Meta-Analysis of NOS3 G894T Polymorphisms with Air Pollution on the Risk of Ischemic Heart Disease Worldwide
Abstract
1. Introduction
2. Materials and Methods
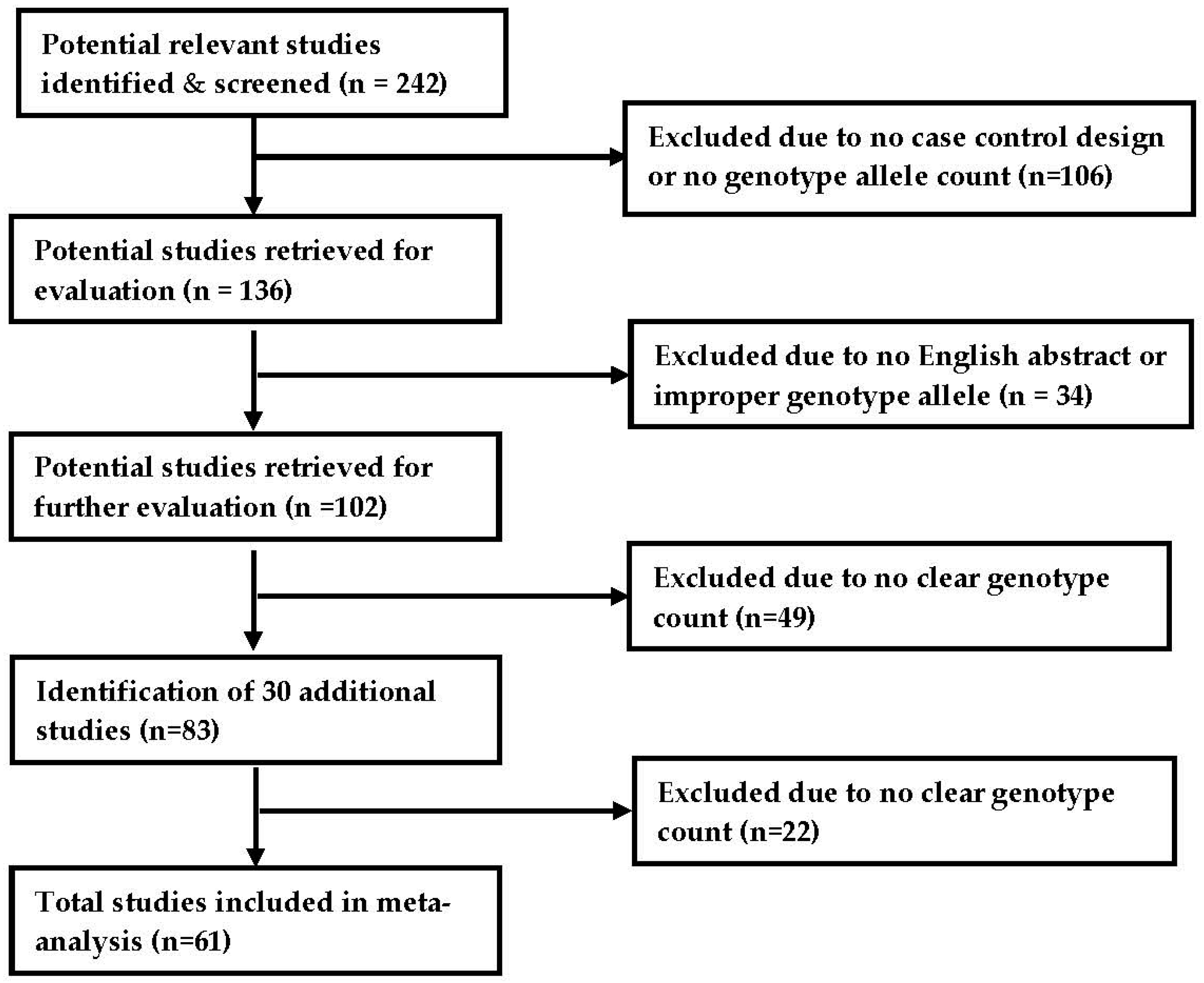
2.1. Search Strategy and Selection Criteria
2.2. Characteristics of Included Studies
2.3. Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
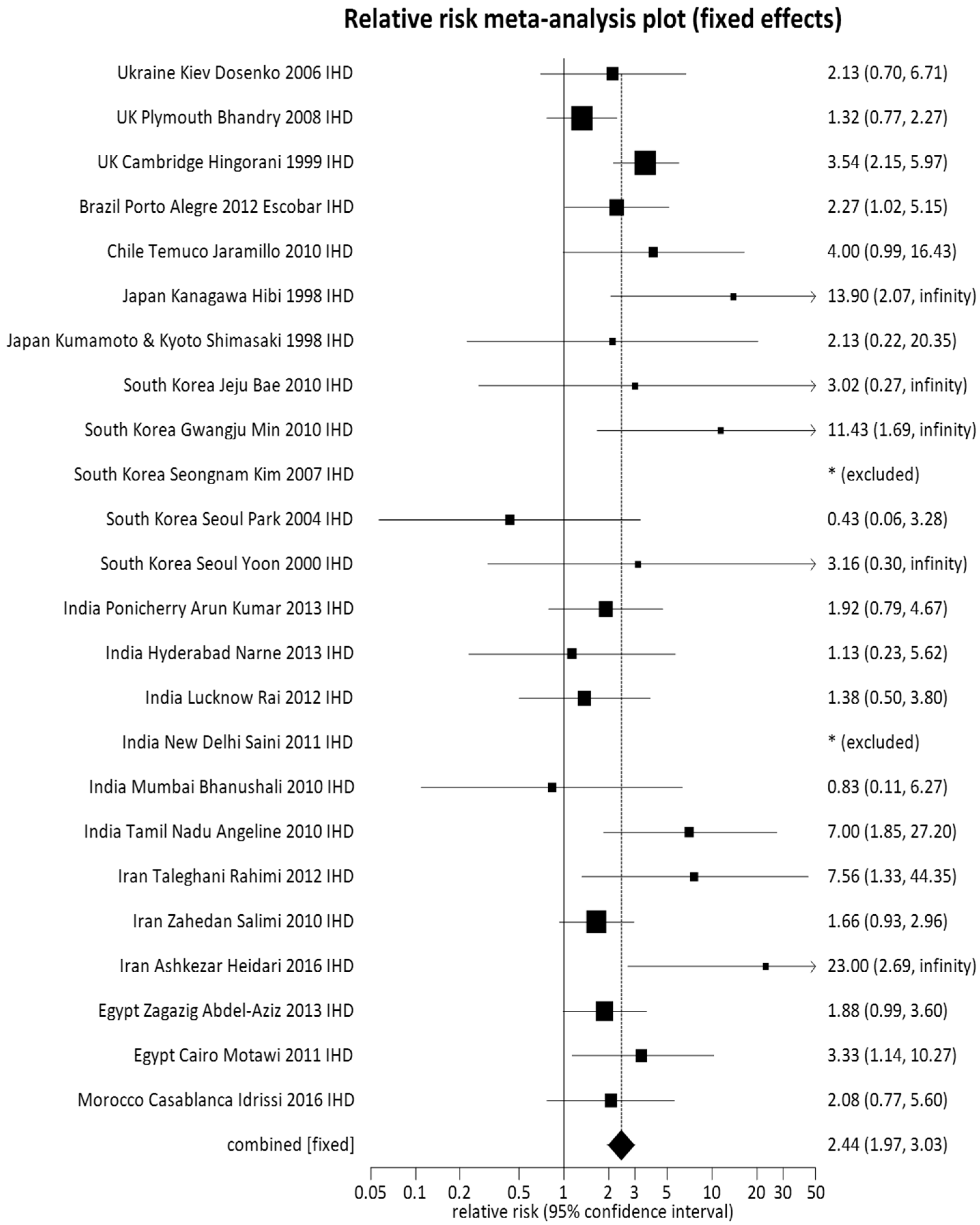
3.1. Pooled Meta-Analysis
3.2. Subgroup Analysis
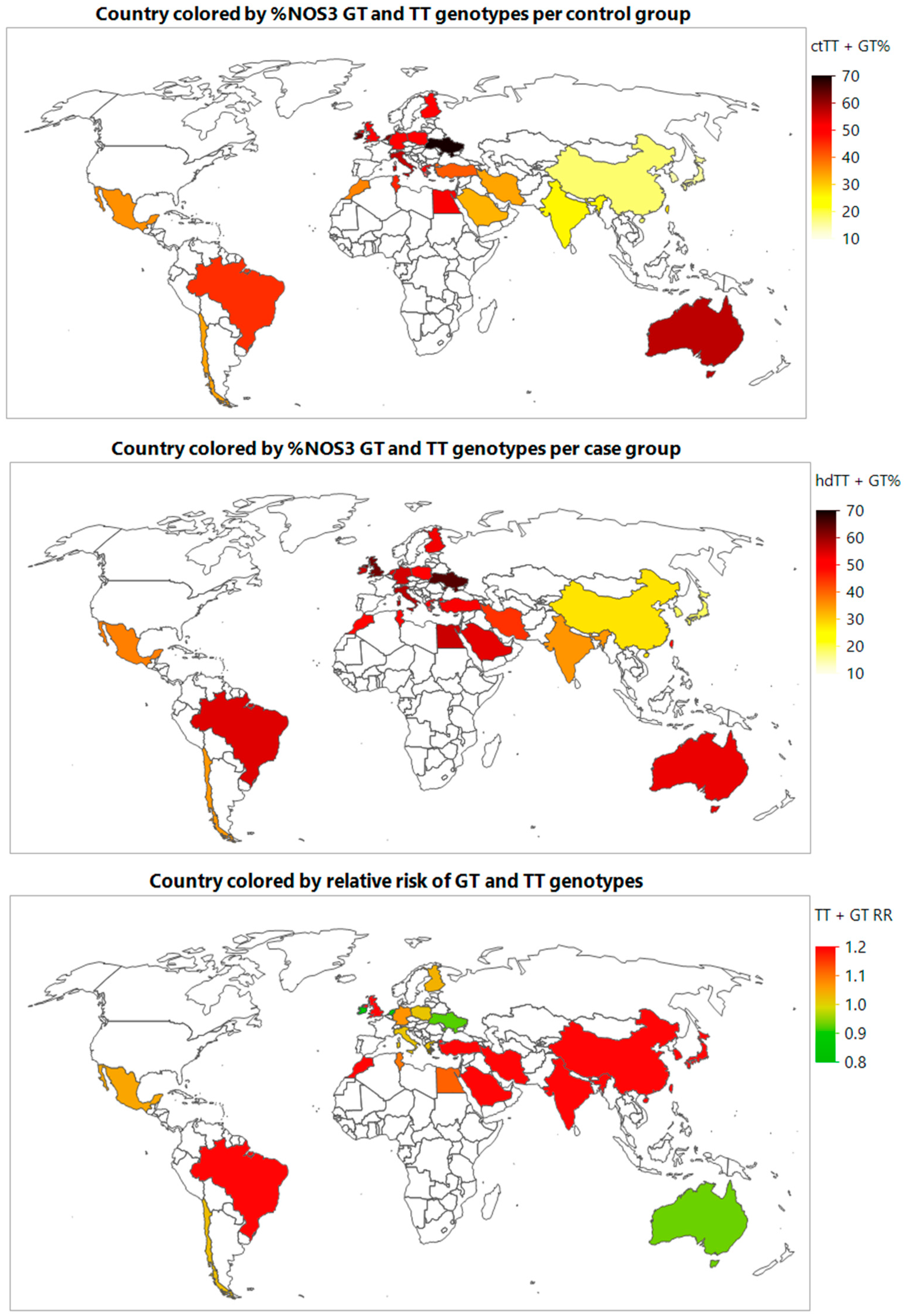
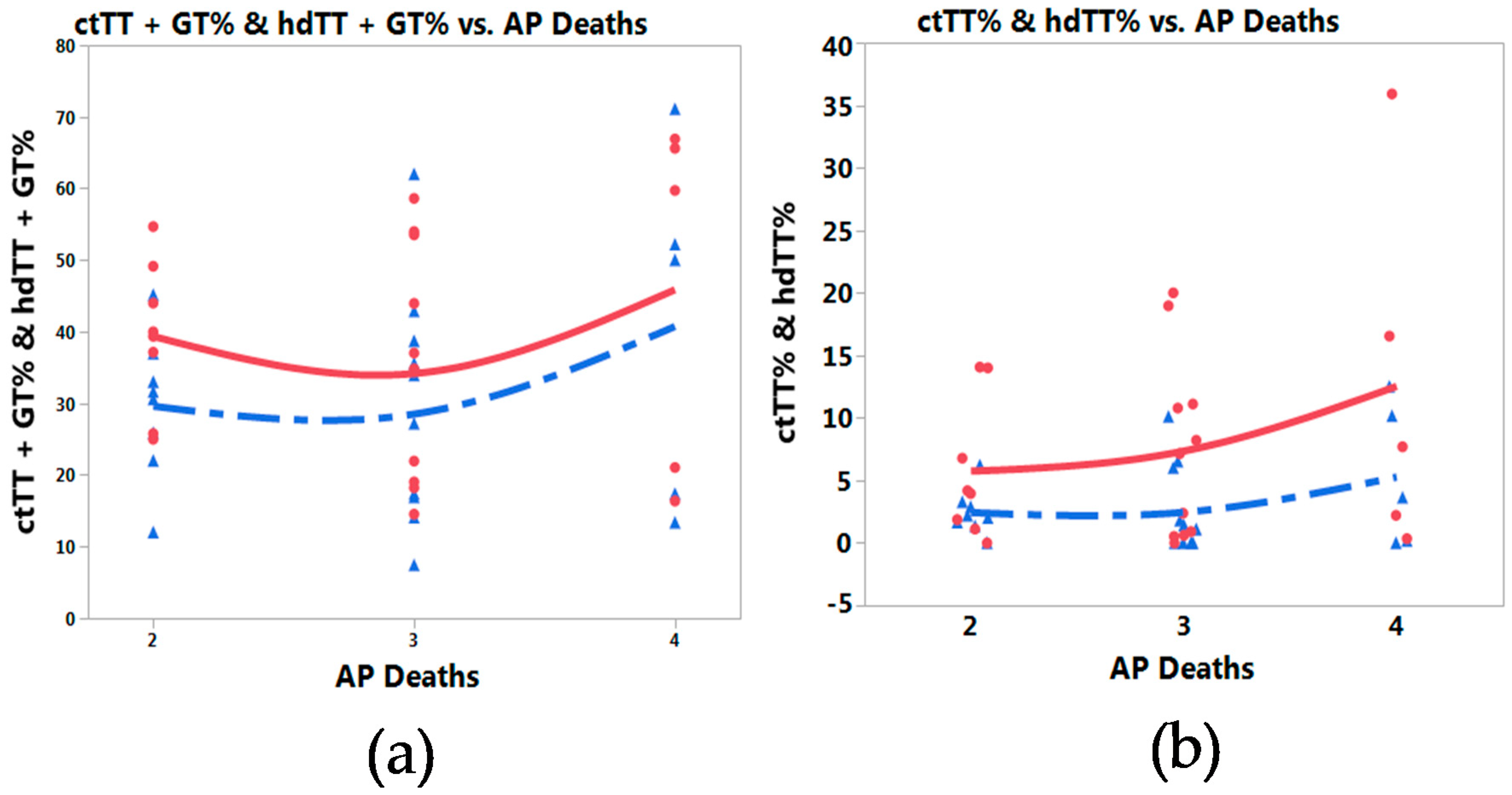
3.3. Meta-Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ferguson, J.F.; Phillips, C.M.; McMonagle, J.; Pérez-Martínez, P.; Shaw, D.I.; Lovegrove, J.A.; Roche, H.M. NOS3 gene polymorphisms are associated with risk markers of cardiovascular disease, and interact with omega-3 polyunsaturated fatty acids. Atherosclerosis 2010, 211, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, P.C.; Munoz, M.A.; Lanas, M.C.; Lanas, Z.F.; Salazar, I.A. Endothelial nitric oxide synthase G894T gene polymorphism in Chilean subjects with coronary artery disease and controls. Clin. Chim. Acta 2006, 371, 102–106. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases. Available online: http://www.who.int/cardiovascular diseases/en/ (accessed on 1 March 2018).
- Center for Disease Control and Prevention. Heart Disease Facts. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 1 March 2018).
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Woo, Y.J. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Cardiovascular Disease: A Costly Burden for Americans. Available online: http://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_491543.pdf (accessed on 1 March 2018).
- Arnett, D.K.; Baird, A.E.; Barkley, R.A.; Basson, C.T.; Boerwinkle, E.; Ganesh, S.K.; O’Donnell, C.J. Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: A scientific statement from the American Heart Association council on epidemiology and prevention, the stroke council, and the functional genomics and translational biology interdisciplinary working group. Circulation 2007, 115, 2878–2901. [Google Scholar] [CrossRef] [PubMed]
- Gluba, A.; Banach, M.; Rysz, J.; Piotrowski, G.; Fendler, W.; Pietrucha, T. Is polymorphism within eNOS gene associated with the late onset of myocardial infarction? A pilot study. Angiology 2009, 60, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.G.; Andreassi, M.G.; Paradossi, U.; Botto, N.; Manfredi, S.; Masetti, S.; Biagini, A. Evidence for association of a common variant of the endothelial nitric oxide synthase gene (Glu298Asp polymorphism) to the presence, extent, and severity of coronary artery disease. Heart 2002, 82, 525–528. [Google Scholar] [CrossRef]
- Gardemann, A.; Lohre, J.; Cayci, S.; Katz, N.; Tillmanns, H.; Haberbosch, W. The T allele of the missense Glu298Asp endothelial nitric oxide synthase gene polymorphism is associated with coronary heart disease in younger individuals with high atherosclerotic risk profile. Atherosclerosis 2002, 160, 167–175. [Google Scholar] [CrossRef]
- Hingorani, A.D.; Liang, C.F.; Fatibene, J.; Lyon, A.; Monteith, S.; Parson, A.; Brown, M.J. A common variant of the endothelial nitric oxide synthase (Glu298–>Asp) is a major risk factor for coronary artery disease in the UK. Circulation 1999, 100, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Rai, H.; Parveen, F.; Kumar, S.; Kapoor, A.; Sinha, N. Association of endothelial nitric oxide synthase gene polymorphisms with coronary artery disease: An updated meta-analysis and systematic review. PLoS ONE 2009, 9, e113363. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Baghdadi, H.; Allam, A.R. Role of genetic changes in the progression of cardiovascular diseases. Int. J. Biomed. Sci. 2011, 7, 238–248. [Google Scholar] [PubMed]
- Gorchakova, O.; Koch, W.; von Beckerath, N.; Mehilli, J.; Schömig, A.; Kastrati, A. Association of a genetic variant of endothelial nitric oxide synthase with the 1 year clinical outcome after coronary stent placement. Eur. Heart J. 2003, 9, 820–827. [Google Scholar] [CrossRef]
- Pereira, T.V.; Rudnicki, M.; Cheung, B.M.Y.; Baum, L.; Yamada, Y.; Oliveira, P.S.L.; Krieger, J.E. Three endothelial nitric oxide (NOS3) gene polymorphisms in hypertensive and normotensive individuals: Meta-analysis of 53 studies reveals evidence of publication bias. J. Hypertens. 2007, 25, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.S.; Mineo, C.; Shaul, P.W.; Bauer, J.A. Biochemical consequences of the NOS3 Glu298Asp variation in human endothelium: Altered caveolar localization and impaired response to shear. FASEB J. 2007, 21, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, K.; Liu, Z.; Lu, X. Endothelial nitric oxide synthase (eNOS) 4b/a gene polymorphisms and coronary artery disease: Evidence from a meta-analysis. Int. J. Mol. Sci. 2014, 7, 7987–8003. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Q.; Wen, J.G.; Zhou, H.H.; Chen, X.P.; Zhang, W. Endothelial nitric oxide synthase gene G894T polymorphism and myocardial infarction: A meta-analysis of 34 studies involving 21068 subjects. PLoS ONE 2014, 9, e87196. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.P.; Bautista, L.E.; Humphries, S.E.; Hingorani, A.D. Endothelial nitric oxide synthase genotype and ischemic heart disease: meta-analysis of 26 studies involving 23028 subjects. Circulation 2004, 109, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Yu, L.; Huang, G.R.; Zhang, L.; Liu, Y.Q.; Wang, T.W.; Xiong, H.Y. Polymorphisms of angiotensin converting enzyme and nitric oxide synthase 3 genes risk factors of high altitude pulmonary edema: A case-control study and meta-analysis. Tohoku J. Exp. Med. 2013, 229, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Bai, P.; Shi, S.; Zhou, B.; Wang, Y.; Song, Y.; Rao, L.; Zhang, L. The G894T polymorphism on endothelial nitric oxide synthase gene is associated with increased coronary heart disease among Asia population: Evidence from a Meta analysis. Thromb. Res. 2012, 130, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 2016, 8, E8–E19. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.; Ezzati, M.; Dockery, D.W. Fine-particulate air pollution and life expectancy in the United States. N. Engl. J. Med. 2009, 360, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J. Particulate air pollution, progression, and survival after myocardial infarction. Environ. Health Perspect. 2007, 115, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D. Evaluating the effects of ambient air pollution on life expectancy. N. Engl. J. Med. 2009, 360, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Rich, D.Q.; Schwartz, J.; Mittleman, M.A.; Link, M.; Luttmann-Gibson, H.; Catalano, P.J.; Dockery, D.W. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am. J. Epidemiol. 2005, 161, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Cassano, P.A.; Litonjua, A.; Park, S.K.; Suh, H.; Sparrow, D.; Vokonas, P.; Schwartz, J. Cardiac autonomic dysfunction: Effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation 2008, 117, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Jensen, S.S.; Ketzel, M.; Sorensen, M.; Hansen, J.; Overvad, K. Traffic air pollution and mortality from cardiovascular disease and all causes: A Danish cohort study. Environ. Health 2012, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.; Yu, C. Meta-prediction of MTHFR gene polymorphism mutations and associated risk for colorectal cancer. Biol. Res. Nurs. 2016, 18, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, Z.; Young, L.; Shiao, P.S. Meta-prediction of the effect of methylenetetrahydrofolate reductase polymorphisms and air pollution on Alzheimer’s disease risk. Int. J. Environ. Res. Public Health 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Nunez, V.; Johns, R.; Shiao, P.S. APOA5 gene polymorphisms and cardiovascular diseases metaprediction in global populations. Nurs. Res. 2017, 66, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.C.; Yu, P.; Shiao, S.P.K. MTHFR gene polymorphism-mutations and air pollution as risk factors for breast cancer: A metaprediction study. Nurs. Res. 2017, 66, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.-Y.A.; Young, L.; Gau, B.-S.; Shiao, S.P. Meta-prediction of MTHFR gene polymorphism-mutations, air pollution, and risks of leukemia among world populations. Oncotarget 2017, 8, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Yang, H.L.; Shiao, S.P. Meta-prediction of MTHFR gene polymorphisms and air pollution on the risk of hypertensive disorders in pregnancy worldwide. Int. J. Environ. Res. Public Health 2018, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Baccarelli, A.; Schwartz, J. Gene-air pollution interaction and cardiovascular disease: A review. Prog. Cardiovasc. Dis. 2011, 53, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Kim, J.H.; Jung, K.; Hong, Y.C. Associations of air pollution exposure with blood pressure and heart rate variability are modified by oxidative stress genes: A repeated-measures panel among elderly urban residents. Environ. Health 2016, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscion, E.; Iaccarino, G.; Sorriento, D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail. Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Schneider, A.; Greven, S.; Bellander, T.; Forastiere, F.; Ibald-Mulli, A.; Sunyer, J. Air pollution and inflammatory response in myocardial infarction survivors: Gene-environment interactions in a high-risk group. Inhal. Toxicol. 2007, 19, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Park, S.K.; Vokonas, P.S.; Sparrow, D.; Wilker, E.; Baccarelli, A.; Suh, H.H.; Tucker, K.L.; Wright, R.O.; Schwartz, J. Air pollution and homocysteine: More evidence that oxidative stress-related genes modify effects of particulate air pollution. Epidemiology 2010, 21, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Muto, E.; Hayashi, T.; Yamada, K.; Esaki, T.; Sagai, M.; Iguchi, A. Endothelial-constitutive nitric oxide synthase exists in airways and diesel exhaust particles inhibit the effect of nitric oxide. Life Sci. 1996, 59, 1563–1570. [Google Scholar] [CrossRef]
- Sun, Q.H.; Yue, P.B.; Ying, Z.K.; Cardounel, A.J.; Brook, R.D.; Devlin, R.; Rajagopalan, S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Panasevich, S.; Leander, K.; Ljungman, P.; Bellander, T.; de Faire, U.; Pershagen, G.; Nyberg, F. Interaction between air pollution exposure and genes in relation to levels of inflammatory markers and risk of myocardial infarction. BMJ Open 2013, 3, e003058. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Ten Have, G.A.; Wolfe, R.R.; Deutz, N.E. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ströhle, A.; Hahn, A. Age-associated changes in the metabolism of vitamin B12 and folic acid: Prevalence, aetiopathogenesis and pathophysiological consequences. Z. Gerontol. Geriatr. 2004, 37, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, O.; Klünemann, H.H.; Sede, F.; Tordjman, S.; Cohen, D.; Walterfang, M. Diagnostic and treatment implications of psychosis secondary to treatable metabolic disorders in adults: A systematic review. Orphanet J. Rare Dis. 2014, 28, 65. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Cook, D.J.; Eastwood, S.; Olkin, I.; Rennie, D.; Stroup, D.F. Improving the quality of reports of meta-analyses of randomized controlled trials: The quorom statement. Onkologie 2000, 23, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomized studies of health care interventions. J. Epidemiol. Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency. Air Quality Index. Available online: http://www.airnow.gov/index.cfm?action=aqibasics.aqi (accessed on 8 December 2017).
- Kenworthy, J.; Laube, F. Urban transport patterns in a global sample of cities and their linkages to transport infrastructure, land use, economics and environment. World Transp. Policy Pract. 2002, 8, 5–19. [Google Scholar]
- World Health Organization. Deaths Attributable to Urban Air Pollution. 2004. Available online: http://www.who.int/heli/risks/urban/en/uapmap.1.pdf?ua=1 (accessed on 8 December 2017).
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. 2009. Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (accessed on 8 December 2017).
- World Health Organization. Global Health Risks: Deaths from Air Pollution. 2012. Available online: https://commons.wikimedia.org/wiki/ File:Deaths from_air_pollution.png (accessed on 8 December 2017).
- World Health Organization. The Urban Environment: A General Directory of Resources. 2015. Available online: http://www.who.int/heli/risks/urban/urbenvdirectory/en/ (accessed on 8 December 2017).
- Sha, Q.; Zhang, S. A test of Hardy-Weinberg equilibrium in structured populations. Genet. Epidemiol. 2011, 35, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Wittke-Thompson, J.K.; Pluzhnikov, A.; Cox, N.J. Rational inferences about departures from Hardy-Weinberg equilibrium. Am. J. Hum. Genet. 2005, 76, 967–986. [Google Scholar] [CrossRef] [PubMed]
- How AICR Recommendations Cuts Colorectal Cancer Risk for Both Men and Women. Available online: http://www.aicr.org/cancer-research-update/2016/11_02/cru-how-AICR-recommendations-cuts-colorectal-cancer-risk-for-men-and-women.html (accessed on 16 March 2018).
- WCRF-AICR Continuous Update Project. Diet, Nutrition, Physical Activity and Colorectal Cancer. 2017. Available online: http://www.aicr.org/continuous-update-project/reports/colorectal-cancer-2017-report.pdf (accessed on 1 July 2018).
- Viera, A.J. Odds ratios and risk ratios: What’s the difference and why does it matter? South. Med. J. 2008, 101, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Scott, I. Interpreting risks and ratios in therapy trials. Aust. Prescr. 2008, 31, 12–16. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.; Altman, D.G. Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J., Green, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 243–296. [Google Scholar]
- Albrecht, J. Key Concepts and Techniques in GIS; Sage: Thousand Oaks, CA, USA, 2007. [Google Scholar]
- Pereira, C.; Denise, A.; Lespinet, O. A meta-approach for improving the prediction and the functional annotation of ortholog groups. BMC Genom. 2014, 15 (Suppl. 6), S16. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Malley, J.; Tutz, G. An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods 2009, 14, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.W.; Wong, T.W.; Wong, A.H.; Hui, D.S. Effect of dust storm events on daily emergency admissions for respiratory diseases. Respirology 2012, 17, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Lai, L. Public health risks of prolonged fine particle events associated with stagnation and air quality index based on fine particle matter with a diameter <2.5 μm in the Kaoping region of Taiwan. Int. J. Biometeorol. 2016, 60, 1907–1917. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Tukey’s honesty significant difference (HSD) test. In Encyclopedia of Research Design; Salkind, N., Ed.; Sage: Thousand Oaks, CA, USA, 2010; pp. 1565–1570. [Google Scholar]
- Mao, W. Leave-one-out cross-validation-based model selection for multi-input multi-output support vector machine. Neural Comput. Appl. 2014, 24, 441–451. [Google Scholar] [CrossRef]
- Yu, C.H. Exploratory data analysis in the context of data mining and resampling. Int. J. Psychol. Res. 2010, 3, 9–22. [Google Scholar] [CrossRef]
- Yu, C.H. Dancing with the Data: The Art and Science of Data Visualization; LAP: Saarbrucken, Germany, 2014. [Google Scholar]
- Cook, E.F.; Goldman, L. Empiric comparison of multivariate analytic techniques: Advantages and disadvantages of recursive partitioning analysis. J. Chronic Dis. 1984, 37, 721–731. [Google Scholar] [CrossRef]
- Kattan, M.W.; Hess, K.R.; Beck, J.R. Experiments to determine whether recursive partitioning (CART) or an artificial neural network overcomes theoretical limitations of Cox proportional hazards regression. Comput. Biomed. Res. 1998, 31, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Prediction and entropy. In A Celebration of Statistics; Atkinson, A.C., Fienberg, S.E., Eds.; Springer: New York, NY, USA, 1985; pp. 1–24. [Google Scholar]
- Faraway, J.J. Extending the Linear Model with r (Texts in Statistical Science); Chapman & Hall/CRC: New York, NY, USA, 2005. [Google Scholar]
- Lu, Y.; Fang, T.B. Examining personal air pollution exposure, intake, and health danger zone using time geography and 3D geovisualization. ISPRS Int. J. Geo-Inf. 2014, 4, 32–46. [Google Scholar] [CrossRef]
- Packard, G. On the use of log-transformation vs. nonlinear regression for analyzing biological power laws. Biol. J. Linn. Soc. 2014, 113, 1167–1178. [Google Scholar] [CrossRef]
- Xiao, X.; White, E.P.; Hooten, M.B.; Durham, S.L. On the use of log-transformation vs. nonlinear regression for analyzing biological power laws. Ecology 2011, 92, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Lee, H.S.; Gan, S.; Brown, E. Nonlinear modeling with big data in SAS and JMP. In Proceedings of the Western Users of SAS Software Conference (paper presented), Long Beach, CA, USA, 22 September 2017. [Google Scholar]
- Health Effects Institute. State of Global Air 2018 Special Report. Health Effects Institute: Boston, MA. Available online: https://www.stateofglobalair.org/sites/default/files/soga-2018-report.pdf (accessed on 21 April 2018).
- Miller, M.; Shaw, C.; Langrish, J. From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012, 8, 577–602. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Guo, Y.; Zhang, Y.; Westerdahl, D.; Mo, Y.; Liang, F.; Pan, X. Spatiotemporal analysis of particulate air pollution and ischemic heart disease mortality in Beijing, China. Environ. Health 2014, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Zhou, J.; Wong, P.W.; Kowalisyn, J.; Strokosch, G. Intermediate homocysteinemia: A thermolabile variant of methylenetetrahydrofolate reductase. Am. J. Hum. Genet. 1998, 43, 414–421. [Google Scholar]
- Leclerc, D.; Sibani, S.; Rozen, R. Molecular biology of methylenetetrahydrofolate reductase (MTHFR) and overview of mutations/polymorphisms. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK6561/ (accessed on 29 May 2018).
- Mahmoodi, K.; Nasehi, L.; Karami, E.; Soltanpour, M.S. Association of nitric oxide levels and endothelial nitric oxide synthase G894T polymorphism with coronary artery disease in the Iranian population. Vasc. Specialist Int. 2016, 32, 105. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Hsiao, J.Y.; Tien, K.J.; Chang, S.J.; Lin, P.C.; Hsu, S.C.; Lin, S.R. The association of endothelial nitric oxide synthase G894T polymorphism with C-reactive protein level and metabolic syndrome in a Chinese study group. Metabolism 2008, 57, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Mackawy, A.M.; Khan, A.A.; Badawy, M.E. Association of the endothelial nitric oxide synthase gene G894T polymorphism with the risk of diabetic nephropathy in Qassim region, Saudi Arabia—A pilot study. Meta Gene 2014, 2, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y. Endothelial nitric oxide synthase G894T gene polymorphism and essential hypertension in the Chinese population: A meta-analysis involving 11,248 subjects. Intern. Med. 2011, 50, 2099–2106. [Google Scholar] [PubMed]
- Angeline, T.; Krithiga, H.R.; Isabel, W.; Asirvatham, A.J.; Poornima, A. Endothelial nitric oxide synthase gene polymorphism (G894T) and diabetes mellitus (type II) among South Indians. Oxid. Med. Cell. Longev. 2011, 2011, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tso, A.W.; Tan, K.C.; Wat, N.M.; Janus, E.D.; Lam, T.H.; Lam, K.S. Endothelial nitric oxide synthase G894T (Glu298Asp) polymorphism was predictive of glycemic status in a 5-year prospective study of Chinese subjects with impaired glucose tolerance. Metabolism 2006, 55, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Li, B.; Li, J.; Sun, Y. The Effects of Age, Period, and Cohort on Mortality from Ischemic Heart Disease in China. Int. J. Environ. Res. Public Health 2017, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Mu, Z.; Jiang, B.; Wang, W.; Yu, H.; Zhang, L.; Li, J. Acute effects of particulate air pollution on ischemic heart disease hospitalizations in Shanghai, China. Int. J. Environ. Res. Public Health 2017, 14, 168. [Google Scholar] [CrossRef] [PubMed]
| Genotype | Cases | Controls | Tests of Association | ||
|---|---|---|---|---|---|
| (Number of Studies) | n = 16,219 (%) | n = 12,222 (%) | Model | RR (95% CI) | p |
| TT (61) | 1551 (9.56) | 759 (6.21) | Random | 1.44 (1.23, 1.67) | 0.0001 |
| Caucasian (25) | 1230 (12.30) | 593 (10.21) | Random | 1.30 (1.08, 1.56) | 0.0051 |
| Hispanic (2) | 33 (6.00) | 17 (4.43) | Fixed | 1.34 (0.76, 2.34) | 0.3044 |
| East Asian (18) | 79 (2.60) | 26 (0.74) | Fixed | 2.17 (1.46, 3.25) | 0.0001 |
| South Asian (6) | 40 (4.16) | 18 (2.08) | Fixed | 2.11 (1.21, 3.65) | 0.0076 |
| Middle East (4) | 65 (9.31) | 25 (4.26) | Fixed | 2.35 (1.47, 3.74) | 0.0003 |
| African (6) | 104 (10.54) | 80 (8.06) | Random | 1.45 (0.87, 2.41) | 0.1457 |
| GT (61) | 6066 (37.40) | 3971 (32.49) | Random | 1.37 (1.18, 1.57) | 0.0001 |
| Caucasian (25) | 4271 (42.71) | 2538 (43.69) | Random | 0.96 (0.91, 1.02) | 0.2571 |
| Hispanic (2) | 175 (31.81) | 126 (32.81) | Fixed | 0.96 (0.79, 1.16) | 0.6801 |
| East Asian (18) | 672 (22.12) | 518 (14.66) | Random | 1.38 (1.19, 1.59) | 0.0001 |
| South Asian (6) | 286 (29.79) | 229 (26.44) | Fixed | 1.17 (1.01, 1.36) | 0.0304 |
| Middle East (4) | 272 (38.96) | 180 (30.61) | Random | 1.25 (0.92, 1.70) | 0.1388 |
| African (6) | 390 (39.55) | 380 (38.27) | Random | 1.02 (0.83, 1.26) | 0.8032 |
| GG (61) | 8613 (53.10) | 7442 (60.89) | Random | 0.92 (0.89, 0.95) | 0.0001 |
| Caucasian (25) | 4498 (44.98) | 2677 (46.09) | Random | 0.95 (0.89, 1.01) | 0.139 |
| Hispanic (2) | 342 (62.18) | 241 (62.76) | Fixed | 0.99 (0.89, 1.10) | 0.9341 |
| East Asian (18) | 2286 (75.27) | 2989 (84.60) | Random | 0.91 (0.87, 0.95) | 0.0001 |
| South Asian (6) | 634 (66.04) | 619 (71.48) | Random | 0.97 (0.84, 1.13) | 0.7656 |
| Middle East (4) | 361 (51.72) | 383 (65.14) | Fixed | 0.77 (0.70, 0.85) | 0.0001 |
| African (6) | 492 (49.90) | 533 (53.68) | Random | 0.91 (0.76, 1.07) | 0.2809 |
| TT + GT (61) | 7617 (46.96) | 4730 (38.70) | Random | 1.15 (1.09, 1.22) | 0.0001 |
| Caucasian (25) | 5501 (55.01) | 3131 (53.90) | Random | 1.03 (0.98, 1.09) | 0.176 |
| Hispanic (2) | 208 (37.81) | 143 (37.24) | Fixed | 1.00 (0.85, 1.19) | 0.9339 |
| East Asian (18) | 751 (24.73) | 544 (15.40) | Random | 1.44 (1.26, 1.64) | 0.0001 |
| South Asian (6) | 326 (33.96) | 247 (28.52) | Fixed | 1.24 (1.08, 1.43) | 0.0018 |
| Middle East (4) | 337 (48.28) | 205 (34.86) | Fixed | 1.42 (1.24, 1.63) | 0.0001 |
| African (6) | 494 (50.10) | 460 (46.32) | Random | 1.10 (0.92, 1.33) | 0.2638 |
| T allele (61) | 4585 (28.20) | 2744 (22.5) | Random | 1.18 (1.11, 1.25) | 0.0001 |
| Caucasian (25) | 3366 (33.66) | 1862 (32.05) | Random | 1.07 (1.00, 1.16) | 0.0466 |
| Hispanic (2) | 120 (21.82) | 80 (20.83) | Fixed | 1.04 (0.81, 1.34) | 0.7399 |
| East Asian (18) | 415 (13.66) | 285 (8.06) | Fixed | 1.48 (1.28, 1.71) | 0.0001 |
| South Asian (6) | 183 (19.06) | 132 (15.24) | Fixed | 1.30 (1.06, 1.60) | 0.0106 |
| Middle East (4) | 201 (28.80) | 115 (19.56) | Fixed | 1.52 (1.24, 1.87) | 0.0001 |
| African (6) | 299 (30.32) | 270 (27.19) | Fixed | 1.10 (0.95, 1.26) | 0.1743 |
| G allele (61) | 11646 (71.80) | 9428 (77.2) | Random | 0.95 (0.93, 0.97) | 0.0001 |
| Caucasian (25) | 6634 (66.34) | 3946 (67.94) | Random | 0.96 (0.92,0.99) | 0.0304 |
| Hispanic (2) | 429 (78.00) | 304 (79.17) | Fixed | 0.98 (0.92, 1.05) | 0.7404 |
| East Asian (18) | 2622 (86.34) | 3248 (91.93) | Fixed | 0.95 (0.93, 0.97) | 0.0001 |
| South Asian (6) | 777 (80.94) | 734 (84.76) | Random | 1.02 (0.89, 1.16) | 0.7493 |
| Middle East (4) | 497 (71.20) | 473 (80.44) | Fixed | 0.87 (0.82, 0.93) | 0.0001 |
| African (6) | 687 (69.68) | 723 (72.81) | Fixed | 0.96 (0.90, 1.01) | 0.1761 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johns, R.; Chen, Z.-F.; Young, L.; Delacruz, F.; Chang, N.-T.; Yu, C.H.; Shiao, S.P.K. Meta-Analysis of NOS3 G894T Polymorphisms with Air Pollution on the Risk of Ischemic Heart Disease Worldwide. Toxics 2018, 6, 44. https://doi.org/10.3390/toxics6030044
Johns R, Chen Z-F, Young L, Delacruz F, Chang N-T, Yu CH, Shiao SPK. Meta-Analysis of NOS3 G894T Polymorphisms with Air Pollution on the Risk of Ischemic Heart Disease Worldwide. Toxics. 2018; 6(3):44. https://doi.org/10.3390/toxics6030044
Chicago/Turabian StyleJohns, Robin, Zhao-Feng Chen, Lufei Young, Flordelis Delacruz, Nien-Tzu Chang, Chong Ho Yu, and S. Pamela K. Shiao. 2018. "Meta-Analysis of NOS3 G894T Polymorphisms with Air Pollution on the Risk of Ischemic Heart Disease Worldwide" Toxics 6, no. 3: 44. https://doi.org/10.3390/toxics6030044
APA StyleJohns, R., Chen, Z.-F., Young, L., Delacruz, F., Chang, N.-T., Yu, C. H., & Shiao, S. P. K. (2018). Meta-Analysis of NOS3 G894T Polymorphisms with Air Pollution on the Risk of Ischemic Heart Disease Worldwide. Toxics, 6(3), 44. https://doi.org/10.3390/toxics6030044






