Intrauterine Exposure to Cadmium Reduces HIF-1 DNA-Binding Ability in Rat Fetal Kidneys
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment of Animals and Tissue Collection
2.2. HIF-1 DNA-Binding Assay
2.3. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.4. VEGF Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Western Blot
2.6. Statisticals
3. Results
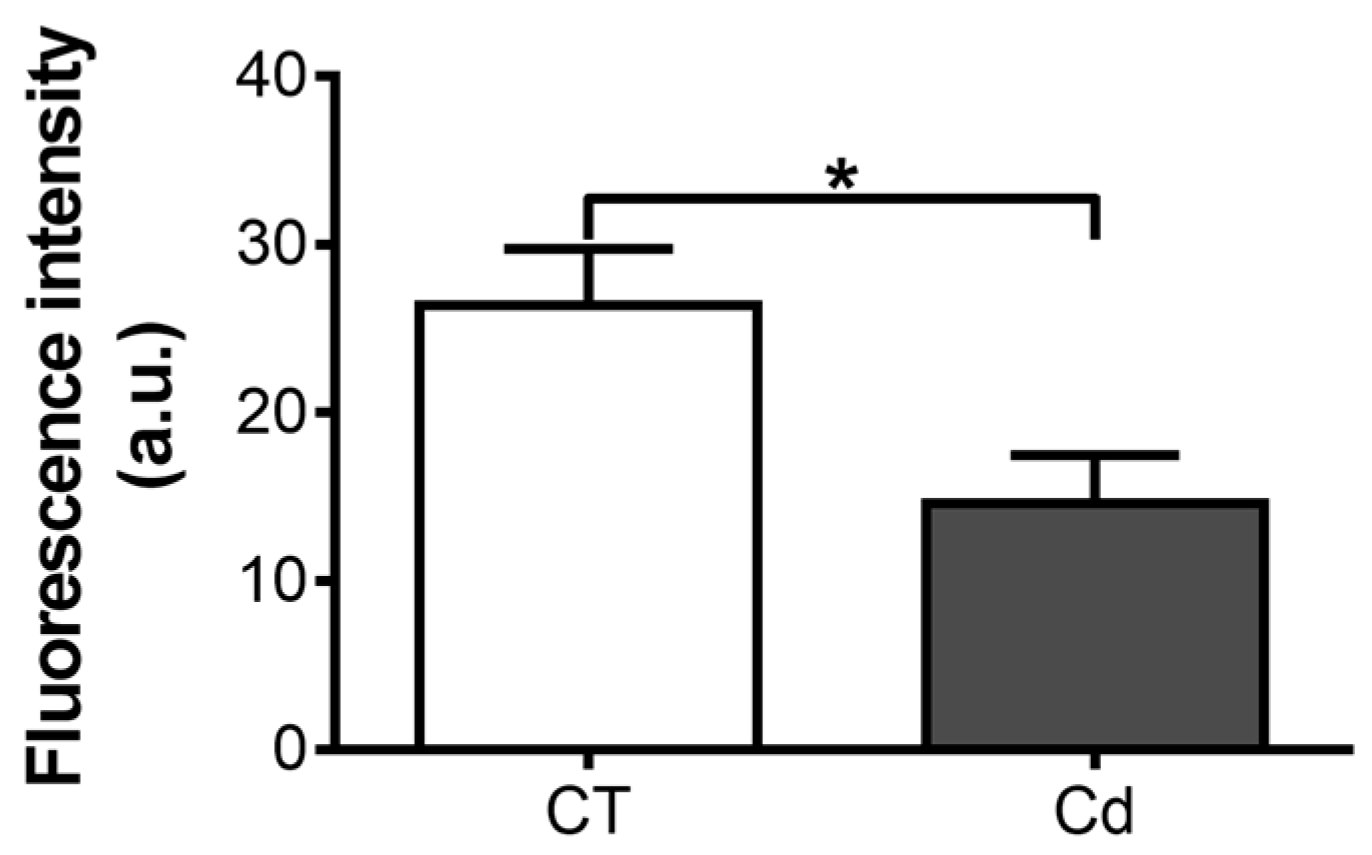
3.1. Effect of Intrauterine Cadmium Exposure on DNA-Binding Ability of HIF-1 in Fetal Kidneys
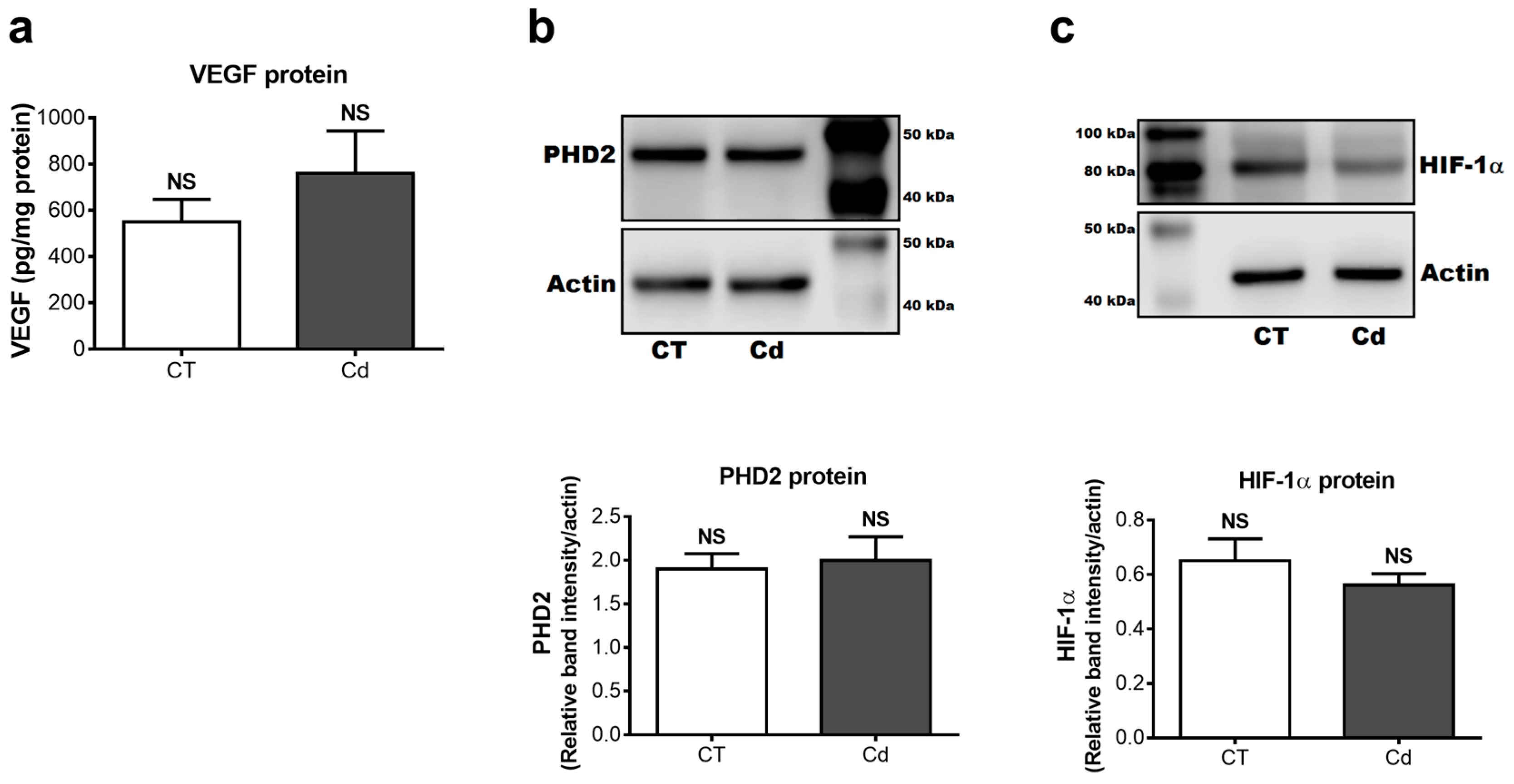
3.2. Effect of Cadmium on the mRNA Expression of VEGF-A, PHD2, and HIF-1α in Fetal Kidneys
3.3. Cadmium-Induced Changes of VEGF, PHD2, and HIF-1α Protein in Fetal Kidneys
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fajersztajn, L.; Veras, M.M. Hypoxia: From placental development to fetal programming. Birth Defects Res. 2017, 109, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, A.R. Embriology and teratology. In The Laboratory Rat; Baker, H.J., Lindsey, J.R., Weisbroth, S.H., Eds.; Academic Press: New York, NY, USA, 1979; Volume 2, pp. 75–101. [Google Scholar]
- Bernhardt, W.M.; Schmitt, R.; Rosenberger, C.; Munchenhagen, P.M.; Grone, H.J.; Frei, U.; Warnecke, C.; Bachmann, S.; Wiesener, M.S.; Willam, C.; et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006, 69, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Dery, M.A.; Michaud, M.D.; Richard, D.E. Hypoxia-inducible factor 1: Regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell Biol. 2005, 37, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Gunaratnam, L.; Bonventre, J.V. HIF in kidney disease and development. J. Am. Soc. Nephrol. 2009, 20, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Mattot, V.; Moons, L.; Lupu, F.; Chernavvsky, D.; Gomez, R.A.; Collen, D.; Carmeliet, P. Loss of the VEGF(164) and VEGF(188) isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J. Am. Soc. Nephrol. 2002, 13, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, Y.; Tokunaga, H.; Tomita, K. Vascular endothelial growth factor is an essential molecule for mouse kidney development: Glomerulogenesis and nephrogenesis. J. Clin. Investig. 1997, 99, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Pagès, G.; Pouyssegur, J. Transcriptional regulation of the vascular endothelial growth factor gene—A concert of activating factors. Cardiovasc. Res. 2005, 65, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohba, K.; Ohta, H. Participation of metal transporters in cadmium transport from mother rat to fetus. J. Toxicol. Sci. 2012, 37, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohba, K.; Suzuki, K.; Ohta, H. Health effects of low-level cadmium intake and the role of metallothionein on cadmium transport from mother rats to fetus. J. Toxicol. Sci. 2012, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Jacquillet, G.; Barbier, O.; Rubera, I.; Tauc, M.; Borderie, A.; Namorado, M.C.; Martin, D.; Sierra, G.; Reyes, J.L.; Poujeol, P.; et al. Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. Am. J. Physiol. Ren. Physiol. 2007, 293, F1450–F1460. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Estrada, T.; Cardenas-Gonzalez, M.; Santoyo-Sanchez, M.; Parada-Cruz, B.; Uria-Galicia, E.; Arreola-Mendoza, L.; Barbier, O. Evaluation of kidney injury biomarkers in rat amniotic fluid after gestational exposure to cadmium. J. Appl. Toxicol. 2016, 36, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Choi, E.; Kim, G.T.; Choi, H.; Kim, C.H.; Lee, M.J.; Kim, M.S.; Park, J.W. Cadmium blocks hypoxia-inducible factor (HIF)-1-mediated response to hypoxia by stimulating the proteasome-dependent degradation of HIF-1alpha. Eur. J. Biochem. 2000, 267, 4198–4204. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, J.; Zhao, Y.; Toselli, P.; Li, W. Hypoxia-response element (hre)-directed transcriptional regulation of the rat lysyl oxidase gene in response to cobalt and cadmium. Toxicol. Sci. 2013, 132, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Kayama, F.; Oguma, E.; Willmore, W.G.; Hradecky, P.; Bunn, H.F. Cadmium and platinum suppression of erythropoietin production in cell culture: Clinical implications. Blood 2000, 96, 3743–3747. [Google Scholar] [PubMed]
- Jing, Y.; Liu, L.Z.; Jiang, Y.; Zhu, Y.; Guo, N.L.; Barnett, J.; Rojanasakul, Y.; Agani, F.; Jiang, B.H. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2012, 125, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Obara, N.; Imagawa, S.; Nakano, Y.; Suzuki, N.; Yamamoto, M.; Nagasawa, T. Suppression of erythropoietin gene expression by cadmium depends on inhibition of HIF-1, not stimulation of GATA-2. Arch. Toxicol. 2003, 77, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Ivanina, A.V.; Sokolov, E.P.; Sokolova, I.M. Effects of cadmium on anaerobic energy metabolism and mRNA expression during air exposure and recovery of an intertidal mollusk Crassostrea virginica. Aquat. Toxicol. 2010, 99, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Piontkivska, H.; Chung, J.S.; Ivanina, A.V.; Sokolov, E.P.; Techa, S.; Sokolova, I.M. Molecular characterization and mRNA expression of two key enzymes of hypoxia-sensing pathways in eastern oysters Crassostrea virginica (Gmelin): Hypoxia-inducible factor alpha (HIF-alpha) and HIF-prolyl hydroxylase (PHD). Comp. Biochem. Physiol. Part D Genomics Proteomics 2011, 6, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Dangre, A.J.; Manning, S.; Brouwer, M. Effects of cadmium on hypoxia-induced expression of hemoglobin and erythropoietin in larval sheepshead minnow, cyprinodon variegatus. Aquat. Toxicol. 2010, 99, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Cadmium; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Prigge, E. Early signs of oral and inhalative cadmium uptake in rats. Arch. Toxicol. 1978, 40, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Baranski, B. Behavioral alterations in offspring of female rats repeatedly exposed to cadmium oxide by inhalation. Toxicol. Lett. 1984, 22, 53–61. [Google Scholar] [CrossRef]
- Trottier, B.; Athot, J.; Ricard, A.C.; Lafond, J. Maternal-fetal distribution of cadmium in the guinea pig following a low dose inhalation exposure. Toxicol. Lett. 2002, 129, 189–197. [Google Scholar] [CrossRef]
- Cabiati, M.; Raucci, S.; Caselli, C.; Guzzardi, M.A.; D’Amico, A.; Prescimone, T.; Giannessi, D.; Del Ry, S. Tissue-specific selection of stable reference genes for real-time pcr normalization in an obese rat model. J. Mol. Endocrinol. 2012, 48, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, L.; Han, Y.; Teng, Y.; Sun, J.; Xie, R.; Cao, J. Testosterone replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing expression of cytokines HIF-1a, SDF-1a and VEGF. Eur. J. Pharmacol. 2012, 684, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Dai, A.G.; Hu, R.C.; Jiang, Y.L. Differential and reciprocal regulation between hypoxia-inducible factor-alpha subunits and their prolyl hydroxylases in pulmonary arteries of rat with hypoxia-induced hypertension. Acta Biochim. Biophys. Sin. 2006, 38, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Katavetin, P.; Miyata, T.; Inagi, R.; Tanaka, T.; Sassa, R.; Ingelfinger, J.R.; Fujita, T.; Nangaku, M. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J. Am. Soc. Nephrol. 2006, 17, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Semczuk, M.; Semczuk-Sikora, A. New data on toxic metal intoxication (Cd, Pb, and Hg in particular) and mg status during pregnancy. Med. Sci. Monit. 2001, 7, 332–340. [Google Scholar] [PubMed]
- Salnikow, K.; Su, W.; Blagosklonny, M.V.; Costa, M. Carcinogenic metals induce hypoxia-inducible factor-stimulated transcription by reactive oxygen species-independent mechanism. Cancer Res. 2000, 60, 3375–3378. [Google Scholar] [PubMed]
- Yao, Y.X.; Lu, Y.H.; Chen, W.C.; Jiang, Y.P.; Cheng, T.; Ma, Y.P.; Lu, L.; Dai, W. Cobalt and nickel stabilize stem cell transcription factor oct4 through modulating its sumoylation and ubiquitination. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, B.; Li, L.; Dong, F.; Chen, X.; Li, Y.; Dong, X.; Wada, Y.; Kapron, C.M.; Liu, J. Low-dose cadmium upregulates vegf expression in lung adenocarcinoma cells. Int. J. Environ. Res. Public Health 2015, 12, 10508–10521. [Google Scholar] [CrossRef] [PubMed]
- Kubis, H.P.; Hanke, N.; Scheibe, R.J.; Gros, G. Accumulation and nuclear import of HIF1 alpha during high and low oxygen concentration in skeletal muscle cells in primary culture. Biochim. Biophys. Acta 2005, 1745, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Minet, E.; Mottet, D.; Michel, G.; Roland, I.; Raes, M.; Remacle, J.; Michiels, C. Hypoxia-induced activation of HIF-1: Role of HIF-1alpha-hsp90 interaction. FEBS Lett. 1999, 460, 251–256. [Google Scholar] [CrossRef]
- Isaacs, J.S.; Jung, Y.J.; Mimnaugh, E.G.; Martinez, A.; Cuttitta, F.; Neckers, L.M. Hsp90 regulates a von hippel lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J. Biol. Chem. 2002, 277, 29936–29944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.; Costa, M.; Gao, J.; Huang, C. JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res. 2010, 70, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Somji, S.; Ann Sens, M.; Garrett, S.H.; Gurel, V.; Todd, J.H.; Sens, D.A. Expression of Hsp90 in the human kidney and in proximal tubule cells exposed to heat, sodium arsenite and cadmium chloride. Toxicol. Lett. 2002, 133, 241–254. [Google Scholar] [CrossRef]
- D’Souza, S.M.; Brown, I.R. Constitutive expression of heat shock proteins Hsp90, Hsc70, Hsp70 and Hsp60 in neural and non-neural tissues of the rat during postnatal development. Cell Stress Chaperones 1998, 3, 188–199. [Google Scholar] [CrossRef]
- Papaconstantinou, A.D.; Brown, K.M.; Noren, B.T.; McAlister, T.; Fisher, B.R.; Goering, P.L. Mercury, cadmium, and arsenite enhance heat shock protein synthesis in chick embryos prior to embryotoxicity. Birth Defects Res. B Dev. Reprod. Toxicol. 2003, 68, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Goering, P.L.; Kish, C.L.; Fisher, B.R. Stress protein synthesis induced by cadmium-cysteine in rat kidney. Toxicology 1993, 85, 25–39. [Google Scholar] [CrossRef]
- Xia, B.; Cao, H.; Luo, J.; Liu, P.; Guo, X.; Hu, G.; Zhang, C. The co-induced effects of molybdenum and cadmium on antioxidants and heat shock proteins in duck kidneys. Biol. Trace Elem. Res. 2015, 168, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hermesz, E.; Abraham, M.; Nemcsok, J. Identification of two Hsp90 genes in carp. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 129, 397–407. [Google Scholar] [CrossRef]
- Madden, E.F.; Akkerman, M.; Fowler, B.A. A comparison of 60, 70, and 90 kDa stress protein expression in normal rat NRK-52 and human HK-2 kidney cell lines following in vitro exposure to arsenite and cadmium alone or in combination. J. Biochem. Mol. Toxicol. 2002, 16, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Dundjerski, J.; Kovac, T.; Pavkovic, N.; Cvoro, A.; Matic, G. Glucocorticoid receptor-Hsp90 interaction in the liver cytosol of cadmium-intoxicated rats. Cell Biol. Toxicol. 2000, 16, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ye, F.; Xue, W.; Zhou, Z.; Kang, Y.J. Copper regulation of hypoxia-inducible factor-1 activity. Mol. Pharmacol. 2009, 75, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kuriwaki, J.; Nishijo, M.; Honda, R.; Tawara, K.; Nakagawa, H.; Hori, E.; Nishijo, H. Effects of cadmium exposure during pregnancy on trace elements in fetal rat liver and kidney. Toxicol. Lett. 2005, 156, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Petering, H.G.; Choudhury, H.; Stemmer, K.L. Some effects of oral ingestion of cadmium on zinc, copper, and iron metabolism. Environ. Health Perspect. 1979, 28, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sowa, B.; Steibert, E. Effect of oral cadmium administration to female rats during pregnancy on zinc, copper, and iron content in placenta, foetal liver, kidney, intestine, and brain. Arch. Toxicol. 1985, 56, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Baranski, B. Effect of cadmium on prenatal development and on tissue cadmium, copper, and zinc concentrations in rats. Environ. Res. 1987, 42, 54–62. [Google Scholar] [CrossRef]
- Gheorghescu, A.K.; Tywoniuk, B.; Duess, J.; Buchete, N.V.; Thompson, J. Exposure of chick embryos to cadmium changes the extra-embryonic vascular branching pattern and alters expression of VEGF-A and VEGF-R2. Toxicol. Appl. Pharmacol. 2015, 289, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Arcondeguy, T.; Lacazette, E.; Millevoi, S.; Prats, H.; Touriol, C. VEGF-A mrna processing, stability and translation: A paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res. 2013, 41, 7997–8010. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobo-Estrada, T.; Cardenas-Gonzalez, M.; Santoyo-Sánchez, M.P.; Thevenod, F.; Barbier, O. Intrauterine Exposure to Cadmium Reduces HIF-1 DNA-Binding Ability in Rat Fetal Kidneys. Toxics 2018, 6, 53. https://doi.org/10.3390/toxics6030053
Jacobo-Estrada T, Cardenas-Gonzalez M, Santoyo-Sánchez MP, Thevenod F, Barbier O. Intrauterine Exposure to Cadmium Reduces HIF-1 DNA-Binding Ability in Rat Fetal Kidneys. Toxics. 2018; 6(3):53. https://doi.org/10.3390/toxics6030053
Chicago/Turabian StyleJacobo-Estrada, Tania, Mariana Cardenas-Gonzalez, Mitzi Paola Santoyo-Sánchez, Frank Thevenod, and Olivier Barbier. 2018. "Intrauterine Exposure to Cadmium Reduces HIF-1 DNA-Binding Ability in Rat Fetal Kidneys" Toxics 6, no. 3: 53. https://doi.org/10.3390/toxics6030053
APA StyleJacobo-Estrada, T., Cardenas-Gonzalez, M., Santoyo-Sánchez, M. P., Thevenod, F., & Barbier, O. (2018). Intrauterine Exposure to Cadmium Reduces HIF-1 DNA-Binding Ability in Rat Fetal Kidneys. Toxics, 6(3), 53. https://doi.org/10.3390/toxics6030053





