Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Isoprostane Analysis
2.3. Phthalate Metabolite Measurements
2.4. Statistical Analyses
3. Results
3.1. CHAMACOS Participants
3.2. Phthalate Exposure
3.3. Isoprostane
3.4. Phthalates and Isoprostane
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CDC | Centers for Disease Control and Prevention |
| CHAMACOS | Center for Health Assessment of Mothers and Children of Salinas |
| CHAP | Chronic Hazard Advisory Panel |
| DBP | dibutyl phthalate |
| DEHP | di-2-ethylhexyl phthalate |
| DEP | diethyl phthalate |
| HMW | high molecular weight |
| LMW | low molecular weight |
| LOD | limit of detection |
| MBP | mono-n-butyl phthalate |
| MBzP | monobenzyl phthalate |
| MCNP | monocarboxynonyl phthalate |
| MCOP | monocarboxyoctyl phthalate |
| MCPP | mono(3-carboxypropyl) phthalate |
| MDA | malondialdehyde |
| MECPP | mono(2-ethyl-5-carboxypentyl) phthalate |
| MEHP | mono(2-ethylhexyl) phthalate |
| MEHHP | mono(2-ethyl-5-hydroxyhexyl) phthalate |
| MEOHP | mono(2-ethyl-5-oxohexyl) phthalate |
| MEP | monoethyl phthalate |
| MiBP | mono-isobutyl phthalate |
| NHANES | National Health and Nutrition Examination Survey |
| SE | standard error |
| SES | socioeconomic status |
| QA/QC | quality assurance/quality control |
| 8-oxo-dG | 8-Oxo-2′-deoxyguanosine |
References
- Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2009. Available online: http://www.cdc.gov/exposurereport/ (accessed on 9 March 2016).
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Malek, N.A.; Hodge, C.C.; Caudill, S.P.; Brock, J.W.; Needham, L.L.; Calafat, A.M. Urinary levels of seven phthalate metabolites in the U.S. Population from the national health and nutrition examination survey (NHANES) 1999–2000. Environ. Health Perspect. 2004, 112, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zheng, W.; Zheng, P.; Wang, S.; Tan, H.; He, G.; Qu, W. Human urinary/seminal phthalates or their metabolite levels and semen quality: A meta-analysis. Environ. Res. 2015, 142, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Mukherjee, B.; Loch-Caruso, R.; Meeker, J.D. Associations between maternal biomarkers of phthalate exposure and inflammation using repeated measurements across pregnancy. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Smarr, M.M.; Grantz, K.L.; Sundaram, R.; Maisog, J.M.; Kannan, K.; Louis, G.M. Parental urinary biomarkers of preconception exposure to bisphenol a and phthalates in relation to birth outcomes. Environ. Health 2015. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Mervish, N.; Moshier, E.L.; Vangeepuram, N.; Galvez, M.P.; Calafat, A.M.; Silva, M.J.; Brenner, B.L.; Wolff, M.S. Associations between phthalate metabolite urinary concentrations and body size measures in new york city children. Environ. Res. 2012, 112, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Whyatt, R.M.; Perzanowski, M.S.; Just, A.C.; Rundle, A.G.; Donohue, K.M.; Calafat, A.M.; Hoepner, L.A.; Perera, F.P.; Miller, R.L. Asthma in inner-city children at 5–11 years of age and prenatal exposure to phthalates: The columbia center for children’s environmental health cohort. Environ. Health Perspect. 2014, 122, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- United States Consumer Product Safety Commission. Chronic Hazard Advisory Panel on Phthalates and Phthalates Alternatives; U.S. Consumer Product Safety Commission: Bethesda, MA, USA, 2014. Available online: http://www.cpsc.gov/PageFiles/169902/CHAP-REPORT-With-Appendices.pdf (accessed on 9 March 2016).
- Hatch, E.E.; Nelson, J.W.; Qureshi, M.M.; Weinberg, J.; Moore, L.L.; Singer, M.; Webster, T.F. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: A cross-sectional study of nhanes data, 1999–2002. Environ. Health 2008. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, R.W.; van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. Males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Roos, V.; Ronn, M.; Johansson, L.; Ahlstrom, H.; Kullberg, J.; Lind, L. Serum concentrations of phthalate metabolites related to abdominal fat distribution two years later in elderly women. Environ. Health 2012. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the framingham study. Arterioscler Thromb Vasc Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.R.; Rosa, J.S.; Milne, G.L.; Pontello, A.M.; Borntrager, H.L.; Heydari, S.; Galassetti, P.R. Increased oxidative stress and altered substrate metabolism in obese children. Int. J. Pediatr. Obes. 2010, 5, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fukuo, K.; Suzuki, K.; Yoshino, G.; Kazumi, T. Relationships of systemic oxidative stress to body fat distribution, adipokines and inflammatory markers in healthy middle-aged women. Endocr. J. 2009, 56, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005, 25, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J., II. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Dobashi, K.; Yamamoto, Y.; Asayama, K.; Kusuhara, K. Increased plasma isoprostane is associated with visceral fat, high molecular weight adiponectin, and metabolic complications in obese children. Eur. J. Pediatr. 2010, 169, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Ostrow, V.; Wu, S.; Aguilar, A.; Bonner, R., Jr.; Suarez, E.; de Luca, F. Association between oxidative stress and masked hypertension in a multi-ethnic population of obese children and adolescents. J. Pediatr. 2011, 158. [Google Scholar] [CrossRef] [PubMed]
- Rossner, P., Jr.; Svecova, V.; Milcova, A.; Lnenickova, Z.; Solansky, I.; Santella, R.M.; Sram, R.J. Oxidative and nitrosative stress markers in bus drivers. Mutat Res. 2007, 617, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Helmersson, J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal 2005, 7, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, A.; Cipollone, F.; Cuccurullo, F. Oxidative stress and cardiovascular complications in diabetes: Isoprostanes as new markers on an old paradigm. Cardiovasc Res. 2000, 47, 475–488. [Google Scholar] [CrossRef]
- Sampson, M.J.; Gopaul, N.; Davies, I.R.; Hughes, D.A.; Carrier, M.J. Plasma F2 isoprostanes: Direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care 2002, 25, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.P.; Pratico, D.; Delanty, N.; DiMinno, G.; Tremoli, E.; Rader, D.; Kapoor, S.; Rokach, J.; Lawson, J.; FitzGerald, G.A. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation 1998, 98, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, L.D.; Sokol, R.J.; Jones, R.H.; Awad, J.A.; Rewers, M.J.; Norris, J.M. Urinary F2-isoprostanes in young healthy children at risk for type 1 diabetes mellitus. Free Radic Biol. Med. 2003, 35, 551–557. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Rachidi, W.; Yuzugullu, O.G.; Giray, B.; Favier, A.; Ozturk, M.; Hincal, F. Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (dehp) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 leydig cells and protection by selenium. Toxicol. Appl. Pharmacol. 2010, 248, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Park, E.Y.; Park, M.S.; Ko, J.A.; Oh, S.Y.; Kim, H.; Lee, K.H.; Leem, J.H.; Ha, E.H. Community level exposure to chemicals and oxidative stress in adult population. Toxicol. Lett. 2009, 184, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P. How dangerous are phthalate plasticizers? Integrated approach to toxicity based on metabolism, electron transfer, reactive oxygen species and cell signaling. Med. Hypotheses 2010, 74, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Rusyn, I.; Peters, J.M.; Cunningham, M.L. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol. 2006, 36, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Kim, K.B.; Kim, Y.J.; Choi, J.Y.; Lee, K.T.; Choi, K.S. Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem. Toxicol. 2004, 42, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Takahashi, K.; Hosokawa, T.; Saito, T.; Kurasaki, M. Diethyl phthalate enhances apoptosis induced by serum deprivation in PC12 cells. Basic Clin. Pharmacol. Toxicol. 2012, 112, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ao, H.; Chen, L.; Sottas, C.M.; Ge, R.S.; Li, L.; Zhang, Y. Mono-(2-ethylhexyl) phthalate affects the steroidogenesis in rat Leydig cells through provoking ROS perturbation. Toxicol. Vitro 2012, 26, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; Loch-Caruso, R.; Meeker, J.D. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: Nhanes 1999–2006. Environ. Res. 2011, 111, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; Loch-Caruso, R.; Meeker, J.D. Exploration of oxidative stress and inflammatory markers in relation to urinary phthalate metabolites: Nhanes 1999–2006. Environ. Sci. Technol. 2012, 46, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kambia, N.; Dine, T.; Gressier, B.; Frimat, B.; Cazin, J.L.; Luyckx, M.; Brunet, C.; Michaud, L.; Gottrand, F. Correlation between exposure to phthalates and concentrations of malondialdehyde in infants and children undergoing cyclic parenteral nutrition. JPEN J. Parenter Enteral Nutr. 2011, 35, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Botelho, G.G.; Bufalo, A.C.; Boareto, A.C.; Muller, J.C.; Morais, R.N.; Martino-Andrade, A.J.; Lemos, K.R.; Dalsenter, P.R. Vitamin C and resveratrol supplementation to rat dams treated with di(2-ethylhexyl)phthalate: Impact on reproductive and oxidative stress end points in male offspring. Arch. Environ. Contam Toxicol. 2009, 57, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Ko, Y.A.; Mukherjee, B.; Meeker, J.D. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int. 2014, 70, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Bradman, A.; Gladstone, E.; Jaramillo, S.; Birch, K.; Holland, N. Chamacos, a longitudinal birth cohort study: Lessons from the fields. J. Child. Healt 2003, 1, 3–27. [Google Scholar] [CrossRef]
- Huen, K.; Calafat, A.; Bradman, A.; Yousefi, P.; Eskenazi, B.; Holland, N. Maternal phthalate exposure during pregnancy is associated with DNA methylation of line-1 and alu repetitive elements in mexican-american children. Environ. Res. in press.
- Silva, M.J.; Samandar, E.; Preau, J.L., Jr.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 860, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Parlett, L.E.; Calafat, A.M.; Swan, S.H. Women’s exposure to phthalates in relation to use of personal care products. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Colt, J.S.; Camann, D.; Davis, S.; Cerhan, J.R.; Severson, R.K.; Bernstein, L.; Hartge, P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health Perspect. 2004, 112, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Calafat, A.M.; Woodruff, T.J. Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010. Environ. Health Perspect. 2014, 122, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sauve, J.F.; Levesque, M.; Huard, M.; Drolet, D.; Lavoue, J.; Tardif, R.; Truchon, G. Creatinine and specific gravity normalization in biological monitoring of occupational exposures. J. Occup. Environ. Hyg. 2015, 12, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Adibi, J.J.; Whyatt, R.M.; Williams, P.L.; Calafat, A.M.; Camann, D.; Herrick, R.; Nelson, H.; Bhat, H.K.; Perera, F.P.; Silva, M.J.; et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 2008, 116, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Hu, H.; Cantonwine, D.E.; Lamadrid-Figueroa, H.; Calafat, A.M.; Ettinger, A.S.; Hernandez-Avila, M.; Loch-Caruso, R.; Tellez-Rojo, M.M. Urinary phthalate metabolites in relation to preterm birth in mexico city. Environ. Health Perspect. 2009, 117, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. Population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, F.W.; Castorina, R.; Maddalena, R.L.; Nishioka, M.G.; McKone, T.E.; Bradman, A. Phthalate exposure and risk assessment in california child care facilities. Environ. Sci. Technol. 2014, 48, 7593–7601. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Upson, K.; Cook, N.R.; Weinberg, C.R. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ. Health Perspect. 2015. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental chemicals in pregnant women in the united states: Nhanes 2003–2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Lien, Y.J.; Ku, H.Y.; Su, P.H.; Chen, S.J.; Chen, H.Y.; Liao, P.C.; Chen, W.J.; Wang, S.L. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan maternal and infant cohort study. Environ. Health Perspect. 2015, 123, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Just, A.C.; Williams, P.L.; Smith, K.W.; Calafat, A.M.; Hauser, R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Biomonitoring California. Available online: http://biomonitoring.ca.gov (accessed on 3 January 2016).
- Braun, J.M.; Smith, K.W.; Williams, P.L.; Calafat, A.M.; Berry, K.; Ehrlich, S.; Hauser, R. Variability of urinary phthalate metabolite and bisphenol a concentrations before and during pregnancy. Environ. Health Perspect. 2012, 120, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Valvi, D.; Monfort, N.; Ventura, R.; Casas, M.; Casas, L.; Sunyer, J.; Vrijheid, M. Variability and predictors of urinary phthalate metabolites in spanish pregnant women. Int. J. Hyg. Environ. Health 2015, 218, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. Comparative assessment of human exposure to phthalate esters from house dust in china and the united states. Environ. Sci. Technol. 2011, 45, 3788–3794. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Sathyanarayana, S.; Hauser, R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013, 25, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.Y.; Su, P.H.; Wen, H.J.; Sun, H.L.; Wang, C.J.; Chen, H.Y.; Jaakkola, J.J.; Wang, S.L. Prenatal and postnatal exposure to phthalate esters and asthma: A 9-year follow-up study of a taiwanese birth cohort. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Harley, K.; Kogut, K.; Madrigal, D.; Cardenas, M.; Vera, I.; Meza-Alfaro, G.; She, J.; Gavin, Q.; Zahedi, R.; Bradman, A.; et al. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: Findings from the HERMOSA intervention study. Environ. Health Perspect. 2016. [Google Scholar] [CrossRef] [PubMed]
- Colacino, J.A.; Harris, T.R.; Schecter, A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ. Health Perspect. 2010, 118, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Diaz, S.; Su, Y.C.; Mitchell, A.A.; Kelley, K.E.; Calafat, A.M.; Hauser, R. Medications as a potential source of exposure to phthalates among women of childbearing age. Reprod. Toxicol. 2013, 37, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vinas, P.; Campillo, N.; Pastor-Belda, M.; Oller, A.; Hernandez-Cordoba, M. Determination of phthalate esters in cleaning and personal care products by dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1376, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Lorber, M.; Christensen, K.L.; Palmke, C.; Koslitz, S.; Bruning, T. Identifying sources of phthalate exposure with human biomonitoring: Results of a 48 h fasting study with urine collection and personal activity patterns. Int. J. Hyg. Environ. Health 2013, 216, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Meeker, J.D.; Singh, N.P.; Silva, M.J.; Ryan, L.; Duty, S.; Calafat, A.M. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum. Reprod. 2007, 22, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Lorber, M.; Angerer, J.; Koch, H.M. A simple pharmacokinetic model to characterize exposure of americans to di-2-ethylhexyl phthalate. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Bruning, T. Assessing exposure to phthalates—The human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, S.; Lee, G.; Lee, S.; Jo, A.; Kwak, K.; Kim, D.; Koh, D.; Kho, Y.L.; Choi, K. Urinary phthalate metabolites among elementary school children of korea: Sources, risks, and their association with oxidative stress marker. Sci. Total Environ. 2014, 472, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Harley, K.; Eskenazi, B.; Block, G. The association of time in the us and diet during pregnancy in low-income women of mexican descent. Paediatr. Perinat Epidemiol. 2005, 19, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Gradaščević Gubaljević, J.; Čaušević, A. Monitoring changes in serum 8-isoprostane concentration as a possible marker of oxidative stress in pregnancy. J. Health Sci. 2013, 3, 227–231. [Google Scholar] [CrossRef]
- Peter Stein, T.; Scholl, T.O.; Schluter, M.D.; Leskiw, M.J.; Chen, X.; Spur, B.W.; Rodriguez, A. Oxidative stress early in pregnancy and pregnancy outcome. Free Radic Res. 2008, 42, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Nash, P. Experimental and Clinical Studies of Oxidative Stress in Preeclampsia. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 13 April 2007. [Google Scholar]
- Larose, J.; Julien, P.; Bilodeau, J.F. Analysis of F2-isoprostanes in plasma of pregnant women by HPLC-MS/MS using a column packed with core-shell particles. J. Lipid Res. 2013, 54, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.T.; Chen, S.F.; Lo, L.M.; Li, M.J.; Yeh, Y.L.; Hung, T.H. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reprod. Sci. 2012, 19, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F., III; Petraglia, F. Inflammation and pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, H.Y.; Bae, S.; Lim, Y.H.; Hong, Y.C. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: A panel study. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.M.; Dodson, R.E.; Engel, C.L.; Gray, J.M.; Rudel, R.A. Temporal variability of urinary di(2-ethylhexyl) phthalate metabolites during a dietary intervention study. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food packaging and bisphenol a and bis(2-ethyhexyl) phthalate exposure: Findings from a dietary intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Mothers with Phthalate Data * (N = 433) N (%) | Mothers with Isoprostane Data* (N = 196) N (%) |
|---|---|---|
| Pre-pregnancy Weight Status | - | - |
| Normal | 156 (36.8) | 59 (30.1) |
| Underweight | 3 (0.7) | 1 (0.5) |
| Overweight | 163 (38.4) | 84 (42.9) |
| Obese | 102 (24.1) | 52 (26.5) |
| Age at Delivery | - | - |
| 18–24 | 189 (43.8) | 70 (35.7) |
| 25–29 | 136 (31.5) | 73 (37.2) |
| 30–34 | 70 (16.2) | 32 (16.3) |
| 35–45 | 37 (8.6) | 21 (10.7) |
| Education | - | - |
| ≤6th grade | 186 (43) | 89 (45.4) |
| 7–12th grade | 154 (35.6) | 71 (36.2) |
| ≥High School Graduate | 93 (21.5) | 36 (18.4) |
| Years in U.S. | - | - |
| ≤1 | 107 (24.7) | 45 (23) |
| 2–5 | 111 (25.6) | 48 (24.5) |
| 6–10 | 98 (22.6) | 60 (30.6) |
| 11+ | 66 (15.2) | 30 (15.3) |
| Entire life | 51 (11.8) | 13 (6.6) |
| Poverty Status | - | - |
| At or below poverty | 270 (62.4) | 132 (67.3) |
| Poverty-200% | 148 (34.2) | 57 (29.1) |
| >200% poverty | 15 (3.5) | 7 (3.6) |
| Alcohol Use during Pregnancy | - | - |
| No | 403 (94.6) | 183 (94.8) |
| Yes | 23 (5.4) | 10 (5.2) |
| Smoking during Pregnancy | - | - |
| No | 410 (94.7) | 188 (95.9) |
| Yes | 23 (5.3) | 8 (4.1) |
| Parity | - | - |
| 0 | 144 (33.3) | 59 (30.1) |
| ≥1 | 289 (66.7) | 137 (69.9) |
| Exposure * | 13 Weeks (N = 432) | 26 Weeks (N = 417) | Correlation | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | 5th | 95th | Median | IQR | 5th | 95th | |||
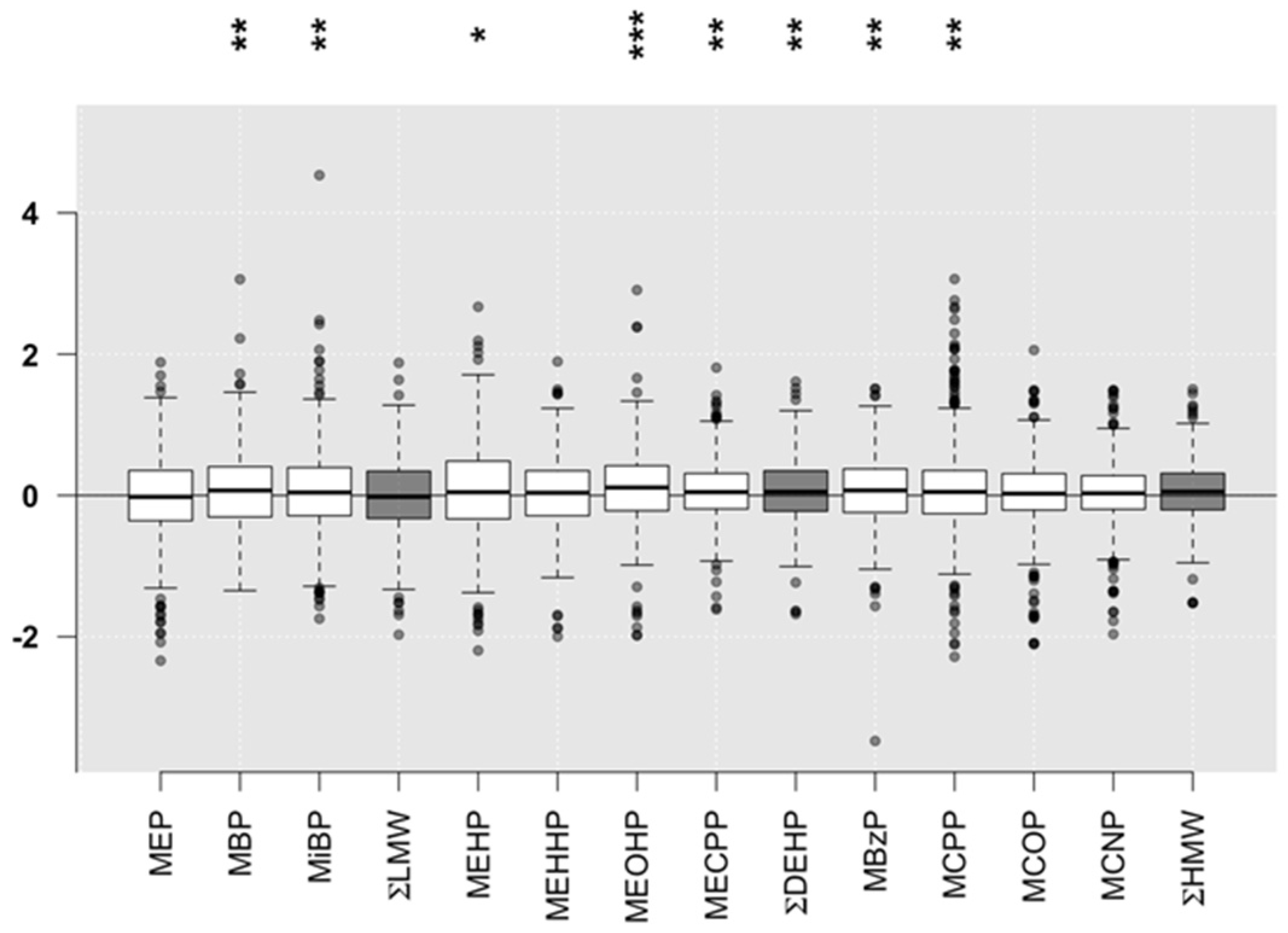
| MEP | 161.8 | (67.3, 435.1) | 24.3 | 1618.6 | 153.3 | (65.6, 376.3) | 22.2 | 1262.1 | 0.389 | 4.44 × 10−16 |
| MBP | 18.4 | (9.1, 37.9) | 3.7 | 102.4 | 22.6 | (11.7, 42.6) | 5.0 | 121.6 | 0.211 | 1.76 × 10−5 |
| MiBP | 2.4 | (1.1, 4.5) | 0.2 | 14.0 | 2.8 | (1.4, 5.2) | 0.4 | 14.4 | 0.267 | 4.46 × 10−8 |
| ΣLMW | 211.9 | (100.7, 524.4) | 40.0 | 1806.8 | 217.8 | (113.7, 451.4) | 43.2 | 1506.3 | 0.371 | 9.77 × 10−15 |
| MEHP | 3.0 | (1.4, 6.4) | 0.2 | 17.7 | 3.6 | (1.9, 6.7) | 0.4 | 18.0 | 0.199 | 5.04 × 10−5 |
| MEHHP | 12.6 | (6.8, 24.7) | 2.5 | 76.9 | 15.7 | (8.3, 28.1) | 3.3 | 66.2 | 0.233 | 2.02 × 10−6 |
| MEOHP | 8.7 | (4.7, 16.7) | 1.6 | 47.2 | 12.1 | (6.8, 21.1) | 2.8 | 48.4 | 0.204 | 3.37 × 10−5 |
| MECPP | 22.0 | (13.7, 39.7) | 5.9 | 104.0 | 25.8 | (16, 45.6) | 8.5 | 97.5 | 0.241 | 8.79 × 10−7 |
| ΣDEHP | 46.0 | (27.6, 84.3) | 11.8 | 242.3 | 57.8 | (33.2, 99.3) | 16.1 | 235.5 | 0.231 | 2.35 × 10−6 |
| MBzP | 6.6 | (3.1, 12.7) | 0.9 | 32.1 | 7.6 | (4.3, 14) | 1.5 | 38.1 | 0.378 | 2.66 × 10−15 |
| MCPP | 1.8 | (1, 2.9) | 0.1 | 6.4 | 2.1 | (1.2, 3.2) | 0.2 | 6.4 | 0.154 | 1.76 × 10−3 |
| MCOP | 2.8 | (1.7, 4.6) | 0.5 | 10.7 | 3.2 | (2.1, 5) | 0.8 | 9.6 | 0.138 | 5.30 × 10−3 |
| MCNP | 1.8 | (1, 2.7) | 0.4 | 7.2 | 1.9 | (1.3, 2.8) | 0.6 | 5.8 | 0.206 | 2.80 × 10−5 |
| ΣHMW | 65.7 | (37.8, 110.4) | 17.6 | 303.8 | 79.5 | (46.8, 126.4) | 24.2 | 270.5 | 0.244 | 6.37 × 10−7 |
| Isoprostane ** | 3.6 | (2.2, 5.0) | 1.0 | 10.8 | 4.6 | (3.1, 6) | 1.2 | 8.9 | 0.176 | 3.34 × 10−2 |
| Phthalate Metabolite (µg/g Creatinine) | 13 Weeks (n = 166) | 26 Weeks (n = 180) | Change (n = 150) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | β | 95% CI | p-Value | |
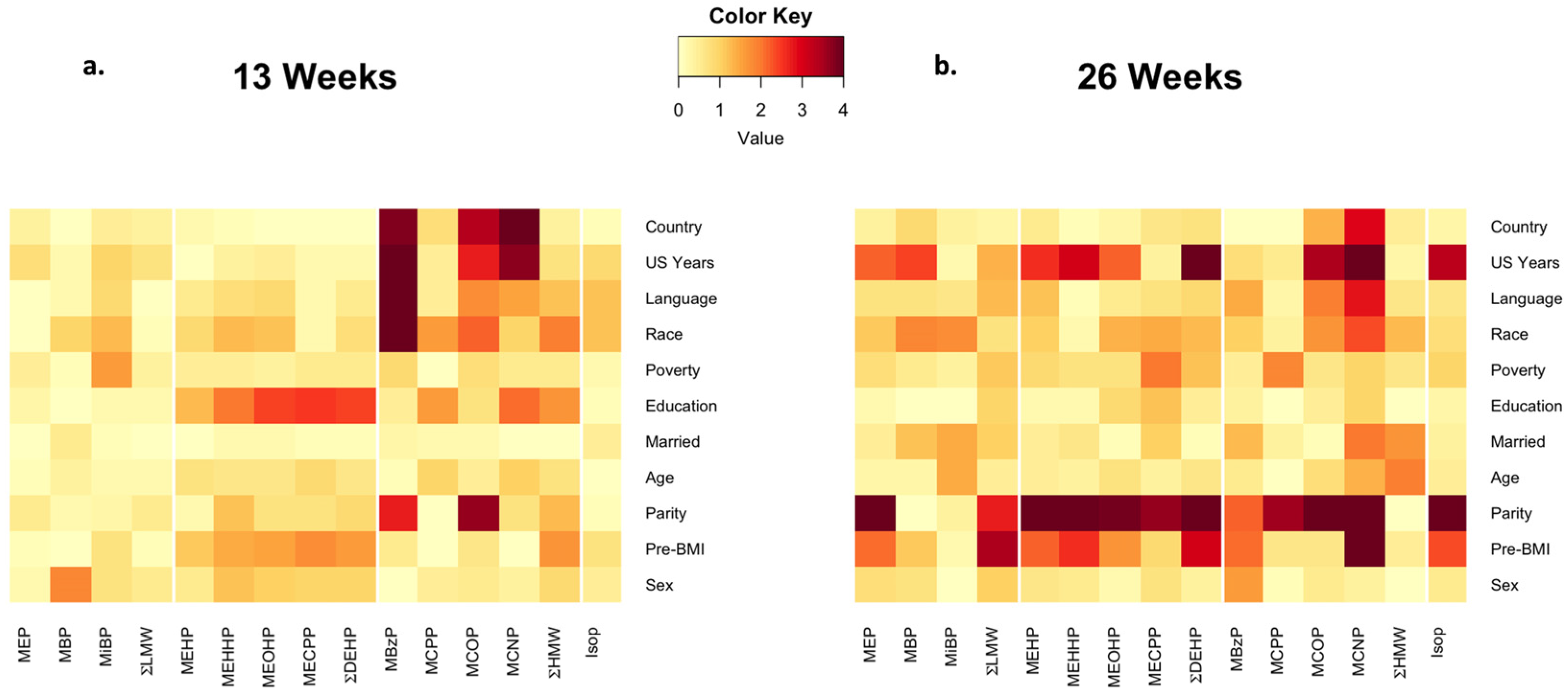
| MEP | 0.045 | (−0.031, 0.121) | 0.2486 | 0.074 | (0.003, 0.145) | 0.0417 | 0.110 | (0.014, 0.206) | 0.0271 |
| MBP | 0.064 | (−0.028, 0.156) | 0.1721 | 0.183 | (0.083, 0.283) | 0.0004 | 0.094 | (−0.006, 0.194) | 0.0684 |
| MiBP | −0.002 | (−0.088, 0.084) | 0.9665 | 0.097 | (0.023, 0.171) | 0.0108 | 0.059 | (-0.031, 0.149) | 0.2012 |
| ΣLMW | 0.056 | (−0.028, 0.140) | 0.2007 | 0.109 | (0.029, 0.189) | 0.0087 | 0.144 | (0.036, 0.252) | 0.0099 |
| MEHP | 0.006 | (−0.080, 0.092) | 0.8949 | 0.057 | (−0.023, 0.137) | 0.1643 | 0.073 | (−0.019, 0.165) | 0.1239 |
| MEHHP | 0.066 | (−0.048, 0.180) | 0.2526 | 0.068 | (−0.018, 0.154) | 0.1271 | 0.101 | (-0.009, 0.211) | 0.0768 |
| MEOHP | 0.064 | (−0.036, 0.164) | 0.2160 | 0.072 | (−0.018, 0.162) | 0.1220 | 0.092 | (-0.014, 0.198) | 0.0910 |
| MECPP | 0.087 | (−0.050, 0.224) | 0.2151 | 0.074 | (−0.040, 0.188) | 0.2002 | 0.130 | (−0.005, 0.265) | 0.0613 |
| ΣDEHP | 0.075 | (−0.052, 0.202) | 0.2499 | 0.080 | (−0.026, 0.186) | 0.1349 | 0.131 | (0.004, 0.258) | 0.0464 |
| MBzP | 0.045 | (−0.059, 0.149) | 0.3983 | 0.012 | (−0.074, 0.098) | 0.7805 | 0.076 | (−0.032, 0.184) | 0.1710 |
| MCPP | 0.033 | (−0.045, 0.111) | 0.4142 | 0.125 | (0.035, 0.215) | 0.0072 | 0.003 | (−0.052, 0.112) | 0.4728 |
| MCOP | 0.178 | (0.062, 0.294) | 0.0031 | 0.090 | (−0.020, 0.200) | 0.1119 | 0.078 | (−0.045, 0.201) | 0.2188 |
| MCNP | 0.152 | (0.029, 0.275) | 0.0166 | 0.136 | (0.021, 0.250) | 0.0211 | 0.110 | (−0.014, 0.233) | 0.0832 |
| ΣHMW | 0.088 | (−0.050, 0.226) | 0.2119 | 0.092 | (−0.026, 0.209) | 0.1300 | 0.142 | (0.002, 0.283) | 0.0487 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holland, N.; Huen, K.; Tran, V.; Street, K.; Nguyen, B.; Bradman, A.; Eskenazi, B. Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy. Toxics 2016, 4, 7. https://doi.org/10.3390/toxics4010007
Holland N, Huen K, Tran V, Street K, Nguyen B, Bradman A, Eskenazi B. Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy. Toxics. 2016; 4(1):7. https://doi.org/10.3390/toxics4010007
Chicago/Turabian StyleHolland, Nina, Karen Huen, Vy Tran, Kelly Street, Brian Nguyen, Asa Bradman, and Brenda Eskenazi. 2016. "Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy" Toxics 4, no. 1: 7. https://doi.org/10.3390/toxics4010007
APA StyleHolland, N., Huen, K., Tran, V., Street, K., Nguyen, B., Bradman, A., & Eskenazi, B. (2016). Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy. Toxics, 4(1), 7. https://doi.org/10.3390/toxics4010007