Variation in Atmospheric 137Cs and the Carriers in Aerosol Samples Obtained from a Heavily Contaminated Area of Fukushima Prefecture
Abstract
1. Introduction
1.1. The Fukushima Daiichi Nuclear Power Plant Accident
1.2. Deposition and Distribution of 137Cs
1.3. Resuspension of 137Cs
2. Materials and Methods
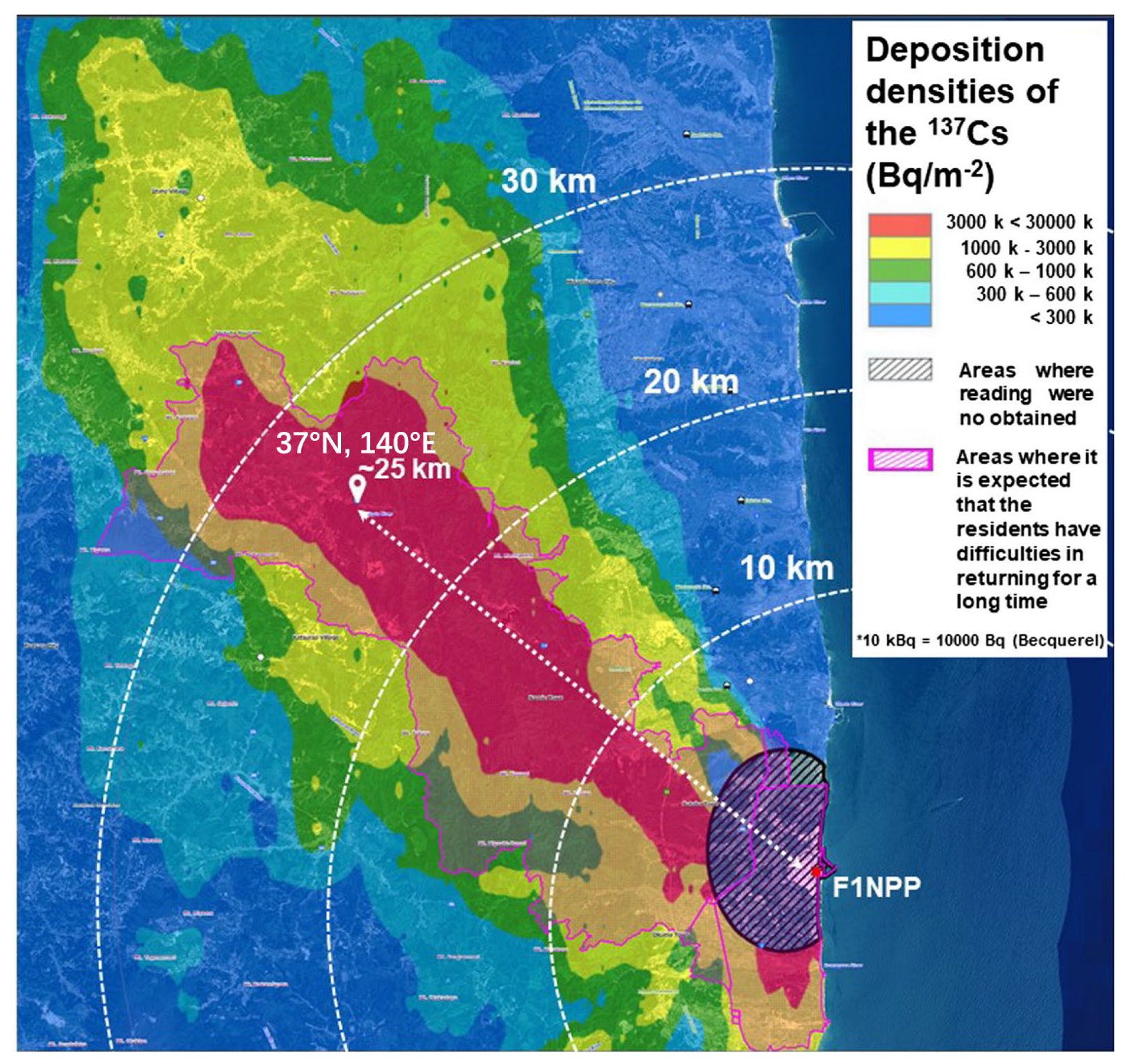
2.1. Sampling Site
2.2. Sampling
2.3. Meteorological Monitoring
2.4. Radioactivity Measurement
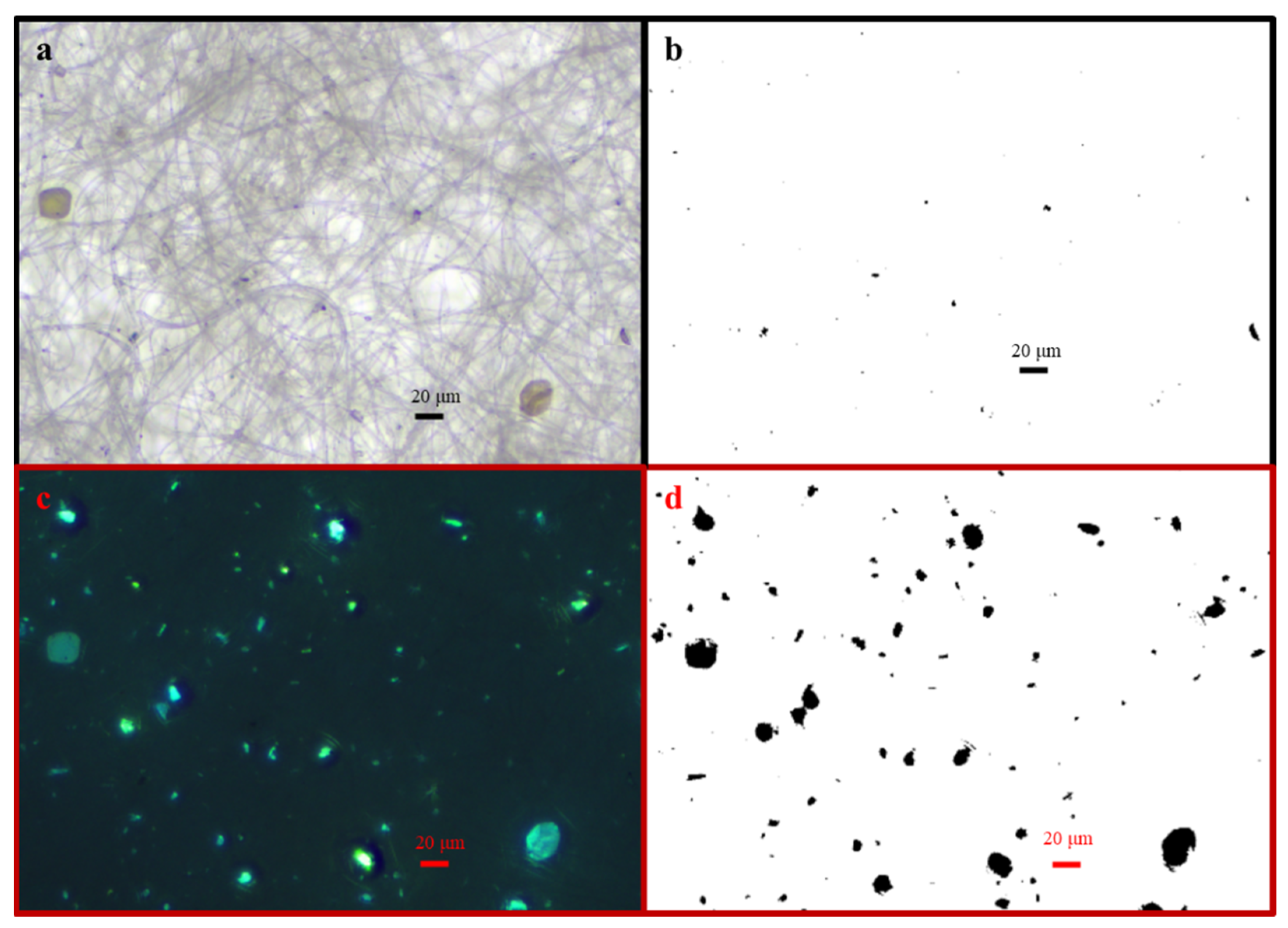
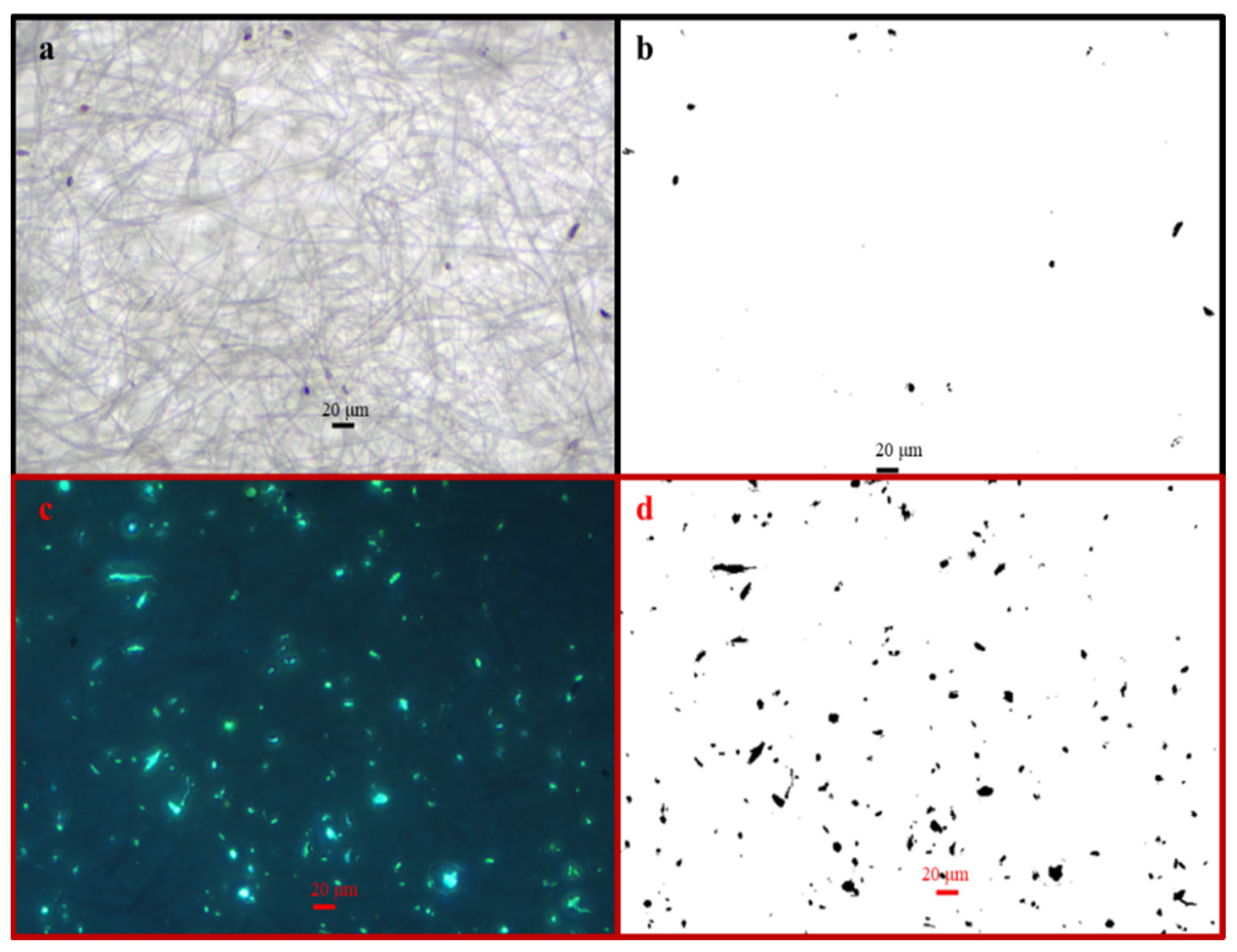
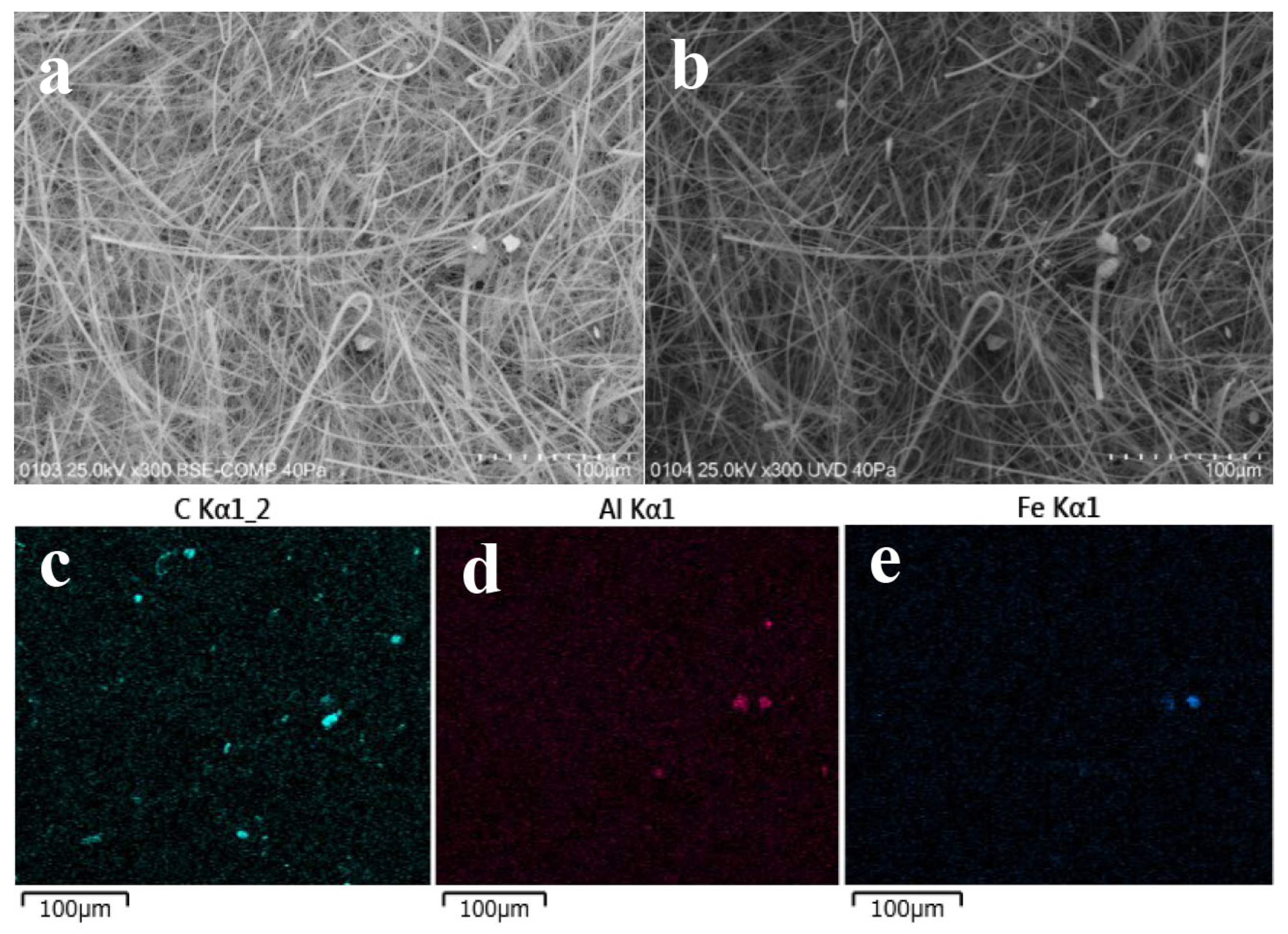
2.5. Microscope Observations
3. Results and Discussions
3.1. Variations in 137Cs in Aerosol Filters Sampled in 2019
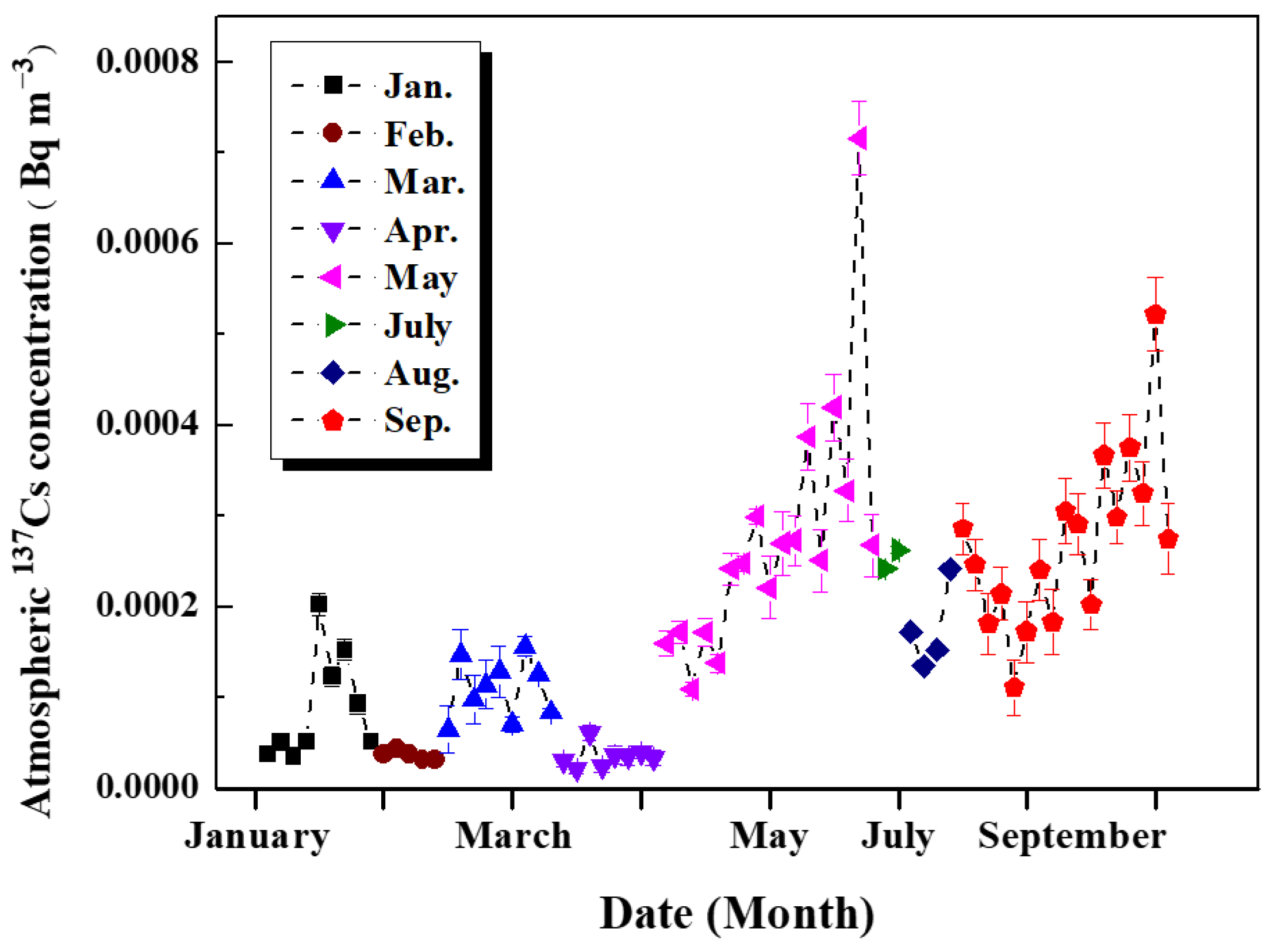
3.1.1. Annual Variations in Atmospheric 137Cs
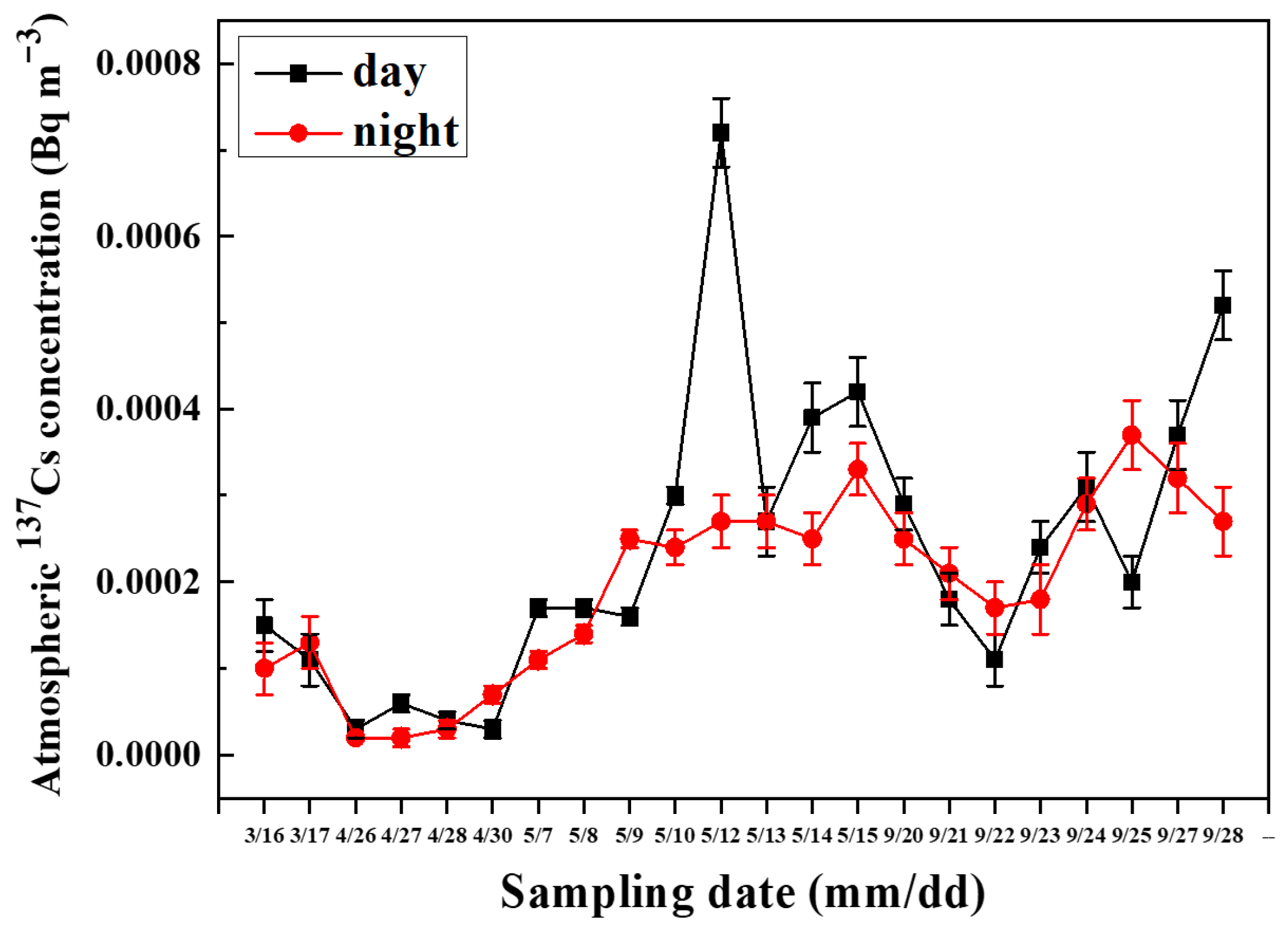
3.1.2. Diurnal Variation in 137Cs
3.2. Carriers of 137Cs in May and September
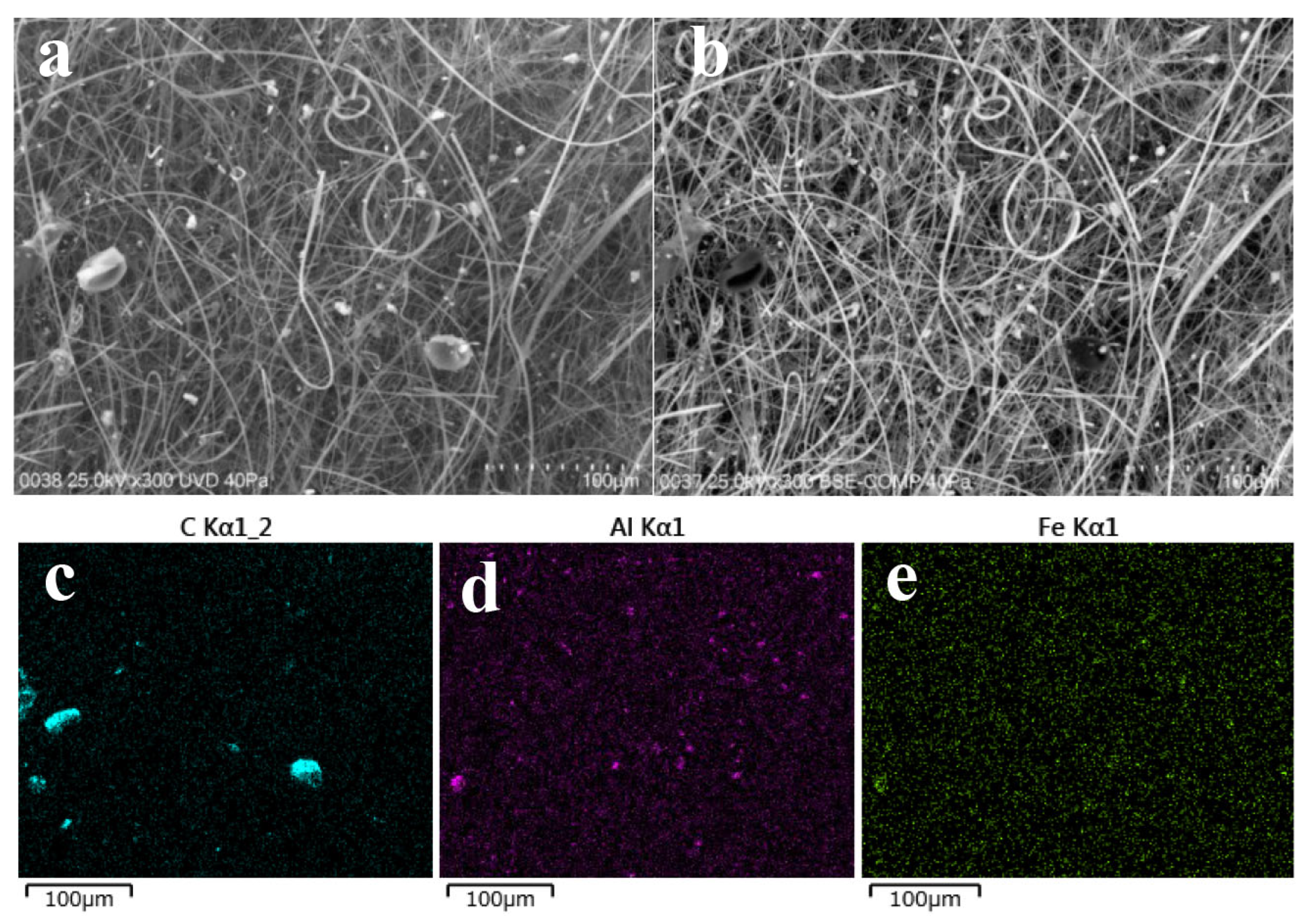
3.2.1. Carriers of 137Cs for the HV Samples Collected in May 2019
3.2.2. Carriers of 137Cs for the HV Samples Collected in September 2019
3.3. Bioaerosol Particles and Their Size Distributions
3.4. Relation Between Aerosol Particles and Atmospheric 137Cs
3.5. Effect Estimation of Weather Conditions on Atmospheric 137Cs and Its Carriers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HERP. Ten Years after the 2011 off the Pacific Coast of Tohoku Earthquake; HERP: Shinagawa, Japan, 2021. [Google Scholar]
- TEPCO. The Record of the Earthquake Intensity Observed at Fukushima Daiichi Nuclear Power Station and Fukushima Daini Nuclear Power Station (Interim Report); TEPCO: Tokyo, Japan, 2011. [Google Scholar]
- TEPCO. Plant Status of Fukushima Daiichi Nuclear Power Station (as of 0 AM March 12th); TEPCO: Tokyo, Japan, 2011. [Google Scholar]
- IAEA. The Fukushima Daiichi Accident; International Atomic Energy Agency: Vienna, Austria, 2015. [Google Scholar]
- Ohara, T.; Morino, Y.; Tanaka, A. Atmospheric behavior of radioactive materials from Fukushima Daiichi nuclear power plant. Hoken Iryo Kagaku 2011, 60, 292–299. [Google Scholar]
- Christoudias, T.; Lelieveld, J. Modelling the global atmospheric transport and deposition of radionuclides from the Fukushima Dai-ichi nuclear accident. Atmos. Chem. Phys. 2013, 13, 1425–1438. [Google Scholar] [CrossRef]
- Sato, I.; Sasaki, J.; Satoh, H.; Deguchi, Y.; Otani, K.; Okada, K. Distribution of radioactive cesium and its seasonal variations in cattle living in the “difficult-to-return zone” of the Fukushima nuclear accident. Anim. Sci. J. 2016, 87, 607–611. [Google Scholar] [CrossRef]
- Onda, Y.; Taniguchi, K.; Yoshimura, K.; Kato, H.; Takahashi, J.; Wakiyama, Y.; Coppin, F.; Smith, H. Radionuclides from the Fukushima Daiichi nuclear power plant in terrestrial systems. Nat. Rev. Earth Environ. 2020, 1, 644–660. [Google Scholar] [CrossRef]
- Hirose, K. Temporal variation of monthly 137Cs deposition observed in Japan: Effects of the Fukushima Daiichi nuclear power plant accident. Appl. Radiat. Isot. 2013, 81, 325–329. [Google Scholar] [CrossRef]
- Tang, P.; Kita, K.; Igarashi, Y.; Satou, Y.; Hatanaka, K.; Adachi, K.; Kinase, T.; Ninomiya, K.; Shinohara, A. Atmospheric resuspension of insoluble radioactive cesium-bearing particles found in the difficult-to-return area in Fukushima. Prog. Earth Planet. Sci. 2022, 9, 17. [Google Scholar] [CrossRef]
- Hirose, K. Atmospheric effects of Fukushima nuclear accident: A review from a sight of atmospheric monitoring. J. Environ. Radioact. 2020, 218, 106240. [Google Scholar] [CrossRef]
- Igarashi, Y.; Aoyama, M.; Hirose, K.; Miyao, T.; Nemoto, K.; Tomita, M.; Fujikawa, T. Resuspension: Decadal monitoring time series of the anthropogenic radioactivity deposition in Japan. J. Radiat. Res. 2003, 44, 319. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y. Anthropogenic radioactivity in aerosol-a review focusing on studies during the 2000s. Jpn. J. Health Phys. 2009, 44, 313. [Google Scholar] [CrossRef][Green Version]
- Kajino, M.; Ishizuka, M.; Igarashi, Y.; Kita, K.; Yoshikawa, C.; Inatsu, M. Long-term assessment of airborne radiocesium after the Fukushima nuclear accident: Re-suspension from bare soil and forest ecosystems. Atmos. Chem. Phys. 2016, 16, 13149–13172. [Google Scholar] [CrossRef]
- Ishizuka, M.; Mikami, M.; Tanaka, T.Y.; Igarashi, Y.; Kita, K.; Yamada, Y.; Yoshida, N.; Toyoda, S.; Satou, Y.; Kinase, T. Use of a size-resolved 1-D resuspension scheme to evaluate resuspended radioactive material associated with mineral dust particles from the ground surface. J. Environ. Radioact. 2017, 166, 436–448. [Google Scholar] [CrossRef]
- Kinase, T.; Kita, K.; Igarashi, Y.; Adachi, K.; Ninomiya, K.; Shinohara, A.; Okochi, H.; Ogata, H.; Ishizuka, M.; Toyoda, S. The seasonal variations of atmospheric 134,137 Cs activity and possible host particles for their resuspension in the contaminated areas of Tsushima and Yamakiya, Fukushima, Japan. Prog. Earth Planet. Sci. 2018, 5, 12. [Google Scholar] [CrossRef]
- Igarashi, Y.; Kita, K.; Maki, T.; Kinase, T.; Hayashi, N.; Hosaka, K.; Adachi, K.; Kajino, M.; Ishizuka, M.; Sekiyama, T.T. Fungal spore involvement in the resuspension of radiocaesium in summer. Sci. Rep. 2019, 9, 1954. [Google Scholar] [CrossRef]
- Kaneyasu, N.; Ohashi, H.; Suzuki, F.; Okuda, T.; Ikemori, F. Sulfate aerosol as a potential transport medium of radiocesium from the Fukushima nuclear accident. Environ. Sci. Technol. 2012, 46, 5720–5726. [Google Scholar] [CrossRef]
- Nakagawa, M.; Yamada, K.; Toyoda, S.; Kita, K.; Igarashi, Y.; Komatsu, S.; Yamada, K.; Yoshida, N. Characterization of hydrocarbons in aerosols and investigation of biogenic sources as a carrier of radiocesium isotopes. Geochem. J. 2018, 52, 163–172. [Google Scholar] [CrossRef]
- Higaki, S.; Yoshida-Ohuchi, H.; Shinohara, N. Radiocesium-bearing microparticles discovered on masks worn during indoor cleaning. Sci. Rep. 2023, 13, 10008. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, G.; Niisoe, T.; Harada, K.H.; Shozugawa, K.; Schneider, S.; Synal, H.-A.; Walther, C.; Christl, M.; Nanba, K.; Ishikawa, H. Post-accident sporadic releases of airborne radionuclides from the Fukushima Daiichi nuclear power plant site. Environ. Sci. Technol. 2015, 49, 14028–14035. [Google Scholar] [CrossRef]
- Maki, T.; Hara, K.; Yamada, M.; Kobayashi, F.; Hasegawa, H.; Iwasaka, Y. Epifluorescent microscopic observation of aerosol. Earozoru Kenkyu 2013, 28, 201–207. [Google Scholar]
- Kita, K.; Igarashi, Y.; Kinase, T.; Hayashi, N.; Ishizuka, M.; Adachi, K.; Koitabashi, M.; Sekiyama, T.T.; Onda, Y. Rain-induced bioecological resuspension of radiocaesium in a polluted forest in Japan. Sci. Rep. 2020, 10, 15330. [Google Scholar] [CrossRef]
- Kimura, M. Seasonal Transitions of Airborne Carriers of Radiocesium in Spring and Early Summer. Master’s Thesis, Ibaraki University, Mito, Japan, 2019. [Google Scholar]
- Stanley, R.G.; Linskens, H.F. Pollen: Biology Biochemistry Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kelly, J.K.; Rasch, A.; Kalisz, S. A method to estimate pollen viability from pollen size variation. Am. J. Bot. 2002, 89, 1021–1023. [Google Scholar] [CrossRef]
- Yamamoto, N.; Bibby, K.; Qian, J.; Hospodsky, D.; Rismani-Yazdi, H.; Nazaroff, W.W.; Peccia, J. Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. ISME J. 2012, 6, 1801–1811. [Google Scholar] [CrossRef]
- Hoorman, J.J. The role of soil bacteria. Ohio State Univ. Ext. Columb. 2011, 2, 1–4. [Google Scholar]
- Guo, L.-C.; Bao, L.-J.; She, J.-W.; Zeng, E.Y. Significance of wet deposition to removal of atmospheric particulate matter and polycyclic aromatic hydrocarbons: A case study in Guangzhou, China. Atmos. Environ. 2014, 83, 136–144. [Google Scholar] [CrossRef]
- Suárez-Contreras, S.; Sánchez-Mendieta, V.; Hernández-Méndez, B.; Sánchez-Meza, J.C.; Balcázar, M. Thirteen-Year Cesium-137 Distribution Environmental Analysis in an Undisturbed Area. Appl. Sci. 2025, 15, 9982. [Google Scholar] [CrossRef]
- Igarashi, Y.; Kogure, T.; Kurihara, Y.; Miura, H.; Okumura, T.; Satou, Y.; Takahashi, Y.; Yamaguchi, N. A review of Cs-bearing microparticles in the environment emitted by the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2019, 205, 101–118. [Google Scholar] [CrossRef]
- Higaki, S.; Kurihara, Y.; Takahashi, Y. Discovery of radiocesium-bearing particles in masks worn by members of the public in Fukushima in spring 2013. Health Phys. 2020, 118, 656–663. [Google Scholar] [CrossRef]
- Nihei, N.; Yoshimura, K.; Okumura, T.; Tanoi, K.; Iijima, K.; Kogure, T.; Nakanishi, T. Secondary radiocesium contamination of agricultural products by resuspended matter. J. Radioanal. Nucl. Chem. 2018, 318, 341–346. [Google Scholar] [CrossRef]
- Otosaka, S.; Kamidaira, Y.; Ikenoue, T.; Kawamura, H. Distribution, dynamics, and fate of radiocesium derived from FDNPP accident in the ocean. J. Nucl. Sci. Technol. 2022, 59, 409–423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, H.; Tang, P.; Kita, K. Variation in Atmospheric 137Cs and the Carriers in Aerosol Samples Obtained from a Heavily Contaminated Area of Fukushima Prefecture. Toxics 2026, 14, 88. https://doi.org/10.3390/toxics14010088
Li H, Tang P, Kita K. Variation in Atmospheric 137Cs and the Carriers in Aerosol Samples Obtained from a Heavily Contaminated Area of Fukushima Prefecture. Toxics. 2026; 14(1):88. https://doi.org/10.3390/toxics14010088
Chicago/Turabian StyleLi, Huihui, Peng Tang, and Kazuyuki Kita. 2026. "Variation in Atmospheric 137Cs and the Carriers in Aerosol Samples Obtained from a Heavily Contaminated Area of Fukushima Prefecture" Toxics 14, no. 1: 88. https://doi.org/10.3390/toxics14010088
APA StyleLi, H., Tang, P., & Kita, K. (2026). Variation in Atmospheric 137Cs and the Carriers in Aerosol Samples Obtained from a Heavily Contaminated Area of Fukushima Prefecture. Toxics, 14(1), 88. https://doi.org/10.3390/toxics14010088





