Constructed Wetlands with Novel Substrate Exposed to Nano-Plastics: Mitigating the Effects of Substrate Enzyme and Ecological Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Modified Basalt Fiber (MBF) and Bio-Nests
2.2. Setup of the Constructed Wetland
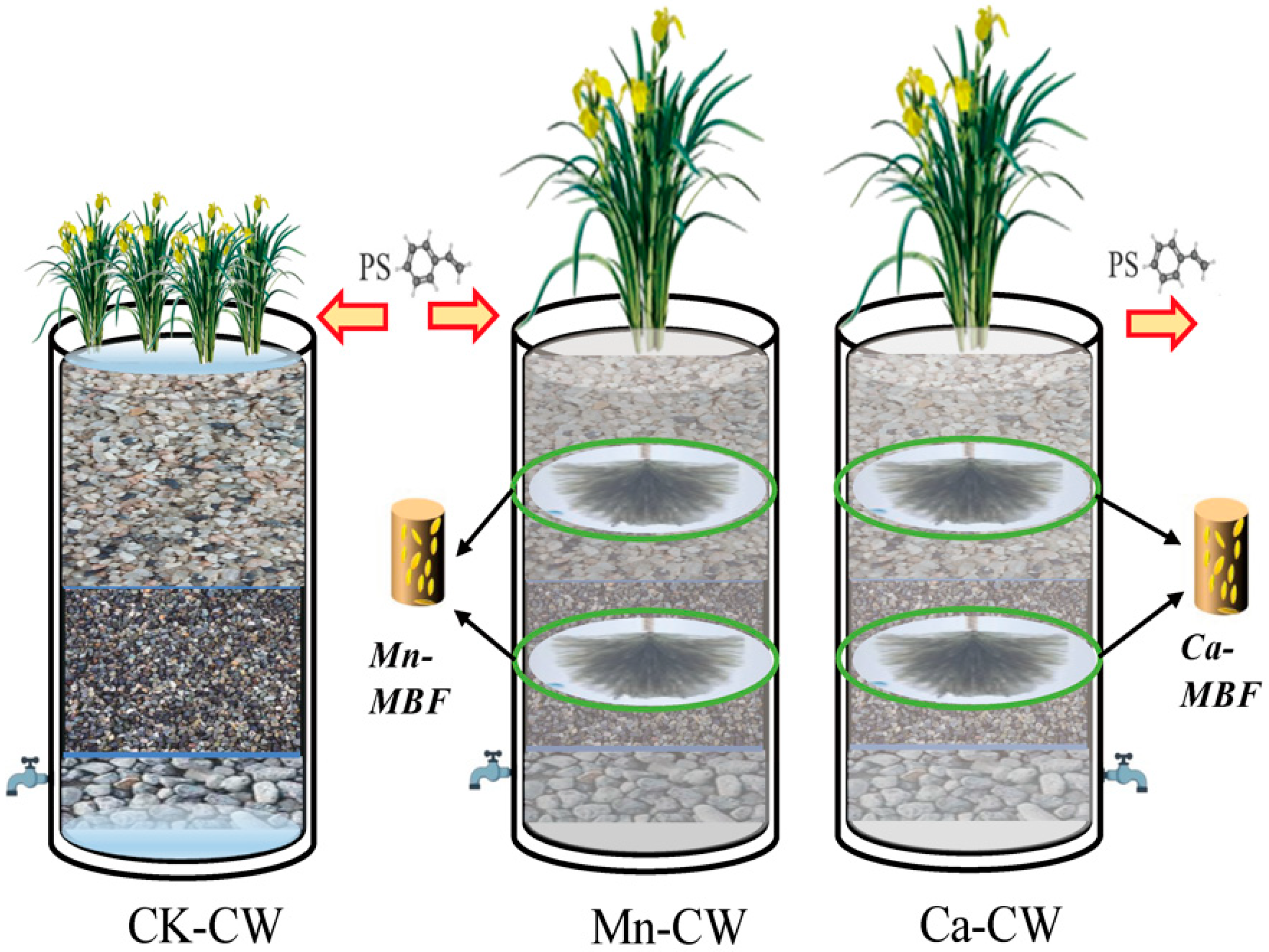
2.3. The Substrate Enzyme
2.4. Microbiological Analyses
2.5. Statistical Analysis
3. Result and Discussion
3.1. Characteristic of MBF and Bio-Nests
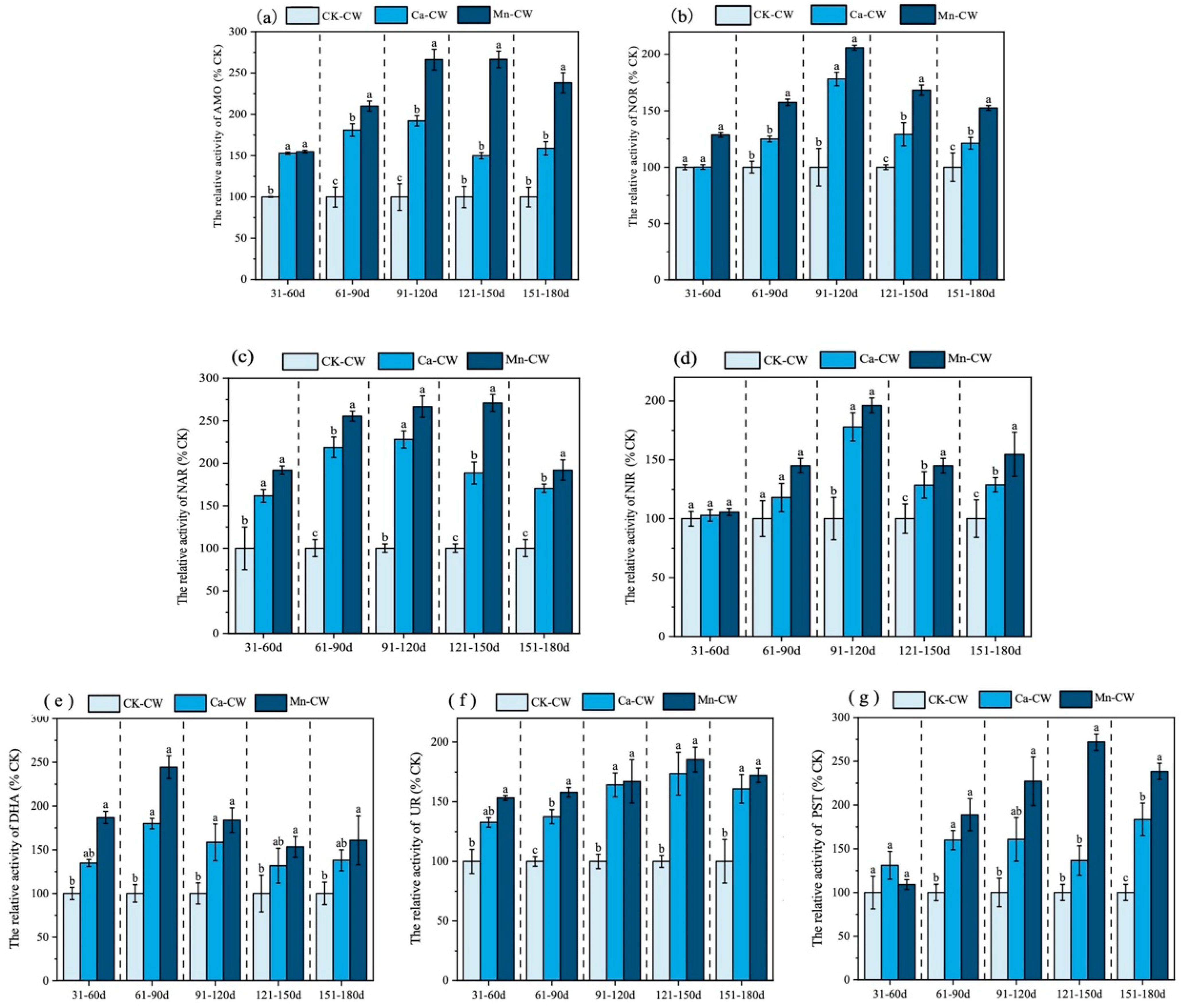
3.2. Effect on Enzyme Activity of Substrate
3.2.1. Ammonia Monooxygenase and Nitrite Oxidoreductase
3.2.2. Effect on Nitrite Reductase and Nitrate Reductase Activities
3.2.3. Dehydrogenases, Ureases, and Phosphatases
3.2.4. Correlation of Substrate Enzymes and Ecological Indicators
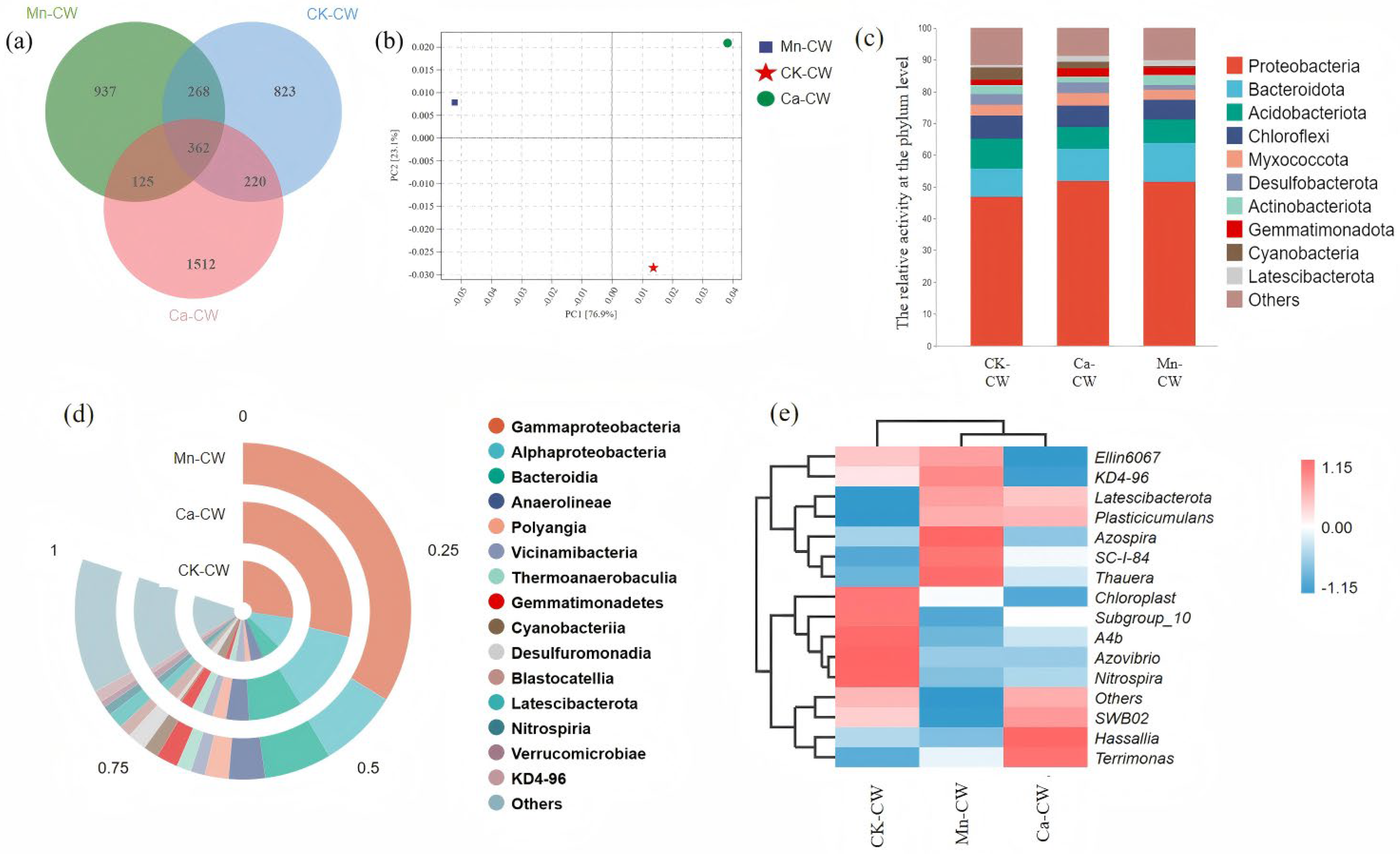
3.3. Microbial Diversity Analysis
3.3.1. Analysis of Community Diversity and Species Difference
3.3.2. The Structure of Microbial Community
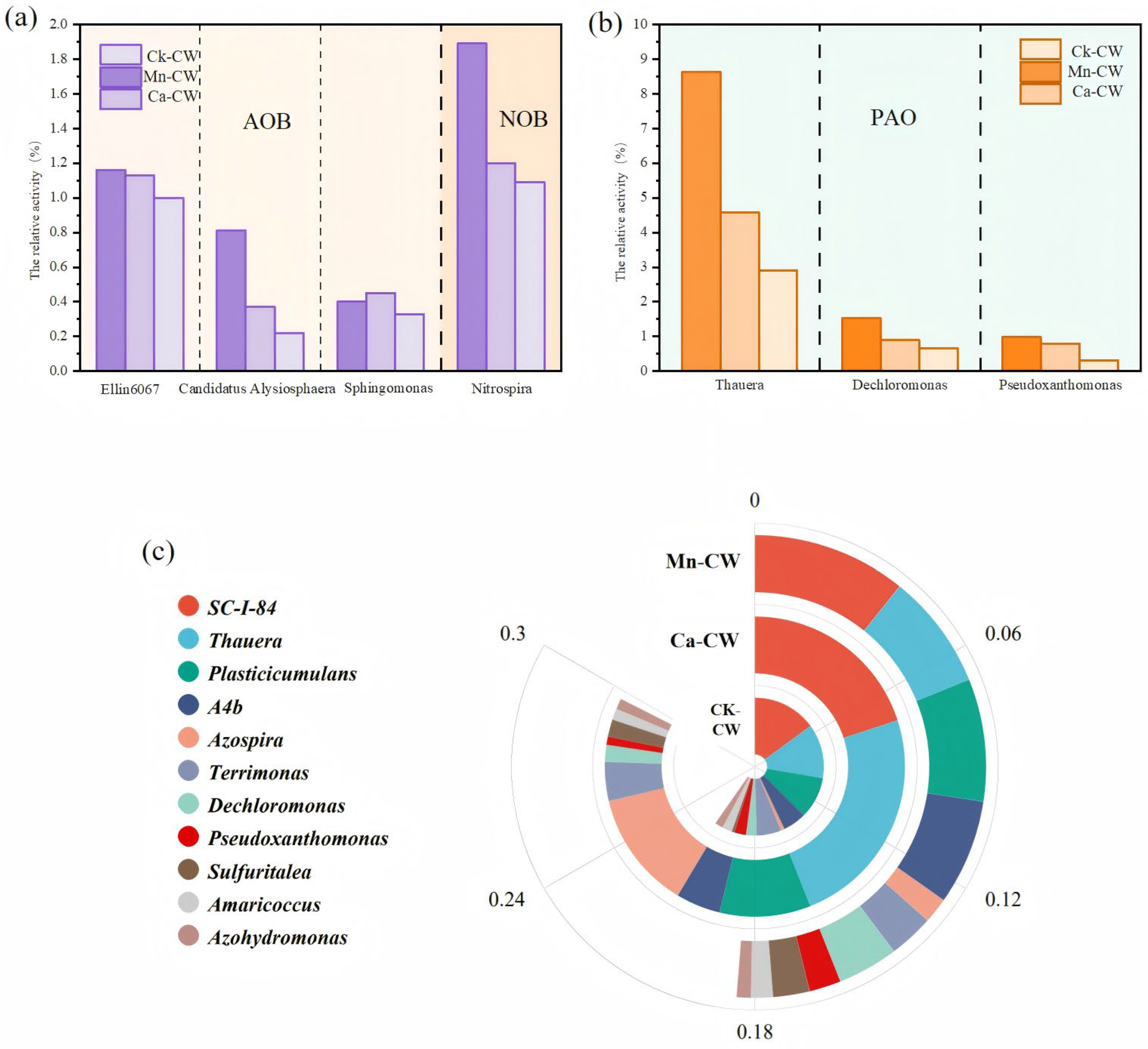
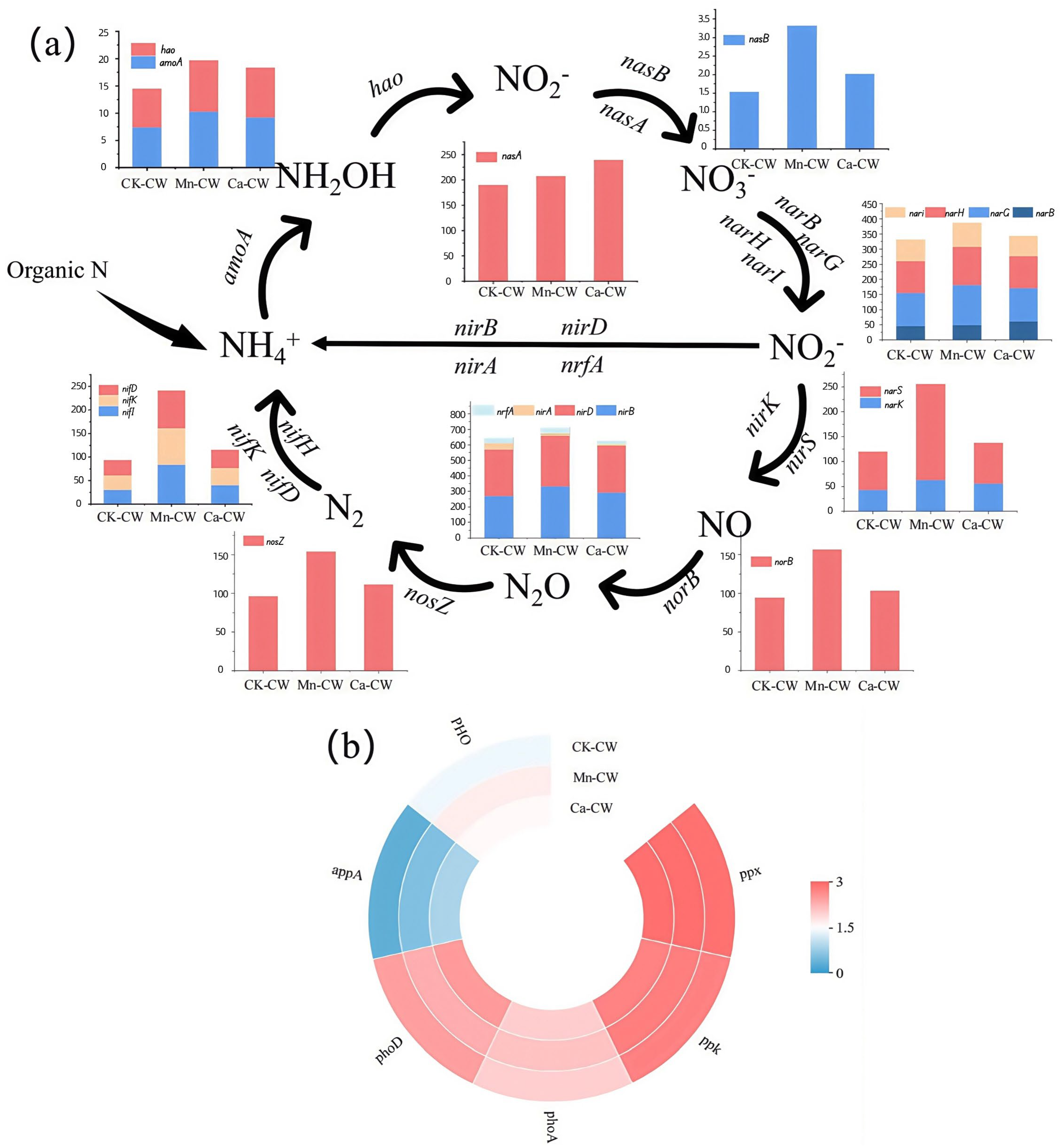
3.4. Functional Microbial Analysis
3.5. Functional Gene Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| NPs | Nano-Plastics |
| CWs | Constructed Wetlands |
| MBF | Modified Basalt Fiber |
| PS | Polystyrene |
| Ca | Calcium |
| Mn | Manganese |
| AMO | Ammonia monooxygenase |
| NOR | Nitrite oxidoreductase |
| NAR | Nitrate reductase |
| NIR | Nitrite reductase |
| PST | Phosphatase |
| UR | Urease |
| DHA | Dehydrogenase |
References
- Lu, S.; Bai, X.; Zhang, J.; Li, J.; Li, W.; Lin, J. Impact of Virtual Water Export on Water Resource Security Associated with the Energy and Food Bases in Northeast China. Technol. Forecast. Soc. Chang. 2022, 180, 121635. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Peng, H.; Skitmore, M.; Bai, X.; Zheng, Z. The Energy-Food-Water Nexus: Water Footprint of Henan-Hubei-Hunan in China. Renew. Sustain. Energy Rev. 2021, 135, 110417. [Google Scholar] [CrossRef]
- Lu, S.; Lu, W.; Xu, M.; Taghizadeh-Hesary, F.; Tang, Y. Water-Energy-Food Security under Green Finance Constraints in Southwest China. Energy Econ. 2023, 118, 106478. [Google Scholar] [CrossRef]
- Parde, D.; Patwa, A.; Shukla, A.; Vijay, R.; Killedar, D.J.; Kumar, R. A Review of Constructed Wetland on Type, Treatment and Technology of Wastewater. Environ. Technol. Innov. 2021, 21, 101261. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J.; O’Connor, D. Remediation of Mercury Contaminated Soil, Water, and Air: A Review of Emerging Materials and Innovative Technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.; Gupta, A.K.; Ghosal, P.S.; Majumder, A. A Review on Performance of Constructed Wetlands in Tropical and Cold Climate: Insights of Mechanism, Role of Influencing Factors, and System Modification in Low Temperature. Sci. Total Environ. 2021, 755, 142540. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Chen, J.; Chang, J. Application of Biochar as an Innovative Substrate in Constructed Wetlands/Biofilters for Wastewater Treatment: Performance and Ecological Benefits. J. Clean. Prod. 2021, 293, 126156. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, S.; Gu, X.; Zhang, M.; Peng, Y.; Yan, P.; He, S. Boosting the Denitrification Efficiency of Iron-Based Constructed Wetlands in-Situ via Plant Biomass-Derived Biochar: Intensified Iron Redox Cycle and Microbial Responses. Water Res. 2024, 253, 121285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, Y.; Yu, G.; Wang, G.; Zhou, Z.; Li, P.; Zhang, Y.; Yang, K.; Wang, S. A Review on Microorganisms in Constructed Wetlands for Typical Pollutant Removal: Species, Function, and Diversity. Front. Microbiol. 2022, 13, 845725. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global Development of Various Emerged Substrates Utilized in Constructed Wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef]
- Qian, X.; Huang, J.; Cao, C.; Yao, J.; Wu, Y.; Wang, L.; Wang, X. Modified Basalt Fiber Filled in Constructed Wetland-Microbial Fuel Cell: Comparison of Performance and Microbial Impacts under PFASs Exposure. J. Hazard. Mater. 2024, 476, 135179. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Huang, J.; Ji, X.; Yan, C.; Cao, C.; Wu, Y.; Wang, X. Modified Basalt Fibers Boost Performance of Constructed Wetlands: Comparison between Surface Coating and Chemical Grafting. Bioresour. Technol. 2024, 397, 130492. [Google Scholar] [CrossRef] [PubMed]
- Fiore, V.; Scalici, T.; Di Bella, G.; Valenza, A. A Review on Basalt Fibre and Its Composites. Compos. Part B Eng. 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Xie, Y.; Rong, X.; Liu, Z.; Xiao, X.; Liang, Z.; Jiang, S.; Wei, J.; Wu, Z. A Sustainable Bio-Carrier Medium for Wastewater Treatment: Modified Basalt Fiber. J. Clean. Prod. 2019, 225, 472–480. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Ni, H.; Rong, X.; Zhang, Q.; Xiao, X.; Huan, H.; Liu, J.F.; Wu, Z. Surface Modification of Basalt Fiber with Organic/Inorganic Composites for Biofilm Carrier Used in Wastewater Treatment. ACS Sustain. Chem. Eng. 2018, 6, 2596–2602. [Google Scholar] [CrossRef]
- Ni, H.; Wang, C.; Arslan, M.; Qian, J.; Liang, Z.; Luo, Z.; Cai, R.; Gamal El-Din, M.; Wu, Z. Enhanced Wastewater Treatment by Modified Basalt Fiber Bio-Carriers: Effect of Etching and Surface Functionalization. J. Clean. Prod. 2022, 343, 130927. [Google Scholar] [CrossRef]
- Qian, X.; Ji, X.; Yan, C.; Huang, J.; Wu, Y.; Wang, X. Superiority of Basalt Fibers with Different Modifications in Contact Oxidation Reactor for Wastewater Treatment: Exploration of Microbial Community and Underlying Mechanism. J. Environ. Chem. Eng. 2024, 12, 112441. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, X.; Ma, Y.; Zhang, X.; Rong, X.; Xiao, X.; Wu, Z.; Wei, J. Calcium Modified Basalt Fiber Bio-Carrier for Wastewater Treatment: Investigation on Bacterial Community and Nitrogen Removal Enhancement of Bio-Nest. Bioresour. Technol. 2021, 335, 125259. [Google Scholar] [CrossRef]
- Xu, L.; Su, J.; Ali, A.; Huang, T.; Yang, Y.; Shi, J.; Liang, E. Magnetite-Loaded Rice Husk Biochar Promoted the Denitrification Performance of Aquabacterium sp. XL4 under Low Carbon to Nitrogen Ratio: Optimization and Mechanism. Bioresour. Technol. 2022, 348, 126802. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, Q. Surface Modification of Carbon Fiber Support by Ferrous Oxalate for Biofilm Wastewater Treatment System. J. Clean. Prod. 2018, 194, 416–424. [Google Scholar] [CrossRef]
- Ni, H.; Qian, J.; Arslan, M.; Zhou, X.; Luo, Z.; Wei, J.; Gamal El-Din, M.; Wu, Z. Treatment of High-Load Organic Wastewater by Novel Basalt Fiber Carrier Media. Sci. Total Environ. 2021, 758, 143760. [Google Scholar] [CrossRef]
- Qian, X.; Huang, J.; Li, X.; Cao, C.; Yao, J. Novel Insights on Ecological Responses of Short- and Long-Chain Perfluorocarboxylic Acids in Constructed Wetlands Coupled with Modified Basalt Fiber Bio-Nest. Chemosphere 2024, 365, 143384. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of Chemical Contaminants with Microplastics: Principles and Perspectives. Sci. Total Environ. 2020, 706, 135978. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Tamayo-Belda, M.; Pulido-Reyes, G.; Amariei, G.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Secondary Nanoplastics Released from a Biodegradable Microplastic Severely Impact Freshwater Environments. Environ. Sci. Nano 2019, 6, 1382–1392. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of Antibiotics on Microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Hüffer, T.; Hofmann, T. Sorption of Non-Polar Organic Compounds by Micro-Sized Plastic Particles in Aqueous Solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Kiran, B.R.; Kopperi, H.; Venkata Mohan, S. Micro/Nano-Plastics Occurrence, Identification, Risk Analysis and Mitigation: Challenges and Perspectives. Rev. Environ. Sci. Biotechnol. 2022, 21, 169–203. [Google Scholar] [CrossRef]
- Gupta, N.; Parsai, T.; Kulkarni, H.V. A Review on the Fate of Micro and Nano Plastics (MNPs) and Their Implication in Regulating Nutrient Cycling in Constructed Wetland Systems. J. Environ. Manag. 2024, 350, 119559. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, J.; Han, T.; Yan, C.; Cao, C.; Cao, M. Comprehensive Metagenomic and Enzyme Activity Analysis Reveals the Negatively Influential and Potentially Toxic Mechanism of Polystyrene Nanoparticles on Nitrogen Transformation in Constructed Wetlands. Water Res. 2021, 202, 117420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, Y.; Liu, Y.; Yang, J.; Guo, M.; Bai, S.; Hou, N.; Zhao, X. A Study on Microbial Mechanism in Response to Different Nano-Plastics Concentrations in Constructed Wetland and Its Carbon Footprints Analysis. Chem. Eng. J. 2024, 480, 148023. [Google Scholar] [CrossRef]
- Yang, X.; He, Q.; Guo, F.; Sun, X.; Zhang, J.; Chen, M.; Vymazal, J.; Chen, Y. Nanoplastics Disturb Nitrogen Removal in Constructed Wetlands: Responses of Microbes and Macrophytes. Environ. Sci. Technol. 2020, 54, 14007–14016. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, L.; Yu, H.; Cao, X.; Peng, J.; Liu, H.; Qu, J. Negative Impacts of Nanoplastics on the Purification Function of Submerged Plants in Constructed Wetlands: Responses of Oxidative Stress and Metabolic Processes. Water Res. 2022, 227, 119339. [Google Scholar] [CrossRef]
- Qian, X.; Huang, J.; Yan, C.; Xiao, J.; Cao, C.; Wu, Y.; Wang, L. Evaluation of Ecological Impacts with Ferrous Iron Addition in Constructed Wetland under Perfluorooctanoic Acid Stress. J. Hazard. Mater. 2024, 469, 134074. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Van Alst, N.; Vollertsen, J. Quantification of Microplastic Mass and Removal Rates at Wastewater Treatment Plants Applying Focal Plane Array (FPA)-Based Fourier Transform Infrared (FT-IR) Imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, J.; Tuo, J.; Li, X.; Xu, J.; Li, X.; Qian, X. Modified Basalt Fibers (MBF) Bio-Nest Boost Constructed Wetland Operating Performance and Plant Activity after Exposure to Nano-Plastics. Process Saf. Environ. Prot. 2025, 201, 107394. [Google Scholar] [CrossRef]
- Bhat, T.; Fortomaris, D.; Kandare, E.; Mouritz, A.P. Properties of Thermally Recycled Basalt Fibres and Basalt Fibre Composites. J. Mater. Sci. 2018, 53, 1933–1944. [Google Scholar] [CrossRef]
- Awet, T.T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.-L.; Ruf, T.; Jost, C.; Drexel, R.; Tunc, E.; Emmerling, C. Effects of Polystyrene Nanoparticles on the Microbiota and Functional Diversity of Enzymes in Soil. Environ. Sci. Eur. 2018, 30, 11. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global Patterns of Phosphatase Activity in Natural Soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op Den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete Nitrification by a Single Microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Liu, R.; Li, S.; Gao, X.; Yu, N.; Zhao, C.; Gao, C.; Lv, W. Single and Combined Impacts of Nickel and Cadmium on the Performance, Microbial Community and Enzymatic Activity of Sequencing Batch Reactors. Sci. Total Environ. 2020, 727, 138571. [Google Scholar] [CrossRef] [PubMed]
- Durvasula, K.; Jantama, K.; Fischer, K.; Vega, A.; Koopman, B.; Svoronos, S.A. Effect of Periplasmic Nitrate Reductase on Diauxic Lag of Paracoccus pantotrophus. Biotechnol. Prog. 2009, 25, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Huang, J.; Yan, C.; Xiao, J. Ecological Restoration Performance Enhanced by Nano Zero Valent Iron Treatment in Constructed Wetlands under Perfluorooctanoic Acid Stress. Sci. Total Environ. 2022, 846, 157413. [Google Scholar] [CrossRef]
- Huang, J.; Li, R.; Ma, Y.; Cao, C.; Li, X.; Han, T.; Cao, M. Effects of Macrophytes on Micro–And Nanoplastic Retention and Cycling in Constructed Wetlands. Environ. Pollut. 2023, 326, 121259. [Google Scholar] [CrossRef] [PubMed]
- Mariano, E.; De Sant Ana Filho, C.R.; Bortoletto-Santos, R.; Bendassolli, J.A.; Trivelin, P.C.O. Ammonia Losses Following Surface Application of Enhanced-Efficiency Nitrogen Fertilizers and Urea. Atmos. Environ. 2019, 203, 242–251. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic Acid Metabolism and Functions: A Comparison of Plants and Mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE Microplastic Films Alter Microbial Community Composition and Enzymatic Activities in Soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Yang, X.; Guo, F.; Su, X.; Chen, Y. Acute and Chronic Responses of Macrophyte and Microorganisms in Constructed Wetlands to Cerium Dioxide Nanoparticles: Implications for Wastewater Treatment. Chem. Eng. J. 2018, 348, 35–45. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Huang, J.; Tuo, J.; Li, X.; Qian, X.; Abolfathi, S. The Operating Performance of Modified Basalt Fibers (MBF) Bio-Nest Reactor Exposed to Nano-Plastics. J. Hazard. Mater. 2024, 480, 136042. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wu, Z.; Cheng, S.; Zhou, Q.; Hu, H. Roles of Substrate Microorganisms and Urease Activities in Wastewater Purification in a Constructed Wetland System. Ecol. Eng. 2003, 21, 191–195. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Wei, W.; Huang, Q.-S.; Wang, C.; Wang, Y.; Ni, B.-J. Insights into the Microbial Response of Anaerobic Granular Sludge during Long-Term Exposure to Polyethylene Terephthalate Microplastics. Water Res. 2020, 179, 115898. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Li, S.; Chu, Y.; Xie, P.; Xie, Y.; Chang, H.; Ho, S.-H. Insights into the Microalgae-Bacteria Consortia Treating Swine Wastewater: Symbiotic Mechanism and Resistance Genes Analysis. Bioresour. Technol. 2022, 349, 126892. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, T.; Jin, S.; Ren, K.; Cai, Z.; Cai, B.; Li, S. The Salinity Survival Strategy of Chenopodium Quinoa: Investigating Microbial Community Shifts and Nitrogen Cycling in Saline Soils. Microorganisms 2023, 11, 2829. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Wu, S.; Yang, Z.-H.; Zhao, F. Bacterial Community Structure of Autotrophic Denitrification Biocathode by 454 Pyrosequencing of the 16S rRNA Gene. Microb. Ecol. 2015, 69, 492–499. [Google Scholar] [CrossRef]
- Shangguan, H.; Liu, J.; Zhu, Y.; Tong, Z.; Wu, Y. Start-up of a Spiral Periphyton Bioreactor (SPR) for Removal of COD and the Characteristics of the Associated Microbial Community. Bioresour. Technol. 2015, 193, 456–462. [Google Scholar] [CrossRef]
- Cai, X.; Wen, P.; Yuan, Y.; Tang, J.; Yu, Z.; Zhou, S. Identification of Nitrogen-Incorporating Bacteria in a Sequencing Batch Reactor: A Combining Cultivation-Dependent and Cultivation-Independent Method. Bioresour. Technol. 2020, 316, 123964. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Z.; Yang, B.; Yang, H.; Zhang, Y.; Zeng, Q.; Yan, C.; He, Y.; Peng, Y.; Wang, W.; et al. The Effect of White Grub (Maladera verticalis) Larvae Feeding on Rhizosphere Microbial Characterization of Aerobic Rice (Oryza sativa L.) in Puer City, Yunnan Province, China. BMC Microbiol. 2024, 24, 123. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, N.; Yang, G.; Xu, Q.; Wang, D.; Tang, L.; Xia, J.; Li, P.; Li, X. Environmentally Relevant Level of Perfluorooctanoic Acid Affect the Formation of Aerobic Granular Sludge. J. Environ. Manag. 2023, 336, 117659. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.; Hu, J.; Chen, S.; Hu, S.; Wu, Y.; Liu, B.; Xiao, K.; Liang, S.; Yang, J.; et al. Efficient Degradation of Refractory Pollutant in a Microbial Fuel Cell with Novel Hybrid Photocatalytic Air-Cathode: Intimate Coupling of Microbial and Photocatalytic Processes. Bioresour. Technol. 2021, 340, 125717. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, G. Deciphering the Effects of Antibiotics on Nitrogen Removal and Bacterial Communities of Autotrophic Denitrification Systems in a Three-Dimensional Biofilm Electrode Reactor. Environ. Pollut. 2022, 315, 120476. [Google Scholar] [CrossRef]
- Wang, S.; Teng, Z.; Li, Y.; Chen, F.; Liu, X.; Liu, S.; He, J.; Wang, W. A Novel Vertical Dual-Loop Reactor for Rapid Start-up of Simultaneous Partial Nitrification and Anammox Process in Treating Landfill Leachate: Performances and Mechanisms. Bioresour. Technol. 2022, 364, 127947. [Google Scholar] [CrossRef]
- Foesel, B.U.; Gößner, A.S.; Drake, H.L.; Schramm, A. Geminicoccus roseus Gen. Nov., Sp. Nov., an Aerobic Phototrophic Alphaproteobacterium Isolated from a Marine Aquaculture Biofilter. Syst. Appl. Microbiol. 2007, 30, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Antwi, P.; Zhang, D.; Su, H.; Luo, W.; Quashie, F.K.; Kabutey, F.T.; Xiao, L.; Lai, C.; Liu, Z.; Li, J. Nitrogen Removal from Landfill Leachate by Single-Stage Anammox and Partial-Nitritation Process: Effects of Microaerobic Condition on Performance and Microbial Activities. J. Water Process Eng. 2020, 38, 101572. [Google Scholar] [CrossRef]
- Ren, T.; Chi, Y.; Wang, Y.; Shi, X.; Jin, X.; Jin, P. Diversified Metabolism Makes Novel Thauera Strain Highly Competitive in Low Carbon Wastewater Treatment. Water Res. 2021, 206, 117742. [Google Scholar] [CrossRef]
- Qiu, G.; Zuniga-Montanez, R.; Law, Y.; Thi, S.S.; Nguyen, T.Q.N.; Eganathan, K.; Liu, X.; Nielsen, P.H.; Williams, R.B.H.; Wuertz, S. Polyphosphate-Accumulating Organisms in Full-Scale Tropical Wastewater Treatment Plants Use Diverse Carbon Sources. Water Res. 2019, 149, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, M.H.M.; Hassan, M.A.; Tokura, M.; Shirai, Y. Indigenous Cellulolytic and Hemicellulolytic Bacteria Enhanced Rapid Co-Composting of Lignocellulose Oil Palm Empty Fruit Bunch with Palm Oil Mill Effluent Anaerobic Sludge. Bioresour. Technol. 2013, 147, 632–635. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Yu, X.; Xie, H.; Häggblom, M.; Liang, S.; Hu, Z. Inorganic Particle Accumulation Promotes Nutrient Removal of Vertical Flow Constructed Wetlands: Mechanisms and Implications. Sci. Total Environ. 2021, 778, 146203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sorokin, D.Y.; Junicke, H.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Plasticicumulans lactativorans sp. Nov., a Polyhydroxybutyrate-Accumulating Gammaproteobacterium from a Sequencing-Batch Bioreactor Fed with Lactate. Int. J. Syst. Evol. Microbiol. 2014, 64, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mikes, M.C.; Martin, T.K.; Moe, W.M. Azospira inquinata sp. Nov., a Nitrate-Reducing Bacterium of the Family Rhodocyclaceae Isolated from Contaminated Groundwater. Int. J. Syst. Evol. Microbiol. 2021, 71, 005172. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Miura, A.; Iwata, T.; Kojima, H.; Fukui, M. Dominance of Sulfuritalea Species in Nitrate-depleted Water of a Stratified Freshwater Lake and Arsenate Respiration Ability within the Genus. Environ. Microbiol. Rep. 2017, 9, 522–527. [Google Scholar] [CrossRef] [PubMed]

| Groups | Observed Species | Chao1 | Shannon | Simpson | Goods Coverage |
|---|---|---|---|---|---|
| CK-CW | 1673.1 | 1675.72 | 9.07418 | 0.993141 | 0.999058 |
| Mn-CW | 2220.6 | 2223.59 | 9.85513 | 0.996969 | 0.999464 |
| Ca-CW | 1692.1 | 1693.18 | 9.38836 | 0.996123 | 0.999291 |
| Functional Type | Genus of Microorganisms | CK-CW | Mn-CW | Ca-CW |
|---|---|---|---|---|
| AOB | Ellin6067 | 1.00 | 1.16 | 1.13 |
| Candidatus Alysiosphaera | 0.22 | 0.81 | 0.37 | |
| Sphingomonas | 0.33 | 0.40 | 0.45 | |
| NOB | Nitrospira | 1.09 | 1.89 | 1.20 |
| PAO | Thauera | 2.91 | 8.62 | 4.59 |
| Dechloromonas | 0.65 | 1.53 | 0.89 | |
| Pseudoxanthomonas | 0.32 | 0.98 | 0.80 |
| Function Types | Microorganisms Genus | CK-CW | Mn-CW | Ca-CW |
|---|---|---|---|---|
| DNB | SC-I-84 | 3.88 | 7.20 | 5.38 |
| Thauera | 2.91 | 8.63 | 4.59 | |
| Plasticicumulans | 3.09 | 3.54 | 3.52 | |
| Azospira | 0.64 | 4.58 | 0.32 | |
| A4b | 1.73 | 2.66 | 1.98 | |
| Terrimonas | 1.14 | 1.52 | 2.08 | |
| Dechloromonas | 0.65 | 1.53 | 0.89 | |
| Amaricoccus | 0.43 | 0.83 | 0.54 | |
| Azohydromonas | 0.42 | 0.69 | 0.36 | |
| Pseudoxanthomonas | 0.32 | 0.98 | 0.80 | |
| Sulfuritalea | 0.24 | 0.67 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Huang, J.; Tuo, J.; Xu, J.; Li, X. Constructed Wetlands with Novel Substrate Exposed to Nano-Plastics: Mitigating the Effects of Substrate Enzyme and Ecological Processes. Toxics 2025, 13, 800. https://doi.org/10.3390/toxics13090800
Wang L, Huang J, Tuo J, Xu J, Li X. Constructed Wetlands with Novel Substrate Exposed to Nano-Plastics: Mitigating the Effects of Substrate Enzyme and Ecological Processes. Toxics. 2025; 13(9):800. https://doi.org/10.3390/toxics13090800
Chicago/Turabian StyleWang, Luming, Juan Huang, Jing Tuo, Jin Xu, and Xinwei Li. 2025. "Constructed Wetlands with Novel Substrate Exposed to Nano-Plastics: Mitigating the Effects of Substrate Enzyme and Ecological Processes" Toxics 13, no. 9: 800. https://doi.org/10.3390/toxics13090800
APA StyleWang, L., Huang, J., Tuo, J., Xu, J., & Li, X. (2025). Constructed Wetlands with Novel Substrate Exposed to Nano-Plastics: Mitigating the Effects of Substrate Enzyme and Ecological Processes. Toxics, 13(9), 800. https://doi.org/10.3390/toxics13090800







