Cytotoxic and Inflammatory Potential of Air Samples from Occupational Settings with Exposure to Organic Dust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Occupational Environments
2.2. Fungal Burden Assessment
2.2.1. Samples Collection
2.2.2. Sample Preparation and Analysis
2.3. Particles Assessment
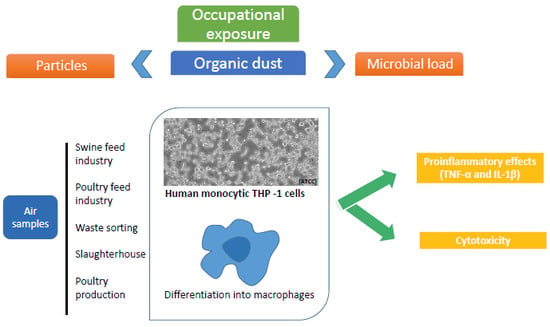
2.4. Cytotoxic and Inflammatory Assessment
2.5. Data Analysis
3. Results
3.1. Fungal Burden
3.1.1. Fungal Load
3.1.2. Fungal Identification
3.1.3. Fungal Detection
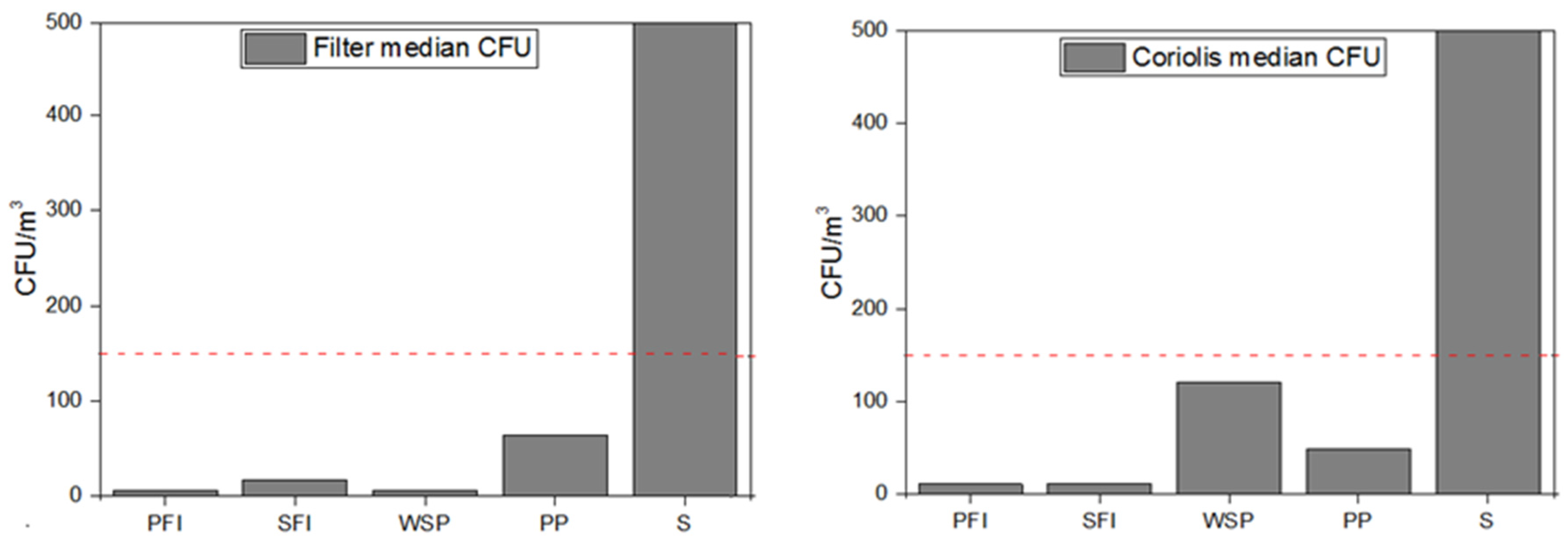
3.2. Particles
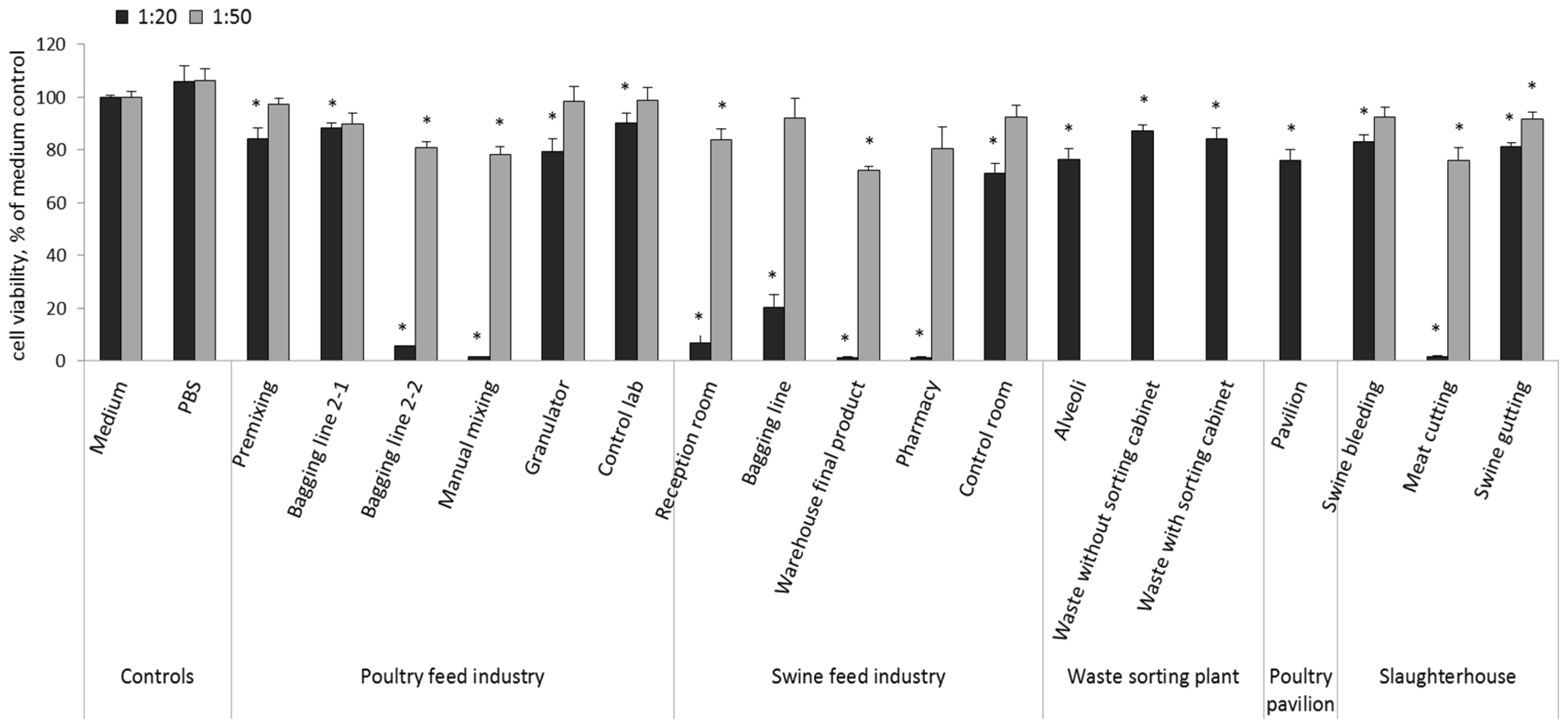
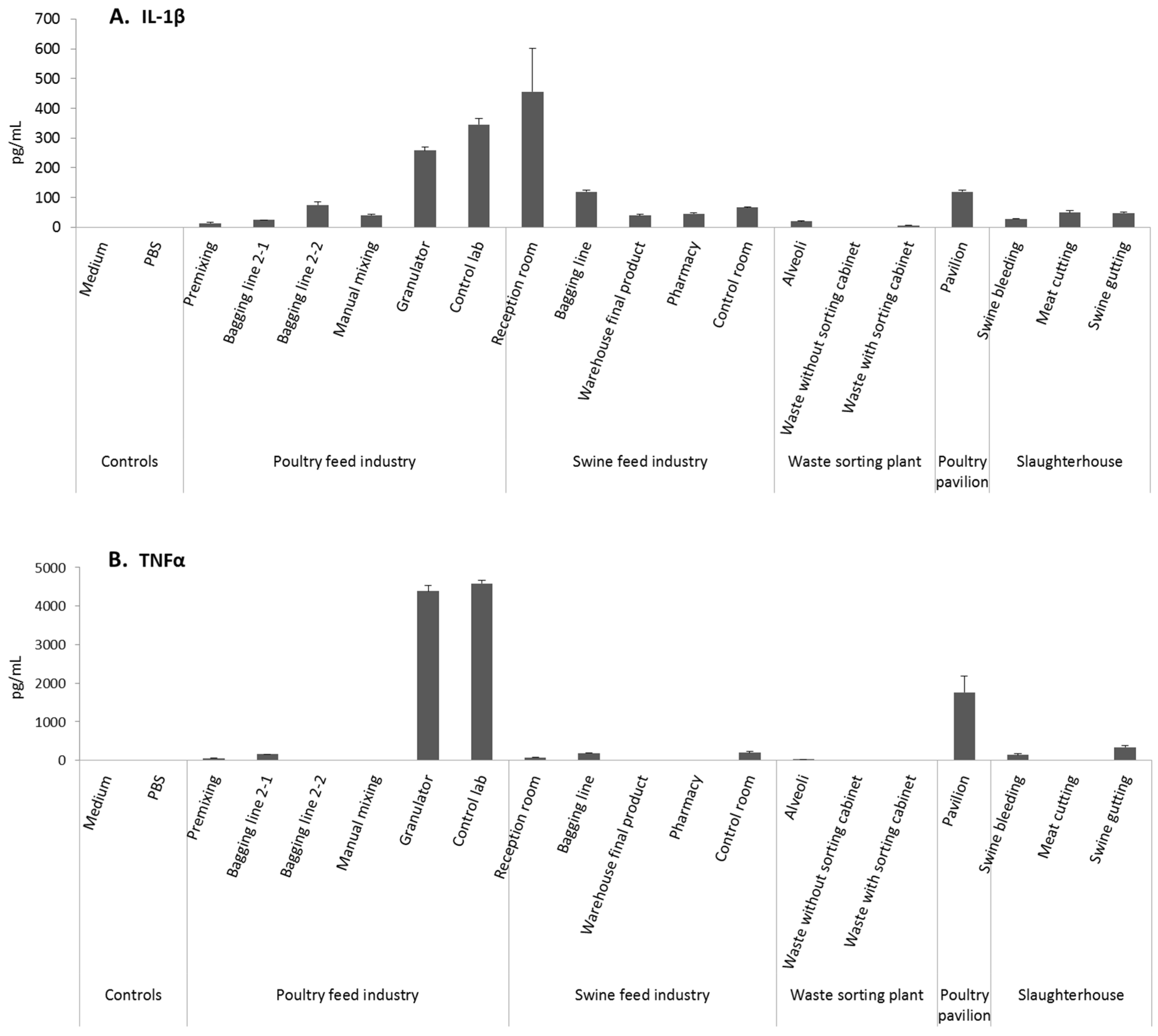
3.3. Cytotoxicity and Pro-Inflammatory Effects
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Review Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [PubMed]
- Wouters, I.M.; Spaan, S.; Douwes, J.; Doekes, G.; Heederik, D. Overview of personal occupational exposure levels to inhalable dust, endotoxin, beta(1→3)-glucan and fungal extracellular polysaccharides in the waste management chain. Ann. Occup. Hyg. 2006, 50, 39–53. [Google Scholar] [PubMed]
- Schenker, M.B.; Christiani, D.; Cormier, Y.; Dimich-Ward, H.; Doekes, G.; Dosman, J.; Douwes, J.; Dowling, K.; Enarson, D.; Green, F. Respiratory health hazards in agriculture. Am. J. Respir. Crit. Care Med. 1998, 158, S1–S76. [Google Scholar]
- Linaker, C.; Smedley, J. Respiratory illness in agricultural workers. Occup. Med. (Lond) 2002, 52, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Sigsgaard, T.; Schlünssen, V. Occupational asthma diagnosis in workers exposed to organic dust. Ann. Agric. Environ. Med. 2004, 11, 1–7. [Google Scholar] [PubMed]
- Cleave, J.; Willson, P.J.; Town, J.; Gordon, J.R. Fractionation of swine barn dust and assessment of its impact on the respiratory tract following repeated airway exposure. J. Toxicol. Environ. Health A 2010, 73, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.J.; Nonnenmann, M.W.; Basinas, I.; Davidson, M.; Elfman, L.; Gordon, J.; Kirychuck, S.; Reed, S.; Schaeffer, J.W.; Schenker, M.B.; et al. Systematic review of respiratory health among dairy workers. Agromedicine 2013, 18, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Basinas, I.; Schlünssen, V.; Heederik, D.; Sigsgaard, T.; Smit, L.A.; Samadi, S.; Omland, O.; Hjort, C.; Madsen, A.M.; Skov, S.; et al. Sensitisation to common allergens and respiratory symptoms in endotoxin exposed workers: A pooled analysis. Occup. Environ. Med. 2012, 69, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Seedorf, J.; Hartung, J.; Schröder, M.; Linkert, K.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.; Pedersen, P.; et al. Concentrations and Emissions of Airborne Endotoxins and Microorganisms in Livestock Buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 97–109. [Google Scholar] [CrossRef]
- Poole, J.A.; Wyatt, T.A.; Von Essen, S.G.; Hervert, J.; Parks, C.; Mathisen, T.; Romberger, D.J. Repeat Organic Dust Exposure–Induced Monocyte Inflammation is Associated with Protein Kinase C Activity. J. Allergy Clin. Immunol. 2007, 120, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Hawley, B.; Schaeffer, J.; Poole, J.A.; Doodley, G.P.; Reynolds, S.; Volckens, J. Differential Response of Human Nasal and Bronchial Epithelial Cells Upon Exposure to Size-Fractionated Dairy Dust. J. Toxicol. Environ. Health Part A Curr. Issues 2015, 78, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Faria, T.; dos Santos, M.; Carolino, E. Task-based approach importance for the occupational risk assessment-the case of particles exposure in feed industry. In Proceedings of the International Symposium on Occupational Safety and Hygiene SHO2016, Guimarães, Portugal, 23–24 March 2016.
- Van Tongeren, M.; van Amelsvoort, L.; Heederik, D. Exposure to organic dusts, endotoxins, and microorganisms in the municipal waste industry. Int. J. Occup. Environ. Health 1997, 3, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, J.A.; Tarkowski, S.; Cyprowski, M.; Szarapińska-Kwaszewska, J.; Dudkiewicz, B. Occupational exposure to organic dust associated with municipal waste collection and management. Int. J. Occup. Med. Environ. Health 2002, 15, 289–301. [Google Scholar] [PubMed]
- Gladding, T.L.; Thorn, J.; Stott, D. Organic dust exposure and work-related effects among recycling workers. Am. J. Ind. Med. 2003, 42, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.H.A. Airborne dust, bacteria, actinomycetes and fungi at a flourmill. Aerobiologia 2007, 23, 59–69. [Google Scholar] [CrossRef]
- Bünger, J.; Schlappler-Scheele, B.; Hilgers, R.; Hallier, E. A 5-year follow-up study on respiratory disorders and lung function in workers exposed to organic dust from composting plants. Int. Arch. Occup. Environ. Health 2007, 80, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Baatjies, R.; Meijster, T.; Heederik, D.; Sander, I.; Jeebhay, M.F. Effectiveness of interventions to reduce flour dust exposures in supermarket bakeries in South Africa. Occup. Environ. Med. 2014, 71, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Thilsing, T.; Madsen, A.M.; Basinas, I.; Schlünssen, V.; Tendal, K.; Bælum, J. Dust, Endotoxin, Fungi, and Bacteria Exposure as Determined by Work Task, Season, and Type of Plant in a Flower Greenhouse. Ann. Occup. Hyg. 2014, 59, 142–157. [Google Scholar] [PubMed]
- Viegas, S.; Veiga, L.; Figueiredo, P.; Almeida, A.; Carolino, E.; Viegas, C. Assessment of Workers’ Exposure to Aflatoxin B1 in a Portuguese Waste Industry. Ann. Occup. Hyg. 2015, 59, 173–181. [Google Scholar] [PubMed]
- Palmberg, L.; Larsson, B.M.; Malmberg, P.; Larsson, K. Airway responses of healthy farmers and non-farmers to exposure in a swine confinement building. Scand. J. Work Environ. Health 2002, 28, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, S.; Romberger, D. The respiratory inflammatory response to the swine confinement building environment: The adaptation to respiratory exposures in the chronically exposed worker. J. Agric. Saf. Health 2003, 9, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.J.; Iversen, M.; Sigsgaard, T.; Omland, O.; Takai, H.; Bonefeld-Jørgensen, E.; Seedorf, J.; Dahl, R. A single exposure to organic dust of non-naive non-exposed volunteers induces long-lasting symptoms of endotoxin tolerance. Arch. Allergy Immunol. 2005, 138, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Thorne, P.S.; Yagla, S.J.; Burmeister, L.F.; Olenchock, S.A.; Watt, J.L. The role of endotoxin in grain dust induced lung disease. Am. J. Respir. Crit. Care Med. 1995, 152, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Spurzem, J.R.; Romberger, D.J.; Von Essen, S.G. Agricultural lung disease. Clin. Chest. Med. 2002, 23, 795–810. [Google Scholar] [CrossRef]
- Rylander, R. Organic dust induced pulmonary disease – the role of mould derived beta-glucan. Ann. Agric. Environ. Med. 2010, 17, 9–13. [Google Scholar] [PubMed]
- Liebers, V.; van Kampen, V.; Bünger, J.; Düser, M.; Stubel, H.; Brüning, T.; Raulf-Heimsoth, M. Assessment of airborne exposure to endotoxin and pyrogenic active dust using electrostatic dust fall collectors (EDCs). J. Toxicol. Environ. Health A 2012, 75, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Eduard, W.; Douwes, J.; Mehl, R.; Heederik, D.; Melbostad, E. Short term exposure to airborne microbial agents during farm work: Exposure response relations with eye and respiratory symptoms. Occup. Environ. Med. 2001, 58, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Donaldson, K. Wool and grain dusts stimulate TNF secretion by alveolar macrophages in vitro. Occup. Environ. Med. 1996, 53, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Pennings, H.J.; Kramer, K.; Bast, A.; Buurman, W.A.; Wouters, E.F. Tumour necrosis factor-alpha induces hyperreactivity in tracheal smooth muscle of the guinea-pig in vitro. Eur. Respir. J. 1998, 12, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Borish, L.; Steinke, J.W. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef] [PubMed]
- Segura, P.; François, M.; Gagnon, C.; Sauvé, S. Review of the occurrence of anti-infectives in contaminated wastewaters and natural and drinking waters. Environ Health Perspect. 2009, 117, 675–684. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Guarro, J.; Gene, J.; Figueras, M.J. Atlas of Clinical Fungi; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2001. [Google Scholar]
- World Health Organization Regional Office for Europe. WHO Guidelines for Indoor Air Quality: Dampness and Mould; WHO Regional Office for Europe: Geneva, Swiss, 2009. [Google Scholar]
- Gerrity, T.R. Regional deposition of gases and particles in the lung: Implications for mixtures. Toxicology 1995, 105, 327–334. [Google Scholar] [CrossRef]
- Adamson, I.Y.R.; Vincent, R.; Bjarnason, S.G. Cell injury and interstitial inflammation in rat lung after inhalation of ozone and urban particulates. Am. J. Respir. Cell Mol. Biol. 1999, 20, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, R.B. Interaction of gaseous and particulate pollutants in the respiratory tract: Mechanisms and modulators. Toxicology 1995, 105, 315–325. [Google Scholar] [CrossRef]
- Korkalainen, M.; Täubel, M.; Naarala, J.; Kirjavainen, P.; Koistinen, A.; Hyvärinen, A.; Komulainen, H.; Viluksela, M. Synergistic proinflammatory interactions of microbial toxins and structural components characteristic to moisture-damaged buildings. Indoor Air 2017, 27, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Granum, B.; Løvik, M. The Effect of Particles on Allergic Immune Responses. Toxicol. Sci. 2002, 65, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Barr, C.D.; Michelle, L.B. Protecting Human Health from Air Pollution shifting from a single-pollutant to a multipollutant approach. Epidemiology 2010, 21, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Austin, E.; Coull, B.A.; Schwartz, J.; Koutrakis, P. Health effects of multi-pollutant profiles. Environ. Int. 2014, 71, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Pirani, M.; Best, N.; Blangiardo, M.; Liverani, S.; Atkinson, R.W.; Fuller, G.W. Analysing the health effects of simultaneous exposure to physical and chemical properties of airborne particles. Environ. Int. 2015, 79, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Pastuszka, J.S.; Nevalainen, A.; Täubel, M.; Hyvärinen, A. Analysis of the known synergistic effects of the exposure to selected air pollutants. In Synergistic Influence of Gaseous, Particulate, and Biological Pollutants on Human Health; Pastuszka, J.S., Ed.; CRC Press, Taylor & Francis Group: New York, NY, USA, 2016; pp. 243–264. [Google Scholar]
- Miller, J.D.; Sun, M.; Gilyan, A.; Roy, J.; Rand, T. Inflammation-associated gene transcription and expression in mouse lungs induced by low molecular weight compounds from fungi from the built environment. Chem. Biol. Interact. 2010, 183, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Donham, K.J.; Cumro, D.; Reynolds, S.J.; Merchant, J.A. Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: Recommendations for exposure limits. J. Occup. Environ. Med. 2000, 42, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.A.; Spaan, S.; Heederik, D. Endotoxin exposure and symptoms in wastewater treatment workers. Am. J. Ind. Med. 2005, 48, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.; Chilosi, M.; Golec, M.; Skórska, C.; Huaux, F.; Yakoub, Y.; Pastena, C.; Daniele, I.; Cholewa, G.; Sitkowska, J.; et al. Mouse model of hypersensitivity pneumonitis after inhalation exposure to different microbial antigens associated with organic dusts. Ann. Agric. Environ. Med. 2011, 18, 159–168. [Google Scholar] [PubMed]
- Mackiewicz, B.; Skórska, C.; Dutkiewicz, J. Relationship between concentrations of microbiological agents in the air of agricultural settings and occurrence of work-related symptoms in exposed persons. Ann. Agric. Environ. Med. 2015, 22, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Cormier, Y.; Tremblay, G.; Meriaux, A.; Brochu, G.; Lavoie, J. Airborne microbial contents in two types of swine confinement buildings in Quebec. Am. Ind. Hyg. Assoc. J. 1990, 51, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.K.; Bark, S.M. Health hazards affecting the animal confinement farm worker. AAOHN J. 1996, 44, 198–204. [Google Scholar] [PubMed]
- May, S.; Romberger, D.J.; Poole, J.A. Respiratory health effects of large animal farming environments. J. Toxicol. Environ. Health Part B 2012, 15, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Neves, O.; Sabino, R.; Viegas, S. Fungi occupational exposure assessment: A methodology to be followed for a more sound health effects discussion. In International Symposium on Occupational Safety and Hygiene SHO2016; Arezes, P., Baptista, J.B., Barroso, M.P., Carneiro, P., Cordeiro, P., Costa, N., Melo, R.B., Miguel, A.S., Perestrelo, G., Eds.; Portuguese Society of Occupational Safety and Hygiene: Guimarães, Portugal, 2016; pp. 374–376. ISBN 978-989-98203-6-4. [Google Scholar]
- Hoffmeyer, F.; van Kampen, V.; Taeger, D.; Deckert, A.; Rosenkranz, N.; Kaßen, M.; Schantora, A.L.; Brüning, T.; Raulf, M.; Bünger, J. Prevalence of and relationship between rhinoconjunctivitis and lower airway diseases in compost workers with current or former exposure to organic dust. Ann. Agric. Environ. Med. 2014, 21, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Arhami, M.; Polidori, A.; Gillen, D.L.; George, S.C.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Associations of Primary and Secondary Organic Aerosols With Airway and Systemic Inflammation in an Elderly Panel Cohort. Epidemiology 2001, 21, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Millner, P.D. Bioaerosols associated with animal production operations. Bioresour. Technol. 2009, 100, 5379–5385. [Google Scholar] [CrossRef] [PubMed]
- Tsapko, V.; Chudnovets, A.; Sterenbogen, M.; Papach, V.; Dutkiewicz, J.; Skórska, C.; Krysinska-Traczyk, E.; Golec, M. Exposure to bioaerosols in the selected agricultural facilities of the Ukraine and Poland—A review. Ann. Agric. Environ. Med. 2011, 18, 19–27. [Google Scholar] [PubMed]
- Ellen, H.H.; Bottcher, R.W.; von Wachenfelt, E.; Takai, H. Dust levels and control methods in poultry houses. J. Agric. Saf. Health 2000, 6, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Dungan, R.S. Airborne endotoxin from Indoor and outdoor environments: Effect of Sample Dilution on the Kinetic Limulus Amebocyte Lysate (LAL) Assay. J. Occup. Environ. Hyg. 2011, 8, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Paba, E.; Tranfo, G.; Corsetti, F.; Marcelloni, A.M.; Iavicoli, S. Indoor Exposure to Airborne Endotoxin: A Review of the Literature on Sampling and Analysis Methods. Ind. Health 2013, 51, 237–255. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Preas, H.L; Pugin, J.; Fiuza, C.; Tropea, M.; Reda, D.; Banks, S.M.; Suffredini, A.F. Local Inflammatory Responses following Bronchial Endotoxin Installation in Humans. Am. J. Respir. Crit. Care Med. 2001, 163, 1591–1598. [Google Scholar]
- Copeland, S.; Warren, H.S.; Lowry, S.F.; Calvano, S.E.; Remick, D. Acute Inflammatory Response to Endotoxin in Mice and Humans. Clin. Diagn. Lab. Immunol. 2005, 12, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Loh, L.C.; Vyas, B.; Kanabar, V.; Kemeny, D.M.; O’Connor, B.J. Inhaled endotoxin in healthy human subjects: A dose-related study on systemic effects and peripheral CD4+ and CD8+ T cells. Respir. Med. 2006, 100, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Janssen, O.; Schaumann, F.; Holz, O.; Lavae-Mokhtari, B.; Welker, L.; Winkler, C.; Biller, H.; Krug, N.; Hohlfeld, J.M. Low-dose endotoxin inhalation in healthy volunteers—A challenge model for early clinical drug development. BMC Pulm. Med. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Rylander, R.; Lin, R.H. (1→3)-beta-d-glucan—Relationship to indoor air-related symptoms, allergy and asthma. Toxicology 2000, 152, 47–52. [Google Scholar] [CrossRef]
- Fogelmark, B.; Thorn, J.; Rylander, R. Inhalation of (1→3)-β-d-glucan causes airway eosinophilia. Mediat. Inflamm. 2001, 10, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Rylander, R.; Jacobs, R.R. Endotoxins in the environment—A criteria document. Int. J. Occup. Environ. Health 1997, 3, 1–48. [Google Scholar]
- Beijer, L.; Thorn, J.; Rylander, R. Effects after inhalation of (1→3)-beta-d-Glucan and relation to mould exposure in the home. Mediat. Inflamm. 2002, 11, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Tercelj, M.; Salobir, B.; Harlander, M.; Rylander, R. Fungal exposure in homes of patients with sarcoidosis —An environmental exposure study. Environ. Health 2011, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Walinder, R.; Norback, D.; Wessen, B.; Venge, P. Nasal lavage biomarkers: Effects of water damage and microbial growth in an office building. Arch. Environ. Occup. Health 2001, 56, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Grinshpun, S.A.; Kim, K.Y.; Iossifova, Y.; Adhikari, A.; Reponen, T. Relationship between indoor and outdoor airborne fungal spores, pollen, and (1→3)-β-d-glucan in homes without visible mold growth. Aerobiologia 2006, 22, 227–236. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food. Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Gqaleni, N. Effects of temperature, water activity, and icubation time on production of aflatoxins and cyclopiazonic acid by an isolate of Aspergillus flavus in surface Agar culture. Appl. Environ. Microbiol. 1997, 63, 1048–1053. [Google Scholar] [PubMed]
- Freire, F.C.O.; Vieira, I.G.P.; Guedes, M.I.F.; Mendes, F.N.P. Micotoxinas: Importância na Alimentação e na Saúde Humana e Animal; Empresa Brasileira de Pesquisa Agropecuária: Documentos 110; Embrapa Agroindústria Tropical: Fortaleza, Ceará, Brazil, 2007; p. 48. [Google Scholar]
- Santurio, J.M. Mycotoxins and Mycotoxicosis in Poultry. Rev. Bras. Cienc. Avic. 2000, 2, 1–12. [Google Scholar]
- Viegas, C.; Garcia, F.; Monteiro, L.; Rodrigues, P.; Henriques, S.; Viegas, S. Occupational exposure to fungi and mycotoxins in swine feed production: Data review. In International Symposium on Occupational Safety and Hygiene SHO2016; Arezes, P., Baptista, J.B., Barroso, M.P., Carneiro, P., Cordeiro, P., Costa, N., Melo, R.B., Miguel, A.S., Perestrelo, G., Eds.; Portuguese Society of Occupational Safety and Hygiene: Guimarães, Portugal, 2016; pp. 377–379. [Google Scholar]
- Viegas, S.; Veiga, L.; Malta-Vacas, J.; Sabino, R.; Figueiredo, P.; Almeida, A.; Viegas, C.; Carolino, E. Occupational exposure to aflatoxin (AFB1) in poultry production. J. Toxicol. Environ. Health A 2012, 75, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Veiga, L.; Verissimo, C.; Sabino, R.; Figueiredo, P.; Almeida, A.; Carolino, E.; Viegas, C. Occupational exposure to aflatoxin B1: The case of poultry and swine production. World Mycotoxin J. 2013, 6, 309–315. [Google Scholar] [CrossRef]
- Jarvis, B.B.; Miller, J.D. Mycotoxins as harmful indoor air contaminants. Appl. Microbiol. Biotechnol. 2005, 66, 367–772. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.; Longhin, E.; Caloni, F.; Camatini, M.; Gualtieri, M. Synergistic inflammatory effect of PM10 with mycotoxin deoxynivalenol on human lung epithelial cells. Toxicon 2015, 104, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.D.; Ovrebø, S. Background, approaches and recent trends for setting health-based occupational exposure limits: A minireview. Regul. Toxicol. Pharmacol. 2008, 51, 253–269. [Google Scholar] [CrossRef] [PubMed]

| Poultry Feed Industry (PFI) | Swine Feed Industry (SFI) | Waste Sorting Plant (WSP) | Poultry Pavilion (PP) | Slaughterhouse (S) |
|---|---|---|---|---|
| Premixing | Reception room | Alveoli (waste discharging area) | Pavilion | Swine bleeding |
| Bagging line 2-1 | Bagging line | Waste without sorting cabinet | - | Meat cutting |
| Bagging line 2-2 | Final product warehouse | Waste with sorting cabinet | - | Swine gutting |
| Manual mixing | Pharmacy | - | - | - |
| Granulator | Control room | - | - | - |
| Control lab | - | - | - | - |
| Aspergillus Sections | Sequence |
|---|---|
| Aspergillus section Flavi (toxigenic strains) | |
| Primer Forward | 5′-GTCCAAGCAACAGGCCAAGT-3′ |
| Primer Reverse | 5′-TCGTGCATGTTGGTGATGGT-3′ |
| Probe | 5′-TGTCTTGATCGGCGCCCG-3′ |
| Aspergillus section Fumigati | |
| Primer Forward | 5′-CGCGTCCGGTCCTCG-3′ |
| Primer Reverse | 5′-TTAGAAAAATAAAGTTGGGTGTCGG-3′ |
| Probe | 5′-TGTCACCTGCTCTGTAGGCCCG-3′ |
| Aspergillus section Circumdati | |
| Primer Forward | 5′-CGGGTCTAATGCAGCTCCAA-3′ |
| Primer Reverse | 5′-CGGGCACCAATCCTTTCA-3′ |
| Probe | 5′-CGTCAATAAGCGCTTTT-3′ |
| Occupational Environments | Conventional Methods | Molecular Biology | Particulate Matter | In Vitro Toxicological Assessment | |
|---|---|---|---|---|---|
| Impinger Method | Filter Method | ||||
| Poultry feed industry (PFI) | 5 | 5 | 5 | 5 | 5 |
| Swine feed industry (SFI) | 6 | 3 | 6 | 3 | 6 |
| Waste sorting plant (WSP) | 3 | 3 | 3 | 3 | 3 |
| Poultry pavilion (PP) | 1 | 1 | 1 | Not assessed | 1 |
| Slaughterhouse (S) | 3 | 3 | 3 | Not assessed | 3 |
| Total of samples | 18 | 15 | 15 | 11 | 18 |
| Settings | Workplace | Mass Average (mg/m3) | mg/m3 | Kruskal–Wallis Test Results | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean Rank | df | p | |||||
| Poultry feed industry (PFI) | Bagging line (5 kg) | 0.181 | Mass average 0.098 (min.–max.) (0.028–0.198) SD 0.061 | 90 | 141,02 | 35,342 | 2 | 2.1 × 10−8 |
| Premixing control room | 0.074 | |||||||
| Control lab | 0.038 | |||||||
| Swine feed industry (SFI) | Reception room | 0.113 | Mass average 0.054 (min.–max.) (0.007–0.143) SD 0.042 | 150 | 149,81 | |||
| Bagging line | 0.053 | |||||||
| Warehouse final product | 0.080 | |||||||
| Pharmacy | 0.014 | |||||||
| Control room | 0.010 | |||||||
| Waste sorting plant (WSP) | Alveoli | 0.049 | Mass average 0.049 (min.–max.) (0.036–0.062) SD 0.007 | 90 | 216,13 | |||
| Pre Screening | 0.044 | |||||||
| Screening | 0.053 | |||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, S.; Caetano, L.A.; Korkalainen, M.; Faria, T.; Pacífico, C.; Carolino, E.; Quintal Gomes, A.; Viegas, C. Cytotoxic and Inflammatory Potential of Air Samples from Occupational Settings with Exposure to Organic Dust. Toxics 2017, 5, 8. https://doi.org/10.3390/toxics5010008
Viegas S, Caetano LA, Korkalainen M, Faria T, Pacífico C, Carolino E, Quintal Gomes A, Viegas C. Cytotoxic and Inflammatory Potential of Air Samples from Occupational Settings with Exposure to Organic Dust. Toxics. 2017; 5(1):8. https://doi.org/10.3390/toxics5010008
Chicago/Turabian StyleViegas, Susana, Liliana Aranha Caetano, Merja Korkalainen, Tiago Faria, Cátia Pacífico, Elisabete Carolino, Anita Quintal Gomes, and Carla Viegas. 2017. "Cytotoxic and Inflammatory Potential of Air Samples from Occupational Settings with Exposure to Organic Dust" Toxics 5, no. 1: 8. https://doi.org/10.3390/toxics5010008
APA StyleViegas, S., Caetano, L. A., Korkalainen, M., Faria, T., Pacífico, C., Carolino, E., Quintal Gomes, A., & Viegas, C. (2017). Cytotoxic and Inflammatory Potential of Air Samples from Occupational Settings with Exposure to Organic Dust. Toxics, 5(1), 8. https://doi.org/10.3390/toxics5010008