Effects of Estrogen, Nitric Oxide, and Dopamine on Behavioral Locomotor Activities in the Embryonic Zebrafish: A Pharmacological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Preparation
2.2. Reagent Preparations
2.2.1. NO/E2 Related Reagents
2.2.2. DA Related Reagents
2.3. Research Protocols
2.3.1. NO Response Systems in Embryonic Zebrafish
2.3.2. NO-sGC-cGMP-Dependent/Independent Pathway Analyses
2.3.3. NO/E2 Rescue and Recovery from 6OHDA and nNOSI-Induced Listless/PD-Like Symptoms
2.4. Sensory-Motor Function Assays
2.5. Data Analysis
3. Results
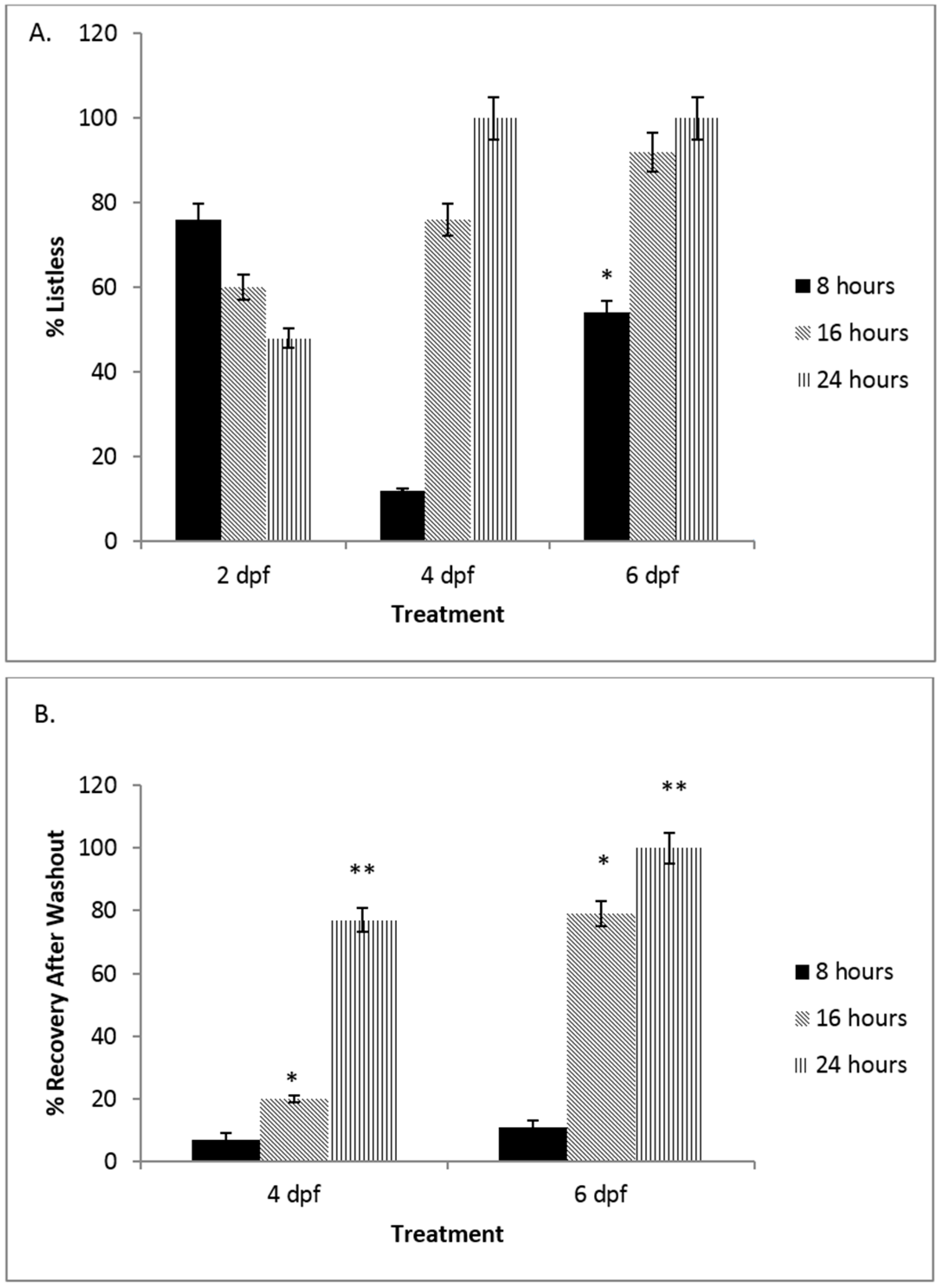
3.1. There Is a Developmental Regulation of the NO Response System in Embryonic Zebrafish
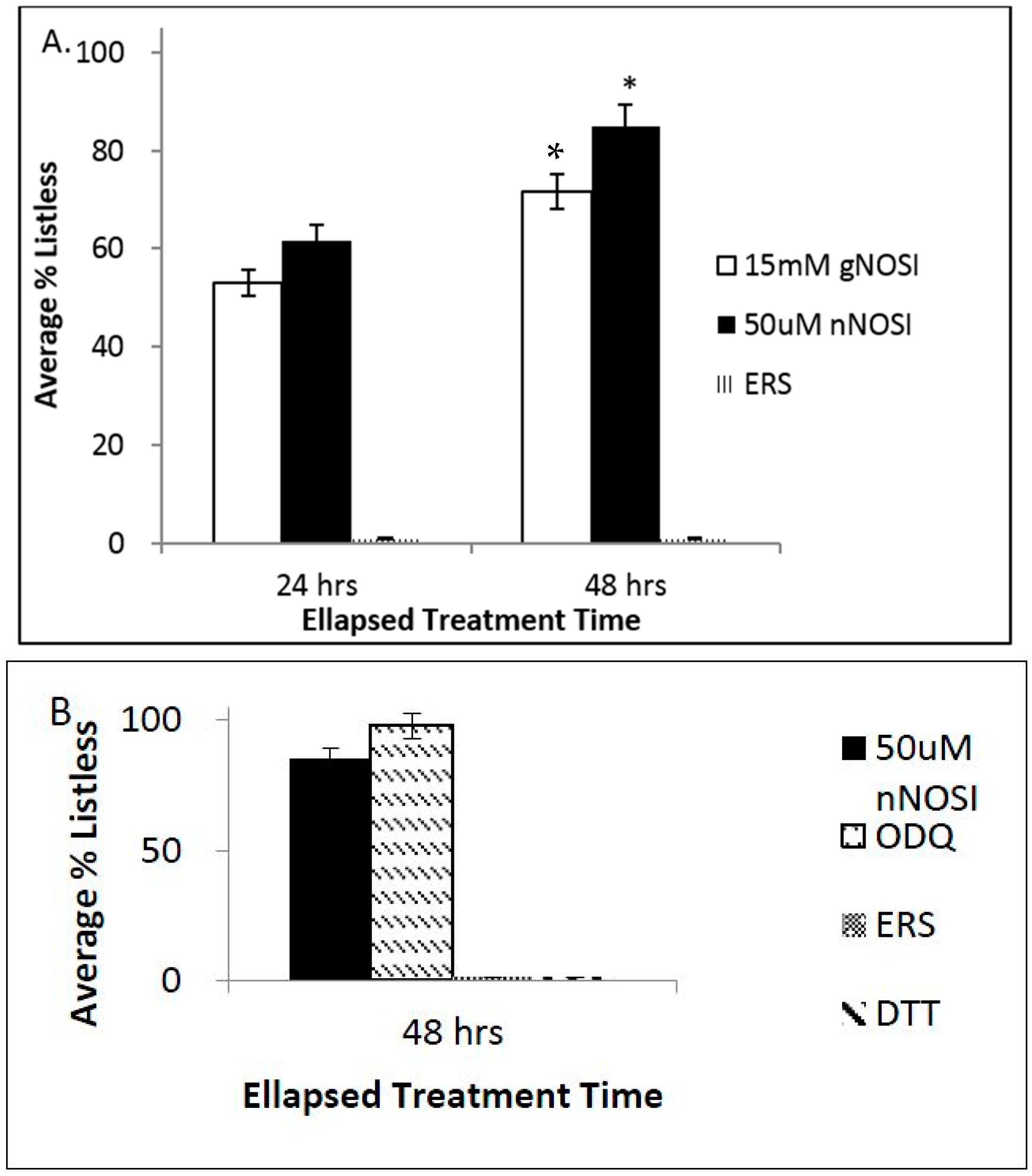
3.2. NO and E2 Deficiencies Cause Severe Sensory-Motor Deficits in the Embryonic Zebrafish Which Act through the NO-sGC-cGMP-Dependent Pathway
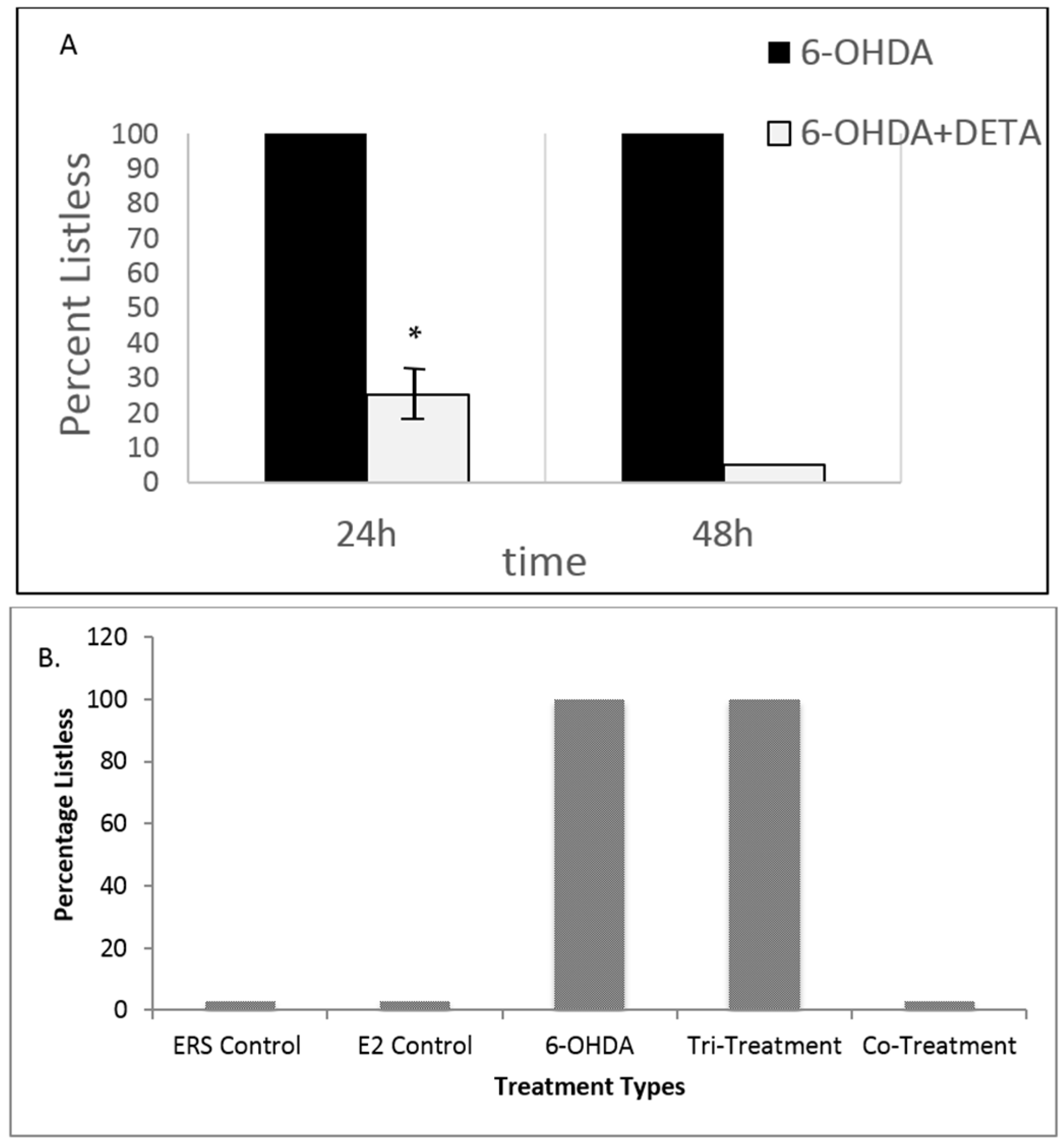
3.3. Both NO and E2 Can Rescue Fish from Severe Sensory-Motor Deficits Elicited by the Neurotoxin 6OHDA; However, E2 Rescue Is Dependent on the Downstream NO Pathway
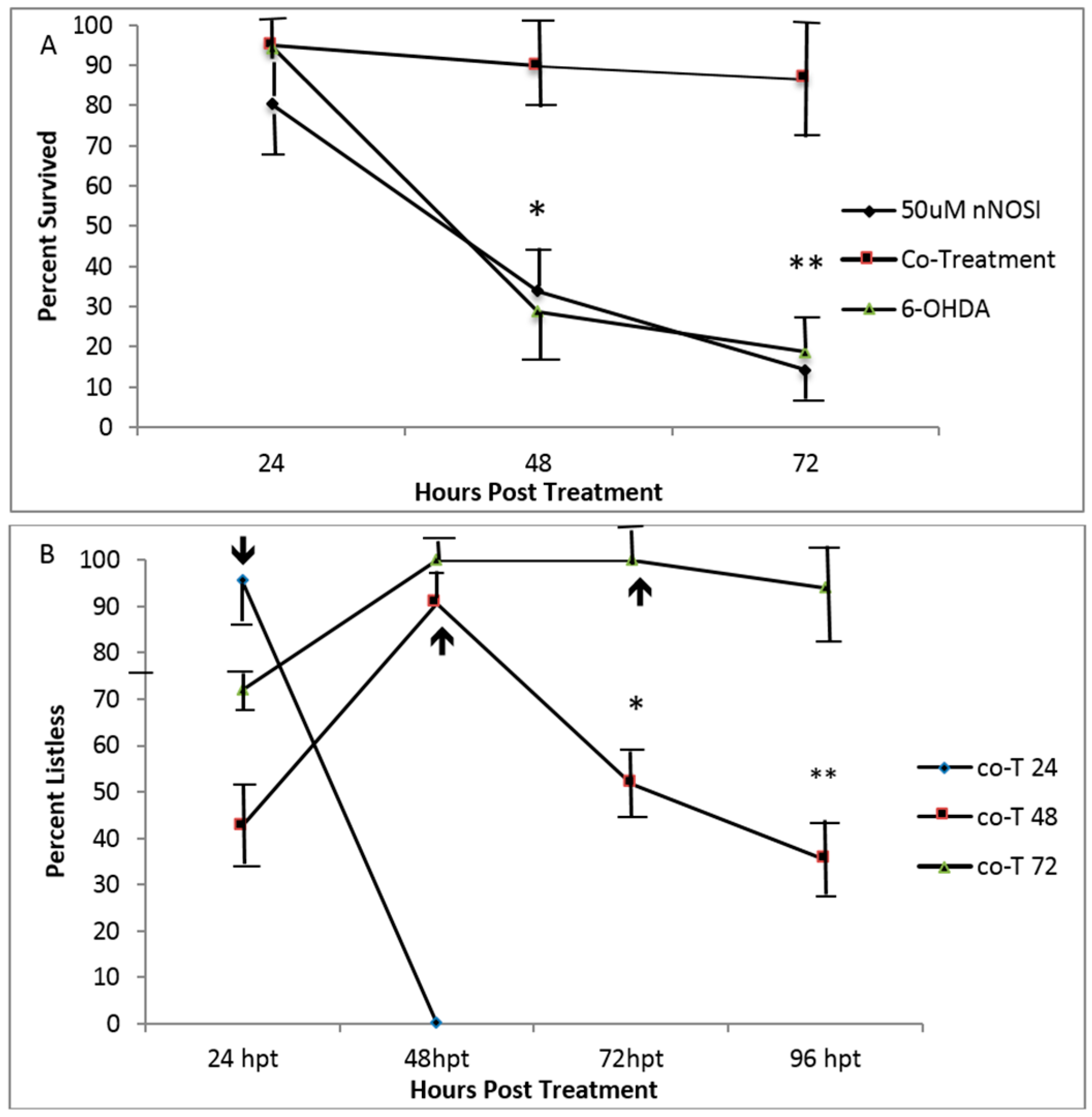
3.4. Embryonic Fish Treated with 6OHDA Recover at Rates Dependent on Exposure Time after Removal of the Neurotoxin and Survive Longer When Co-Treated with nNOSI
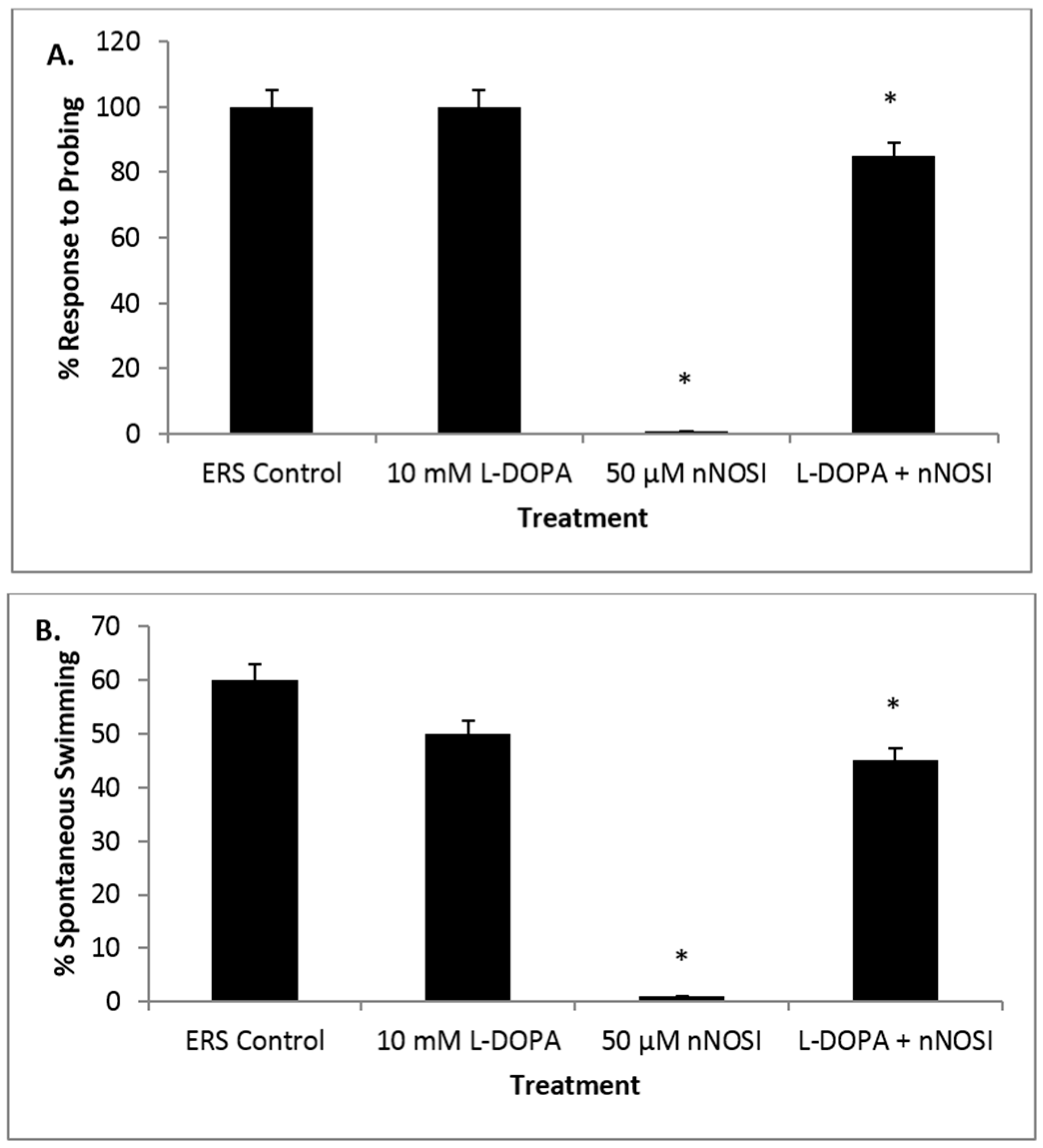
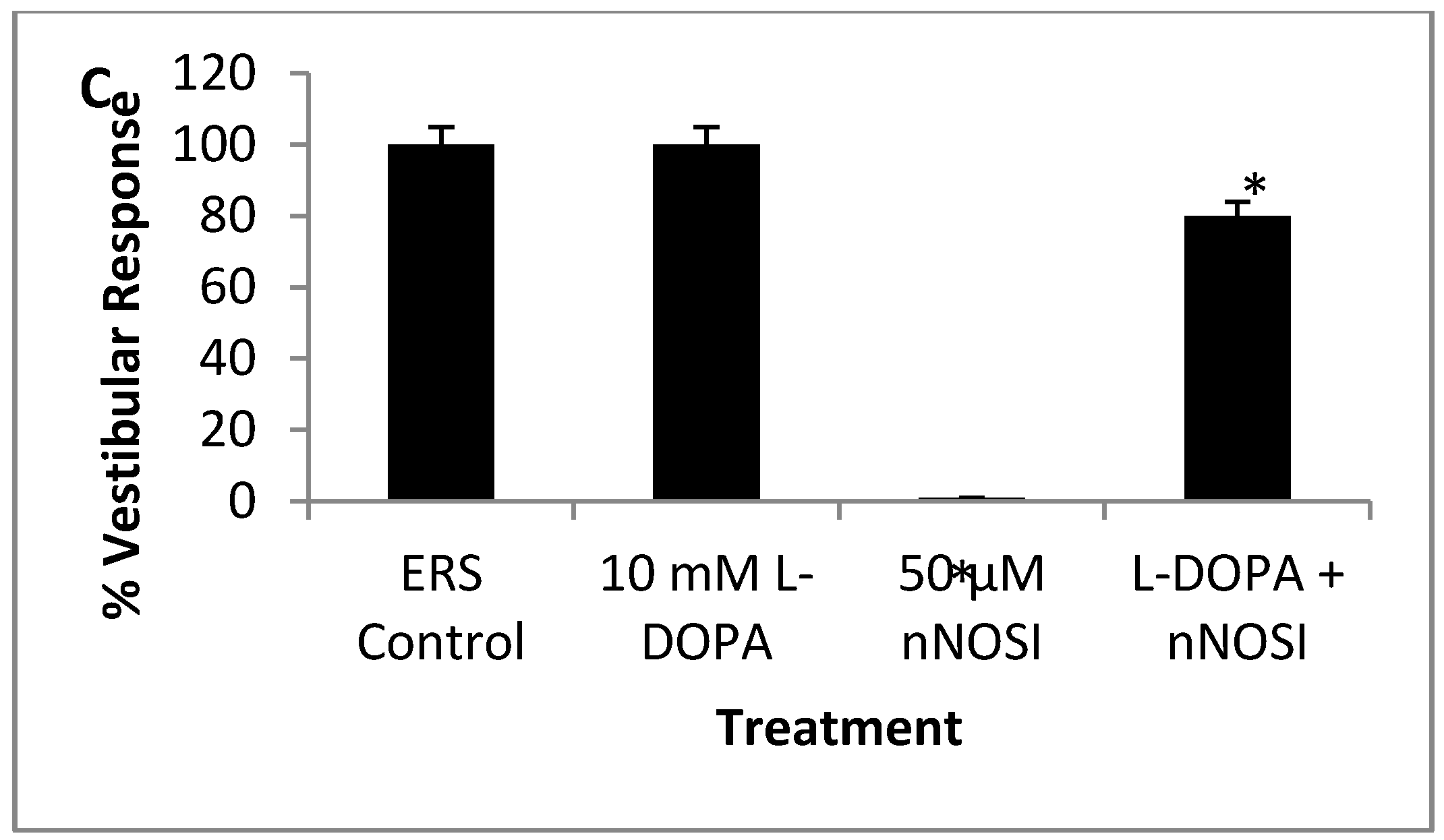
3.5. l-DOPA Treatment Restores Sensory-Motor Deficits Initiated in NO Deficient Fish
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lisman, J. Two-phase model of the basal ganglia: Implications for discontinuous control of the motor system. J. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Stephenson-Jones, M.; Ericsson, J.; Robertson, B.; Grillner, S.J. Evolution of the basal ganglia: Dual-output pathways conserved throughout vertebrate phylogeny. J. Comp. Neurol. 2012, 520, 2957–2973. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, L.; Worbe, Y.; Thobois, S.; Sgambato-Faure, V.; Féger, J. Selective dysfunction of basal ganglia subterritories: From movement to behavioral disorders. J. Mov. Disord. 2015, 30, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Coffey, R.J. Deep brain stimulation devices: A brief technical history and review. Artif. Organs 2009, 33, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.; Forrester, M.T.; Hess, D.T.; Stamler, J.S. S-nitrosylation in cardiovascular signaling. J. Am. Heart Assoc. 2010, 106, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Pelster, B.; Grillitsch, S.; Schwerte, T. NO as a mediator during the early development of the cardiovascular system in the zebrafish. Comp. Biochem. Physiol. A 2005, 142, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.; Tossell, K.; Lockley, R.; McDearmid, J.R. Nitric oxide synthase regulates morphogenesis of zebrafish spinal cord motor neurons. J. Neurosci. 2010, 30, 16818–16831. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Balligand, J.L. Nitric Oxide synthase and cyclic GMP signaling in cardiac myocytes: From contractility to remodeling. J. Mol. Cell. Cardiol. 2011, 52, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric Oxide synthases: Regulation and function. Europ. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Padovan-Neto, F.E.; Echeverry, M.B.; Chiavegatto, S.; Del-Bel, E. Nitric Oxide Synthase Inhibitor Improves De Novo and Long-Term l-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats. Front. Syst. Neurosci. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Karacay, B.; Bonthius, D.J. The neuronal nitric oxide synthase (nNOS) gene and neuroprotection against alcohol toxicity. Cell. Mol. Neurobiol. 2015, 35, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Kurauchi, Y.; Hisatsune, A.; Isohama, Y.; Sawa, T.; Akaike, T.; Katsuki, H. Nitric oxide/soluble guanylyl cyclase signaling mediates depolarization-induced protection of rat mesencephalic dopaminergic neurons from MPP⁺ cytotoxicity. Neuroscience 2013, 231, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, T.; McKay, J.S.; Quinn, J.P.; Morris, R. Nitric oxide, a biological double-faced janus—Is this good or bad? Histol. Histopathol. 2006, 21, 445–458. [Google Scholar] [PubMed]
- Gao, Y. The multiple actions of NO. Eur. J. Physiol. 2010, 459, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Tota, B.; Amelio, D.; Pelligrino, D.; Ip, Y.K.; Cerra, M.C. NO modulation of myocardial performance in fish hearts. Comp. Biochem. Physiol. 2005, 142, 164–177. [Google Scholar]
- Derbyshire, E.R.; Marletta, M.A. Structure and Regulation of Soluble Guanylyl Cyclase. Ann. Rev. Biochem. 2012, 81, 533–559. [Google Scholar] [CrossRef] [PubMed]
- Lorenc-Koci, E.; Czarnecka, A. Role of nitric oxide in the regulation of motor function. An overview of behavioral, biochemical and histological studies in animal models. Pharmacol. Rep. 2013, 65, 1043–1055. [Google Scholar] [CrossRef]
- Turgeon, J.L.; Carr, M.C.; Maki, P.M.; Mendelsohn, M.E.; Wise, P.M. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr. Rev. 2006, 27, 575–605. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Dahodwala, N. Sex differences in Parkinson’s disease and other movement disorders. Exp. Neurol. 2014, 259, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Chambliss, K.L.; Shaul, P.W. Rapid activation of endothelial NO synthase by estrogen: Evidence for a steroid receptor fast-action complex (SRFC) in caveolae. Steroids 2002, 67, 413–419. [Google Scholar] [CrossRef]
- Rink, E.; Wullimann, M.F. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 2001, 19, 316–330. [Google Scholar] [CrossRef]
- McKinley, E.T.; Baranowski, T.C.; Blavo, D.O.; Cato, C.; Doan, T.N.; Rubinstein, A.L. Neuroprotection of MPTP-induced toxicity in zebrafish dopaminergic neurons. Brain Res. Mol. Brain Res. 2005, 141, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Parng, C.; Roy, N.M.; Ton, C.; Lin, Y.; McGrath, P. Neurotoxicity assessment using zebrafish. J. Pharmacol. Toxicol. Methods 2007, 55, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Flinn, L.; Bretaud, S.; Lo, C.; Ingham, P.W.; Bandmann, O. Zebrafish as a new animal model for movement disorders. J. Neurochem. 2008, 106, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.; Henriet, R.P.; Holt, A.W.; Bopp, K.C.; Houser, A.P.; Allgood, O.E.; Turner, J.E. The role of estrogen on the developmental appearance of sensory-motor behaviors in the zebrafish (Danio rerio): The characterization of the “listless” mode. Brain Res. 2008, 1222, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Houser, A.; McNair, C.; Piccinini, R.; Luxhoj, A.; Bell, W.E.; Turner, J.E. Effects of estrogen on the neuromuscular system in the embryonic zebrafish (Danio rerio). Brain Res. 2011, 1381, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Allgood, O.E.; Hamad, A.; Fox, J.; DeFrank, A.; Gilley, R.; Dawson, F.; Sykes, B.; Underwood, T.J.; Naylor, R.C.; Briggs, A.A.; et al. Estrogen prevents cardiac and vascular failure in the ‘listless’ zebrafish (Danio rerio) developmental model. Gen. Comp. Endocrinol. 2013, 189, 33–42. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Zon, L.I. Transparent Adult Zebrafish as a Tool for in vivo Transplantation Analysis. Cell Stem Cell 2018, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L.; Choudhuri, R.; Zhang, H.T.; Donnini, S.; Granger, H.J.; Bicknell, R. Nitric Oxide Synthase Lies Downstream from Vascular Endothelial Growth Factor-induced but not Basic Fibroblast Growth Factor-induced Angiogenesis. J. Clin. Investig. 1997, 99, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Jay, M.; Bradley, S.; McDearmid, J.R. Effects of nitric oxide on neuromuscular properties of developing zebrafish embryos. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, N.J.; Rose, B.; Kobayashi, K.; Guo, S. Antipsychotics produce locomotor impairment in larval zebrafish. Neurotoxicol. Teratol. 2006, 28, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Qu, D.; Kwok, K.H.; Ng, S.S.; Lim, A.Y.; Aw, S.S.; Lee, C.W.; Sung, W.K.; Tan, E.K.; Lufkin, T.; et al. Deletion of the WD40 domain of LRRK2 in Zebrafish causes Parkinsonism-like loss of neurons and locomotive defect. PLoS Genet. 2010, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickels, T.J.; Reed, G.W.; Drummond, J.T.; Blevins, D.E.; Lutz, M.C.; Wilson, D.F. Does nitric oxide modulate transmitter release at the mammalian neuromuscular junction? Clin. Exp. Pharmacol. Physiol. 2007, 34, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; Gemaque, J.; Benzecry, R.; Lima, M.G.; Batista Ede, J.; Picanço-Diniz, D.W.; Oliveira, K.R.; Herculano, A.M. Role of nitric oxide in the behavioral and neurochemical effects of IB-MECA in zebrafish. Psychopharmacology 2015, 232, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Anichtchik, O.V.; Kaslin, J.; Peitsaro, N.; Scheinin, M.; Panula, P. Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurochem. 2004, 88, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.W.; Wen, Z.H.; Huang, S.Y.; Hung, H.C.; Chen, C.H.; Yang, S.N.; Chen, N.F.; Wang, H.M.; Hsiao, C.D.; Chen, W.F. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish 2014, 11, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, B.; Ellingsen, B.; Forsell, J.; Zhdanova, I.; Alm, P. The early ontogeny of neuronal nitric oxide synthase systems in the zebrafish. J. Exp. Biol. 2004, 207, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Nakaso, K.; Horikoshi, Y.; Takahashi, T.; Hanaki, M.; Kitagawa, Y.; Koike, T.; Matsura, T. Estrogen receptor-mediated effect of δ-tocotreienol prevents neurotoxicity and motor deficit in the MPTP mouse model of Parkinson’s disease. Neurosci. Lett. 2016, 610, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kang, M.J.; Kim, K.H.; Han, P.L.; Ha, J.Y.; Son, J.H. Nitric oxide induction of Parkin translocation in PTEN-induced putative kinase 1 (PINK1) deficiency; functional role of neuronal nitric oxide synthase during mitophagy. J. Biol. Chem. 2015, 290, 10325–10335. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murcia, V.; Johnson, L.; Baldasare, M.; Pouliot, B.; McKelvey, J.; Barbery, B.; Lozier, J.; Bell, W.E.; Turner, J.E. Effects of Estrogen, Nitric Oxide, and Dopamine on Behavioral Locomotor Activities in the Embryonic Zebrafish: A Pharmacological Study. Toxics 2016, 4, 24. https://doi.org/10.3390/toxics4040024
Murcia V, Johnson L, Baldasare M, Pouliot B, McKelvey J, Barbery B, Lozier J, Bell WE, Turner JE. Effects of Estrogen, Nitric Oxide, and Dopamine on Behavioral Locomotor Activities in the Embryonic Zebrafish: A Pharmacological Study. Toxics. 2016; 4(4):24. https://doi.org/10.3390/toxics4040024
Chicago/Turabian StyleMurcia, Vania, Luke Johnson, Meredith Baldasare, Bridgette Pouliot, John McKelvey, Brandon Barbery, Julie Lozier, Wade E. Bell, and James E. Turner. 2016. "Effects of Estrogen, Nitric Oxide, and Dopamine on Behavioral Locomotor Activities in the Embryonic Zebrafish: A Pharmacological Study" Toxics 4, no. 4: 24. https://doi.org/10.3390/toxics4040024
APA StyleMurcia, V., Johnson, L., Baldasare, M., Pouliot, B., McKelvey, J., Barbery, B., Lozier, J., Bell, W. E., & Turner, J. E. (2016). Effects of Estrogen, Nitric Oxide, and Dopamine on Behavioral Locomotor Activities in the Embryonic Zebrafish: A Pharmacological Study. Toxics, 4(4), 24. https://doi.org/10.3390/toxics4040024





