Heavy Metals and Microplastics as Emerging Contaminants in Bangladesh’s River Systems: Evidence from Urban–Industrial Corridors
Abstract
1. Introduction
2. Methodology
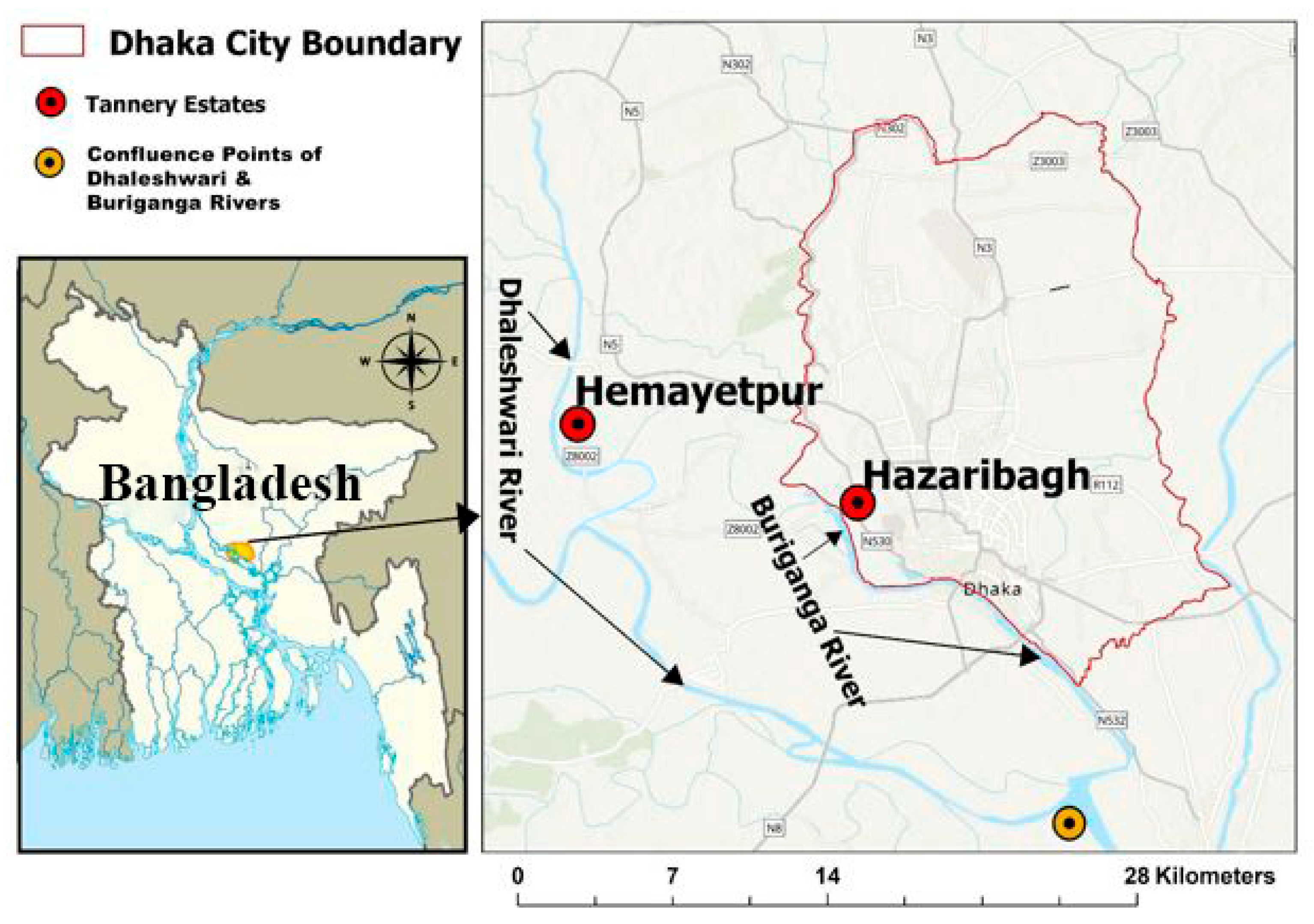
2.1. Geographic and Socioeconomic Context
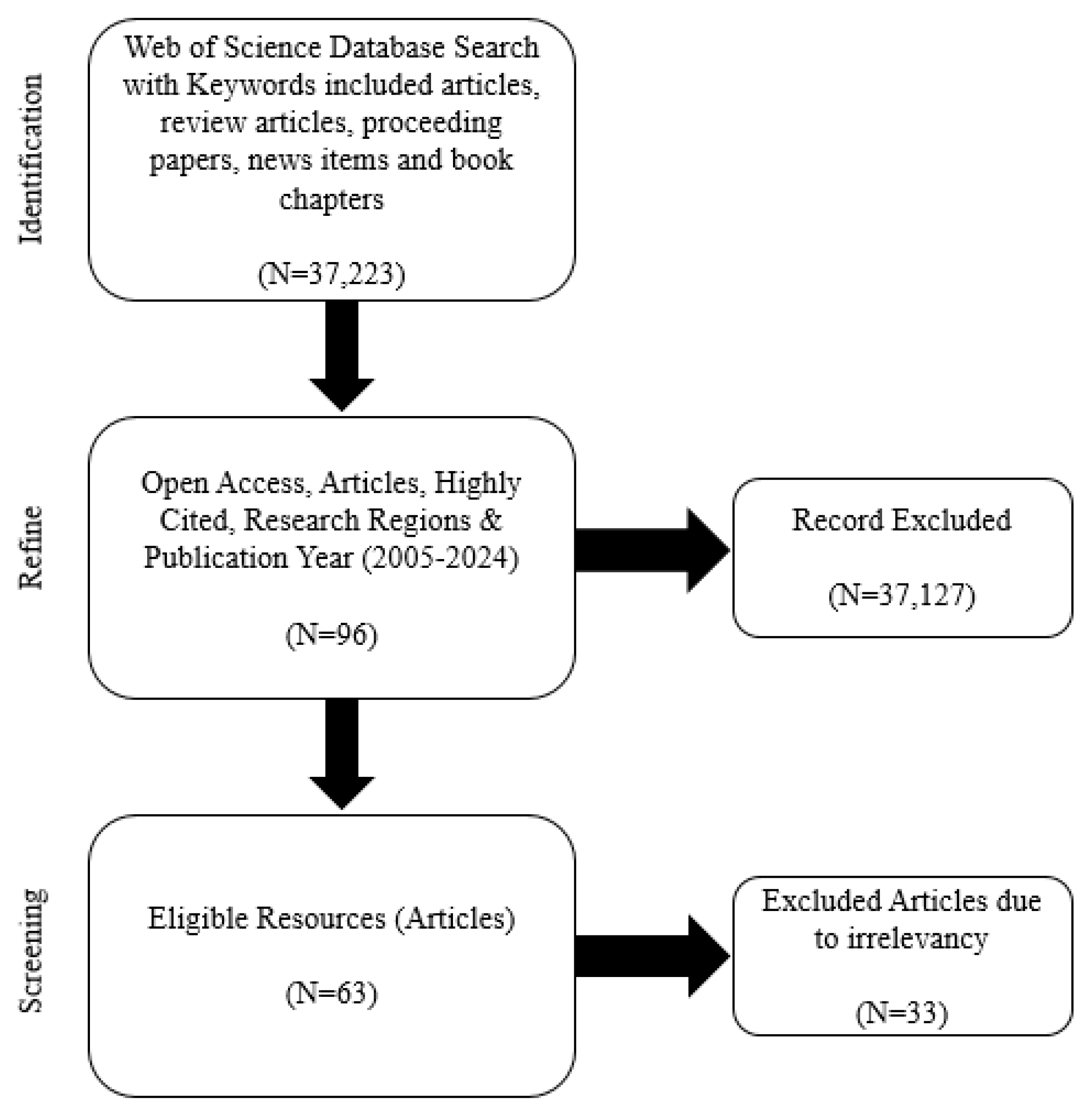
2.2. Systematic Critical Review
2.3. Bibliometric Analysis
3. Results
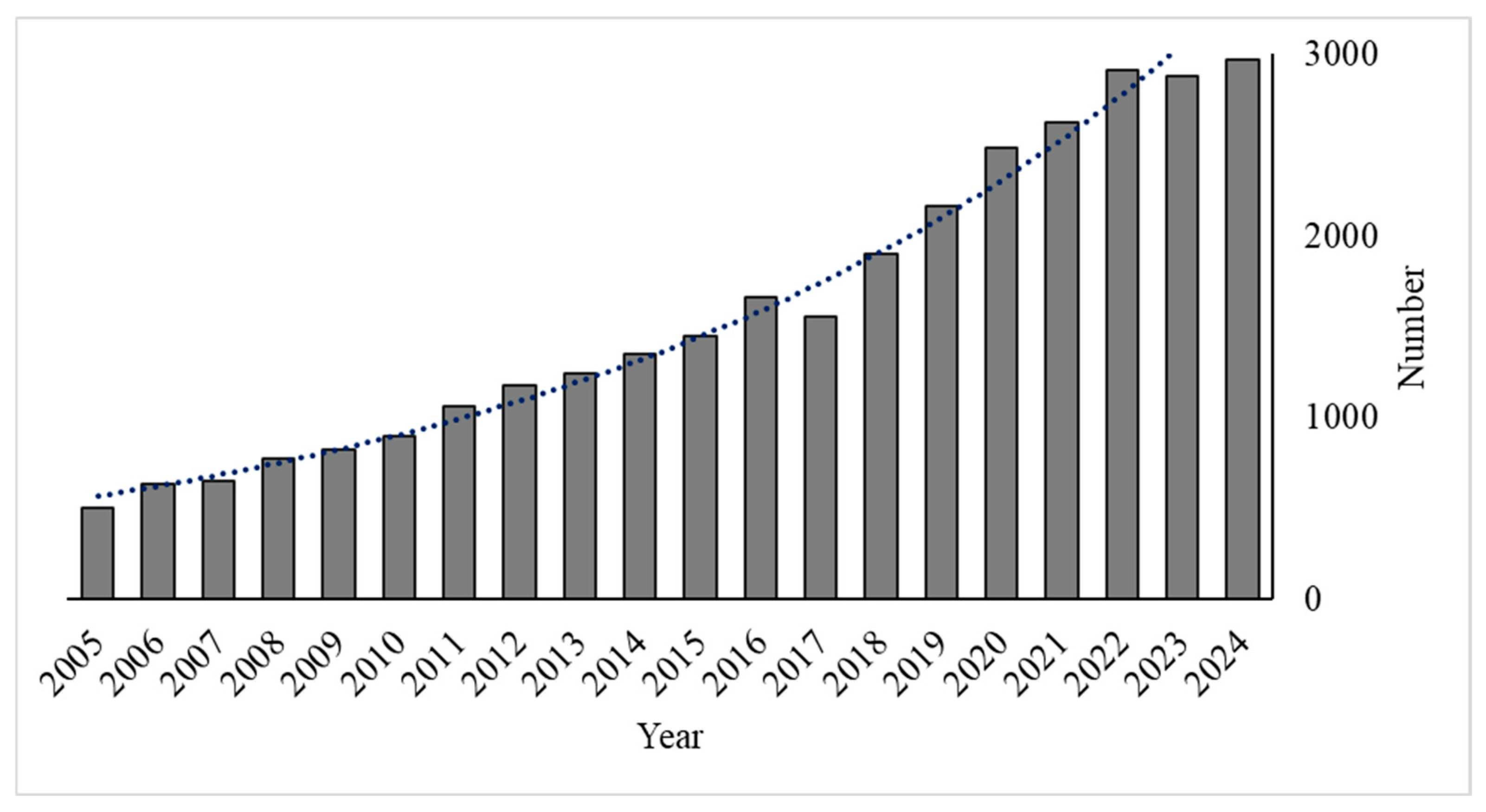
3.1. Bibliometric Analysis
3.2. Occurrence of Heavy Metals (HMs)
| Sampling Period | Number of Samples | Cr | Cd | Pb | Ni | Zn | Hg | As | Mn | Cu | Fe | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buriganga River | ||||||||||||
| Sum 2010 | 264 | 35,330–167,160 | 10–70 | 1180–3830 | - | - | 40–360 | - | 120–1920 | - | - | [48] |
| Sum and Win 2017 | - | 19–57 | ND—5 | ND—114 | 38–80 | 50–670 | 5–421 | ND | - | 160–565 | - | [12] |
| November 2014–December 2015 | - | 9.8–13 | <3.0 | 15–21 | 0.55–10.85 | 8.36–14.77 | - | <3–8.25 | - | <5.0 | - | [46] |
| - | - | 2–270 | - | 1 | - | 1000–4000 | <1 | - | - | - | - | [49] |
| August 2019–February 2020 | 210 | 27.7 | 12.7 | 29.9 | 22.2 | 65.1 | - | 16.5 | 238 | 26 | - | [50] |
| March 2010 | 30 | 12–180 | 30–90 | 100–210 | 90–400 | 110–900 | - | 2–220 | 60–310 | 100–990 | 90–2200 | [51] |
| Sum and Win in 2018–2019 | 20 | 1990 | - | 280 | 1050 | 1060 | - | - | - | 690 | 1231 | [26] |
| - | 16 | - | - | 58 | - | 113 | - | 10 | 230 | 94 | 1400 | [52] |
| - | 40 | 59 | 22.9 | 182 | 136 | 23 | - | 192 | - | - | - | |
| March–May 2014 | - | - | 100–500 | 300–500 | 2500–3500 | 60,000–90,000 | - | - | 150,000–200,000 | 1500–2500 | 20,000–30,000 | [33] |
| Mon and Win 2021–2022 | 168 | 128–170 | 48.7–79.1 | 65–97.2 | 112–151 | 201–273 | 20.1–27.7 | 46.6–77.6 | 134–167 | 132–186 | 478–620 | [53] |
| Sum and Win in 2009 | 20 | 1430–1960 | 160–220 | 230–500 | 150–170 | 220–260 | - | 240–400 | - | 1710–2740 | - | [54] |
| April 2016 | 10 | 365 | 3.19 | 40.66 | - | 660 | 1.61 | - | - | - | 1230 | [55] |
| Pre-Mon, Mon and Post-Mon | - | BDL-224 | BDL-1 | BDL-24 | 8–27 | 20–120 | 13–270 | - | - | - | - | [41] |
| Over 2011 | 84 | 2.2–20 | - | 30–254 | - | - | - | - | - | - | - | [35] |
| December 2017 and January 2018 | 78 | 0.57–3.17 | 0.004–0.07 | 0.004–0.08 | 1.22–3.18 | 3.14–19.92 | BDL–0.02 | 2.70–5.27 | 0.11–96.59 | - | 1.91–12.78 | [56] |
| December 2017 and January 2018 | 72 | 0.1–3.17 | BDL—0.07 | 0.004–0.16 | 0.22–3.18 | 2.27–19.92 | BDL—0.02 | 0.72–5.83 | BDL–96.59 | 0.38–9.05 | 0.68–12.78 | [57] |
| Dhaleshwari River | ||||||||||||
| September 2022 | 8 | 177 | 24 | 122 | BDL | - | - | - | - | - | - | [29] |
| Mon, Post-Mon, and Win in 2018–2019 | 32 | 20–900 | 2 | 50 | 20 | 10–30 | - | - | - | 10 | 490–6040 | [47] |
| Mon, post-Mon, and Win in 2018–2019 | 24 | 40–370 | 2 | 5 | 20 | 5–20 | - | - | - | 10 | 2440–2730 | [58] |
| April 2021–January 2022 | 30 | 1704–2215 | 276–422 | 343–456 | 1057–1732 | 344–438 | 14–16 | 164–184 | 3336–4509 | 614–1506 | 190–341 | [31,59] |
| - | - | 710 | 190 | - | 620 | 180 | - | - | - | - | - | [14] |
| Mon and Win | - | 2590–3350 | 1520–1890 | 1020–1320 | - | - | - | 410–750 | - | 1010–1090 | - | [24] |
| - | 41 | - | 130–420 | 630–3900 | - | 1600–5490 | - | - | - | - | - | [34] |
| October 2018 | - | 653 | - | 225 | 395 | 1341 | - | - | - | - | - | [45] |
| August–September 2019 | - | 0.18–0.64 | 0.27–0.45 | - | - | 6.68–8.17 | - | 2.81–3.01 | - | - | - | [57] |
| Standard Value (μg/L) | ||||||||||||
| WHO | 50 | 3 | 10 | 20 | 3000 | 1 | 10 | 500 | 2000 | 300 | [60] | |
| Bangladesh (Drinking water) | 50 | 3 | 10 | 50 | 5000 | 1 | 50 | 400 | 1500 | 300–1000 | [61] | |
| Bangladesh (Industrial Effluents) | 500 | 2000 | 100 | 1000 | 5000 | 10 | 200 | 2000 | 3000 | 3000 | [61] |
3.2.1. Chromium (Cr)
| Sampling Period | Number of Samples | Cr | Cd | Pb | Ni | Zn | Hg | As | Mn | Cu | Fe | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buriganga River | ||||||||||||
| Sum and Win, 2017 | - | 21.22–22.47 | 0.27–0.28 | 107.76–110.64 | 19.71–21.04 | 208.777–222.30 | 0.61–0.62 | 2.03–2.15 | - | 159.07–168.96 | - | [12] |
| - | 5 | 101.2 | 0.82 | 79.4 | - | 502.26 | - | - | - | 184.52 | - | [63] |
| Mar 2010 | 15 | 1019–1884 | 4.21–11.28 | 50.12–80.2 | 35–59 | 45.45–60.5 | - | 11.82–26.4 | 368–692 | 50.12–80.2 | 9480–15,435 | [51] |
| Sum and Win, 2018–2019 | 20 | 106 | - | 17 | 33 | 29 | - | - | - | 31 | 4655 | [26] |
| February–March 2013 and August–September 2013 | 36 | 17–841 | 2–19 | 43–3312 | 62–539 | - | - | 7.6–67 | - | 62–712 | - | [37] |
| Sum and Win, 2009 | 20 | 105–4249 | 3.5–9.5 | 56–1592 | 56–244 | 129–3002 | - | 9–34 | - | 53–743 | - | [54] |
| Pre-Mon, Mon, Post-Mon | - | BDL—103.58 | BDL < −0.16 | 2–8.9 | BDL–21.93 | 26.2–71.9 | - | - | - | - | - | [41] |
| 7 August 2015 and 5 February 2016 | 7 | 39.70–41.45 | 0.21–0.23 | 10.41–11.40 | 6.39–7.14 | 36.73–40.71 | 0.001–0.02 | 0.18–0.21 | - | 14.07–15.93 | 37.58–39.06 | [64] |
| - | 9 | 119–2050 | - | <0.36–93 | - | 61.0–210 | 0.09 | 0.91–23 | 423–701 | <1.95–83.3 | 2.21–2.82 | [39] |
| Dhaleshwari River | ||||||||||||
| Sum | 5 | BDL—282.4 | BDL—4.4 | BDL—414.6 | 85.1–264.5 | - | - | - | - | - | 11,800–14,375 | [36] |
| Mon and Win | - | 96.02–118.37 | 1.56–1.67 | 23.69–26 | - | - | - | 5.15–6.59 | - | 21.15–24.15 | - | [24] |
| September 2019 | 24 | 186 | - | 8.78 | - | - | - | - | 3.12 | 1.76 | 42.7 | [38] |
| Standard Value (μg/g) | ||||||||||||
| ASV a | 90 | 0.3 | 20 | 68 | 95 | 0.4 | 13 | 850 | 45 | 47,200 | [65] | |
| TRV b | 26 | 0.6 | 31 | 16 | 110 | - | 6 | - | 16 | - | [66] | |
| LEL c | 26 | 0.6 | 31 | 16 | 120 | 0.2 | 6 | 460 | 16 | 2% | [67] | |
| SEL d | 110 | 10 | 250 | 75 | 820 | 2 | 33 | 1100 | 110 | 4% | [67] |
3.2.2. Cadmium (Cd)
3.2.3. Lead (Pb)
3.2.4. Nickel (Ni)
3.2.5. Zinc (Zn)
3.2.6. Mercury (Hg)
3.2.7. Arsenic (As)
| Sampling Period | Number of Samples | Cr | Cd | Pb | Ni | Zn | Hg | As | Mn | Cu | Fe | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buriganga River | ||||||||||||
| - | 6 | 5–32 | 0.01–0.08 | 0.25–0.9 | 0.05–0.8 | 6–17 | - | - | - | 1–6.3 | - | [40] |
| Pre-Mon, Mon, Post-Mon | - | 0.95–163.1 | 0.23–0.32 | 1.29–6 | 0.66–27.75 | 18.68–244 | - | - | - | - | - | [41] |
| Dhaleshwari River | ||||||||||||
| September 2022 | 13 | 211.70–270.55 | 1.24–3.42 | 6.21–82.89 | 9.75–22.87 | - | - | - | - | - | - | [29] |
| - | 27 | 1.06–10.7 | 0.58–1.21 | 0.45–1.65 | 0.20–0.35 | 58.3–118 | - | - | - | 2.79–4.59 | - | [13] |
| Standard Value (μg/g) | ||||||||||||
| WHO/FAO | - | 2.3 | 0.05 | 0.1 | 10 | 9.4 | - | 0.002 | 500 | 40 | 425.5 | [78] |
3.2.8. Manganese (Mn)
3.2.9. Copper (Cu)
| Sampling Period | Sampling Species | Analyzed Tissues | Number of Samples | Cr | Cd | Pb | Ni | Zn | Hg | As | Mn | Cu | Fe | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buriganga | ||||||||||||||
| 1 year in 2010 | Anabas testudineus, Channa punctata, and Oreochromis mossambicus | Whole body | - | 2.7 | 0.12 | 0.72 | - | - | 0.56 | - | 0.53 | - | - | [48] |
| September 2015–December 2015 | Puntius ticto, Heteropneustes fossilis, and | Liver, intestine, kidney, and muscles | - | 0.51–7.75 | <0.1–13.43 | 1.65–5.35 | 8.66–90.49 | - | - | <0.1–0.12 | - | 0.09–31.04 | - | [46] |
| Channa punctatus | ||||||||||||||
| April– | Heteropneustes fossilis | Muscle, gill, stomach, | 30 | 1.40–8.65 | 0.3–5.50 | 1.79–18.79 | - | 16.82–67.8 | - | 0.2–3.99 | - | 6.22–47.3 | - | [42] |
| May-11 | intestine, and liver | |||||||||||||
| January–February 2018 and May–June 2019 | Heteropneustes fossilis, Channa punctatus, and Channa striata | Muscle | 12 | 87.01–187.07 | - | 1.2–5.07 | 0.07–3.01 | 11.08–35.12 | - | - | - | 2.07–3.51 | 19.66–39.07 | [26] |
| November 2017 | Heteropnuestes | Whole body | - | BDL–0.35 | 0.21–0.73 | 1.14–10.14 | 0.35–1.14 | 77.26–271.33 | - | BDL | 2.02–16.46 | 0.19–1.7 | - | [73] |
| fossilis, Channa punctatus, Notopterus notopterus, Channa striata, and Colisa fasciata | ||||||||||||||
| November–December 2017 | Batasio batasio, Colisa fasciata, Gonialosa manmina, Amblypharyngodon microlepis, Awaous | Edible portions | - | 0.41–0.94 | - | 0.32–0.65 | - | 82.07–189.64 | 0.33–1.36 | 0.41–0.51 | 0.31–1.31 | 16.09–26.70 | 59.63–98.58 | [44] |
| guamensis, Heteropneustes fossilis, Otolithoides | ||||||||||||||
| pama, Mastacembelus armatus, Labeo calbasu, and Channa punctata | ||||||||||||||
| Mon, Win, and Sum | Labeo rohita | Muscle | - | 6.69–8.24 | 1.02–2.44 | 5.25–7.38 | - | - | - | - | - | 20.74–25.22 | - | [81] |
| Dhaleshwari | ||||||||||||||
| Mon and Win | Labeo rohita, Catla | Muscle tissue, guts, and gills | - | 0.78–2.23 | 0.24–0.51 | 0.27–1.87 | - | - | - | ND | - | 0.42–2.68 | - | [24] |
| catla, and Bagarius bagarius | ||||||||||||||
| October 2018 | Heteropneustes fossillis, Channa punctata, Nandus nandus, Chanda | body (skin) | 84 | 1.60–116.7 | - | 1.5–11.5 | 0.45–8.5 | 57.09–314.47 | - | - | - | - | - | [45] |
| nama, Anabas testudineus, Mystus gulio, and Colisa fasciata | scales, and muscle) and head (no gills) | |||||||||||||
| Standard Value | ||||||||||||||
| WHO | - | 0.05 | 0.05 | 0.2 | 0.5 | 50 | 0.5 | 0.001 | 0.01 | 30 | 50 | [82] | ||
3.2.10. Iron (Fe)
3.3. Occurrence of Microplastics (MPs)
| Sampling Period | Number of Samples | Conc of Microplastics (MPs) | Type of Polymers | References |
|---|---|---|---|---|
| Buriganga River | ||||
| March 2021 | 11 (Sediment) | 165.45 ± 127.87 items/kg (sediment) | Polyethylene (PE), Polypropylene (PP), and Polyethylene terephthalate (PET) | [43] |
| - | - | 4.33–43.67 items/L (Surface water); 17.33–133.67 items/kg (Sediments) | Polystyrene (PS), Polyvinylchloride (PVC), Polyethylene terephthalate (PET), Polyester, and Polyamide | [85] |
| Sum and Win | 9 (Water), 31 (Sediment), and 79 (Aquatic Species) | 0.25–0.12 MPs/mL (Water); 3.50–8.16 MPs/g (Sediments); 0.94–5.34 MPs/g (Aquatic Species) | Ethylene-vinyl acetate (EVA), Polyethylene terephthalate (PETE), Acrylonitrile butadiene styrene (ABS), High-density polyethylene (HDPE), Cellulose acetate (CA), and Nylon | [87] |
4. Discussion
4.1. Sources of Emerging Contaminants in Buriganga and Dhaleshawari
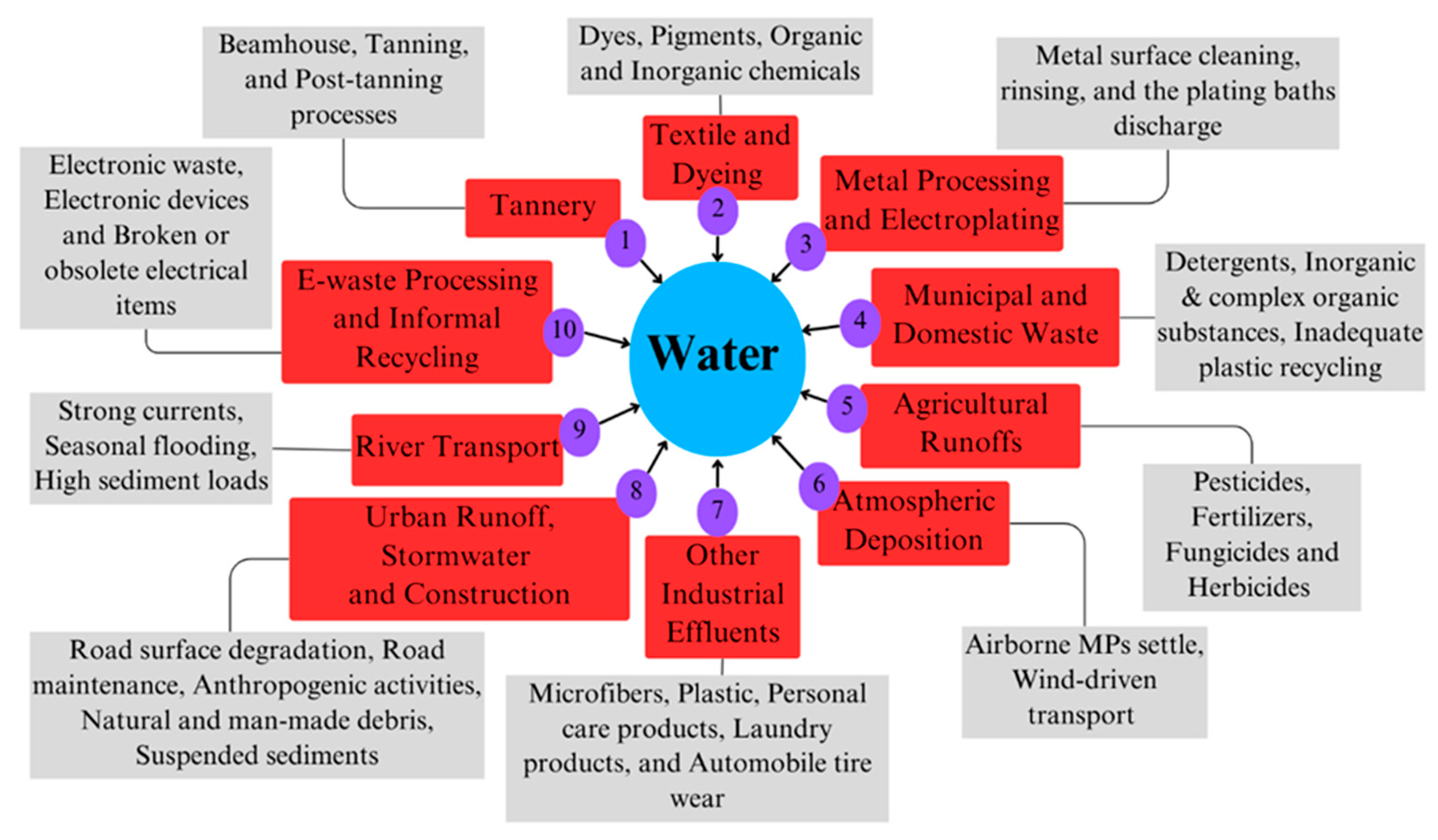
4.1.1. Sources of Heavy Metals (HMs)
Tannery Industries
Textile and Dyeing Industries
Metal Processing and Electroplating Facilities
Municipal and Domestic Waste
Agricultural Runoff and Pesticide Use
Urban Runoff and Construction Activities
E-Waste Processing and Informal Recycling
4.1.2. Sources of Microplastics (MPs)
Industrial Effluents
Municipal Waste and Poor Waste Management
Agricultural Runoff
Urban Runoff and Stormwater
Atmospheric Deposition
River Transport
4.2. Effect of Different Emerging Contaminants on the Environment
4.2.1. Toxic Effect of Heavy Metals
| Heavy Metals | Reference Dosage (RfD) (mg/kg/day) | Main Toxic Effects | References |
|---|---|---|---|
| Cr (vi) | 0.003 | Carcinogenesis, reproductive impairment, damage to the respiratory, renal, and hepatic systems. | [16,70,141,153,154] |
| Cd | 0.0005 | DNA alteration, kidney dysfunction, metabolic disruption, and hormonal interaction. | [142,143,153,154] |
| Pb | 0.0035 | Headaches, muscle tremors, shallowness, irritability, reduced attention span, memory decline, hallucinations, and reproductive system damage. | [33,66,101,153,154] |
| Ni | 0.02 | Chronic bronchitis, respiratory cancer, lung cancer, asthma, mutagenesis, and pulmonary cell hyperplasia. | [67,101,153,154] |
| Zn | 0.3 | Ionic zinc (Zn2+) can be highly toxic to neurons, glial cells, and other cell types when it exceeds safe levels. | [68,153,154] |
| Hg as Methylmercury (MeHg) | 0.0001 | Vision impairments and unstable motion, especially in fetuses. | [71,143,153,154] |
| As | 0.0003 | Cardiovascular, skin, nervous, hepatic, kidney, respiratory, and genitourinary systems. | [101,143,153,154] |
| Mn | 0.14 | Amounts exceeding the RfD may cause symptoms of neurotoxicity. | [75,153,154] |
| Cu | 0.04 | Atherosclerosis, cardiovascular disease, diabetes, cancer progression, and neurological disorders. | [153,154,155] |
| Fe | 0.7 | Excessive Fe may cause homeostasis, DNA damage, myocardial infarction, coronary disease, and tissue damage. | [153,154,155] |
| Plant Samples | BCFCr | BCFCd | BCFPb | BCFNi | BCFZn | BCFHg | BCFAs | BCFMn | BCFCu | BCFFe | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enhydra fluctuens, Lemnoideae | 0.024–1 | - | 0.0301 | - | 0.0998 | - | - | - | - | - | [85] |
| Amaranthus cruentus, Spanacia oleracea, Corchorus capsularies, Lagenaria siceraria, Brassica juncea, and Impomoea aquatica | 0.04–0.07 | 0.01–0.09 | 0.01–0.03 | 0.00–0.02 | 0.01–0.10 | - | - | - | 0.03–0.15 | - | [37] |
| Amaranthus lividus, Lagenaria siceraria, Basella alba, Corchorus olitorius, Cucurbita moschata, Ipomoea aquatica, Brassica oleracea, Amaranthus gangeticus, Capsicum species, Spinacia oleracea, Trichosanthes anguina, Abelmoschus esculentus, and Solanum melongena | 0–0.0005 | 0–0.172 | 0–0.718 | 0–0.015 | 0.243–0.678 | - | - | - | 0.104–0.286 | - | [54] |
4.2.2. Toxic Effects of Microplastics
4.3. Future Directions and Recommendations
- Conduct systematic assessments of seasonal fluctuations in EC levels, with emphasis on the pollutant loads from industrial effluents and urban runoff [170].
- Evaluate how water, sediments, plants, and aquatic organisms (especially fish) are crucial to understanding the bioaccumulation and trophic transfer of ECs in river ecosystems [33].
- Address the scarcity of data on persistent contaminants, such as PFAS, PPCPs, and EDCs, and investigate their concentrations, sources, and behavior across different environmental compartments by comparing studies on other regional rivers, which can inform broader mitigation strategies.
- Develop sensitive detection methods and cost-effective wastewater treatment technologies tailored to the leather, textile, and chemical industries.
- Investigate EC uptake in aquatic plants and fish, their movement across trophic levels, and potential risks to consumers, including humans.
- Evaluate the long-term impacts of EC exposure on aquatic life and communities dependent on river water for drinking and subsistence. Include a special focus on MP contamination in aquatic systems, including underwater communities [156].
- Determine MP concentrations in fish muscle tissue to assess the human health implications of contaminated seafood [156].
- Examine how ecological and geographic factors influence MP ingestion and HM accumulation in freshwater organisms [172].
- Assess MP absorption, retention, and physiological effects on aquatic and riparian vegetation in river ecosystems.
- Establish a national database under the Department of Environment, Forests, and Climate Change to track sources, transport, and concentrations of ECs, MPs, and organic pollutants, particularly those linked to industrial activities.
- Inform regulatory development and promote sustainable industrial practices to reduce EC contamination in the Buriganga and Dhaleshwari rivers [49].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | Polyethylene |
| PP | Polypropylene |
| PET | Polyethylene Terephthalate |
| ECs | Emerging Contaminants |
| HMs | Heavy Metals |
| MPs | Microplastics |
| PPCPs | Pharmaceuticals and Personal Care Products |
| PFAS | Per- and Poly-Fluoroalkyl Substances |
| EDCs | Endocrine-Disrupting Chemicals |
| ETP | Effluent Treatment Plant |
| CETP | Common Effluent Treatment Plant |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| POPs | Persistent Organic Pollutants |
| BDL | Below Detection Limit |
| ND | Not Detected |
| WHO | World Health Organization |
| ASV | Average Share Value |
| TRV | Toxicity Reference Value |
| LEL | Lowest Effect Level |
| SEL | Severe Effect Level |
| FAO | Food And Agriculture Organization |
| MeHg | Methyl Mercury |
| ECR | Environmental Conservation Rules |
| PBDEs | Polybrominated Diphenyl Ethers |
| BPA | Bisphenol A |
| PVC | Polyvinylchloride |
| ABS | Acrylonitrile Butadiene Styrene |
| HDPE | High-Density Polyethylene |
| TDS | Total Dissolved Solids |
| IEDS | Industrial Effluent Discharge Standards |
| CE | Conformité Européenne |
| EU | European Union |
| SS | Suspended Solids |
| BOD | Biochemical Oxygen Demand |
| COD | Chemical Oxygen Demand |
| DO | Dissolved Oxygen |
| PCBs | Printed Circuit Boards |
| LDPE | Low-Density Polyethylene |
| IAs | Inorganic Arsenic |
| SR | Social Relevance |
References
- Williams, M.; Kookana, R.S.; Mehta, A.; Yadav, S.K.; Tailor, B.L.; Maheshwari, B. Emerging contaminants in a river receiving untreated wastewater from an Indian urban centre. Sci. Total Environ. 2019, 647, 1256–1265. [Google Scholar] [CrossRef]
- Alam, M.S.; Tahriri, F.; Chen, G. Trends, challenges, and research pathways in emerging contaminants: A comprehensive bibliometric analysis. Integr. Environ. Assess. Manag. 2025, 21, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Altahaan, Z.; Dobslaw, D. Assessment of the Impact of War on Concentrations of Pollutants and Heavy Metals and Their Seasonal Variations in Water and Sediments of the Tigris River in Mosul/Iraq. Environments 2024, 11, 10. [Google Scholar] [CrossRef]
- Das, S.; Parida, V.K.; Tiwary, C.S.; Gupta, A.K.; Chowdhury, S. Emerging Contaminants in the Aquatic Environment: Fate, Occurrence, Impacts, and Toxicity. In Bioremediation of Emerging Contaminants in Water. Volume 1; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2024; Volume 1475, pp. 1–32. [Google Scholar]
- Pereira, L.C.; de Souza, A.O.; Bernardes, M.F.F.; Pazin, M.; Tasso, M.J.; Pereira, P.H.; Dorta, D.J. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ. Sci. Pollut. Res. 2015, 22, 13800–13823. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Ali, M.H. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Talib, A.; Randhir, T.O. Managing Emerging Contaminants: Status, Impacts, and Watershed-Wide Strategies. Expo. Health 2016, 8, 143–158. [Google Scholar] [CrossRef]
- Gomes, I.B.; Maillard, J.Y.; Simoes, L.C.; Simoes, M. Emerging contaminants affect the microbiome of water systems-strategies for their mitigation. Npj Clean Water 2020, 3, 39. [Google Scholar] [CrossRef]
- Pal, A.; He, Y.L.; Jekel, M.; Reinhard, M.; Gin, K.Y.H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeong, Y.K. Urban river pollution in Bangladesh during last 40 years: Potential public health and ecological risk, present policy, and future prospects toward smart water management. Heliyon 2021, 7, e06107. [Google Scholar] [CrossRef]
- Ali, M.S.; Begum, S.; Rabbi, F.M.; Sumaia; Hasan, M.K.; Rahman, M.A.; Rahman, M.M.; Rahaman, M.H. Multivariate analysis of water quality in the Dhaleshwari River, Bangladesh: Identifying pollution sources and environmental implications. Water Pract. Technol. 2024, 19, 4128–4147. [Google Scholar] [CrossRef]
- Akbor, M.A.; Rahman, M.M.; Bodrud-Doza, M.; Haque, M.M.; Siddique, M.A.; Ahsan, M.A.; Bondad, S.E.C.; Uddin, M.K. Metal pollution in water and sediment of the Buriganga River, Bangladesh: An ecological risk perspective. Desalination Water Treat. 2020, 193, 284–301. [Google Scholar] [CrossRef]
- Mizan, A.; Mamun, M.A.H.; Islam, M.S. Metal contamination in soil and vegetables around Savar tannery area, Dhaka, Bangladesh: A preliminary study for risk assessment. Heliyon 2023, 9, e13856. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Hossain, M.E.; Majed, N. Assessment of Physicochemical Properties and Comparative Pollution Status of the Dhaleshwari River in Bangladesh. Earth 2021, 2, 696–714. [Google Scholar] [CrossRef]
- Hoque, S.F.; Peters, R.; Whitehead, P.; Hope, R.; Hossain, M.A. River pollution and social inequalities in Dhaka, Bangladesh. Environ. Res. Commun. 2021, 3, 095003. [Google Scholar] [CrossRef]
- Md, H.-A.-M.; Islam, M.M.; Sadman, S.; Susmita, I.; Mir Mohammad, A.; Md. Hasan, F. Occurrence, sources and ecological risk assessment of per- and polyfluoroalkyl substances (PFASs) in water and sediment from urban rivers in Dhaka, Bangladesh. J. Transl. Genet. Genom. 2025, 4, 24. [Google Scholar] [CrossRef]
- DoE. Environmental Impact Study of Two Tannery Estates on the Buriganga and the Dhaleshwari Rivers; Ministry of Environment, Forest and Climate Change: Dhaka, Bangladesh, 2018.
- Alam, M.S.; Han, B.S.; Al-Mizan; Pichtel, J. Assessment of soil and groundwater contamination at a former Tannery district in Dhaka, Bangladesh. Environ. Geochem. Health 2020, 42, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Ishraq, R.; Nehal, K.A.; Urbi, F.B.S.; Almas, M. Current Scenario of Buriganga and Dhaleswari Rivers After the Shiftment of the Tannery Industry: A WQI Study; Islamic University of Technology: Gazipur, Bangladesh, 2023; Available online: http://repository.iutoic-dhaka.edu/items/74a21431-2290-424e-a3b5-e134cfdd5300 (accessed on 19 August 2025).
- Velusamy, S.; Roy, A.; Sundaram, S.; Mallick, T.K. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Xu, X.; Hou, Q.T.; Xue, Y.G.; Jian, Y.; Wang, L.P. Pollution characteristics and fate of microfibers in the wastewater from textile dyeing wastewater treatment plant. Water Sci. Technol. 2018, 78, 2046–2054. [Google Scholar] [CrossRef]
- Shengtao, M.; Meiqing, L.; Jian, T.; Ranran, L.; Yan, Y.; Yingxin, Y.; Guiying, L.; Taicheng, A. Occurrence and fate of polycyclic aromatic hydrocarbons from electronic waste dismantling activities: A critical review from environmental pollution to human health. J. Hazard. Mater. 2022, 424, 127683. [Google Scholar] [CrossRef]
- Chakraborty, P.; Gadhavi, H.; Prithiviraj, B.; Mukhopadhyay, M.; Khuman, S.N.; Nakamura, M.; Spak, S.N. Passive Air Sampling of PCDD/Fs, PCBs, PAEs, DEHA, and PAHs from Informal Electronic Waste Recycling and Allied Sectors in Indian Megacities. Environ. Sci. Technol. 2021, 55, 9469–9478. [Google Scholar] [CrossRef]
- Lipy, E.P.; Hakim, M.; Mohanta, L.C.; Islam, D.; Lyzu, C.; Roy, D.C.; Jahan, I.; Akhter, S.; Raknuzzaman, M.; Abu Sayed, M. Assessment of Heavy Metal Concentration in Water, Sediment and Common Fish Species of Dhaleshwari River in Bangladesh and their Health Implications. Biol. Trace Elem. Res. 2021, 199, 4295–4307. [Google Scholar] [CrossRef]
- López, A.P.A.; Trilleras, J.; Arana, V.A.; Garcia-Alzate, L.S.; Grande-Tovar, C.D. Atmospheric microplastics: Exposure, toxicity, and detrimental health effects. RSC Adv. 2023, 13, 7468–7489. [Google Scholar] [CrossRef]
- Hossain, M.N.; Rahaman, A.; Hasan, M.J.; Uddin, M.M.; Khatun, N.; Shamsuddin, S.M. Comparative seasonal assessment of pollution and health risks associated with heavy metals in water, sediment and Fish of Buriganga and Turag River in Dhaka City, Bangladesh. SN Appl. Sci. 2021, 3, 509. [Google Scholar] [CrossRef]
- Alberghini, L.; Truant, A.; Santonicola, S.; Colavita, G.; Giaccone, V. Microplastics in Fish and Fishery Products and Risks for Human Health: A Review. Int. J. Environ. Res. Public Health 2023, 20, 789. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Aurnab, I.T.; Uddin, M.J.; Kabir, A. Risk assessment of four toxic heavy metals in terrestrial and aquatic ecosystems around BSCIC tannery industrial estate of Savar, Dhaka, Bangladesh. Environ. Sci. Pollut. Res. 2024, 31, 34124–34143. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Meenar, M. Just Sustainability in the Global South: A Case Study of the Megacity of Dhaka. J. Dev. Soc. 2018, 34, 401–424. [Google Scholar] [CrossRef]
- Hassan, M.M. Monitoring land use/land cover change, urban growth dynamics and landscape pattern analysis in five fastest urbanized cities in Bangladesh. Remote Sens. Appl. Soc. Environ. 2017, 7, 69–83. [Google Scholar] [CrossRef]
- Alam, M.S.; Chen, G. Per- and Poly-fluoroalkyl Substances Regulatory Frameworks, Sources, and Occurrence: Trend, Concern, and Implication. Int. J. Environ. Res. 2025, 5, 176. [Google Scholar] [CrossRef]
- Kibria, G.; Hossain, M.M.; Mallick, D.; Lau, T.C.; Wu, R. Monitoring of metal pollution in waterways across Bangladesh and ecological and public health implications of pollution. Chemosphere 2016, 165, 1–9. [Google Scholar] [CrossRef]
- Majed, N.; Islam, M.A. Contaminant Discharge From Outfalls and Subsequent Aquatic Ecological Risks in the River Systems in Dhaka City: Extent of Waste Load Contribution in Pollution. Front. Public Health 2022, 10, 880399. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Sarker, D.C. Evaluation of surface water quality of the Buriganga River. J. Water Reuse Desalination 2013, 3, 160–168. [Google Scholar] [CrossRef]
- Islam, A.; Hossain, M.E.; Nahar, K.; Majed, N. Assessment of Environmental Hazard and Heavy Metal Contamination in Dhaleshwari River Sediment: A Toxicity based Study on Pollution. Pollution 2022, 9, 67–83. [Google Scholar] [CrossRef]
- Islam, M.S.; Proshad, R.; Ahmed, S. Ecological risk of heavy metals in sediment of an urban river in Bangladesh. Hum. Ecol. Risk Assess. 2018, 24, 699–720. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ahmed, Z.; Seefat, S.M.; Alam, R.; Islam, A.M.T.; Choudhury, T.R.; Begum, B.A.; Idris, A.M. Assessment of heavy metal contamination in sediment at the newly established tannery industrial Estate in Bangladesh: A case study. Environ. Chem. Ecotoxicol. 2022, 4, 1–12. [Google Scholar] [CrossRef]
- Tamim, U.; Khan, R.; Jolly, Y.N.; Fatema, K.; Das, S.; Naher, K.; Islam, M.A.; Islam, S.M.A.; Hossain, S.M. Elemental distribution of metals in urban river sediments near an industrial effluent source. Chemosphere 2016, 155, 509–518. [Google Scholar] [CrossRef]
- Islam, M.D.; Hasan, M.M.; Rahaman, A.; Haque, P.; Islam, M.S.; Rahman, M.M. Translocation and bioaccumulation of trace metals from industrial effluent to locally grown vegetables and assessment of human health risk in Bangladesh. SN Appl. Sci. 2020, 2, 1315. [Google Scholar] [CrossRef]
- Majed, N.; Real, M.I.H.; Redwan, A.; Azam, H.M. How dynamic is the heavy metals pollution in the Buriganga River of Bangladesh? A spatiotemporal assessment based on environmental indices. Int. J. Environ. Sci. Technol. 2022, 19, 4181–4200. [Google Scholar] [CrossRef]
- Begum, A.; Mustafa, A.I.; Amin, M.N.; Chowdhury, T.R.; Quraishi, S.B.; Banu, N. Levels of heavy metals in tissues of shingi fish (Heteropneustes fossilis) from Buriganga River, Bangladesh. Environ. Monit. Assess. 2013, 185, 5461–5469. [Google Scholar] [CrossRef]
- Islam, M.S.; Karim, M.R.; Islam, M.T.; Oishi, H.T.; Tasnim, Z.; Das, H.; Kabir, A.H.M.E.; Sekine, M. Abundance, characteristics, and ecological risks of microplastics in the riverbed sediments around Dhaka city. Sci. Total Environ. 2023, 877, 162866. [Google Scholar] [CrossRef] [PubMed]
- Jolly, Y.N.; Surovi, S.A.; Rahman, S.M.M.; Kabir, J.; Akter, S.; Mamun, K.M.; Rahman, A. A Probabilistic-Deterministic Approach Towards Human Health Risk Assessment and Source Apportionment of Potentially Toxic Elements (PTEs) in Some Contaminated Fish Species. Biol. Trace Elem. Res. 2023, 201, 1996–2010. [Google Scholar] [CrossRef]
- Wahiduzzaman, M.; Islam, M.M.; Sikder, A.H.F.; Parveen, Z. Bioaccumulation and Heavy Metal Contamination in Fish Species of the Dhaleswari River of Bangladesh and Related Human Health Implications. Biol. Trace Elem. Res. 2022, 200, 3854–3866. [Google Scholar] [CrossRef] [PubMed]
- Baki, M.A.; Shojib, M.F.H.; Sehrin, S.; Chakraborty, S.; Choudhury, T.R.; Bristy, M.S.; Ahmed, M.K.; Yusoff, S.B.; Khan, M.F. Health risk assessment of heavy metal accumulation in the Buriganga and Turag River systems for Puntius ticto, Heteropneustes fossilis, and Channa punctatus. Environ. Geochem. Health 2020, 42, 531–543. [Google Scholar] [CrossRef]
- Hasan, M.M.; Ahmed, M.S.; Adnan, R. Assessment of physico-chemical characteristics of river water emphasizing tannery industrial park: A case study of Dhaleshwari River, Bangladesh. Environ. Monit. Assess. 2020, 192, 807. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Hasan, I.; Rajia, S.; Khan, N.; Kabir, K.A. Impact of tannery effluents on the aquatic environment of the Buriganga River in Dhaka, Bangladesh. Toxicol. Ind. Health 2016, 32, 1106–1113. [Google Scholar] [CrossRef]
- Bashar, T.; Fung, I.W.H. Water Pollution in a Densely Populated Megapolis, Dhaka. Water 2020, 12, 2124. [Google Scholar] [CrossRef]
- Bashar, M.K.; Noro, K.; Wang, Q.; Tokumura, M.; Mori, I.; Raknuzzaman, M.; Hossain, A.; Amagai, T. Spatiotemporal distribution and pollution assessment of trace metals in the Buriganga River, Bangladesh. J. Water Health 2023, 21, 815–825. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.H.; Dampare, S.B.; Suzuki, M.A.I.S. Source apportionment and pollution evaluation of heavy metals in water and sediments of Buriganga River, Bangladesh, using multivariate analysis and pollution evaluation indices. Environ. Monit. Assess. 2015, 187, 4075. [Google Scholar] [CrossRef]
- Jolly, Y.N.; Rakib, M.R.J.; Kumar, R.; Sultana, S.; Rahman, S.M.M.; Kabir, J.; Akter, S.; Mamun, K.M.; Fatema, K.J.; Mehnaz, M.; et al. Evaluation of surface water quality near pollution sources in Buriganga River and deciphering their probable emergence, ecological, and health risk aspects. Reg. Stud. Mar. Sci. 2023, 63, 102988. [Google Scholar] [CrossRef]
- Kormoker, T.; Islam, M.S.; Siddique, M.A.; Kumar, S.; Phoungthong, K.; Kabir, M.H.; Iqubal, K.F.; Kumar, R.; Ali, M.M.; Islam, A.M.T. Layer-wise physicochemical and elemental distribution in an urban river water, Bangladesh: Potential pollution, sources, and human health risk assessment. Environ. Sci.-Adv. 2023, 2, 1382–1398. [Google Scholar] [CrossRef]
- Mohiuddin, K.M.; Ogawa, Y.; Zakir, H.M.; Otomo, K.; Shikazono, N. Heavy metals contamination in water and sediments of an urban river in a developing country. Int. J. Environ. Sci. Technol. 2011, 8, 723–736. [Google Scholar] [CrossRef]
- Mottalib, M.; Roy, S.; Ahmed, M.; Khan, M.; Al-Razee, A. Comparative study of water quality of Buriganga and Balu river, Dhaka, Bangladesh. Int. J. Curr. Res 2017, 9, 59132–59137. [Google Scholar]
- Rampley, C.P.N.; Whitehead, P.G.; Softley, L.; Hossain, M.A.; Jin, L.; David, J.; Shawal, S.; Das, P.; Thompson, I.P.; Huang, W.E.; et al. River toxicity assessment using molecular biosensors: Heavy metal contamination in the Turag-Balu-Buriganga river systems, Dhaka, Bangladesh. Sci. Total Environ. 2020, 703, 134760. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Bussi, G.; Peters, R.; Hossain, M.A.; Softley, L.; Shawal, S.; Jin, L.; Rampley, C.P.N.; Holdship, P.; Hope, R.; et al. Modelling heavy metals in the Buriganga River System, Dhaka, Bangladesh: Impacts of tannery pollution control. Sci. Total Environ. 2019, 697, 134090. [Google Scholar] [CrossRef]
- Hasan, M.M.; Ahmed, M.S.; Adnan, R.; Shafiquzzaman, M. Water quality indices to assess the spatiotemporal variations of Dhaleshwari river in central Bangladesh. Environ. Sustain. Indic. 2020, 8, 100068. [Google Scholar] [CrossRef]
- Hassan, B.H.; Moniruzzaman, M.; Majumder, R.K.; Ahmed, F.; Bhuiyan, M.A.Q.; Ahsan, M.A.; Al-Asad, H. Impacts of seasonal variations and wastewater discharge on river quality and associated human health risks: A case of northwest Dhaka, Bangladesh. Heliyon 2023, 9, e18171. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004506-4. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 19 August 2025).
- ECR. Bangladesh Environmental Conservation Rules; Bangladesh Ministry of Environment, Forest and Climate Change: Dhaka, Bangladesh, 2023.
- Das, T.; Baroi, S.; Saikia, R.; Dutta, R.K. A hybrid method for simultaneous removal of hexavalent chromium and fluoride from drinking water in plug-flow mode. Sep. Sci. Technol. 2025, 60, 220–233. [Google Scholar] [CrossRef]
- Saha, P.K.; Hossain, M.D. Assessment of Heavy Metal Contamination and Sediment Quality in the Buriganga River, Bangladesh. Environ. Sci. Technol. 2011, 6 Pt 1, Vi384–Vi388. [Google Scholar]
- Nargis, A.; Sultana, S.; Raihan, M.J.; Haque, M.E.; Sadique, A.B.M.R.; Sarkar, M.S.I.; Un-Nabie, M.M.; Zhai, W.; Cai, M.; Habib, A. Multielement analysis in sediments of the River Buriganga (Bangladesh): Potential ecological risk assessment. Int. J. Environ. Sci. Technol. 2019, 16, 1663–1676. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. GSA Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- USEPA. Screening Level Ecological Risk Assessment Protocol; Appendix E: Toxicity Reference Values; Office of Solid Waste: Washington, DC, USA, 1999.
- Persaud, D.; Jaagumagi, R.; Hayton, A. Guidelines for the Protection and Management of Aquatic Sediment Quality in Ontario; Ministry of Environment and Energy: Toronto, ON, Canada, 1993. Available online: http://hdl.handle.net/10214/15797 (accessed on 19 August 2025).
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Mitra, A.K.; Haque, A.; Islam, M.; Bashar, S. Lead poisoning: An alarming public health problem in Bangladesh. Int. J. Environ. Res. Public Health 2009, 6, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Heijerick, D.G.; De Schamphelaere, K.A.C.; Janssen, C.R. Predicting acute zinc toxicity for Daphnia magna as a function of key water chemistry characteristics: Development and validation of a biotic ligand model. Environ. Toxicol. Chem. 2002, 21, 1309–1315. [Google Scholar] [CrossRef]
- Islam, T.T.; Chakma, S.K.; Akter, L.; Mondol, A.S.; Bari, F.S. A cross sectional study on consumption pattern and heavy metal content in Buriganga River fish. Heliyon 2023, 9, e22714. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Wang, Q.; Kim, D.; Dionysiou, D.D.; Sorial, G.A.; Timberlake, D. Sources and remediation for mercury contamination in aquatic systems—A literature review. Environ. Pollut. 2004, 131, 323–336. [Google Scholar] [CrossRef]
- Regnell, O.; Watras, C.J. Microbial Mercury Methylation in Aquatic Environments: A Critical Review of Published Field and Laboratory Studies. Environ. Sci. Technol. 2019, 53, 4–19. [Google Scholar] [CrossRef]
- Islam, M.; Phoungthong, K.; Islam, A.; Ali, M.; Sarker, A.; Kabir, M.; Idris, A. Present status and mitigation approaches of arsenic in the environment of Bangladesh: A critical review. Int. J. Environ. Sci. Technol. 2023, 20, 13883–13894. [Google Scholar] [CrossRef]
- WHO/FAO. Report of the 33rd Session of the Codex Committee on Food Additives and Contaminants; Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme: Geneva, Switzerland, 2001.
- Rahman, M.A.; Hashem, M.A.; Rana, M.S.; Islam, M.R. Manganese in potable water of nine districts, Bangladesh: Human health risk. Environ. Sci. Pollut. Res. 2021, 28, 45663–45675. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Mohanta, L.C.; Huque, A.; Islam, D.; Roy, D.C.; Hakim, M.; Akhter, S.; Lyzu, C.; Lipy, E.P.; Nabi, M.R. Accumulation of Heavy Metals in Long-Evans Rat Through Feeding Fishes of Buriganga River and Their Histopathological Evaluation. Biol. Trace Elem. Res. 2023, 201, 3928–3940. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. Available online: https://www.who.int/publications/i/item/9789241548151 (accessed on 19 August 2025).
- Pernil, R.; Schleiff, E. Metalloproteins in the Biology of Heterocysts. Life 2019, 9, 32. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Islam, M.S.; Islam, Z.; Hasan, M.R. Pervasiveness and characteristics of microplastics in surface water and sediment of the Buriganga River, Bangladesh. Chemosphere 2022, 307, 135945. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Haque, M.R.; Ali, M.M.; Ahmed, W.; Siddique, M.A.; Akbor, M.A.; Islam, M.S.; Rahman, M.M. Assessment of microplastics pollution in aquatic species (fish, crab, and snail), water, and sediment from the Buriganga River, Bangladesh: An ecological risk appraisals. Sci. Total Environ. 2023, 857, 159344. [Google Scholar] [CrossRef]
- Das, R.K.; Mizan, A.; Zohra, F.T.; Ahmed, S.; Ahmed, K.S.; Hossain, H. Extraction of a novel tanning agent from indigenous plant bark and its application in leather processing. J. Leather Sci. Eng. 2022, 4, 18. [Google Scholar] [CrossRef]
- Al, M.; Juel, M.A.I.; Alam, M.S.; Pichtel, J.; Ahmed, T. Environmental and health risks of metal-contaminated soil in the former tannery area of Hazaribagh, Dhaka. SN Appl. Sci. 2020, 2, 1915. [Google Scholar] [CrossRef]
- Hoque, M.M.M.; Sarker, A.; Sarker, M.E.; Kabir, M.H.; Ahmed, F.T.; Yeasmin, M.; Islamd, M.S.; Idris, A.M. Heavy metals in sediments of an urban river at the vicinity of tannery industries in Bangladesh: A preliminary study for ecological and human health risk. Int. J. Environ. Anal. Chem. 2023, 103, 7909–7927. [Google Scholar] [CrossRef]
- Uddin, M.A.; Begum, M.S.; Ashraf, M.; Azad, A.K.; Adhikary, A.C.; Hossain, M.S. Water and chemical consumption in the textile processing industry of Bangladesh. PLoS Sustain. Transform. 2023, 2, e0000072. [Google Scholar] [CrossRef]
- Hossain, L.; Sarker, S.K.; Khan, M.S. Evaluation of present and future wastewater impacts of textile dyeing industries in Bangladesh. Environ. Dev. 2018, 26, 23–33. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Uthman, T.O. A review of history, properties, classification, applications and challenges of natural and synthetic dyes. Heliyon 2024, 10, e33646. [Google Scholar] [CrossRef]
- Nahar, K.; Chowdhury, M.A.K.; Chowdhury, M.A.H.; Rahman, A.; Mohiuddin, K.M. Heavy metals in handloom-dyeing effluents and their biosorption by agricultural byproducts. Environ. Sci. Pollut. Res. 2018, 25, 7954–7967. [Google Scholar] [CrossRef]
- Nargis, A.; Habib, A.; Rashid, H.O.; Harun, H.B.; Sarker, M.S.I.; Jin, R.; Liu, G.R.; Liu, W.B.; Al-Razee, A.N.M.; Chen, K.; et al. Status of multielement in water of the river Buriganga, Bangladesh: Aquatic chemistry of metal ions in polluted river water. Emerg. Contam. 2021, 7, 99–115. [Google Scholar] [CrossRef]
- Jolly, Y.N.; Rakib, M.R.J.; Kumar, R.; Islam, A.M.T.; Rabby, A.; Mamun, K.M.; Akter, S.; Kabir, J.; Bhuiyan, T.J.; Chowdhury, A.M.S.; et al. Deciphering the source of heavy metals in industrially affected river sediment of Shitalakshya river, Bangladesh, and potential ecological and health implications. J. Hazard. Mater. Adv. 2023, 10, 100268. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.G.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Cui, F.; Zhu, M.Y.; Shen, L.Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Yin, H.L.; Islam, M.S.; Ju, M.D. Urban river pollution in the densely populated city of Dhaka, Bangladesh: Big picture and rehabilitation experience from other developing countries. J. Clean. Prod. 2021, 321, 129040. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Haynes, R.J. Sorption of Heavy Metals by Inorganic and Organic Components of Solid Wastes: Significance to Use of Wastes as Low-Cost Adsorbents and Immobilizing Agents. Crit. Rev. Environ. Sci. Technol. 2010, 40, 909–977. [Google Scholar] [CrossRef]
- Smith, S.R. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ. Int. 2009, 35, 142–156. [Google Scholar] [CrossRef]
- Beinabaj, S.M.H.; Heydariyan, H.; Mohammad Aleii, H.; Hosseinzadeh, A. Concentration of heavy metals in leachate, soil, and plants in Tehran’s landfill: Investigation of the effect of landfill age on the intensity of pollution. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Fonseka, S.; Fernando, A.; Jayalath, K.; Amarasinghe, M.; Siribaddana, S.; Gunatilake, S.; Paranagama, P. Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. Springerplus 2015, 4, 90. [Google Scholar] [CrossRef]
- Kari, T.; Ylivainio, K.; Keskinen, R.; Sarvi, M.; Eurola, M.; Rinne, M.; Ketoja, E.; Mannio, J.; Suomi, J.; Kivirinta, H. Assessment of Risks Related to Increasing Heavy Metal Limits for Fertilizers in Finland. 2018. Available online: https://julkaisut.valtioneuvosto.fi/handle/10024/160703 (accessed on 19 August 2025).
- Aktaruzzaman, M.; Zakir, H.M.; Quadir, Q.F.; Rashid, M.H.; Mallick, S.; Biswas, P.; Nayeem, S.M.M.R. Toxic heavy metals content in different agrochemicals available in markets of Bangladesh and their loads to the agricultural lands. Environ. Monit. Assess. 2024, 196, 1053. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Han, B.; Pichtel, J. Irrigation suitability of White River in Indiana, Midwestern USA. Environ. Geochem. Health 2021, 43, 4179–4200. [Google Scholar] [CrossRef]
- Dippong, T.; Resz, M.A.; Tanaselia, C.; Cadar, O. Assessing microbiological and heavy metal pollution in surface waters associated with potential human health risk assessment at fish ingestion exposure. J. Hazard. Mater. 2024, 476, 135187. [Google Scholar] [CrossRef]
- Islam, M.M.; Karim, M.R.; Zheng, X.; Li, X. Heavy metal and metalloid pollution of soil, water and foods in bangladesh: A critical review. Int. J. Environ. Res. Public Health 2018, 15, 2825. [Google Scholar] [CrossRef]
- Järlskog, I.; Strömvall, A.-M.; Magnusson, K.; Galfi, H.; Björklund, K.; Polukarova, M.; Garção, R.; Markiewicz, A.; Aronsson, M.; Gustafsson, M.; et al. Traffic-related microplastic particles, metals, and organic pollutants in an urban area under reconstruction. Sci. Total Environ. 2021, 774, 145503. [Google Scholar] [CrossRef]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Qi, W. Assessing the water quality in urban river considering the influence of rainstorm flood: A case study of Handan city, China. Ecol. Indic. 2024, 160, 111941. [Google Scholar] [CrossRef]
- Pradhan, J.K.; Kumar, S. Informal e-waste recycling: Environmental risk assessment of heavy metal contamination in Mandoli industrial area, Delhi, India. Environ. Sci. Pollut. Res. 2014, 21, 7913–7928. [Google Scholar] [CrossRef]
- Mowla, M.; Rahman, E.; Islam, N.; Aich, N. Assessment of heavy metal contamination and health risk from indoor dust and air of informal E-waste recycling shops in Dhaka, Bangladesh. J. Hazard. Mater. Adv. 2021, 4, 100025. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, C.; Zhang, H.; Dong, Q. Heavy Metal Contamination from Electronic Waste Recycling at Guiyu, Southeastern China. J. Environ. Qual. 2009, 38, 1617–1626. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021, 780, 146551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Fraser, M.A.; Huang, W.; Ge, C.J.; Wang, Y.; Zhang, C.F.; Guo, P. Microplastic pollution in water, sediment, and specific tissues of crayfish (Procambarus clarkii) within two different breeding modes in Jianli, Hubei province, China. Environ. Pollut. 2021, 272, 115939. [Google Scholar] [CrossRef]
- Wu, P.F.; Tang, Y.Y.; Dang, M.; Wang, S.Q.; Jin, H.B.; Liu, Y.S.; Jing, H.; Zheng, C.M.; Yi, S.P.; Cai, Z.W. Spatial-temporal distribution of microplastics in surface water and sediments of Maozhou River within Guangdong-Hong Kong-Macao Greater Bay Area. Sci. Total Environ. 2020, 717, 135187. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Liu, Y.G.; Chen, Y.; Zhang, W.; Zhao, J.M.; He, S.Y.; Yang, C.P.; Zhang, T.; Tang, C.F.; Zhang, C.; et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2021, 766, 142572. [Google Scholar] [CrossRef]
- Waldschläger, K.; Lechthaler, S.; Stauch, G.; Schüttrumpf, H. The way of microplastic through the environment—Application of the source-pathway-receptor model (review). Sci. Total Environ. 2020, 713, 136584. [Google Scholar] [CrossRef]
- Reddy, A.S.; Abhilash, T.N. The fate of microplastics in wastewater treatment plants: An overview of source and remediation technologies. Environ. Technol. Innov. 2022, 28, 102815. [Google Scholar] [CrossRef]
- Haque, M.M.; Nupur, F.Y.; Parvin, F.; Tareq, S.M. Occurrence and characteristics of microplastic in different types of industrial wastewater and sludge: A potential threat of emerging pollutants to the freshwater of Bangladesh. J. Hazard. Mater. Adv. 2022, 8, 100166. [Google Scholar] [CrossRef]
- Long, Z.X.; Pan, Z.; Wang, W.L.; Ren, J.Y.; Yu, X.G.; Lin, L.Y.; Lin, H.; Chen, H.Z.; Jin, X.L. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef]
- Haque, M.M.; Kabir, A.T.; Latifi, E.M.; Mahmud, D.M.S.; Hossain, M.R.; Himu, H.A.; Fatema, U.K.; Tareq, S.M. Microfiber prevalence and removal efficiency of textile effluent treatment plants in Bangladesh. J. Hazard. Mater. Adv. 2024, 14, 100436. [Google Scholar] [CrossRef]
- Odora, A.T.; Aysha, S.; Sultan, M.B.; Bhuiyan, M.A.R. Evaluating the sources of microplastic contamination and quantifying its abundance in the Balu River, Dhaka, Bangladesh. Environ. Monit. Assess. 2024, 196, 867. [Google Scholar] [CrossRef] [PubMed]
- Bodzek, M.; Pohl, A. Possibilities of removing microplastics from the aquatic environment using membrane processes. Desalination Water Treat. 2023, 288, 104–120. [Google Scholar] [CrossRef]
- Afrin, S.; Uddin, M.K.; Rahman, M.M. Microplastics contamination in the soil from Urban Landfill site, Dhaka, Bangladesh. Heliyon 2020, 6, e05572. [Google Scholar] [CrossRef]
- Hossain, S.; Rahman, M.A.; Chowdhury, M.A.; Mohonta, S.K. Plastic pollution in Bangladesh: A review on current status emphasizing the impacts on environment and public health. Environ. Eng. Res. 2021, 26, 200535. [Google Scholar] [CrossRef]
- Mourshed, M.; Masud, M.H.; Rashid, F.; Joardder, M.U.H. Towards the effective plastic waste management in Bangladesh: A review. Environ. Sci. Pollut. Res. 2017, 24, 27021–27046. [Google Scholar] [CrossRef]
- Islam, T.; Li, Y.L.; Rob, M.M.; Cheng, H.F. Microplastic pollution in Bangladesh: Research and management needs. Environ. Pollut. 2022, 308, 119697. [Google Scholar] [CrossRef]
- Ngoc, U.N.; Schnitzer, H. Sustainable solutions for solid waste management in Southeast Asian countries. Waste Manag. 2009, 29, 1982–1995. [Google Scholar] [CrossRef]
- Fayshal, M.A. Current practices of plastic waste management, environmental impacts, and potential alternatives for reducing pollution and improving management. Heliyon 2024, 10, e40838. [Google Scholar] [CrossRef]
- Sharmin, S.; Wang, Q.Y.; Islam, M.R.; Wang, W.Q.; Enyoh, C.E. Microplastic Contamination of Non-Mulched Agricultural Soils in Bangladesh: Detection, Characterization, Source Apportionment and Probabilistic Health Risk Assessment. J. Xenobiotics 2024, 14, 812–826. [Google Scholar] [CrossRef]
- Filipe, S.; Mourao, P.M.; Couto, N.; Tranchida, D. Towards a Sustainable Future: Advancing an Integrated Approach for the Recycling and Valorization of Agricultural Plastics. Polymers 2023, 15, 4529. [Google Scholar] [CrossRef]
- Rana, M.M.; Haque, M.R.; Tasnim, S.S.; Rahman, M.M. The potential contribution of microplastic pollution by organic fertilizers in agricultural soils of Bangladesh: Quantification, characterization, and risk appraisals. Front. Environ. Sci. 2023, 11, 1205603. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Beriot, N.; Corradini, F.; Silva, V.; Yang, X.M.; Baartman, J.; Rezaei, M.; Van Schaik, L.; Riksen, M.; Geissen, V. Review of microplastic sources, transport pathways and correlations with other soil stressors: A journey from agricultural sites into the environment. Chem. Biol. Technol. Agric. 2022, 9, 20. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; González-Fernández, D.; Fornier, M.; Schmidt, N.; Sempéré, R. Macro-litter in surface waters from the Rhone River: Plastic pollution and loading to the NW Mediterranean Sea. Mar. Pollut. Bull. 2019, 146, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; O’Connor, D.; Wang, L.; Wu, W.-M.; Luo, J.; Hou, D. Microplastics in urban runoff: Global occurrence and fate. Water Res. 2022, 225, 119129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ding, W.C.; Zou, G.Y.; Wang, X.X.; Zhao, M.; Guo, S.; Chen, Y.H. Urban pipeline rainwater runoff is an important pathway for land-based microplastics transport to inland surface water: A case study in Beijing. Sci. Total Environ. 2023, 861, 160619. [Google Scholar] [CrossRef]
- Werbowski, L.M.; Gilbreath, A.N.; Munno, K.; Zhu, X.; Grbic, J.; Wu, T.N.; Sutton, R.; Sedlak, M.D.; Deshpande, A.D.; Rochman, C.M. Urban Stormwater Runoff: A Major Pathway for Anthropogenic Particles, Black Rubbery Fragments, and Other Types of Microplastics to Urban Receiving Waters. ACS EST Water 2021, 1, 1420–1428. [Google Scholar] [CrossRef]
- Österlund, H.; Blecken, G.; Lange, K.; Marsalek, J.; Gopinath, K.; Viklander, M. Microplastics in urban catchments: Review of sources, pathways, and entry into stormwater. Sci. Total Environ. 2023, 858, 159781. [Google Scholar] [CrossRef]
- Long, X.; Fu, T.M.; Yang, X.; Tang, Y.Y.; Zheng, Y.; Zhu, L.; Shen, H.Z.; Ye, J.H.; Wang, C.; Wang, T.; et al. Efficient Atmospheric Transport of Microplastics over Asia and Adjacent Oceans. Environ. Sci. Technol. 2022, 56, 6243–6252. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, G.; Revell, L.E.; Zhang, J.; Zuo, C.; Wang, D.; Le Ru, E.C.; Wu, G.; Mitrano, D.M. Long-range atmospheric transport of microplastics across the southern hemisphere. Nat. Commun. 2023, 14, 7898. [Google Scholar] [CrossRef]
- Meijer, L.J.J.; van Emmerik, T.; van der Ent, R.; Schmidt, C.; Lebreton, L. More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci. Adv. 2021, 7, eaaz5803. [Google Scholar] [CrossRef]
- Strokal, M.; Vriend, P.; Bak, M.P.; Kroeze, C.; van Wijnen, J.; van Emmerik, T. River export of macro- and microplastics to seas by sources worldwide. Nat. Commun. 2023, 14, 4842. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Panigrahi, A.K. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol. Rep. 2018, 5, 440–447. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, K. Microplastics induced developmental toxicity with microcirculation dysfunction in zebrafish embryos. Chemosphere 2022, 286, 131868. [Google Scholar] [CrossRef]
- Das, R.K.; Mızan, A.; Başaran, B.; Ahmed, S. Application of indigenous plant-based vegetable tanning agent extracted from xylocarpus granatum in semi-chrome and chrome retanned leather production. Text. Appar. 2022, 32, 258–264. [Google Scholar] [CrossRef]
- NTP. Cadmium and Cadmium Compounds: 15th Report on Carcinogens; National Toxicology Program: Research Triangle Park, NC, USA, 2021. [CrossRef]
- Parida, L.; Patel, T.N. Systemic impact of heavy metals and their role in cancer development: A review. Environ. Monit. Assess. 2023, 195, 766. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, M.Z.; Liu, Y.; Fahad, S.; Qayyum, A.; Jadoon, S.A.; Chen, Y.L.; Zhu, G.P. Nickel toxicity alters growth patterns and induces oxidative stress response in sweetpotato. Front. Plant Sci. 2022, 13, 1054924. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Qin, A.Z.; Zain, M.; Mushtaq, Z.; Mehmood, F.; Riaz, L.; Naveed, S.; Ansari, M.J.; Saeed, M.; Ahmad, I.; et al. Pb uptake, accumulation, and translocation in plants: Plant physiological, biochemical, and molecular response: A review. Heliyon 2024, 10, e27724. [Google Scholar] [CrossRef]
- USDOE. The Risk Assessment Information System (RAIS); U.S. Department of Energy’s Oak Ridge Operations Office (ORO): Oak Ridge, TN, USA, 2011.
- USEPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- Klaudia, J.; Marian, V. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Khan, M.B.; Urmy, S.Y.; Setu, S.; Kanta, A.H.; Gautam, S.; Eti, S.A.; Rahman, M.M.; Sultana, N.; Mahmud, S.; Baten, M.A. Abundance, distribution and composition of microplastics in sediment and fish species from an Urban River of Bangladesh. Sci. Total Environ. 2023, 885, 163876. [Google Scholar] [CrossRef]
- Wang, S.; Mintenig, S.M.; Cheng, C.; Wu, J.; Koelmans, A.A. Extent and risks of microplastic pollution in the Yangtze River. State of the science. Sci. Total Environ. 2024, 910, 168538. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Zhang, J.; Li, Q.; Wang, Y.; Wang, M.; Huang, X. Distribution and characteristics of microplastics in urban waters of seven cities in the Tuojiang River basin, China. Environ. Res. 2020, 189, 109893. [Google Scholar] [CrossRef] [PubMed]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2015, 5, 17988. [Google Scholar] [CrossRef] [PubMed]
- Kittner, M.; Kerndorff, A.; Ricking, M.; Bednarz, M.; Obermaier, N.; Lukas, M.; Asenova, M.; Bordós, G.; Eisentraut, P.; Hohenblum, P.; et al. Microplastics in the Danube River Basin: A First Comprehensive Screening with a Harmonized Analytical Approach. ACS EST Water 2022, 2, 1174–1181. [Google Scholar] [CrossRef]
- Townsend, K.R.; Lu, H.-C.; Sharley, D.J.; Pettigrove, V. Associations between microplastic pollution and land use in urban wetland sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size matters more than shape: Ingestion of primary and secondary microplastics by small predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Zhang, C.N.; Wang, J.; Zhou, A.G.; Ye, Q.; Feng, Y.Y.; Wang, Z.L.; Wang, S.D.; Xu, G.H.; Zou, J.X. Species-specific effect of microplastics on fish embryos and observation of toxicity kinetics in larvae. J. Hazard. Mater. 2021, 403, 123948. [Google Scholar] [CrossRef]
- Hasan, A.K.M.M.; Hamed, M.; Hasan, J.; Martyniuk, C.J.; Niyogi, S.; Chivers, D.P. A review of the neurobehavioural, physiological, and reproductive toxicity of microplastics in fishes. Ecotoxicol. Environ. Saf. 2024, 282, 116712. [Google Scholar] [CrossRef]
- Ekere, N.R.; Yakubu, N.M.; Oparanozie, T.; Ihedioha, J.N. Levels and risk assessment of polycyclic aromatic hydrocarbons in water and fish of Rivers Niger and Benue confluence Lokoja, Nigeria. J. Environ. Health Sci. Eng. 2019, 17, 383–392. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, E.S.; Sung, H.C. Microplastics as an emerging threat to amphibians: Current status and future perspectives. Heliyon 2024, 10, e28220. [Google Scholar] [CrossRef]
- Richard, F.J.; Southern, I.; Gigauri, M.; Bellini, G.; Rojas, O.; Runde, A. Warning on nine pollutants and their effects on avian communities. Glob. Ecol. Conserv. 2021, 32, e01898. [Google Scholar] [CrossRef]
- He, H.; Wen, H.-P.; Liu, J.-P.; Wu, C.-C.; Mai, L.; Zeng, E.Y. Hydrophobic organic contaminants affiliated with polymer-specific microplastics in urban river tributaries and estuaries. Sci. Total Environ. 2023, 899, 166415. [Google Scholar] [CrossRef]
- Aydin, R.B.; Yozukmaz, A.; Sener, I.; Temiz, F.; Giannetto, D. Occurrence of Microplastics in Most Consumed Fruits and Vegetables from Turkey and Public Risk Assessment for Consumers. Life 2023, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Kibuye, F.A.; Gall, H.E.; Veith, T.L.; Elkin, K.R.; Elliott, H.A.; Harper, J.P.; Watson, J.E. Influence of hydrologic and anthropogenic drivers on emerging organic contaminants in drinking water sources in the Susquehanna River Basin. Chemosphere 2020, 245, 125583. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Abbasi, A.; Chen, G. Fate, distribution, and transport dynamics of Per- and Polyfluoroalkyl Substances (PFASs) in the environment. J. Environ. Manag. 2024, 371, 123163. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.S.; Chakraborty, T.K.; Ghosh, G.C.; Nice, M.S.; Zaman, S.; Khan, A.S. Microplastics and heavy metals in freshwater fish species in the southwestern region of Bangladesh: An emerging concern for public health. Emerg. Contam. 2024, 10, 100325. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, R.K.; Marma, M.; Mizan, A.; Chen, G.; Alam, M.S. Heavy Metals and Microplastics as Emerging Contaminants in Bangladesh’s River Systems: Evidence from Urban–Industrial Corridors. Toxics 2025, 13, 803. https://doi.org/10.3390/toxics13090803
Das RK, Marma M, Mizan A, Chen G, Alam MS. Heavy Metals and Microplastics as Emerging Contaminants in Bangladesh’s River Systems: Evidence from Urban–Industrial Corridors. Toxics. 2025; 13(9):803. https://doi.org/10.3390/toxics13090803
Chicago/Turabian StyleDas, Raju Kumar, Mongsathowai Marma, Al Mizan, Gang Chen, and Md Shahin Alam. 2025. "Heavy Metals and Microplastics as Emerging Contaminants in Bangladesh’s River Systems: Evidence from Urban–Industrial Corridors" Toxics 13, no. 9: 803. https://doi.org/10.3390/toxics13090803
APA StyleDas, R. K., Marma, M., Mizan, A., Chen, G., & Alam, M. S. (2025). Heavy Metals and Microplastics as Emerging Contaminants in Bangladesh’s River Systems: Evidence from Urban–Industrial Corridors. Toxics, 13(9), 803. https://doi.org/10.3390/toxics13090803









