito, Y.; Yamada, T. Evaluation of preservatives contents in foods and the daily intake deduced from the results of the official inspection in Japan in FY 1994. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1997, 38, 145–154. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, C.-D.; Huang, J.; Zhou, R.-Q.; Liao, X.-P. Analysis of volatile compounds in Chinese soy sauces moromi cultured by different fermentation processes. Food Sci. Biotechnol. 2013, 22, 605–612. [Google Scholar] [CrossRef]
- Moos, R.K.; Angerer, J.; Wittsiepe, J.; Wilhelm, M.; Bruning, T.; Koch, H.M. Rapid determination of nine parabens and seven other environmental phenols in urine samples of German children and adults. Int. J. Hyg. Environ. Health 2014, 217, 845–853. [Google Scholar] [CrossRef]
- Smith, K.W.; Braun, J.M.; Williams, P.L.; Ehrlich, S.; Correia, K.F.; Calafat, A.M.; Ye, X.; Ford, J.; Keller, M.; Meeker, J.D.; et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ. Health Perspect. 2012, 120, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Zhang, W.; Kannan, K. Characteristic profiles of urinary p-hydroxybenzoic acid and its esters (parabens) in children and adults from the United States and China. Environ. Sci. Technol. 2013, 47, 2069–2076. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Widespread occurrence of benzophenone-type UV light filters in personal care products from China and the United States: An assessment of human exposure. Environ. Sci. Technol. 2014, 48, 4103–4109. [Google Scholar] [CrossRef]
- Manová, E.; von Goetz, N.; Keller, C.; Siegrist, M.; Hungerbühler, K. Use patterns of leave-on personal care products among Swiss-German children, adolescents, and adults. Int. J. Environ. Res. Public Health 2013, 10, 2778–2798. [Google Scholar] [CrossRef]
- Berger, K.P.; Kogut, K.R.; Bradman, A.; She, J.; Gavin, Q.; Zahedi, R.; Parra, K.L.; Harley, K.G. Personal care product use as a predictor of urinary concentrations of certain phthalates, parabens, and phenols in the HERMOSA study. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 21–32. [Google Scholar] [CrossRef]
- Błędzka, D.; Gromadzińska, J.; Wąsowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Long, F.; Lu, P.; Lei, B.; Jiang, Z.A.; Liu, G.; Zhang, J.; Ma, S.; Yu, Y. Benzophenone-UV filters in personal care products and urine of schoolchildren from Shenzhen, China: Exposure assessment and possible source. Sci. Total Environ. 2018, 640–641, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Coull, B.A.; Gaskins, A.J.; Williams, M.A.; Skakkebaek, N.E.; Ford, J.B.; Ye, X.; Calafat, A.M.; Braun, J.M.; Hauser, R. Personal Care Product Use in Men and Urinary Concentrations of Select Phthalate Metabolites and Parabens: Results from the Environment And Reproductive Health (EARTH) Study. Environ. Health Perspect. 2017, 125, 087012. [Google Scholar] [CrossRef] [PubMed]
- Gosens, I.; Delmaar, C.J.; Ter Burg, W.; De Heer, C.; Schuur, A.G. Aggregate exposure approaches for parabens in personal care products: A case assessment for children between 0 and 3 years old. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 208–214. [Google Scholar] [CrossRef] [PubMed]
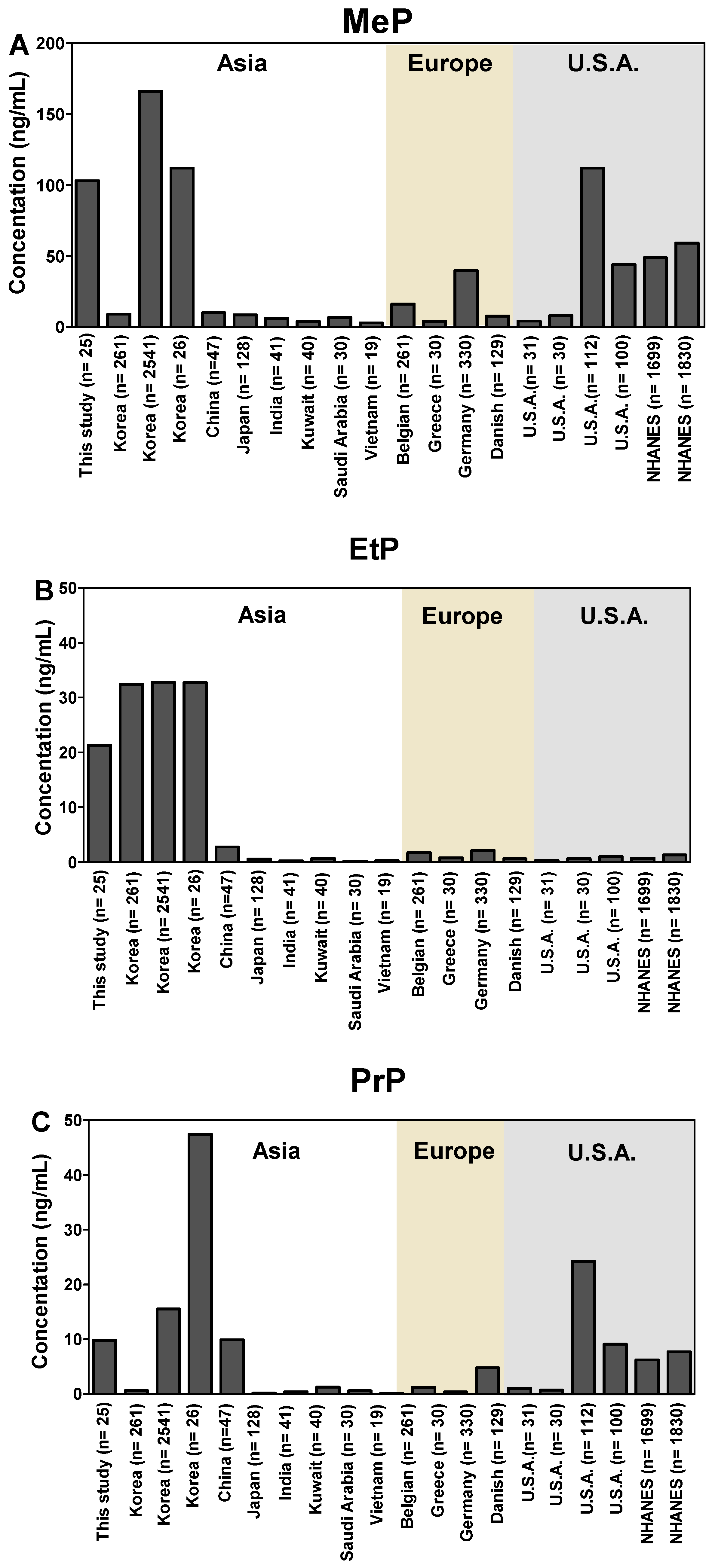
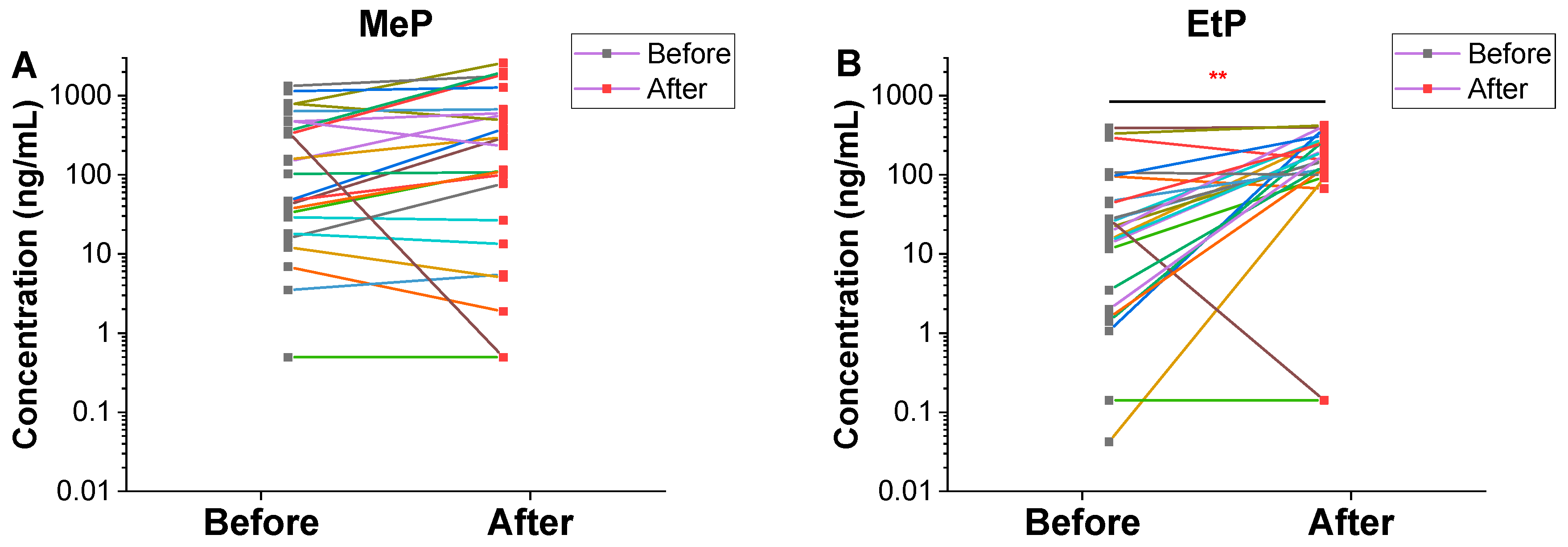
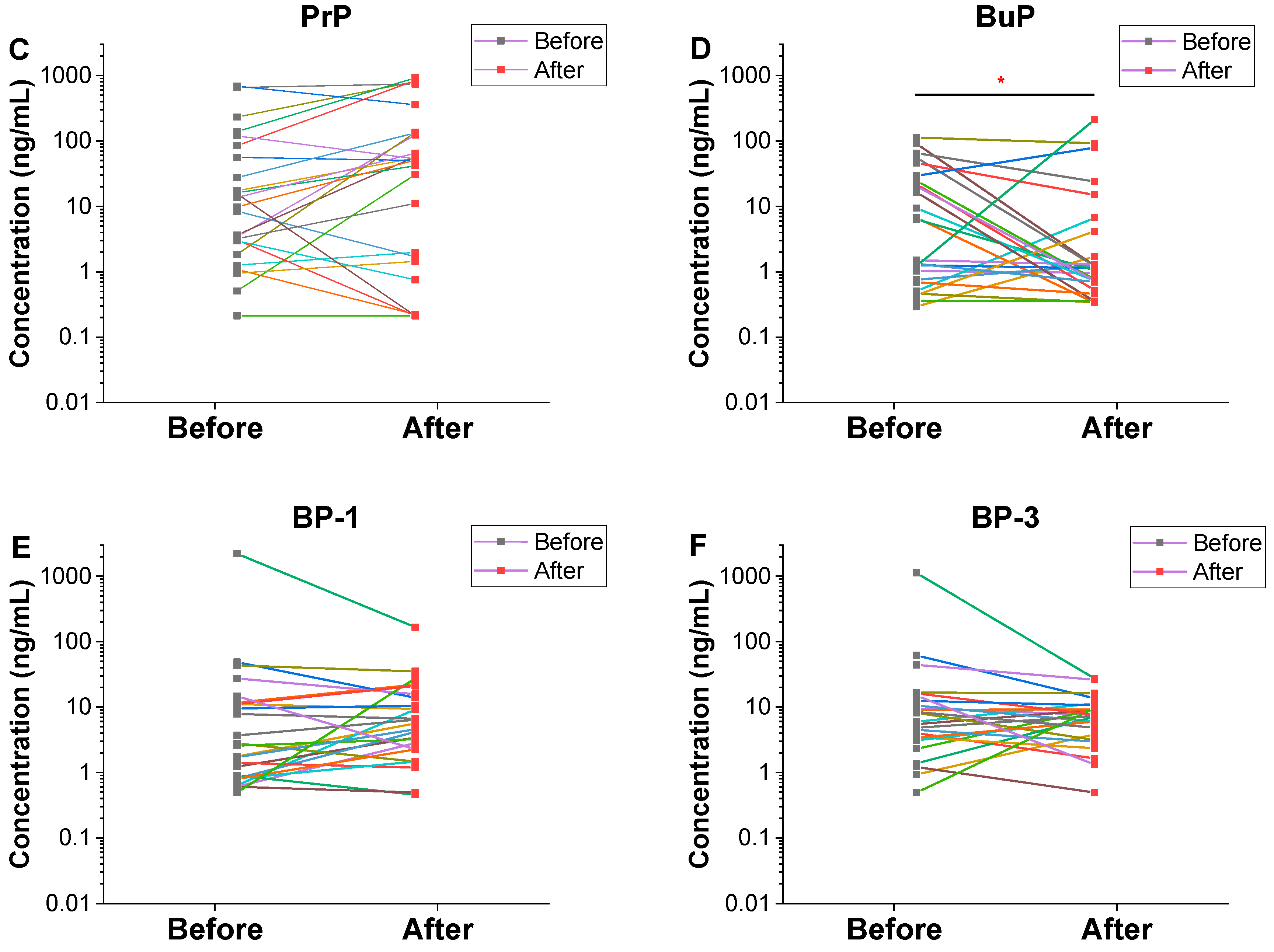
| Chemical | Value | Total | Male (n = 16) | Female (n = 9) | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| MeP | DF | 96.0 | 92.0 | 100 | 93.8 | 88.9 | 88.9 |
| GM | 84.9 | 109 | 51.2 | 53.7 | 208 | 384 | |
| P50 | 103 | 231 | 44.2 | 104 | 481 | 1270 | |
| P95 | 1072 | 1962 | 551 | 580 | 1248 | 2350 | |
| EtP | DF | 96.0 | 92.0 | 100 | 93.8 | 88.9 | 88.9 |
| GM | 14.0 | 105 | 14.7 | 122 | 12.9 | 81.3 | |
| P50 | 21.3 | 154 | 20.1 | 176 ** | 42.9 | 130 * | |
| P95 | 323 | 410 | 321 | 400 | 240 | 379 | |
| PrP | DF | 96.0 | 88.0 | 100 | 87.5 | 88.9 | 88.9 |
| GM | 11.0 | 21.1 | 4.3 | 7.5 | 58.9 | 133.2 | |
| P50 | 9.80 | 51.2 | 3.30 | 20.9 | 120 | 359 | |
| P95 | 573 | 838 | 27.1 | 127 | 678 | 893 | |
| BuP | DF | 96.0 | 92.0 | 100 | 93.8 | 88.9 | 88.9 |
| GM | 4.71 | 2.06 | 3.60 | 1.03 | 7.57 | 7.11 | |
| P50 | 6.40 | 1.10 | 3.90 | 1.10 * | 20.4 | 15.0 | |
| P95 | 86.3 | 89.8 | 64.7 | 4.80 | 93.9 | 164 | |
| BP-1 | DF | 96.0 | 96.0 | 100 | 93.8 | 88.9 | 100 |
| GM | 4.12 | 5.74 | 2.60 | 3.78 | 9.34 | 12.1 | |
| P50 | 2.58 | 5.64 | 1.58 | 3.81 | 9.52 | 10.5 | |
| P95 | 47.7 | 33.7 | 32.8 | 17.4 | 1349 | 114 | |
| BP-3 | DF | 96.0 | 96.0 | 100 | 93.8 | 88.9 | 100 |
| GM | 7.51 | 6.47 | 5.38 | 6.19 | 13.57 | 6.99 | |
| P50 | 6.00 | 7.30 | 5.14 | 7.23 | 12.5 | 7.65 | |
| P95 | 58.5 | 24.1 | 48.8 | 16.7 | 685 | 22.8 | |
| MeP | DF | 96.0 | 92.0 | 100 | 93.8 | 88.9 | 88.9 |
| GM | 52.7 | 54.5 | 30.4 | 25.9 | 140 | 205 | |
| P50 | 67.8 | 85.6 | 23.9 | 75.7 | 243 | 560 | |
| P95 | 604 | 858 | 347 | 248 | 964 | 1218 | |
| EtP | DF | 96.0 | 92.0 | 100 | 93.8 | 88.9 | 88.9 |
| GM | 8.72 | 52.6 | 8.73 | 58.6 | 8.70 | 43.5 | |
| P50 | 12.1 | 85.1 | 11.2 | 90.6 * | 17.4 | 85.1 | |
| P95 | 180 | 204 | 173.4 | 171 | 149 | 314 | |
| PrP | DF | 96.0 | 88.0 | 100 | 87.5 | 88.9 | 88.9 |
| GM | 6.83 | 10.5 | 2.54 | 3.59 | 39.7 | 72.3 | |
| P50 | 7.80 | 21.1 | 1.80 | 9.40 | 67.1 | 131 | |
| P95 | 321 | 350 | 12.0 | 61.0 | 507 | 551 | |
| BuP | DF | 96.0 | 92.0 | 100 | 93.8 | 88.9 | 88.9 |
| GM | 2.93 | 1.03 | 2.14 | 0.49 | 5.10 | 3.80 | |
| P50 | 4.20 | 0.50 | 2.40 | 0.40 * | 10.3 | 5.10 | |
| P95 | 55.7 | 26.5 | 25.5 | 2.70 | 61.5 | 104 | |
| BP-1 | DF | 96.0 | 96.0 | 100 | 93.8 | 88.9 | 100 |
| GM | 2.56 | 2.87 | 1.54 | 1.82 | 6.29 | 6.44 | |
| P50 | 1.50 | 2.37 | 0.89 | 1.46 | 5.08 | 5.51 | |
| P95 | 21.5 | 14.3 | 10.4 | 9.07 | 653 | 78.6 | |
| BP-3 | DF | 96.0 | 96.0 | 100 | 93.8 | 88.9 | 100 |
| GM | 4.67 | 3.23 | 3.20 | 2.98 | 9.14 | 3.74 | |
| P50 | 5.20 | 2.70 | 2.64 | 3.18 | 7.58 | 2.63 | |
| P95 | 16.2 | 8.46 | 14.3 | 7.01 | 333 | 14.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, A.; Kim, S.; Ji, K.; Kho, Y.; Choi, K. Influence of Vegetarian Dietary Intervention on Urinary Paraben Concentrations: A Pilot Study with ‘Temple Stay’ Participants. Toxics 2020, 8, 3. https://doi.org/10.3390/toxics8010003
Jo A, Kim S, Ji K, Kho Y, Choi K. Influence of Vegetarian Dietary Intervention on Urinary Paraben Concentrations: A Pilot Study with ‘Temple Stay’ Participants. Toxics. 2020; 8(1):3. https://doi.org/10.3390/toxics8010003
Chicago/Turabian StyleJo, Areum, Sunmi Kim, Kyunghee Ji, Younglim Kho, and Kyungho Choi. 2020. "Influence of Vegetarian Dietary Intervention on Urinary Paraben Concentrations: A Pilot Study with ‘Temple Stay’ Participants" Toxics 8, no. 1: 3. https://doi.org/10.3390/toxics8010003
APA StyleJo, A., Kim, S., Ji, K., Kho, Y., & Choi, K. (2020). Influence of Vegetarian Dietary Intervention on Urinary Paraben Concentrations: A Pilot Study with ‘Temple Stay’ Participants. Toxics, 8(1), 3. https://doi.org/10.3390/toxics8010003







