Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-poly(ε-caprolactone) Nanocapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production and Characterization of Polymeric Nanocapsules
2.2.1. Production of Polymeric Nanocapsules
2.2.2. Photon Correlation Spectroscopy
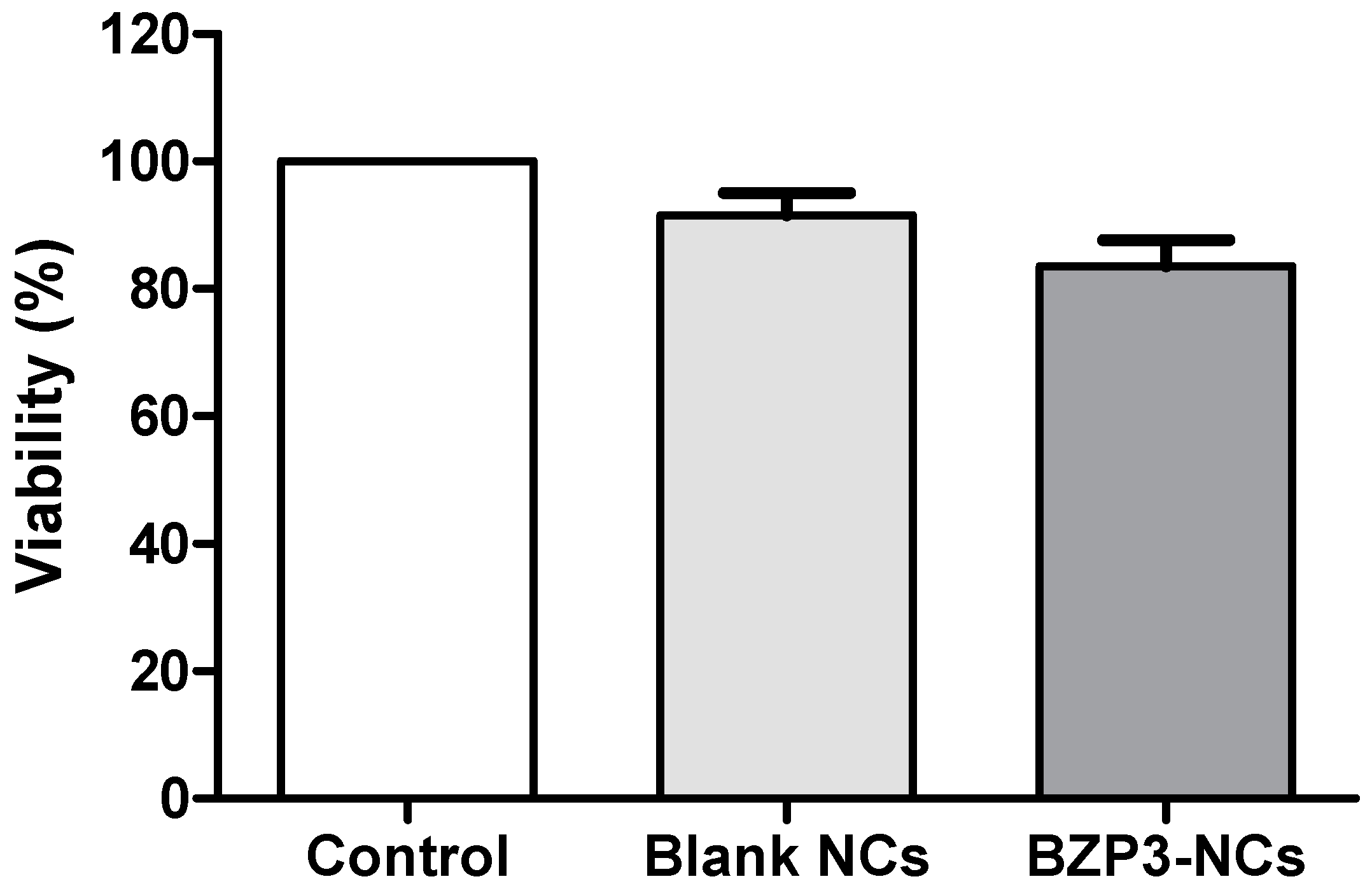
2.2.3. Cytotoxicity
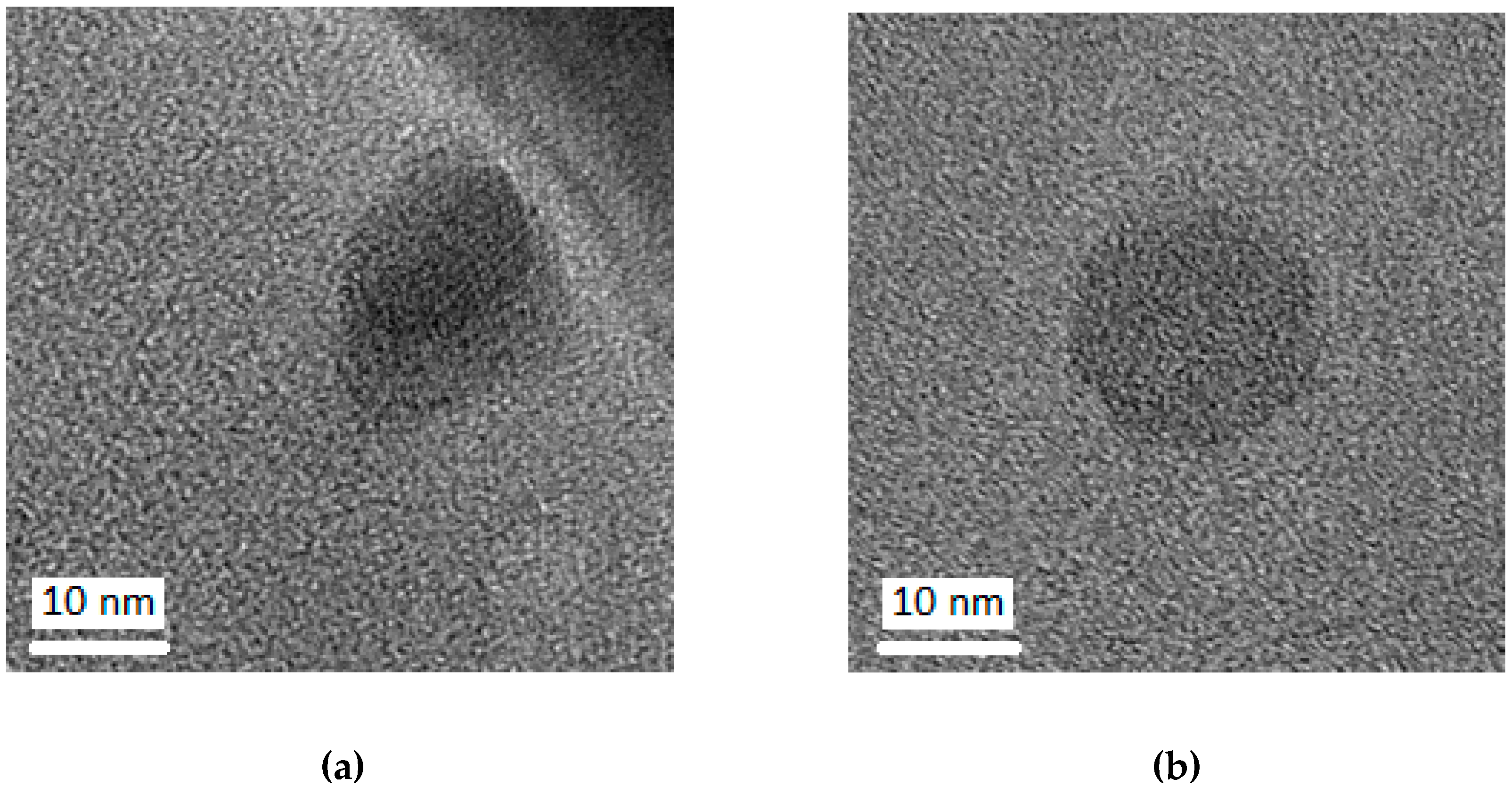
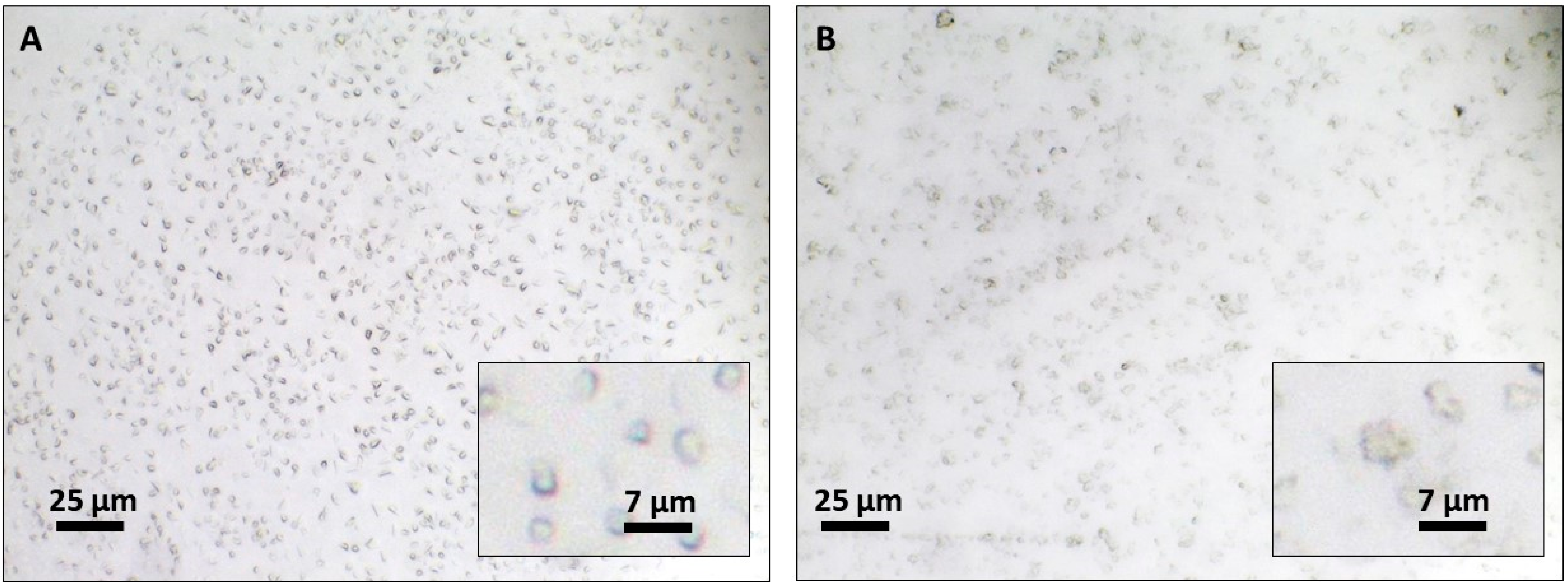
2.2.4. Transmission Electron Microscopy
2.3. Production and Characterization of the Semi-Solid Sunscreen
2.3.1. Production of the Semi-Solid Sunscreen
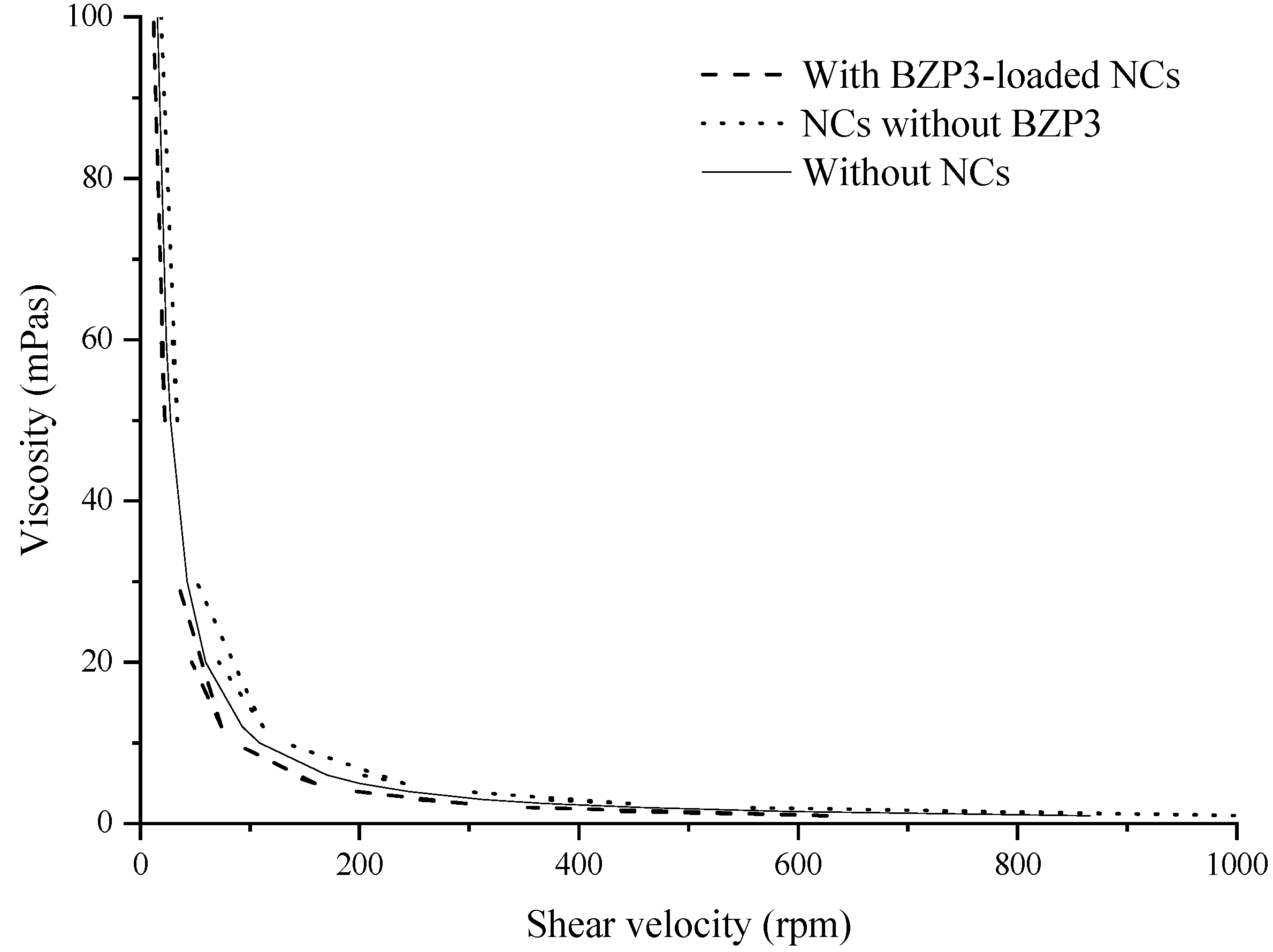
2.3.2. Viscosity Analysis
2.3.3. Stability Tests
2.3.4. Sun Protection Factor (SPF)
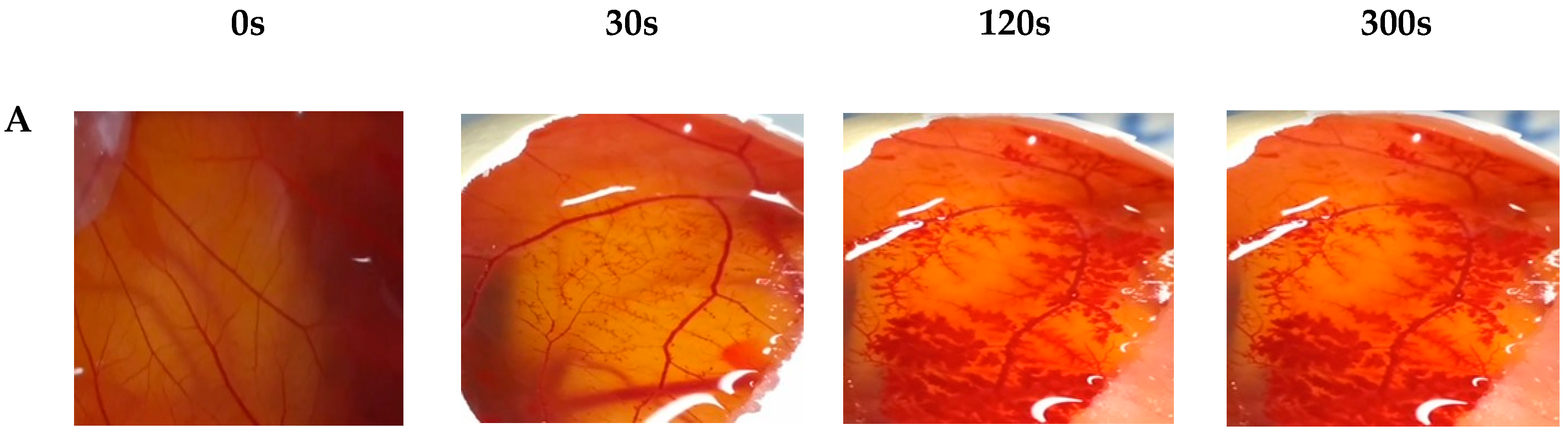
2.3.5. In Vitro Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM)
2.3.6. Morphological Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tampucci, S.; Burgalassi, S.; Chetoni, P.; Monti, D. Cutaneous permeation and penetration of sunscreens: Formulation strategies and in vitro methods. Cosmetics 2018, 5, 1. [Google Scholar] [CrossRef]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Gabros, S.; Zito, P.M. Sunscreens and Photoprotection. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Suh, H.W.; Lewis, J.; Fong, L.; Ramseier, J.Y.; Carlson, K.; Peng, Z.H.; Yin, E.S.; Saltzman, W.M.; Girardi, M. Biodegradable bioadhesive nanoparticle incorporation of broad-spectrum organic sunscreen agents. Bioeng. Transl. Med. 2019, 4, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Moraes, L.F.; Zanchetta, B.; Souto, E.B.; Santana, M.H. Elastic liposomes containing benzophenone-3 for sun protection factor enhancement. Pharm. Dev. Technol. 2012, 17, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Eberlin, S.; da Silva Goncalves, F.C.; Fernandes, A.R.; Marto, J.; Ribeiro, H.M.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. In vitro SPF and Photostability Assays of Emulsion Containing Nanoparticles with Vegetable Extracts Rich in Flavonoids. AAPS PharmSciTech 2018, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Anselmi, C.; Centini, M.; Muller, R.H. Preparation and characterization of n-dodecyl-ferulate-loaded solid lipid nanoparticles (SLN). Int. J. Pharm. 2005, 295, 261–268. [Google Scholar] [CrossRef]
- Xia, Q.; Saupe, A.; Muller, R.H.; Souto, E.B. Nanostructured lipid carriers as novel carrier for sunscreen formulations. Int. J. Cosmet. Sci. 2007, 29, 473–482. [Google Scholar] [CrossRef]
- Wawrzynczak, A.; Feliczak-Guzik, A.; Nowak, I. Nanosunscreens: From nanoencapsulated to nanosized cosmetic active forms. In Nanobiomaterials in Galenic Formulations and Cosmetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 25–46. [Google Scholar]
- Krause, M.; Frederiksen, H.; Sundberg, K.; Jørgensen, F.; Jensen, L.; Nørgaard, P.; Jørgensen, C.; Ertberg, P.; Juul, A.; Drzewiecki, K. Presence of benzophenones commonly used as UV filters and absorbers in paired maternal and fetal samples. Environ. Int. 2018, 110, 51–60. [Google Scholar] [CrossRef]
- Niakousari, M.; Damueh, M.; Gahruie, H.H.; Bekhit, A.; Greiner, R.; Roohiejad, S. Conventional emulsions. In Emulsion-Based Systems for Delivery of Food Active Compounds: Formation, Application, Health and Safety, 1st ed.; Wiley: Croydon, UK, 2018; pp. 1–27. [Google Scholar]
- Gilbert, E.; Roussel, L.; Serre, C.; Sandouk, R.; Salmon, D.; Kirilov, P.; Haftek, M.; Falson, F.; Pirot, F. Percutaneous absorption of benzophenone-3 loaded lipid nanoparticles and polymeric nanocapsules: A comparative study. Int. J. Pharm. 2016, 504, 48–58. [Google Scholar] [CrossRef]
- Marcato, P.D.; Caverzan, J.; Rossi-Bergmann, B.; Pinto, E.F.; Machado, D.; Silva, R.A.; Justo, G.Z.; Ferreira, C.V.; Duran, N. Nanostructured polymer and lipid carriers for sunscreen. Biological effects and skin permeation. J. Nanosci. Nanotechnol. 2011, 11, 1880–1886. [Google Scholar] [CrossRef]
- Paese, K.; Jager, A.; Poletto, F.S.; Pinto, E.F.; Rossi-Bergmann, B.; Pohlmann, A.R.; Guterres, S.S. Semisolid formulation containing a nanoencapsulated sunscreen: Effectiveness, in vitro photostability and immune response. J. Biomed. Nanotechnol. 2009, 5, 240–246. [Google Scholar] [CrossRef]
- Siqueira, N.M.; Contri, R.V.; Paese, K.; Beck, R.C.; Pohlmann, A.R.; Guterres, S.S. Innovative sunscreen formulation based on benzophenone-3-loaded chitosan-coated polymeric nanocapsules. Skin Pharmacol. Physiol. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Teixeira, Z.; Dreiss, C.A.; Lawrence, M.J.; Heenan, R.K.; Machado, D.; Justo, G.Z.; Guterres, S.S.; Duran, N. Retinyl palmitate polymeric nanocapsules as carriers of bioactives. J. Colloid Interface Sci. 2012, 382, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Jorge Murillo, G.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Piñón-Segundo, E.; Llera-Rojas, V.G.; Leyva-Gómez, G.; Urbán-Morlán, Z.; Mendoza-Muñoz, N.; Quintanar-Guerrero, D. The emulsification-diffusion method to obtain polymeric nanoparticles: Two decades of research. In Nanoscale Fabrication, Optimization, Scale-up and Biological Aspects of Pharmaceutical Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–83. [Google Scholar]
- Santos, E.P.; Freitas, Z.M.; Souza, K.R.; Garcia, S.; Vergnanini, A. In vitro and in vivo determinations of sun protection factors of sunscreen lotions with octylmethoxycinnamate. Int. J. Cosmet. Sci. 1999, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; Garcia, M.L. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen-in vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef]
- Araujo, J.; Vega, E.; Lopes, C.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloids Surf. B Biointerfaces 2009, 72, 48–56. [Google Scholar] [CrossRef] [PubMed]
- De Brum, T.L.; Fiel, L.A.; Contri, R.V.; Guterres, S.S.; Pohlmann, A.R. Polymeric Nanocapsules and Lipid-Core Nanocapsules Have Diverse Skin Penetration. J. Nanosci. Nanotechnol. 2015, 15, 773–780. [Google Scholar] [CrossRef]
- Davaeifar, S.; Modarresi, M.H.; Mohammadi, M.; Hashemi, E.; Shafiei, M.; Maleki, H.; Vali, H.; Zahiri, H.S.; Noghabi, K.A. Synthesizing, characterizing, and toxicity evaluating of Phycocyanin-ZnO nanorod composites: A back to nature approaches. Colloids Surf. B Biointerfaces 2019, 175, 221–230. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Hiraishi, R.; Kishimoto, K.; Hamada, S.; Abe, S.; Bekki, Y.; Kamemura, N. Cytotoxicity of benzophenone-3, an organic ultraviolet filter, caused by increased intracellular Zn2+ levels in rat thymocytes. Chem. Biol. Interact. 2019, 298, 52–56. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rigon, R.B.; Goncalez, M.L.; Severino, P.; Alves, D.A.; Santana, M.H.A.; Souto, E.B.; Chorilli, M. Solid lipid nanoparticles optimized by 22 factorial design for skin administration: Cytotoxicity in NIH3T3 fibroblasts. Colloids Surf. B Biointerfaces 2018, 171, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Lohani, A.; Mishra, A.K.; Verma, A. Formulation and evaluation of carrot seed oil-based cosmetic emulsions. J. Cosmet. Laser 2019, 21, 99–107. [Google Scholar] [CrossRef]
- Ohman, H.; Vahlquist, A. In vivo studies concerning a pH gradient in human stratum corneum and upper epidermis. Acta Derm. Venereol. 1994, 74, 375–379. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop(R) and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef]

| Formulation | F1 * [wt.%] | F2 ** [wt.%] |
|---|---|---|
| Organic Phase | ||
| Poly(ε-caprolactone) | 0.017 | 0.017 |
| Carrot oil | 0.005 | 0.005 |
| Benzophenone-3 | 0.0005 | - |
| Acetone | 10 | 10 |
| Aqueous phase | ||
| Pluronic® F68 | 0.02 | 0.02 |
| Distilled water (q.s.) | 20 mL | 20 mL |
| Formulation | Z-Ave [nm] ± SD | PDI [−]± SD | ZP [mV] ± SD |
|---|---|---|---|
| NCs-BZP3 | 418.60 ± 56.28 | 0.366 ± 0.062 | +11.30 ± 0.79 |
| NCs | 287.43 ± 13.60 | 0.189 ± 0.079 | +6.11 ± 0.87 |
| Carrot Oil | Blank NCs | BZP3-NCs | Semi-Solid Sunscreen |
|---|---|---|---|
| 6.80 | 6.84 | 8.64 | 8.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, T.C.; Nascimento, L.É.D.; Bani, C.; Almeida, T.; Nery, M.; Santos, R.S.; Menezes, L.R.d.O.; Zielińska, A.; Fernandes, A.R.; Cardoso, J.C.; et al. Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-poly(ε-caprolactone) Nanocapsules. Toxics 2019, 7, 51. https://doi.org/10.3390/toxics7040051
Barbosa TC, Nascimento LÉD, Bani C, Almeida T, Nery M, Santos RS, Menezes LRdO, Zielińska A, Fernandes AR, Cardoso JC, et al. Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-poly(ε-caprolactone) Nanocapsules. Toxics. 2019; 7(4):51. https://doi.org/10.3390/toxics7040051
Chicago/Turabian StyleBarbosa, Thallysson Carvalho, Lívia Éven Dias Nascimento, Cristiane Bani, Taline Almeida, Marcelo Nery, Rafael Silva Santos, Luana Renyelle de Oliveira Menezes, Aleksandra Zielińska, Ana Rita Fernandes, Juliana Cordeiro Cardoso, and et al. 2019. "Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-poly(ε-caprolactone) Nanocapsules" Toxics 7, no. 4: 51. https://doi.org/10.3390/toxics7040051
APA StyleBarbosa, T. C., Nascimento, L. É. D., Bani, C., Almeida, T., Nery, M., Santos, R. S., Menezes, L. R. d. O., Zielińska, A., Fernandes, A. R., Cardoso, J. C., Jäger, A., Jäger, E., Sanchez-Lopez, E., Nalone, L., Souto, E. B., & Severino, P. (2019). Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-poly(ε-caprolactone) Nanocapsules. Toxics, 7(4), 51. https://doi.org/10.3390/toxics7040051









