Determinants of Hair Manganese, Lead, Cadmium and Arsenic Levels in Environmentally Exposed Children
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Experimental
2.3. Data Analyses
3. Results
3.1. Hair Metal Levels in Children Vary by Several Orders of Magnitude between Subjects and Were Highly Correlated
3.2. Variance in Hair Metal Levels between-Subjects is Much Greater than within-Subjects, and Hair Mn, Pb, and Cd, but Not As Concentrations Increase from Proximal to Distal Segments
3.3. Hair Metal Levels Vary Seasonally
4. Discussion
4.1. Correlations between Metals Suggests Some Shared Exposure Sources/Pathways
4.2. Hair Metal Levels Vary Substantially More between Subjects than within Subjects, and Hair Mn, Pb, and Cd, but Not As Concentrations Increase from Proximal to Distal Segments
4.3. Hair Metal Levels Vary by Season of Collection
4.4. Hair Metal Concentrations Reported Here Are Generally Lower than other Studies in Children
4.5. Hair Metal Levels as a Biomarker of Exposure and Associated Health Effects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, D.; Gwiazda, R.; Bowler, R.; Roels, H.; Park, R.; Taicher, C.; Lucchini, R. Biomarkers of Mn Exposure in Humans. Am. J. Ind. Med. 2007, 50, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Haynes, E.N.; Sucharew, H.; Kuhnell, P.; Alden, J.; Barnas, M.; Wright, R.O.; Parsons, P.J.; Aldous, K.M.; Praamsma, M.L.; Beidler, C.; et al. Manganese Exposure and Neurocognitive Outcomes in Rural School-Age Children: The Communities Actively Researching Exposure Study (Ohio, USA). Environ. Health Perspect. 2015, 123. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, D.J.; McGovern, P.M.; Rao, R.; Harnack, L.J.; Georgieff, M.K.; Stepanov, I. Measuring the Impact of Manganese Exposure on Children’s Neurodevelopment: Advances and Research Gaps in Biomarker-Based Approaches. Environ. Health 2016, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Austin, C.; Sarrafpour, B.; Hernańdez-Ávila, M.; Hu, H.; Wright, R.O.; Tellez-Rojo, M.M. Determining Prenatal, Early Childhood and Cumulative Long-Term Lead Exposure Using Micro-Spatial Deciduous Dentine Levels. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.P.; Claus Henn, B.; Wright, R.O. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr. Environ. Health Rep. 2015, 2, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Zoni, S.; Lucchini, R.G. Manganese Exposure: Cognitive, Motor and Behavioral Effects on Children: A Review of Recent Findings. Curr. Opin. Pediatr. 2013, 25, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Bergdahl, I.A.; Skerfving, S. Biomonitoring of Lead Exposure—Alternatives to Blood. J. Toxicol. Environ. Health Part A 2008, 71, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.A. Monitoring of Human Populations for Early Markers of Cadmium Toxicity: A Review. Toxicol. Appl. Pharmacol. 2009, 238, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Roels, H.A.; Hoet, P.; Lison, D. Usefulness of Biomarkers of Exposure to Inorganic Mercury, Lead, or Cadmium in Controlling Occupational and Environmental Risks of Nephrotoxicity. Ren. Fail. 1999, 21, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-Level Environmental Lead Exposure and Children’s Intellectual Function: An International Pooled Analysis. Environ. Health Perspect. 2005, 113, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Measuring Lead Exposure in Infants, Children, and other Sensitive Populations; National Academies Press: Washington, DC, USA, 1993.
- Lucas, E.L.; Bertrand, P.; Guazzetti, S.; Donna, F.; Peli, M.; Jursa, T.P.; Lucchini, R.; Smith, D.R. Impact of Ferromanganese Alloy Plants on Household Dust Manganese Levels: Implications for Childhood Exposure. Environ. Res. 2015, 138, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Sauvé, S.; Barbeau, B.; Legrand, M.; Brodeur, M.È.; Bouffard, T.; Limoges, E.; Bellinger, D.C.; Mergler, D. Intellectual Impairment in School-Age Children Exposed to Manganese from Drinking Water. Environ. Health Perspect. 2011, 119, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Eastman, R.R.; Jursa, T.P.; Benedetti, C.; Lucchini, R.G.; Smith, D.R. Hair as a Biomarker of Environmental Manganese Exposure. Environ. Sci. Technol. 2013, 47, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Crinella, F.M. Does Soy-Based Infant Formula Cause ADHD? Update and Public Policy Considerations. Expert Rev. Neurother. 2012, 12, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Caito, S.; Farina, M.; Teixeira da Rocha, J.; Aschner, M.; Carvalho, C. Biomarkers of Mercury Toxicity: Past, Present, and Future Trends. J. Toxicol. Environ. Health Part B 2017, 20, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Skröder, H.; Kippler, M.; Nermell, B.; Tofail, F.; Levi, M.; Rahman, S.M.; Raqib, R.; Vahter, M. Major Limitations in Using Element Concentrations in Hair as Biomarkers of Exposure to Toxic and Essential Trace Elements in Children. Environ. Health Perspect. 2017, 125, 67021. [Google Scholar] [CrossRef] [PubMed]
- Harkey, M.R. Anatomy and Physiology of Hair. Forensic Sci. Int. 1993, 63, 9–18. [Google Scholar] [CrossRef]
- Robbins, C.R. Chemical and Physical Behavior of Human Hair, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Razagui, I.B.-A. A Comparative Evaluation of Three Washing Procedures for Minimizing Exogenous Trace Element Contamination in Fetal Scalp Hair of Various Obstetric Outcomes. Biol. Trace Elem. Res. 2008, 123, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Filho, J.A.; de Carvalho-Vivas, C.F.; Viana, G.F.S.; Ferreira, J.R.D.; Nunes, L.S.; Mergler, D.; Abreu, N. Elevated Manganese Exposure and School-Aged Children’s Behavior: A Gender-Stratified Analysis. Neurotoxicology 2014, 45, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bonilla, D.; Schilmann, A.; Montes, S.; Rodríguez-Agudelo, Y.; Rodríguez-Dozal, S.; Solís-Vivanco, R.; Ríos, C.; Riojas-Rodríguez, H. Environmental Exposure to Manganese and Motor Function of Children in Mexico. Neurotoxicology 2011, 32, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; Mergler, D.; Barbeau, B.; Bellinger, D.C.; Bouffard, T.; Brodeur, M.-È.; Saint-Amour, D.; Legrand, M.; Sauvé, S.; Bouchard, M.F. Neurobehavioral Function in School-Age Children Exposed to Manganese in Drinking Water. Environ. Health Perspect. 2014, 122, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Dongarrà, G.; Lombardo, M.; Tamburo, E.; Varrica, D.; Cibella, F.; Cuttitta, G. Concentration and Reference Interval of Trace Elements in Human Hair from Students Living in Palermo, Sicily (Italy). Environ. Toxicol. Pharmacol. 2011, 32, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hanh, H.T.; Kim, K.-W.; Bang, S.; Hoa, N.M. Community Exposure to Arsenic in the Mekong River Delta, Southern Vietnam. J. Environ. Monit. 2011, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Stauber, J.L.; Florence, T.M. Manganese in Scalp Hair: Problems of Exogenous Manganese and Implications for Manganese Monitoring in Groote Eylandt Aborigines. Sci. Total Environ. 1989, 83, 85–98. [Google Scholar] [CrossRef]
- Stein, C.R.; Savitz, D.A.; Bellinger, D.C. Perfluorooctanoate and Neuropsychological Outcomes in Children. Epidemiology 2013, 24, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Rugless, F.; Bhattacharya, A.; Succop, P.; Dietrich, K.N.; Cox, C.; Alden, J.; Kuhnell, P.; Barnas, M.; Wright, R.; Parsons, P.J.; et al. Childhood Exposure to Manganese and Postural Instability in Children Living near a Ferromanganese Refinery in Southeastern Ohio. Neurotoxicol. Teratol. 2014, 41, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Health Consultation: Marietta Area Air Investigation Marietta, Ohio; ATSDR: Atlanta, GA, USA, 2009. [Google Scholar]
- Da Silva, J.J.R.F.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Torrente, M.; Colomina, M.T.; Domingo, J.L. Metal Concentrations in Hair and Cognitive Assessment in an Adolescent Population. Biol. Trace Elem. Res. 2005, 104, 215–222. [Google Scholar] [CrossRef]
- Molina-Villalba, I.; Lacasaña, M.; Rodríguez-Barranco, M.; Hernández, A.F.; Gonzalez-Alzaga, B.; Aguilar-Garduño, C.; Gil, F. Biomonitoring of Arsenic, Cadmium, Lead, Manganese and Mercury in Urine and Hair of Children Living near Mining and Industrial Areas. Chemosphere 2015, 124, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Filho, J.A.; de Sousa Viana, G.F.; Paes, C.R. Determinants of Lead Exposure in Children on the Outskirts of Salvador, Brazil. Environ. Monit. Assess. 2012, 184, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, H.; Yang, L.; Li, Y.; Wei, B.; Wang, W.; Gong, H.; Guo, M.; Nima, C.; Zhao, S.; et al. Trace Element Levels in Scalp Hair of School Children in Shigatse, Tibet, an Endemic Area for Kaschin-Beck Disease (KBD). Biol. Trace Elem. Res. 2017, 180, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Evrenoglou, L.; Partsinevelou, S.A.; Stamatis, P.; Lazaris, A.; Patsouris, E.; Kotampasi, C.; Nicolopoulou-Stamati, P. Children Exposure to Trace Levels of Heavy Metals at the North Zone of Kifissos River. Sci. Total Environ. 2013, 443, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Llorente Ballesteros, M.T.; Navarro Serrano, I.; Izquierdo Álvarez, S. Reference Levels of Trace Elements in Hair Samples from Children and Adolescents in Madrid, Spain. J. Trace Elem. Med. Biol. 2017, 43, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Drobyshev, E.J.; Solovyev, N.D.; Ivanenko, N.B.; Kombarova, M.Y.; Ganeev, A.A. Trace Element Biomonitoring in Hair of School Children from a Polluted Area by Sector Field Inductively Coupled Plasma Mass Spectrometry. J. Trace Elem. Med. Biol. 2017, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Filho, J.A.; Novaes, C.d.O.; Moreira, J.C.; Sarcinelli, P.N.; Mergler, D. Elevated Manganese and Cognitive Performance in School-Aged Children and Their Mothers. Environ. Res. 2011, 111, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Torres-Agustín, R.; Rodríguez-Agudelo, Y.; Schilmann, A.; Solís-Vivanco, R.; Montes, S.; Riojas-Rodríguez, H.; Cortez-Lugo, M.; Ríos, C. Effect of Environmental Manganese Exposure on Verbal Learning and Memory in Mexican Children. Environ. Res. 2013, 121, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.; Mergler, D.; Baldwin, M.; Panisset, M.; Bowler, R.; Roels, H.A. Neurobehavioral Functioning after Cessation of Manganese Exposure: A Follow-up after 14 Years. Am. J. Ind. Med. 2007, 50, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.O.; Amarasiriwardena, C.; Woolf, A.D.; Jim, R.; Bellinger, D.C. Neuropsychological Correlates of Hair Arsenic, Manganese, and Cadmium Levels in School-Age Children Residing near a Hazardous Waste Site. Neurotoxicology 2006, 27, 210–216. [Google Scholar] [CrossRef] [PubMed]
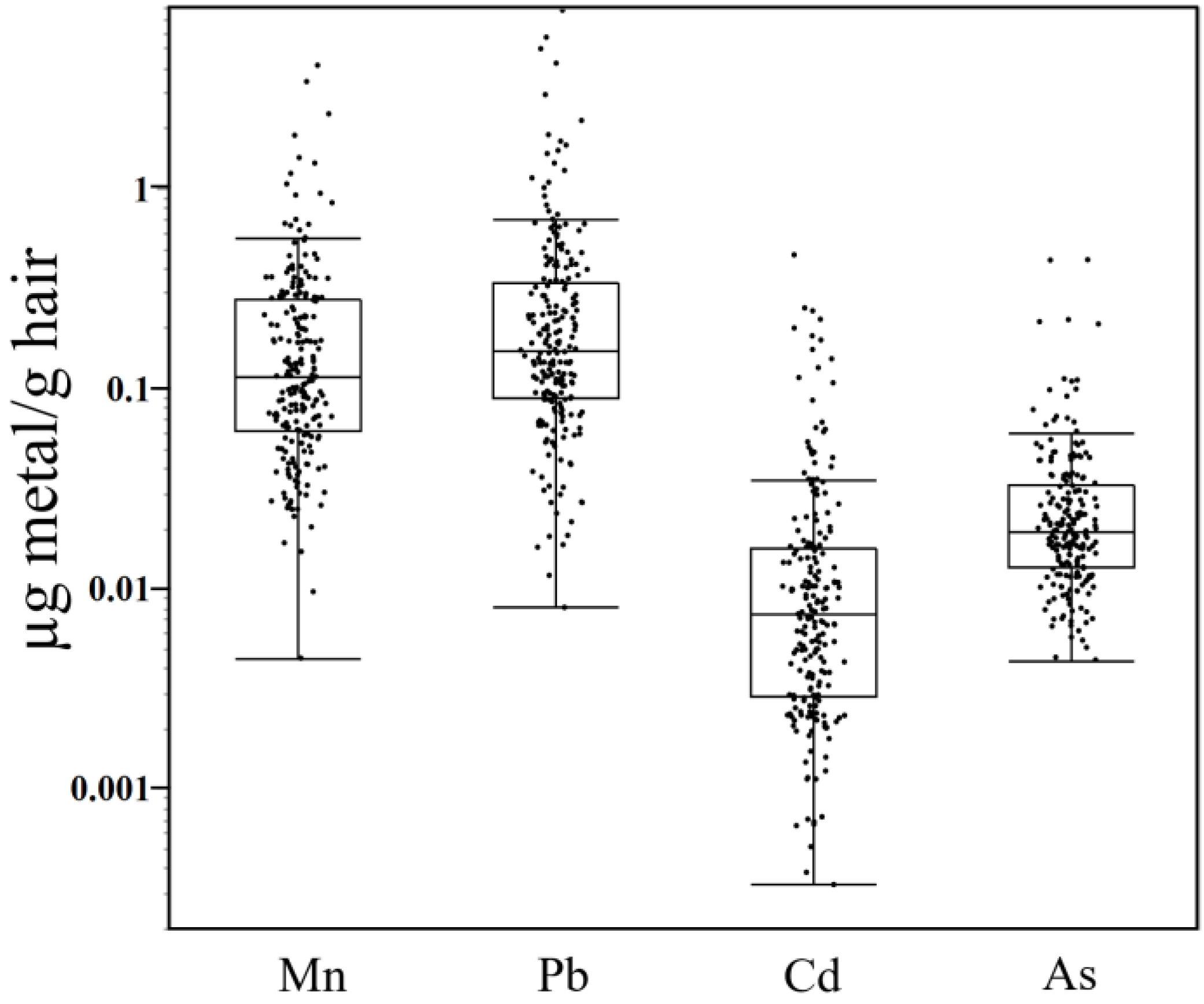
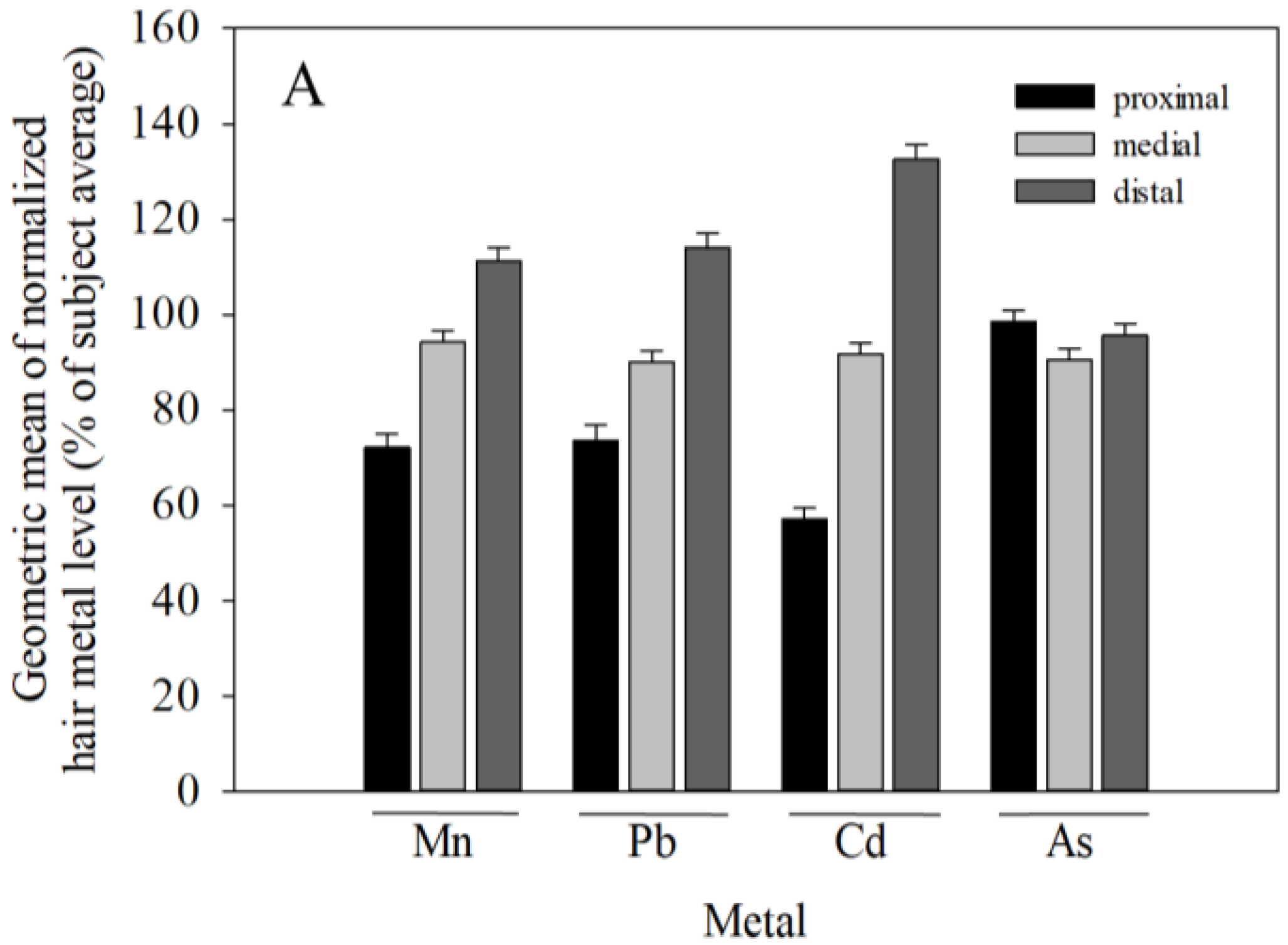
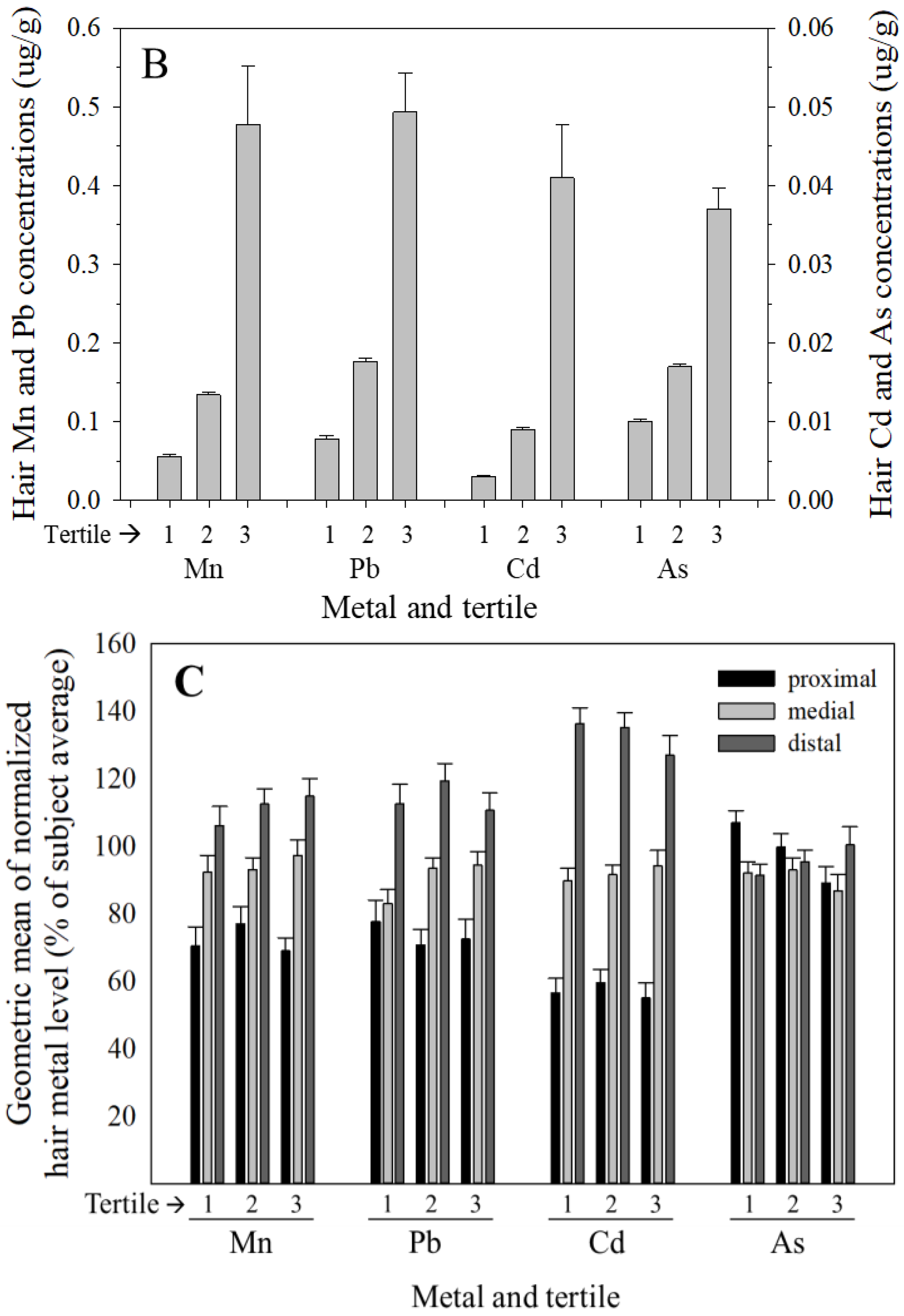
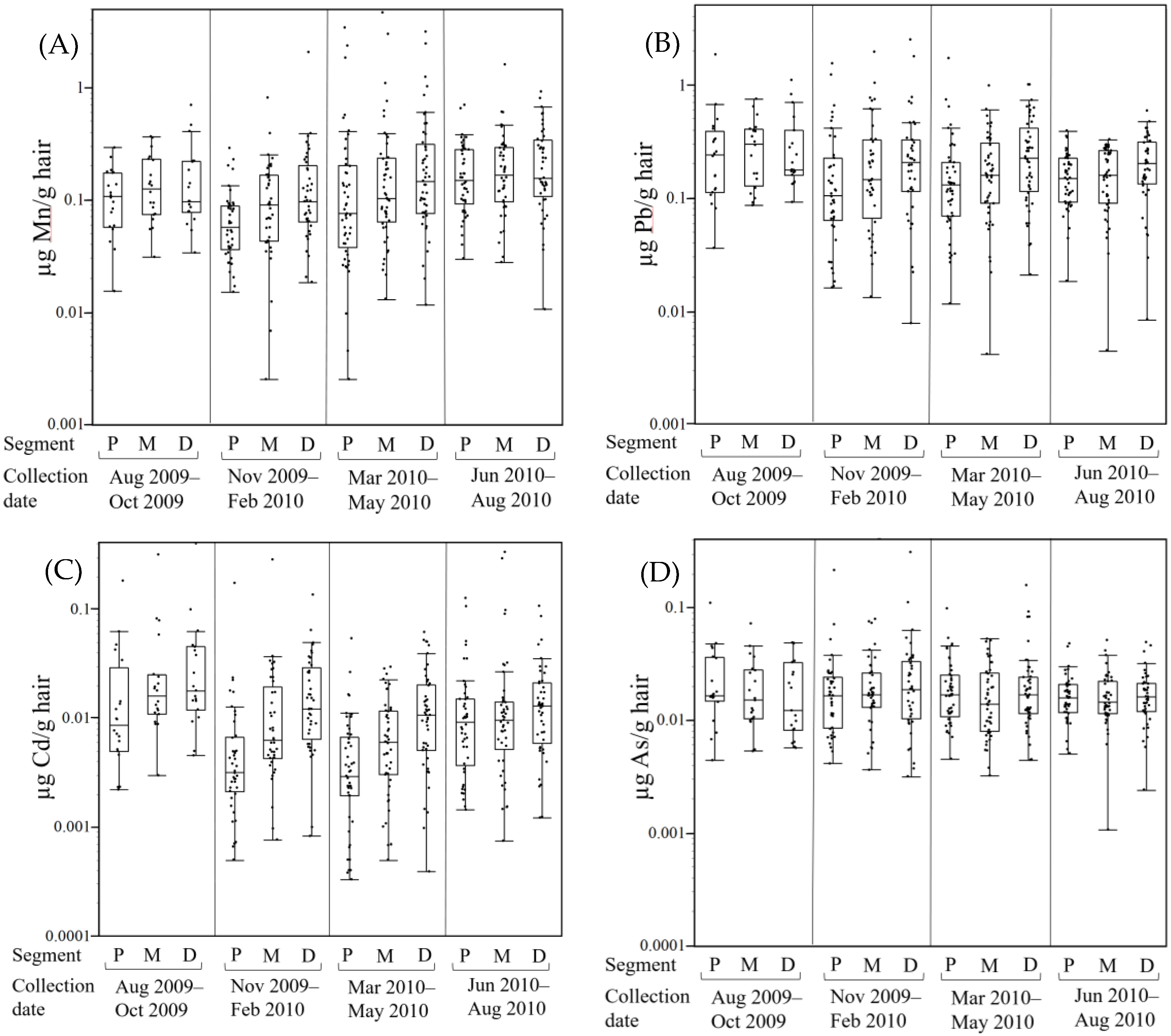
| Sex | Metal | Segment | N | Mean | Median | Range | S.D. |
|---|---|---|---|---|---|---|---|
| Males | Mn | proximal | 53 | 0.406 | 0.232 | 0.0248–4.10 | 0.616 |
| Pb | 52 | 0.829 | 0.414 | 0.0415–7.73 | 1.38 | ||
| Cd | 51 | 0.0540 | 0.0188 | 0.0039–0.463 | 0.0861 | ||
| As | 45 | 0.0630 | 0.0366 | 0.0061–0.438 | 0.0893 | ||
| Females | Mn | proximal | 169 | 0.184 | 0.0948 | 0.0045–3.40 | 0.356 |
| medial | 157 | 0.226 | 0.126 | 0.0067–4.63 | 0.463 | ||
| distal | 155 | 0.253 | 0.135 | 0.0184–3.12 | 0.388 | ||
| Pb | proximal | 169 | 0.242 | 0.134 | 0.0080–5.66 | 0.493 | |
| medial | 156 | 0.229 | 0.163 | 0.0042–1.95 | 0.229 | ||
| distal | 153 | 0.284 | 0.208 | 0.0078–2.49 | 0.298 | ||
| Cd | proximal | 169 | 0.0119 | 0.0050 | 0.0005–0.182 | 0.0242 | |
| medial | 157 | 0.0202 | 0.0088 | 0.0005–0.335 | 0.0491 | ||
| distal | 155 | 0.0215 | 0.0128 | 0.0004–0.401 | 0.0369 | ||
| As | proximal | 169 | 0.0227 | 0.0165 | 0.0044–0.214 | 0.0259 | |
| medial | 157 | 0.0197 | 0.0155 | 0.0011–0.0789 | 0.0143 | ||
| distal | 155 | 0.0238 | 0.0170 | 0.0024–0.310 | 0.0307 |
| Mn | |||
|---|---|---|---|
| Pb | 0.3513 (530) | Pb | |
| Cd | 0.4475 (532) | 0.5780 (529) | Cd |
| As | 0.1872 (526) | 0.1814 (523) | 0.2081 (526) |
| Component | Mn | Pb | Cd | As |
|---|---|---|---|---|
| Between-subject | 72.8 | 71.5 | 70.3 | 65.1 |
| Within-subject | 4.1 | 3.8 | 12.4 | 0.1 |
| Interaction | 23.1 | 24.7 | 17.3 | 34.8 |
| Location (Study) * | Cleaning Method # | Sub-Population (N) & | Mn µg/g | Pb µg/g | Cd µg/g | As µg/g |
|---|---|---|---|---|---|---|
| U.S., Ohio (this study) a | T/S, N/S | all (222) | 0.109 (0.441) | 0.152 (0.829) | 0.007 (0.050) | 0.018 (0.049) |
| Italy [12], a | T/S, N/S | all (501) | 0.098 (0.139) | NA | NA | NA |
| Spain [32], b | T/S, E/S | males (96) | NA | NA | 0.003 (0.003-0.004) | NA |
| females (124) | NA | NA | 0.006 (0.004-0.007) | NA | ||
| combined (220) | 0.137 (NA) | 0.14 (NA) | NA | 0.017 (NA) | ||
| Brazil [21], a | T/S | exposed males (34) | 12.1 (9.9) | NA | NA | NA |
| exposed females (36) | 12.4 (13.4) | NA | NA | NA | ||
| Brazil [33], a | T/S | referents (44) | NA | 2.09 (2.06) | NA | NA |
| exposed (88) | NA | 1.26 (3.70) | NA | NA | ||
| Spain [31], c | T/S | urban area (45) | 0.26 (0.90) | 0.32 (0.30) | <0.03 | NA |
| industrial area (54) | 0.18 (0.28) | 1.59 (3.01) | <0.03 | NA | ||
| Tibet [34], b | T | exposed (22) | 4.28 (5.36) | NA | NA | NA |
| unexposed 1 (24) | 2.87 (3.05) | NA | NA | NA | ||
| unexposed 2 (24) | 2.44 (3.00) | NA | NA | NA | ||
| Bangladesh [17], d | T | all (207) | 5.0 (1.4–23) | 1.6 (0.50–6.4) | 0.029 (0.0008–0.150) | 0.53 (0.14–2.9) |
| U.S., Ohio [2], b | T | all (370) | 0.417 (0.002) | NA | NA | NA |
| Greece [35], b | T | urban area 1 (11) | NA | 0.78 (1.47) | 0.014 (0.028) | 0.020 (0.029) |
| urban area 2 (21) | NA | 1.29 (6.86) | 0.023 (0.021) | 0.036 (0.011) | ||
| suburban area (19) | NA | 0.60 (0.67) | 0.015 (0.037) | 0.026 (0.009) | ||
| Mexico [22], b | T | unexposed (93) | 0.57 (0.49–0.66) | NA | NA | NA |
| exposed (79) | 12 (10.7–13.8) | NA | NA | NA | ||
| Spain [36], d | A/S | all (648) | 0.33 (0.12–0.94) | 0.70 (0.17–4.28) | 0.018 (0.004–0.079) | 0.07 (<0.05–0.26) |
| Italy [24], a | A/S | males (130) | 0.27 (0.25) | 0.78 (0.76) | 0.03 (0.05) | <0.01 |
| females (94) | 0.31 (0.27) | 0.79 (0.80) | 0.03 (0.05) | <0.01 | ||
| Russia [37], a | A | unexposed (84) | 2.25 (3.77) | 1.55 (2.98) | 0.12 (0.18) | 0.030 (0.034) |
| exposed (82) | 1.60 (3.51) | 2.48 (4.20) | 0.11 (0.14) | 0.020 (0.042) | ||
| Vietnam [25], c | A | control males (5) | NA | NA | NA | 0.31 (0.07) |
| control females (4) | NA | NA | NA | 0.36 (0.17) | ||
| exposed males (22) | NA | NA | NA | 2.76 (2.54) | ||
| exposed female (44) | NA | NA | NA | 5.59 (7.90) | ||
| Quebec [23], b | U | males (148) | 0.75 (NA) | NA | NA | NA |
| females (164) | 0.8 (NA) | NA | NA | NA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jursa, T.; Stein, C.R.; Smith, D.R. Determinants of Hair Manganese, Lead, Cadmium and Arsenic Levels in Environmentally Exposed Children. Toxics 2018, 6, 19. https://doi.org/10.3390/toxics6020019
Jursa T, Stein CR, Smith DR. Determinants of Hair Manganese, Lead, Cadmium and Arsenic Levels in Environmentally Exposed Children. Toxics. 2018; 6(2):19. https://doi.org/10.3390/toxics6020019
Chicago/Turabian StyleJursa, Thomas, Cheryl R. Stein, and Donald R. Smith. 2018. "Determinants of Hair Manganese, Lead, Cadmium and Arsenic Levels in Environmentally Exposed Children" Toxics 6, no. 2: 19. https://doi.org/10.3390/toxics6020019
APA StyleJursa, T., Stein, C. R., & Smith, D. R. (2018). Determinants of Hair Manganese, Lead, Cadmium and Arsenic Levels in Environmentally Exposed Children. Toxics, 6(2), 19. https://doi.org/10.3390/toxics6020019




