Effects of Nitrogen and Phosphorus on Estuarine Phytoplankton Communities in Aquatic Microcosms
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Aquatic Microcosm Systems
2.2. Monitoring of Water Quality Parameters
2.3. Phytoplankton Sampling and Identification Methods
2.4. Statistical Methods and Data Analysis
2.4.1. Assessment of Phytoplankton Community Abundance and Diversity
- (1)
- Phytoplankton abundance
- (2)
- Diversity of the phytoplankton community
2.4.2. Statistical Methods
- (1)
- Principal Response Curve analysis (PRC)
- (2)
- Threshold Species Indicator Method (TITAN)
3. Results
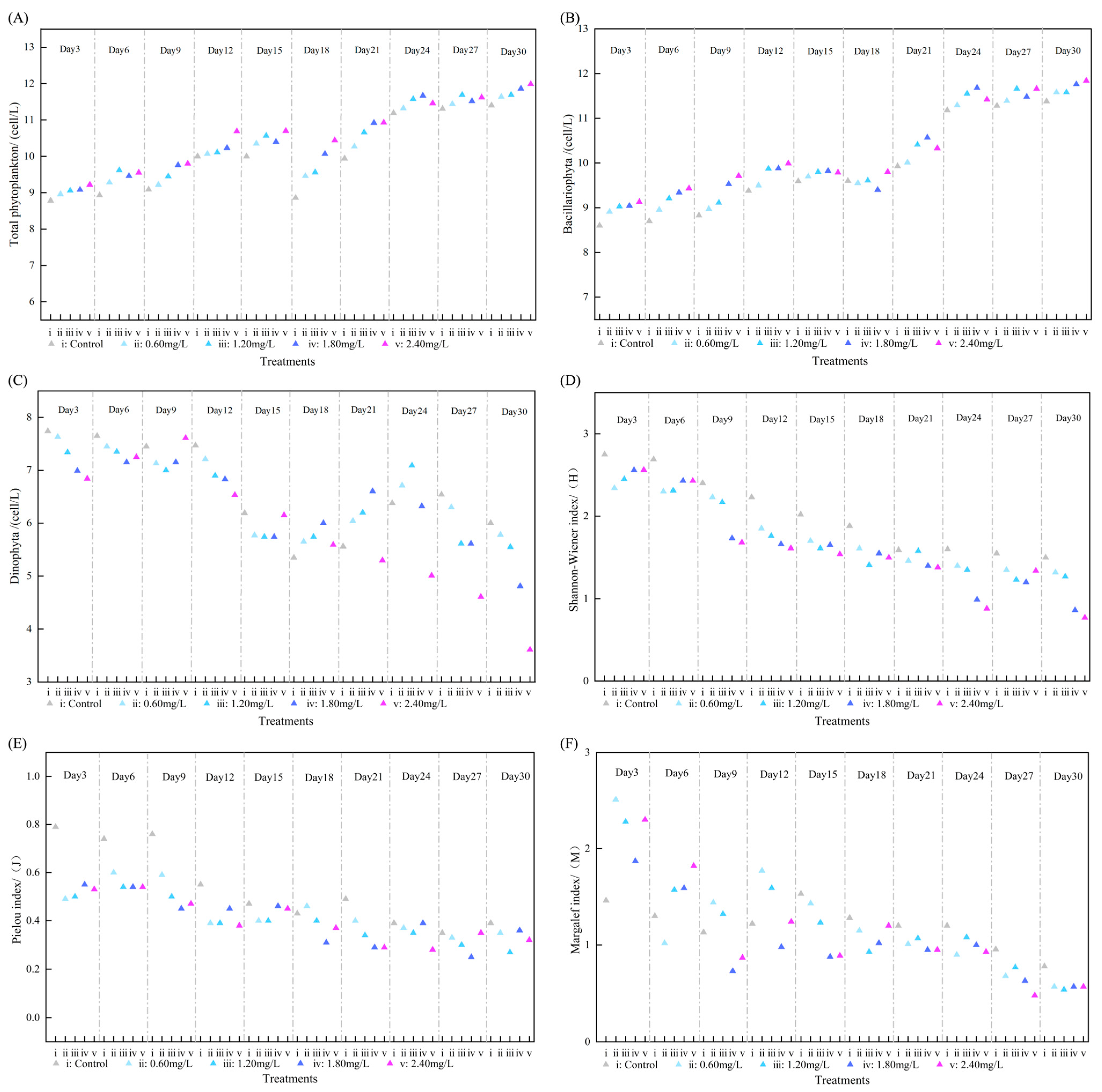
3.1. Community Effects of DIN on Phytoplankton
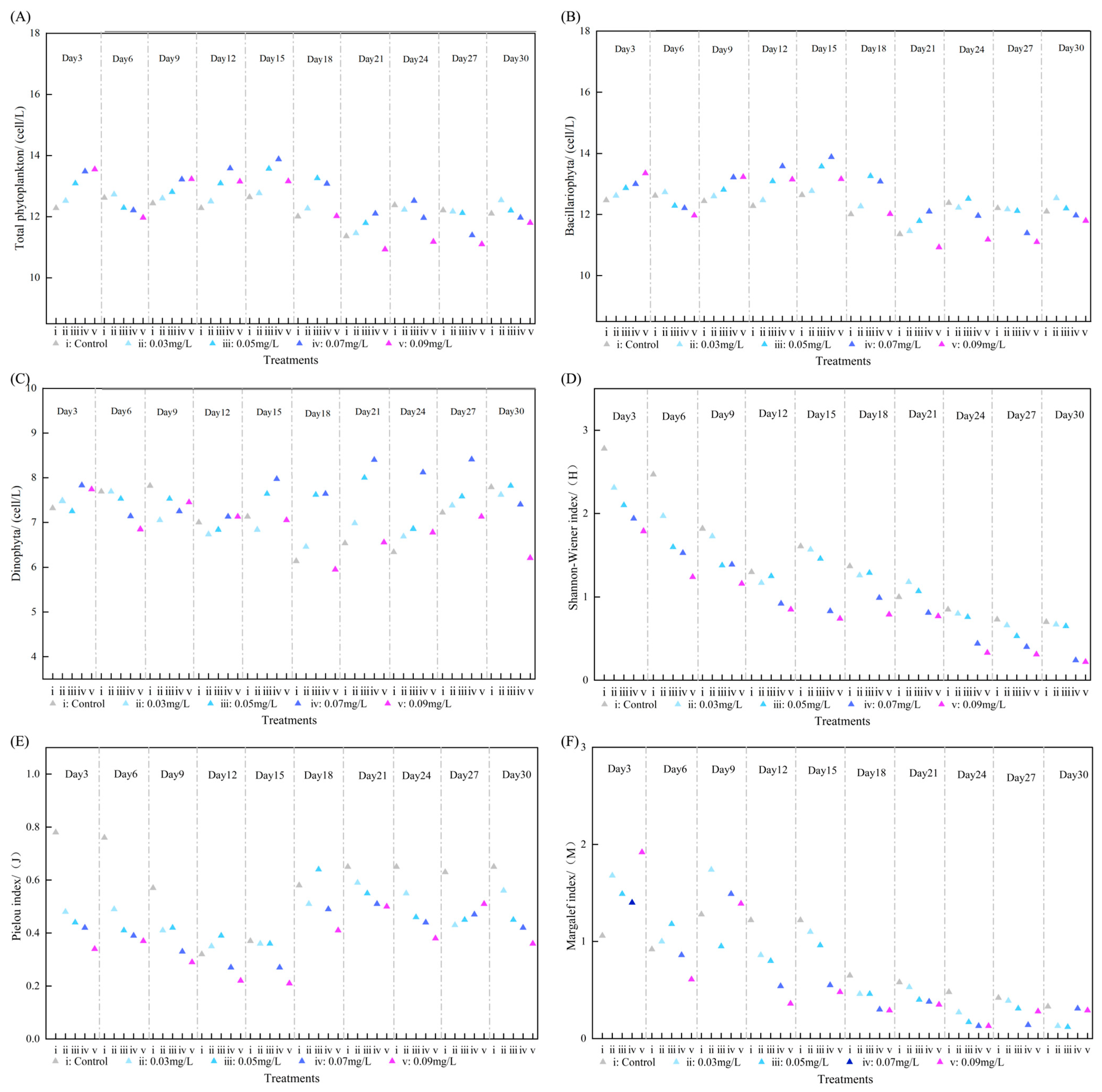
3.2. Community Effects of SRP on Phytoplankton
3.3. Effects of DIN on Phytoplankton Community Structure
3.4. Effects of SRP on Phytoplankton Community Structure
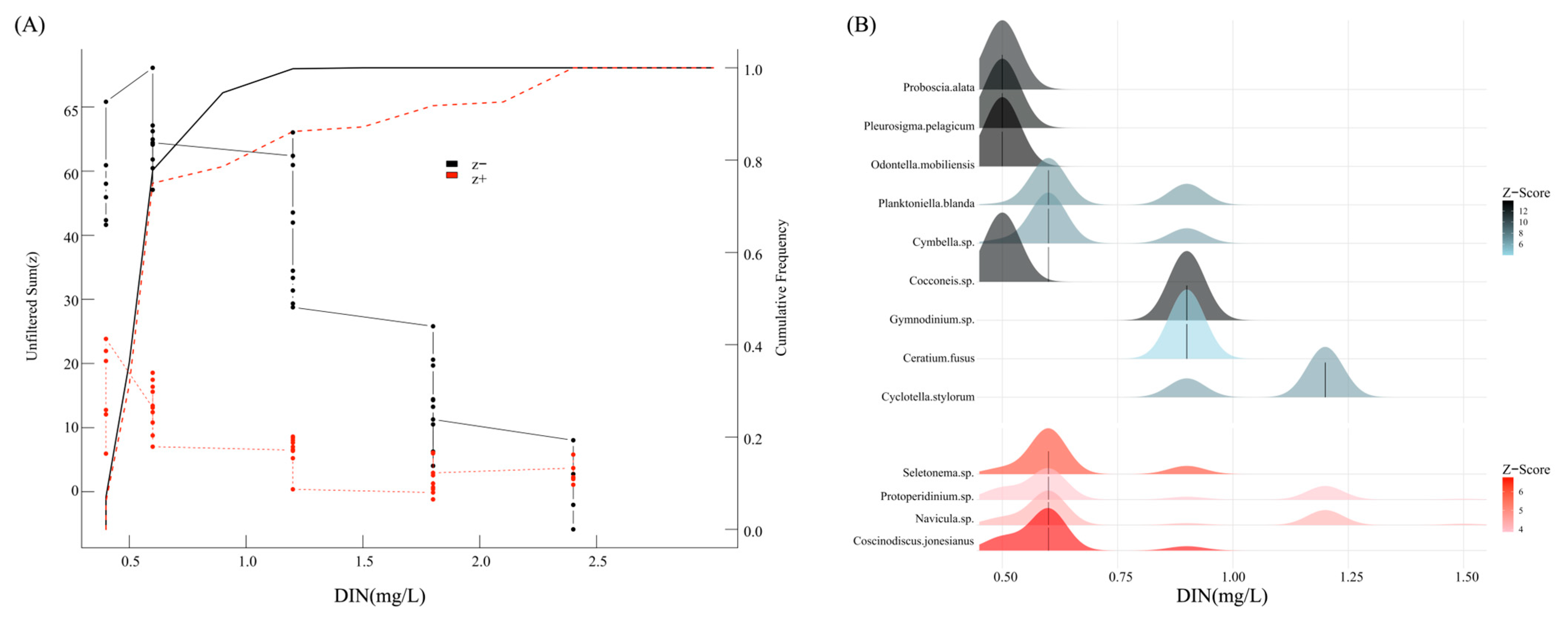
3.5. Ecological Response Thresholds of DIN in Microcosm
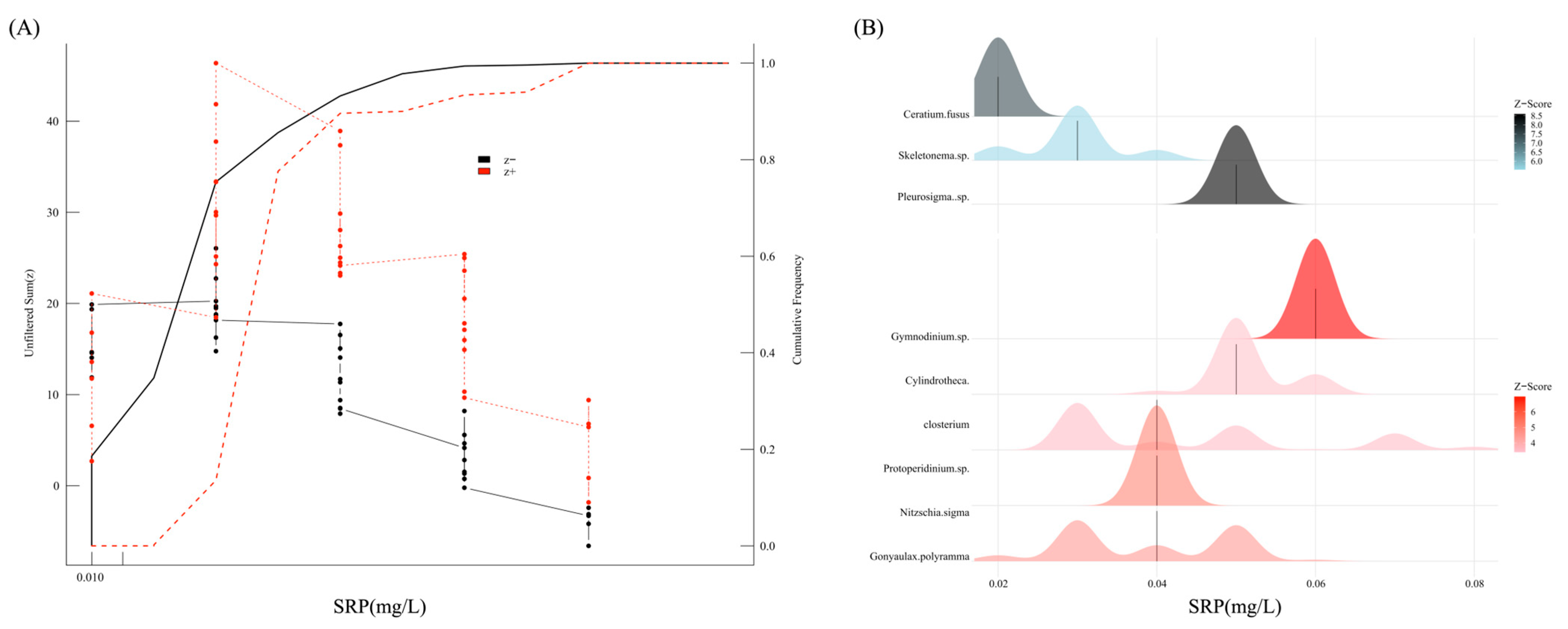
3.6. Ecological Response Thresholds of SRP in Microcosm
4. Discussion
4.1. Phosphorus-Induced Shift from Diatoms to Dinoflagellates
4.2. Ecological Response Thresholds Lower than Stress-Response Values
4.3. Strengths, Limitations, and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havens, K.E. Lake Eutrophication and Plankton Food Webs. In Eutrophication: Causes, Consequences and Control; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 73–80. [Google Scholar]
- Zhou, Y.; Wang, L.; Zhou, Y.; Mao, X. Eutrophication Control Strategies for Highly Anthropogenic Influenced Coastal Waters. Sci. Total Environ. 2020, 705, 135760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, S.; Yan, Z.; Zhao, X.; Feng, M.; Wang, J.; Zhou, Q. eDNA of Zooplankton Reveals the Ecological Community Thresholds for Key Environmental Factors in the Baiyangdian Lake Aquatic Ecosystem. Environ. Sci. Eur. 2023, 35, 56. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Yang, Q.; Liu, Y.; Fan, J.; Guo, H. Disentangling Effects of River Inflow and Marine Diffusion in Shaping the Planktonic Communities in a Heavily Polluted Estuary. Environ. Pollut. 2020, 267, 115414. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, J.; Jiang, D.; Sun, J.; Huang, Y.; Luan, S. Establishment of Stream Nutrient Criteria by Comparing Reference Conditions with Ecological Thresholds in a Typical Eutrophic Lake Basin. Environ. Sci. Process. Impacts 2017, 19, 1554–1562. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Li, Y.; Huang, Y.; Wei, J.; Norgbey, E.; Lai, Q.; Sarpong, L.; Wang, K.; Ji, D.; Yang, Z. Nutrient Addition Bioassay and Phytoplankton Community Structure Monitored during Autumn in Xiangxi Bay of Three Gorges Reservoir, China. Chemosphere 2020, 247, 125960. [Google Scholar] [CrossRef]
- Shi, F.; Wei, X.; Feng, J.; Zhu, L. Nitrogen Uptake Kinetics of a Diatom and Model Prediction Analysis in Different Inorganic Nitrogen Conditions. J. Agro-Environ. Sci. 2018, 37, 1833–1841. [Google Scholar]
- Bai, X.; Jiang, Z.; Fang, Y.; Zhu, L.; Feng, J. Effects of Environmental Concentrations of Total Phosphorus on the Plankton Community Structure and Function in a Microcosm Study. Int. J. Environ. Res. Public Health 2022, 19, 8412. [Google Scholar] [CrossRef]
- Qing, H.; Tao, H.; Zhang, J.; Zhang, X.; Song, X.; GU, W.; Wang, K.; Gai, Y. Effects of Multiple Environmental Factors Such as Nutrients and Heavy Metals on Phytoplankton inLaizhou Bay. Trans. Oceanol. Limnol. 2023, 45, 134–141. [Google Scholar]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L. Marine Harmful Algal Blooms (HABs) in the United States: History, Current Status and Future Trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Ding, Y.; Qin, B.; Deng, J.; Ma, J. Effects of Episodic Sediment Resuspension on Phytoplankton in Lake Taihu: Focusing on Photosynthesis, Biomass and Community Composition. Aquat. Sci. 2017, 79, 617–629. [Google Scholar] [CrossRef]
- Wu, P.; Lu, Y.; Lu, Y.; Dai, J.; Huang, T. Response of the Photosynthetic Activity and Biomass of the Phytoplankton Community to Increasing Nutrients during Cyanobacterial Blooms in Meiliang Bay, Lake Taihu. Water Environ. Res. 2020, 92, 138–148. [Google Scholar] [CrossRef]
- Zhou, Q.; Kong, F.; Zhu, L. Ecotoxicology; Science Press: Beijing, China, 2004. [Google Scholar]
- Li, W.; Yu, H.; Rittmann, B. Chemistry: Reuse Water Pollutants. Nature 2015, 528, 29–31. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, X.Y.; Zeng, J.N.; Shou, L.; Chen, Q.Z.; Jiang, Z.B. Microcosm Experiments on the Influence of Different N/P Ratios on Phytoplankton Community Growth in the East China Sea. Huanjing Kexue 2012, 33, 1832–1838. [Google Scholar] [PubMed]
- Liu, H.; Zhou, Y.; Xiao, W.; Ji, L.; Cao, X.; Song, C. Shifting Nutrient-Mediated Interactions between Algae and Bacteria in a Microcosm: Evidence from Alkaline Phosphatase Assay. Microbiol. Res. 2012, 167, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tsiola, A.; Pitta, P.; Fodelianakis, S.; Pete, R.; Magiopoulos, I.; Mara, P.; Psarra, S.; Tanaka, T.; Mostajir, B. Nutrient Limitation in Surface Waters of the Oligotrophic Eastern Mediterranean Sea: An Enrichment Microcosm Experiment. Microb. Ecol. 2016, 71, 575–588. [Google Scholar] [CrossRef]
- Baker, M.E.; King, R.S. A New Method for Detecting and Interpreting Biodiversity and Ecological Community Thresholds. Methods Ecol. Evol. 2010, 1, 25–37. [Google Scholar] [CrossRef]
- Hu, X.; Zuo, D.; Xu, Z.; Huang, Z.; Liu, B.; Han, Y.; Bi, Y. Response of Macroinvertebrate Community to Water Quality Factors and Aquatic Ecosystem Health Assessment in a Typical River in Beijing, China. Environ. Res. 2022, 212, 113474. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Liao, J.; Sun, J.; Huang, Y. The Threshold Responses of Phytoplankton Community to Nutrient Gradient in a Shallow Eutrophic Chinese Lake. Ecol. Indic. 2016, 61, 258–267. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Qian, T.; Yin, X.; Ruan, R. Quantitative Analysis of Nitrogen and Phosphorus Nutrients for Periphyton Population in Taizi River. Asian J. Ecotoxicol. 2019, 14, 104–117. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, G.; Li, J.; Zhao, Y.; Yang, W. Ecological Threshold of Phosphorus Load in Baiyangdian Lake Based on a PCLake Model and Ecological Network Analysis. Sci. Total Environ. 2024, 915, 170091. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, P.X.; Zhou, H.H.; Xia, L.H. Evaluation of the Biodiversity Variation and Ecosystem Health Assessment in Changjiang Estuary during the Past 15 Years. Acta Ecol. Sin. 2020, 40, 8892–8904. [Google Scholar]
- GB 17378.4-1998; Specification for Marine Monitoring—Part 4: Seawater Analysis. China Standards Press: Beijing, China, 1998.
- GB 3097-1997; Chinese Seawater Quality Requirements. China Standards Press: Beijing, China, 1997.
- Ministry of Ecology and Environment. Water Quality-Determination of Chlorophyll a-Spectrophotometric Method; China Environmental Science Press: Beijing, China, 2017; pp. 3–4.
- Zhang, Z. Microcosm Construction Centered on Silver Carp, Bighead Carp and Xenocypris spp. and the Migration and Transformation of Nitrogen and Phosphorus; Nanjing University of Information Science & Technology: Nanjing, China, 2021. [Google Scholar]
- Van den Brink, P.J.; Hattink, J.; Bransen, F.; Van Donk, E.; Brock, T.C. Impact of the Fungicide Carbendazim in Freshwater Microcosms. II. Zooplankton, Primary Producers and Final Conclusions. Aquat. Toxicol. 2000, 48, 251–264. [Google Scholar] [CrossRef]
- Van den Brink, P.J.; Ter Braak, C.J. Multivariate Analysis of Stress in Experimental Ecosystems by Principal Response Curves and Similarity Analysis. Aquat. Ecol. 1998, 32, 163–178. [Google Scholar] [CrossRef]
- Bai, X.; Jiang, Y.; Jiang, Z.; Zhu, L.; Feng, J. Nutrient Potentiate the Responses of Plankton Community Structure and Metabolites to Cadmium: A Microcosm Study. J. Hazard. Mater. 2022, 430, 128506. [Google Scholar] [CrossRef]
- Gowen, R.; Collos, Y.; Tett, P.; Scherer, C.; Bec, B.; Abadie, E.; Allen, M.; O’brien, T. Response of Diatom and Dinoflagellate Lifeforms to Reduced Phosphorus Loading: A Case Study in the Thau Lagoon, France. Estuar. Coast. Shelf Sci. 2015, 162, 45–52. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, G.; Wan, A.; Zhao, Z.; Wang, S.; Liu, Q. Nutrient-Limitation Induced Diatom-Dinoflagellate Shift of Spring Phytoplankton Community in an Offshore Shellfish Farming Area. Mar. Pollut. Bull. 2019, 141, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Ling, J.; Jiang, Z.; Huang, W.; Shou, L.; Zeng, J. Distribution and diversityof net-collected phytoplankton in the waters nearbyZhoushan Islands. J. Mar. Sci. 2016, 34, 76–83. [Google Scholar]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gaoa, G. Nitrogen and Phosphorus Inputs Control Phytoplankton Growth in Eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Park, S.; Choi, C.; Kim, B.; Kim, J. Landslide Susceptibility Mapping Using Frequency Ratio, Analytic Hierarchy Process, Logistic Regression, and Artificial Neural Network Methods at the Inje Area, Korea. Environ. Earth Sci. 2013, 68, 1443–1464. [Google Scholar] [CrossRef]
- Warren, D.B.; Bergstrom, C.A.; Benameur, H.; Porter, C.J.; Pouton, C.W. Evaluation of the Structural Determinants of Polymeric Precipitation Inhibitors Using Solvent Shift Methods and Principle Component Analysis. Mol. Pharm. 2013, 10, 2823–2848. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Yang, S.; Zhao, D.; Hu, C.; Xu, W.; Anderson, D.M.; Li, Y.; Song, X.-P.; Boyce, D.G.; Gibson, L. Coastal Phytoplankton Blooms Expand and Intensify in the 21st Century. Nature 2023, 615, 280–284. [Google Scholar] [CrossRef]
- Jiang, M.; Nakano, S. The Crucial Influence of Trophic Status on the Relative Requirement of Nitrogen to Phosphorus for Phytoplankton Growth. Water Res. 2022, 222, 118868. [Google Scholar] [CrossRef]
- You, Y.; Sun, X.; Ma, M.; He, J.; Li, L.; Porto, F.W.; Lin, S. Trypsin Is a Coordinate Regulator of N and P Nutrients in Marine Phytoplankton. Nat. Commun. 2022, 13, 4022. [Google Scholar] [CrossRef]
- Li, X. The Impact of Nitrogen and Phosphorus Nutrients on the Community Structure of Phytoplankton in Jiaozhou Bay; Shanghai Ocean University: Shanghai, China, 2021. [Google Scholar]
- Lü, J.; Zhang, G.; Zhao, Z. Seawater Silicate Fertilizer Facilitated Nitrogen Removal via Diatom Proliferation. Mar. Pollut. Bull. 2020, 157, 111331. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, K.; Li, M.; Jiang, H.; Gao, W.; Zhao, J.; Li, K. Diatom-Dinoflagellate Succession in the Bohai Sea: The Role of N/P Ratios and Dissolved Organic Nitrogen Components. Water Res. 2024, 251, 121150. [Google Scholar] [CrossRef]
- Hou, Y.; Zheng, X.; Liu, X.; Ling, J.; Wei, C.; Zhao, Z. Typical Plankton Toxicity and Water Quality Criteria Study of Short-Chain Chlorinated Paraffins. Res. Environ. Sci. 2023, 36, 1435. [Google Scholar]
- Cai, Y. Research on the Biodiversity of Phytoplankton in Hangzhou Bay; Ocean University of China: Dalian, China, 2006. [Google Scholar]
- Lai, J.; Yu, Z.; Song, X.; Han, X.; Cao, X.; Yuan, Y. Phytoplankton Pigment Patterns and Community Structure in the Yangtze Estuary and Its Adjacent Areas. Huanjing Kexue 2013, 34, 3405–3415. [Google Scholar] [PubMed]
- Pinckney, J.L.; Knotts, E.R.; Kibler, K.J.; Smith, E.M. Nutrient Breakpoints for Estuarine Phytoplankton Communities. Limnol. Oceanogr. 2020, 65, 2999–3016. [Google Scholar] [CrossRef]
- Xiang, C.; Li, Y.; Ke, Z.; Li, G.; Huang, Y.; Su, X.; Huang, L.; Song, X. Effects of Daily Nitrogen and Phosphorus Input on Planktonic Community Metabolism in a Semi-Enclosed Bay by Mesocosm Experiment. Acta Oceanol. Sin. 2022, 41, 99–110. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Lee, J.-S.; Kim, H.C.; Lee, W.C.; Shin, K.-H. Algal Photosynthetic Responses to Toxic Metals and Herbicides Assessed by Chlorophyll a Fluorescence. Ecotoxicol. Environ. Saf. 2014, 104, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Van der Plas, F. Biodiversity and Ecosystem Functioning in Naturally Assembled Communities. Biol. Rev. 2019, 94, 1220–1245. [Google Scholar] [CrossRef] [PubMed]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Optimal Nitrogen-to-Phosphorus Stoichiometry of Phytoplankton. Nature 2004, 429, 171–174. [Google Scholar] [CrossRef]
- Lomas, M.W.; Baer, S.E.; Mouginot, C.; Terpis, K.X.; Lomas, D.A.; Altabet, M.A.; Martiny, A.C. Varying Influence of Phytoplankton Biodiversity and Stoichiometric Plasticity on Bulk Particulate Stoichiometry across Ocean Basins. Commun. Earth Environ. 2021, 2, 143. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, W.; Zeng, J.; Jiang, Z.; Liu, J.; Xu, X.; Chen, Q. Effects of Nitrogen and Phosphorus Ratios on Phytoplankton Community Structure in Winter. Chin. J. Appl. Environ. Biol. 2013, 19, 293–299. [Google Scholar]
- Guildford, S.J.; Hecky, R.E. Total Nitrogen, Total Phosphorus, and Nutrient Limitation in Lakes and Oceans: Is There a Common Relationship? Limnol. Oceanogr. 2000, 45, 1213–1223. [Google Scholar] [CrossRef]
- Wei, C.; Ling, J.; Yan, Z.; An, L.; Deng, B.; Ou, M.; Zheng, X. Research on Nutritional Criteria of Dissoloved Inorganic Nitrogen and Active Phosphate in Hangzhou Bay. Res. Environ. Sci. 2024, 37, 1412. [Google Scholar]
- Xiao, W.; Liu, X.; Irwin, A.J.; Laws, E.A.; Wang, L.; Chen, B.; Zeng, Y.; Huang, B. Warming and Eutrophication Combine to Restructure Diatoms and Dinoflagellates. Water Res. 2018, 128, 206–216. [Google Scholar] [CrossRef]
- Pan, J.; Yang, Y.; Han, B. Responses of Phytoplankton Communities to N/P Ratio Changes in Phosphorus-Limited Subtropical Reservoir in Southern China. J. Trop. Subtrop. Bot. 2021, 29, 20–30. [Google Scholar]
- Chen, W.; Guo, F.; Huang, W.; Wang, J.; Zhang, M.; Wu, Q. Advances in Phytoplankton Population Ecology in the Pearl River Estuary. Front. Environ. Sci. 2023, 11, 1084888. [Google Scholar] [CrossRef]
- Cheng, P.; Meng, F.; Wang, Y.; Zhang, L.; Yang, Q.; Xue, H. Thresholds of Water Quality Indicators for Macroinvertebrate in Different Topographic Zones of Songhua River Basin. Res. Environ. Sci. 2020, 33, 2061. [Google Scholar]
- Wei, Y.; Luan, Q.; Shan, X.; Cui, H.; Qu, K.; Cui, Z.; Sun, J. Temperature and Nutrients Drive Distinct Successions between Diatoms and Dinoflagellates over the Past 40 Years: Implications for Climate Warming and Eutrophication. Sci. Total Environ. 2024, 931, 172997. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Song, J.; Xing, J.; Li, X.; Li, N.; Duan, L.; Qu, B.; Wang, Q. Spatial and Seasonal Variations, Partitioning and Fluxes of Dissolved and Particulate Nutrients in Jiaozhou Bay. Cont. Shelf Res. 2018, 171, 140–149. [Google Scholar] [CrossRef]
- Chan, F.; Marino, R.L.; Howarth, R.W.; Pace, M.L. Ecological Constraints on Planktonic Nitrogen Fixation in Saline Estuaries. II. Grazing Controls on Cyanobacterial Population Dynamics. Mar. Ecol. Prog. Ser. 2006, 309, 41–53. [Google Scholar] [CrossRef]
- Armitage, A.R.; Gonzalez, V.L.; Fong, P. Decoupling of Nutrient and Grazer Impacts on a Benthic Estuarine Diatom Assemblage. Estuar. Coast. Shelf Sci. 2009, 84, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Browning, T.J.; Moore, C.M. Global Analysis of Ocean Phytoplankton Nutrient Limitation Reveals High Prevalence of Co-Limitation. Nat. Commun. 2023, 14, 5014. [Google Scholar] [CrossRef]
- Fernández-González, C.; Tarran, G.A.; Schuback, N.; Woodward, E.M.S.; Arístegui, J.; Marañón, E. Phytoplankton Responses to Changing Temperature and Nutrient Availability Are Consistent across the Tropical and Subtropical Atlantic. Commun. Biol. 2022, 5, 1035. [Google Scholar] [CrossRef]



| Experimental Group | DIN (mg/L) | SRP (mg/L) |
|---|---|---|
| Control group 0.4 mg/L | 0.4 mg/L | 0.010 mg/L |
| DIN 0.6 mg/L | 0.6 mg/L | |
| DIN 1.2 mg/L | 1.2 mg/L | |
| DIN 1.8 mg/L | 1.8 mg/L | |
| DIN 2.4 mg/L | 2.4 mg/L |
| Experimental Group | SRP (mg/L) | DIN (mg/L) |
|---|---|---|
| Control group 0.010 mg/L | 0.010 mg/L | 0.40 mg/L |
| SRP 0.030 mg/L | 0.030 mg/L | |
| SRP 0.050 mg/L | 0.050 mg/L | |
| SRP 0.070 mg/L | 0.070 mg/L | |
| SRP 0.090 mg/L | 0.090 mg/L |
| Nutrient | Negative Response Threshold (mg/L) | Positive Response Threshold (mg/L) | Ecological Response Threshold (mg/L) |
|---|---|---|---|
| DIN | 0.50 | 0.60 | 0.50 |
| SRP | 0.030 | 0.040 | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, J.; Wei, C.; Yang, D.; Zeng, J.; Cheng, F.; Zheng, X.; Yang, Z. Effects of Nitrogen and Phosphorus on Estuarine Phytoplankton Communities in Aquatic Microcosms. Toxics 2025, 13, 798. https://doi.org/10.3390/toxics13090798
Ling J, Wei C, Yang D, Zeng J, Cheng F, Zheng X, Yang Z. Effects of Nitrogen and Phosphorus on Estuarine Phytoplankton Communities in Aquatic Microcosms. Toxics. 2025; 13(9):798. https://doi.org/10.3390/toxics13090798
Chicago/Turabian StyleLing, Jianan, Chao Wei, Dongning Yang, Jiangning Zeng, Fangping Cheng, Xin Zheng, and Zhanhong Yang. 2025. "Effects of Nitrogen and Phosphorus on Estuarine Phytoplankton Communities in Aquatic Microcosms" Toxics 13, no. 9: 798. https://doi.org/10.3390/toxics13090798
APA StyleLing, J., Wei, C., Yang, D., Zeng, J., Cheng, F., Zheng, X., & Yang, Z. (2025). Effects of Nitrogen and Phosphorus on Estuarine Phytoplankton Communities in Aquatic Microcosms. Toxics, 13(9), 798. https://doi.org/10.3390/toxics13090798






