Levels of Cadmium in Human Mandibular Bone
Abstract
1. Introduction
2. Materials and Methods
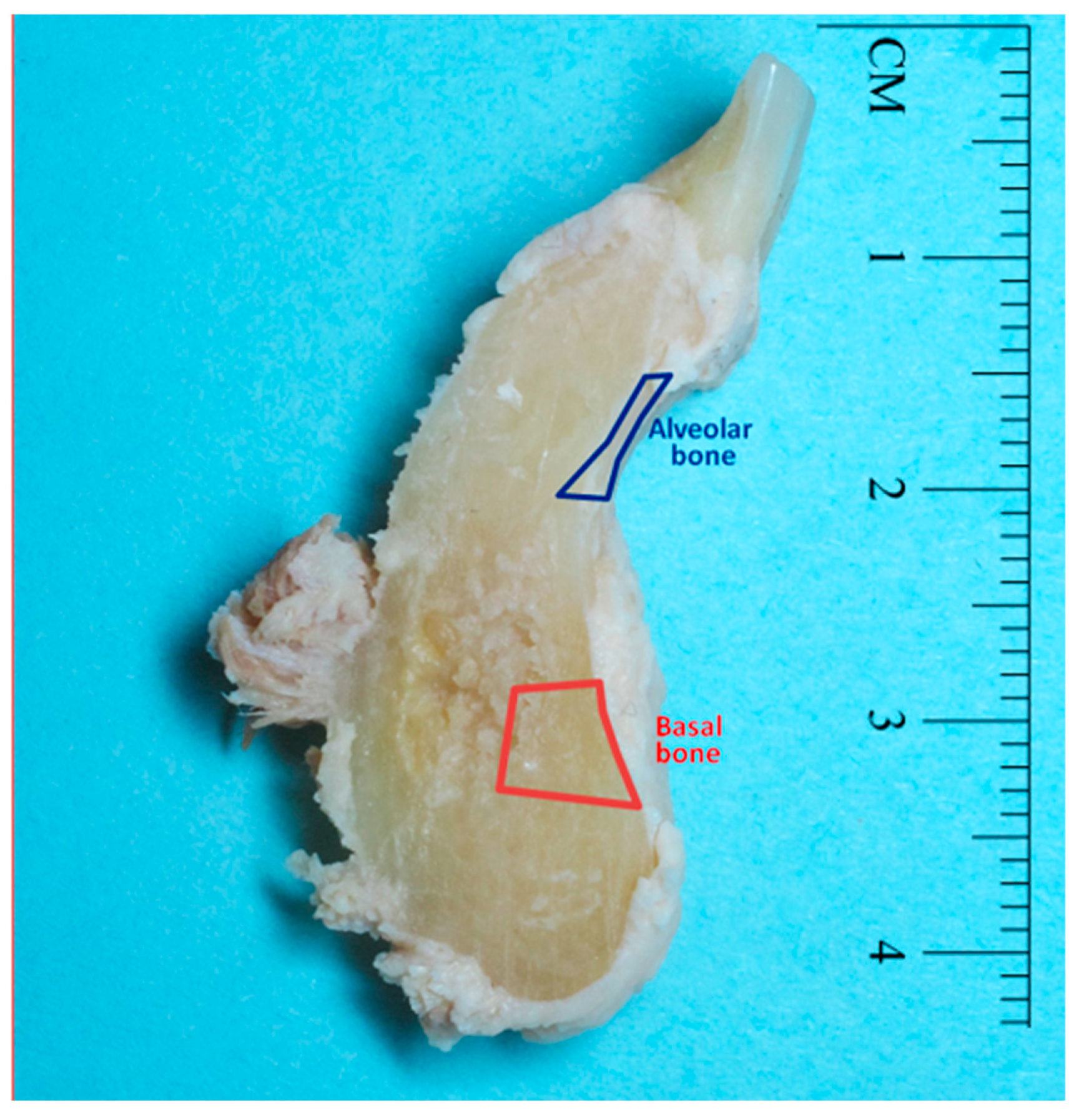
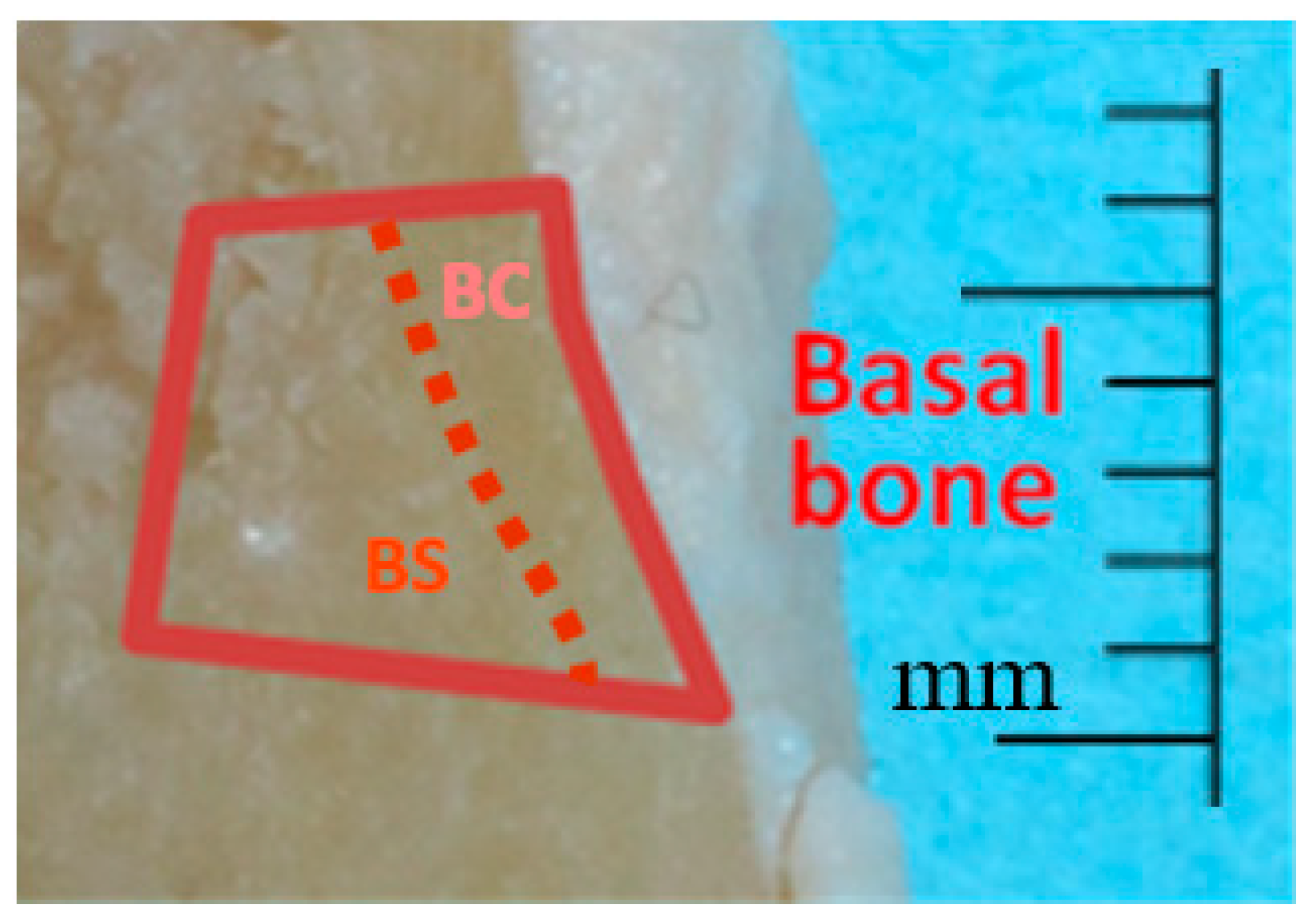
2.1. Sample Collection
2.2. Cd analysis
2.3. Statistics
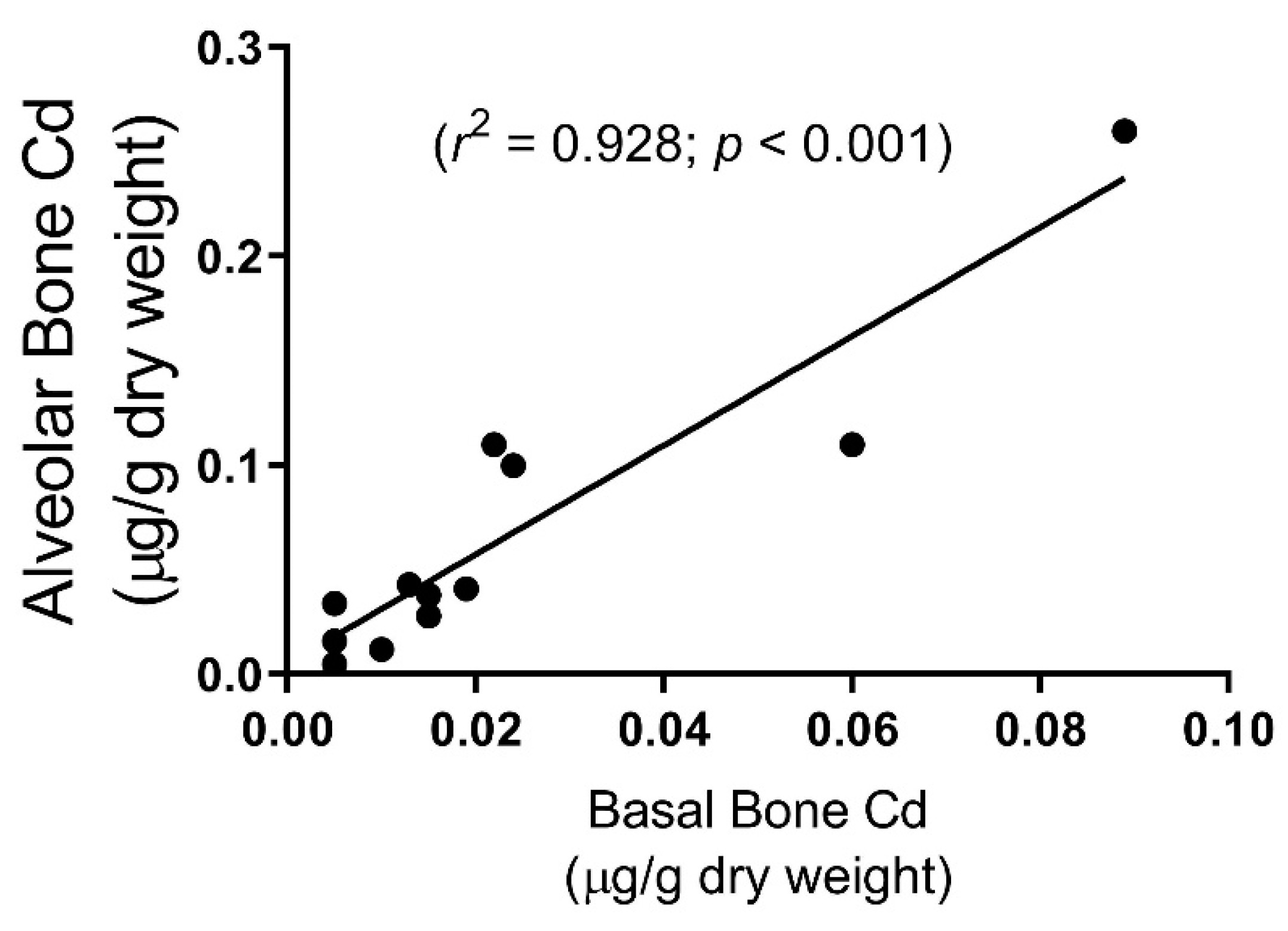
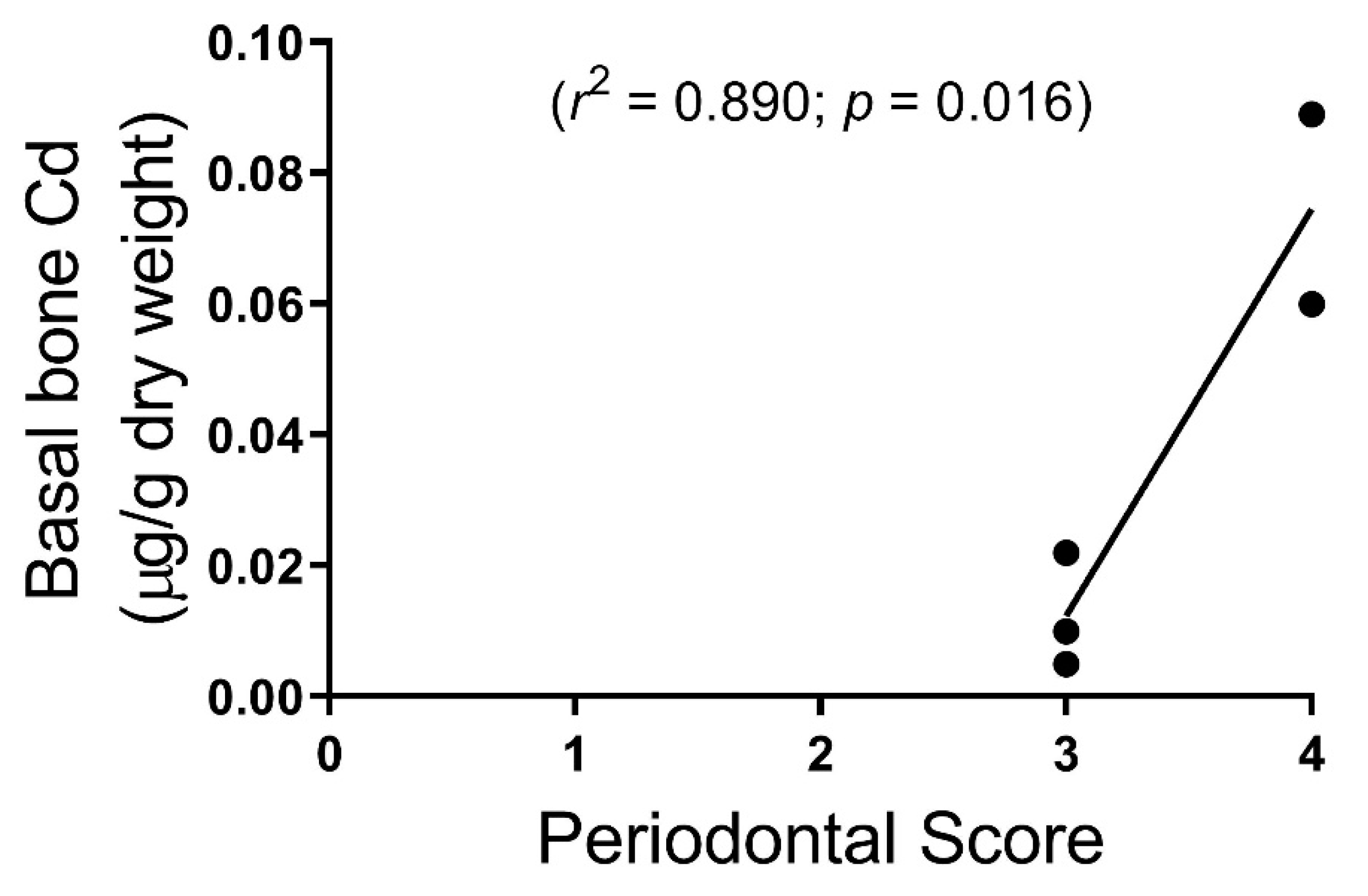
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Paschal, D.C.; Burt, V.; Caudill, S.P.; Gunter, E.W.; Pirkle, J.L.; Sampson, E.J.; Miller, D.T.; Jackson, R.J. Exposure of the U.S. population aged 6 years and older to cadmium: 1988–1994. Arch. Environ. Contam. Toxicol. 2000, 38, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Böhlandt, A.; Schierl, R.; Diemer, J.; Koch, C.; Bolte, G.; Kiranoglu, M.; Fromme, H.; Nowak, D. High concentrations of cadmium, cerium and lanthanum in indoor air due to environmental tobacco smoke. Sci. Total Environ. 2012, 414, 738–741. [Google Scholar] [CrossRef]
- Uchida, M.; Teranishi, H.; Aoshima, K.; Katoh, T.; Kasuya, M.; Inadera, H. Elevated urinary levels of vitamin D-binding protein in the inhabitants of a cadmium polluted area, Jinzu River basin, Japan. Tohoku J. Exp. Med. 2007, 211, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Schutte, R.; Nawrot, T.S.; Richart, T.; Thijs, L.; Vanderschueren, D.; Kuznetsova, T.; Van Hecke, E.; Roels, H.A.; Staessen, J.A. Bone resorption and environmental exposure to cadmium in women: A population study. Environ. Health Perspect. 2008, 116, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.H. Cadmium osteotoxicity in experimental animals: Mechanisms and relationship to human exposures. Toxicol. Appl. Pharmacol. 2009, 238, 258–265. [Google Scholar] [CrossRef]
- Oliver, R.C.; Brown, L.J. Periodontal diseases and tooth loss. Periodontol 2000 1993, 2, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Browar, A.W.; Koufos, E.B.; Wei, Y.; Leavitt, L.L.; Prozialeck, W.C.; Edwards, J.R. Cadmium Exposure Disrupts Periodontal Bone in Experimental Animals: Implications for Periodontal Disease in Humans. Toxics 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Position Paper: Diagnosis of Periodontal Diseases. J. Periodontol. 2003, 74, 1237–1247. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Cadmium levels in the lung, liver, kidney cortex, and urine samples from Australians without occupational exposure to metals. Arch. Environ. Health 2002, 57, 69–77. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, P.C.; Couttenye, M.M.; Lamberts, L.V.; Elseviers, M.M.; Goodman, W.G.; Schrooten, I.; Cabrera, W.E.; De Broe, M.E. Aluminum, iron, lead, cadmium, copper, zinc, chromium, magnesium, strontium, and calcium content in bone of end-stage renal failure patients. Clin. Chem. 1999, 45, 1548–1556. [Google Scholar] [PubMed]
- Carlsson, L.; Lundholm, C.E. Characterisation of the effects of cadmium on the release of calcium and on the activity of some enzymes from neonatal mouse calvaria in culture. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 115, 251–256. [Google Scholar] [CrossRef]
- Romare, A.; Lundholm, C.E. Cadmium-induced calcium release and prostaglandin E2 production in neonatal mouse calvaria are dependent on cox-2 induction and protein kinase C activation. Arch. Toxicol. 1999, 73, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Morita, I.; Yamane, Y.; Murota, S. Cadmium stimulates prostaglandin E2 production and bone resorption in cultured fetal mouse calvaria. Biochem. Biophys. Res. Commun. 1989, 158, 508–513. [Google Scholar] [CrossRef]
- Marth, E.; Barth, S.; Jelovcan, S. Influence of cadmium on the immune system. Description of stimulating reactions. Cent. Eur. J. Public Health 2000, 8, 40–44. [Google Scholar]
- Marth, E.; Jelovcan, S.; Kleinhappl, B.; Gutschi, A.; Barth, S. The effect of heavy metals on the immune system at low concentrations. Int. J. Occup. Med. Environ. Health 2001, 14, 375–386. [Google Scholar]
- Hemdan, N.Y.; Emmrich, F.; Sack, U.; Wichmann, G.; Lehmann, J.; Adham, K.; Lehmann, I. The in vitro immune modulation by cadmium depends on the way of cell activation. Toxicology 2006, 222, 37–45. [Google Scholar] [CrossRef]
- Burt, B. Position paper: Epidemiology of periodontal diseases. J. Periodontol. 2005, 76, 1406–1419. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Environmental exposure to cadmium at a level insufficient to induce renal tubular dysfunction does not affect bone density among female Japanese farmers. Environ. Res. 2005, 97, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wood, N.; Johnson, R.B. Recovery of periodontopathogenic bacteria from embalmed human cadavers. Clin. Anat. 2005, 18, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Karhunen, V.; Forss, H.; Goebeler, S.; Huhtala, H.; Ilveskoski, E.; Kajander, O.; Mikkelsson, J.; Penttila, A.; Perola, M.; Ranta, H.; et al. Radiographic assessment of dental health in middle-aged men following sudden cardiac death. J. Dent. Res. 2006, 85, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nambunmee, K.; Suvagandha, D.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishino, Y. Gender-Specific Impact of Cadmium Exposure on Bone Metabolism in Older People Living in a Cadmium-Polluted Area in Thailand. Int. J. Environ. Res. Public Health 2017, 14, 401. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Apinan, R.; Satarug, S.; Ruengweerayut, R.; Mahavorasirikul, W.; Na-Bangchang, K. The influence of iron stores on cadmium body burden in a Thai population. Environ. Geochem. Health 2010, 32, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Vignery, A.; Baron, R. Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat. Rec. 1980, 196, 191–200. [Google Scholar] [CrossRef] [PubMed]
- King, G.J.; Keeling, S.D.; Wronski, T.J. Histomorphometric study of alveolar bone turnover in orthodontic tooth movement. Bone 1991, 12, 401–409. [Google Scholar] [CrossRef]
- Waddington, R.J.; Embery, G.; Last, K.S. The glycosaminoglycan constituents of alveolar and basal bone of the rabbit. Connect. Tissue Res. 1988, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
| Cadmium µg/g Dry Weight | ||
|---|---|---|
| Alveolar Bone n = 12 | # 0.06 ± 0.02 | |
| Male n = 8 | 0.03 ± 0.01 | |
| Female n = 4 | * 0.13 ± 0.05 | |
| Basal Bone n = 12 | 0.02 ± 0.01 | |
| Male n = 8 | 0.01 ± 0.01 | |
| Female n = 4 | * 0.04 ± 0.02 | |
| Cortical n = 6 | 0.04 ± 0.01 | |
| Spongy n = 6 | 0.03 ± 0.01 | |
| Kidney Cortex n = 6 | 1.83 ± 0.45 | |
| Male n = 4 | 1.97 ± 0.50 | |
| Female n = 2 | 1.55 ± 0.03 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browar, A.W.; Leavitt, L.L.; Prozialeck, W.C.; Edwards, J.R. Levels of Cadmium in Human Mandibular Bone. Toxics 2019, 7, 31. https://doi.org/10.3390/toxics7020031
Browar AW, Leavitt LL, Prozialeck WC, Edwards JR. Levels of Cadmium in Human Mandibular Bone. Toxics. 2019; 7(2):31. https://doi.org/10.3390/toxics7020031
Chicago/Turabian StyleBrowar, Andrew W., Landon L. Leavitt, Walter C. Prozialeck, and Joshua R. Edwards. 2019. "Levels of Cadmium in Human Mandibular Bone" Toxics 7, no. 2: 31. https://doi.org/10.3390/toxics7020031
APA StyleBrowar, A. W., Leavitt, L. L., Prozialeck, W. C., & Edwards, J. R. (2019). Levels of Cadmium in Human Mandibular Bone. Toxics, 7(2), 31. https://doi.org/10.3390/toxics7020031






