Health Symptoms Related to Polycyclic Aromatic Hydrocarbon (PAH) Exposure in Chiang Mai, Thailand: Associations with Biomarkers of Exposure and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethical Approval and Consent
2.3. Questionnaire and Data Collection
2.4. PM2.5 Exposure Measurement
2.5. Biomarker Collection and Laboratory Analysis
2.5.1. Urine Sampling and 1-OHP Measurement
2.5.2. Serum CC16 and 8-iso-PGF2α
2.6. Statistical Analysis
3. Results
3.1. Demographic and Exposure Characteristics of Participants
3.2. Associations Between Participant Characteristics and Biomarker Levels
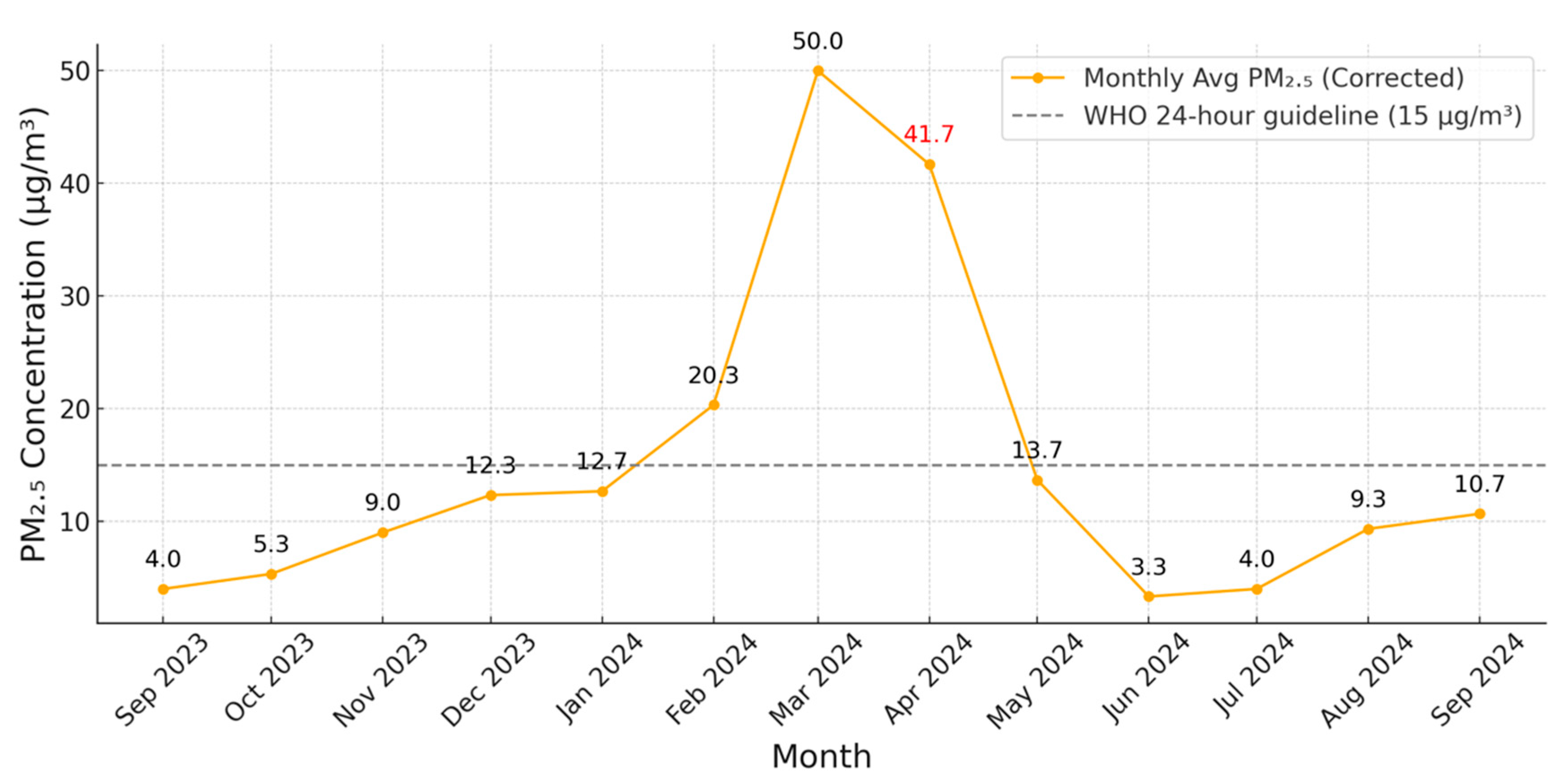
3.3. Monthly Variation in PM2.5 Concentrations (September 2023–September 2024)
3.4. Associations Between Biomarker Levels and Self-Reported Symptoms
3.5. Adjusted Associations Between Self-Reported Respiratory Symptoms and Biomarker Levels (CC16, 8-iso-PGF2α, and 1-OHP)
3.6. Association Between Respiratory Rate and CC16 After Adjustment for Covariates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PM2.5 | Particulate Matter with an aerodynamic diameter ≤ 2.5 µm |
| 1-OHP | 1-Hydroxypyrene |
| CC16 | Club Cell Secretory Protein 16 |
| 8-iso-PGF2α | 8-iso-Prostaglandin F2α |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HPLC | High-Performance Liquid Chromatography |
| BMI | Body Mass Index |
| NTAQHI | Northern Thailand Air Quality Health Index |
References
- Carpentieri, M.; Hayden, P.; Robins, A.G. Wind tunnel measurements of pollutant turbulent fluxes in urban intersections. Atmos. Environ. 2012, 46, 669–674. [Google Scholar] [CrossRef]
- Yang, C.; Meng, X.; Chen, R.; Cai, J.; Zhao, Z.; Wan, Y.; Kan, H. Long-term variations in the association between ambient temperature and daily cardiovascular mortality in Shanghai, China. Sci. Total Environ. 2015, 538, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Inlaung, K.; Chotamonsak, C.; Macatangay, R.; Surapipith, V. Assessment of Transboundary PM2.5 from Biomass Burning in Northern Thailand Using the WRF-Chem Model. Toxics 2024, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2. 5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- John, E.M.; Keegan, T.H.; Terry, M.B.; Koo, J.; Ingles, S.A.; Nguyen, J.T.; Thomsen, C.; Santella, R.M.; Nguyen, K.; Yan, B. Urinary biomarkers of polycyclic aromatic hydrocarbons and timing of pubertal development: The California PAH study. Epidemiology 2022, 33, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.L.; Balmes, J.R.; Lutzker, L.; Mann, J.K.; Margolis, H.G.; Tyner, T.; Holland, N.; Noth, E.M.; Lurmann, F.; Hammond, S.K. Traffic-related air pollution, biomarkers of metabolic dysfunction, oxidative stress, and CC16 in children. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 530–537. [Google Scholar] [CrossRef]
- Woo, S.-D.; Park, H.S.; Yang, E.-M.; Ban, G.-Y.; Park, H.-S. 8-Iso-prostaglandin F2α as a biomarker of type 2 low airway inflammation and remodeling in adult asthma. Ann. Allergy Asthma Immunol. 2024, 133, 73–80.e2. [Google Scholar] [CrossRef]
- Kausar, S.; Cao, X.; Yadoung, S.; Wongta, A.; Zhou, K.; Kosashunhanan, N.; Hongsibsong, S. Associations Between Individual Health Risk Perceptions and Biomarkers of PAH Exposure Before and After PM2.5 Pollution in the Suburbs of Chiang Mai Province. Toxics 2025, 13, 491. [Google Scholar] [CrossRef]
- Sutan, K.; Naksen, W.; Prapamontol, T. A simple high-performance liquid chromatography coupled to fluorescence detection method using column-switching technique for measuring urinary 1-hydroxypyrene from environmental exposure. Chiang Mai J. Sci. 2017, 44, 1441–1452. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.3.2; R Foundation for Statistical Computing: Vienna, Austria, 2025.
- Sutherland, C.; Hare, D.; Johnson, P.J.; Linden, D.W.; Montgomery, R.A.; Droge, E. Practical advice on variable selection and reporting using Akaike information criterion. Proc. R. Soc. B 2023, 290, 20231261. [Google Scholar] [CrossRef]
- Suriyawong, P.; Chuetor, S.; Samae, H.; Piriyakarnsakul, S.; Amin, M.; Furuuchi, M.; Hata, M.; Inerb, M.; Phairuang, W. Airborne particulate matter from biomass burning in Thailand: Recent issues, challenges, and options. Heliyon 2023, 9, e14261. [Google Scholar] [CrossRef]
- World Health Organization. WHO Ambient Air Quality Database: 2022 Update. Status Report; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Sooktawee, S.; Kanchanasuta, S.; Bunplod, N. Assessment of 24-h moving average PM2.5 concentrations in Bangkok, Thailand against WHO guidelines. Sustain. Environ. Res. 2023, 33, 3. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, C.-W.; Lee, H.-L.; Wu, C.; Wang, C.; Sung, F.-C.; Su, T.-C. Global DNA methylation mediates the association between urine mono-2-ethylhexyl phthalate and serum apoptotic microparticles in a young Taiwanese population. Sci. Total Environ. 2022, 808, 152054. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Lv, S.; Liu, Y.; Li, Y. Biomarkers for the adverse effects on respiratory system health associated with atmospheric particulate matter exposure. J. Hazard. Mater. 2022, 421, 126760. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Liu, H.-Y.; He, Q.-Y.; Liu, Y.; Lv, L.-P.; Fei, J.; Fu, L. Cobalt exposure and pulmonary function reduction in chronic obstructive pulmonary disease patients: The mediating role of club cell secretory protein. Respir. Res. 2024, 25, 324. [Google Scholar] [CrossRef]
- López Valdez, N.; Rojas Lemus, M.; Bizarro-Nevares, P.; González Villalva, A.E.; Casarrubias Tabarez, B.; Cervantes Valencia, M.E.; Ustarroz-Cano, M.; Morales Ricardes, G.; Mendoza Martínez, S.; Guerrero Palomo, G. The multiple facets of the club cell in the pulmonary epithelium. Histol Histopathol. 2024, 39, 969–982. [Google Scholar]
- Rostami, M.R.; LeBlanc, M.G.; Strulovici-Barel, Y.; Zuo, W.; Mezey, J.G.; O’Beirne, S.L.; Kaner, R.J.; Leopold, P.L.; Crystal, R.G. Smoking shifts human small airway epithelium club cells toward a lesser differentiated population. NPJ Genom. Med. 2021, 6, 73. [Google Scholar]
- Jung, C.G.; Cao, T.B.T.; Quoc, Q.L.; Yang, E.M.; Ban, G.Y.; Park, H.S. Role of club cell 16-kDa secretory protein in asthmatic airways. Clin. Exp. Allergy 2023, 53, 648–658. [Google Scholar]
- Nauwelaerts, S.J.; Van Goethem, N.; Ureña, B.T.; De Cremer, K.; Bernard, A.; Saenen, N.D.; Nawrot, T.S.; Roosens, N.H.; De Keersmaecker, S.C. Urinary CC16, a potential indicator of lung integrity and inflammation, increases in children after short-term exposure to PM2.5/PM10 and is driven by the CC16 38GG genotype. Environ. Res. 2022, 212, 113272. [Google Scholar]
- Neumann, S.; Casjens, S.; Hoffmeyer, F.; Rühle, K.; Gamrad-Streubel, L.; Haase, L.-M.; Rudolph, K.K.; Giesen, J.; Neumann, V.; Taeger, D. Club cell protein (CC16) in serum as an effect marker for small airway epithelial damage caused by diesel exhaust and blasting fumes in potash mining. Int. Arch. Occup. Environ. Health 2024, 97, 121–132. [Google Scholar] [CrossRef]
- Laing, I.A. CC16: A Biomarker of Pollutant Exposure and Future Lung Disease? Am. J. Respir. Crit. Care Med. 2019, 200, 529–530. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, G.-D. Particulate matter-induced emerging health effects associated with oxidative stress and inflammation. Antioxidants 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Hongsibsong, S.; Chuljerm, H.; Parklak, W.; Ounjaijean, S.; Fakfum, P.; Kausar, S.; Kulprachakarn, K. Assessment of urinary oxidative stress biomarkers associated with fine particulate matter (PM2.5) exposure in Chiang Mai, Thailand. PeerJ 2025, 13, e19047. [Google Scholar] [CrossRef] [PubMed]
- Elfsmark, L.; Ågren, L.; Akfur, C.; Bucht, A.; Jonasson, S. 8-Isoprostane is an early biomarker for oxidative stress in chlorine-induced acute lung injury. Toxicol. Lett. 2018, 282, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.H.; El-Emshaty, H.M.; Alamodi, H.S.; Nasif, W.A. The activity of serum 8-iso-prostaglandin F2α as oxidative stress marker in patients with diabetes mellitus type 2 and associated dyslipidemic hyperglycemia. J. Diabetes Mellit. 2016, 6, 318. [Google Scholar]
- Ma, N.; Zhang, Y.; Liu, B.; Jia, X.; Wang, R.; Lu, Q. Associations of plasma 8-iso-prostaglandin F2α levels with fasting blood glucose (FBG) and intra-abdominal fat (IAF) area in various Glycometabolism populations. BMC Endocr. Disord. 2021, 21, 215. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; Rafieian-Kopaei, M. Oxidative stress and antioxidants in diabetes mellitus. Asian Pac. J. Trop. Med. 2020, 13, 431–438. [Google Scholar] [CrossRef]
- Gorini, F.; Sabatino, L.; Gaggini, M.; Chatzianagnostou, K.; Vassalle, C. Oxidative stress biomarkers in the relationship between type 2 diabetes and air pollution. Antioxidants 2021, 10, 1234. [Google Scholar] [CrossRef]
- Hou, G.; Dong, Y.; Jiang, Y.-H.; Wenbo, Z.; Zhou, L.; Cao, S.; Li, W. Immune Inflammation and Metabolic Interactions in the Pathogenesis of Diabetic Nephropathy. Front. Endocrinol. 2025, 16, 1602594. [Google Scholar] [CrossRef]
- Kheniser, K.; Kashyap, S.; Kasumov, T. A systematic review: The appraisal of the effects of metformin on lipoprotein modification and function. Obes. Sci. Pract. 2019, 5, 36–45. [Google Scholar] [CrossRef]

| Variables | Total (N = 127) |
|---|---|
| Age (years, mean ± SD) | 59.7 ± 7.9 |
| Sex: Male (n, %) | 94 (74.0%) |
| Sex: Female (n, %) | 33 (26.0%) |
| Smoking history: Yes (n, %) | 20 (15.7%) |
| Body Mass Index (mean ± SD) | 24.28 ± 8.14 |
| Urinary 1-OHP (μmol/mol Cre) | 0.88 ± 1.26 |
| Serum CC16 (ng/mL, mean ± SD) | 101 ± 48 |
| Serum 8-iso-PGF2α (ng/mL, mean ± SD) | 52.5 ± 20.6 |
| Variables | CC16 (ng/mL) | 8-iso-PGF2α Mean ± SD (ng/mL) | 1-OHP Mean ± SD | |||
|---|---|---|---|---|---|---|
| Mean ± SD | p Value | Mean ± SD | p Value | Mean ± SD | p Value | |
| Sex a | ||||||
| Male (74%) | 100.1 ± 39.6 | p > 0.05 | 51.3 ± 14.1 | p > 0.05 | 0.87 ± 1.15 | p > 0.05 |
| Female (26%) | 107.8 ± 67.2 | 56.0 ± 32.9 | 0.96 ± 1.39 | |||
| Age a | ||||||
| Age ≥ 60 (56.8%) | 101.1 ± 39.8 | p > 0.05 | 52.5 ± 20.5 | p > 0.05 | 0.88 ± 1.26 | p > 0.05 |
| 45 < Age < 60 (39%) | 101.3 ± 60.4 | 51.3 ± 14.3 | 0.98 ± 1.27 | |||
| Age ≤ 45 (4.2%) | 102.5 ± 22.5 | 45.6 ± 12.3 | 1.61 ± 2.31 | |||
| BMI a | ||||||
| BMI > 25 (31.4%) | 92.9 ± 40.6 | p > 0.05 | 47.9 ± 12.5 | p > 0.05 | 0.72 ± 0.81 | p > 0.05 |
| 23 < BMI < 25 (28%) | 108.8 ± 67.5 | 54.7 ± 32.2 | 0.95 ± 1.63 | |||
| BMI < 23 (50.6%) | 102.5 ± 36.1 | 54.3 ± 13.9 | 0.92 ± 1.27 | |||
| Smoke a | ||||||
| No | 96.3 ± 39.9 | p < 0.05 * | 50.4 ± 13.8 | p > 0.05 | 0.82 ± 1.18 | p > 0.05 |
| Yes | 130.0 ± 65.3 | 62.7 ± 34.1 | 1.24 ± 1.74 | |||
| Alcohol a | ||||||
| No | 101.5 ± 62.7 | p > 0.05 | 50.8 ± 12.9 | p > 0.05 | 0.91 ± 1.23 | p > 0.05 |
| Yes | 101.7 ± 37.7 | 55.4 ± 29.7 | 0.81 ± 1.33 | |||
| Symptoms | Urinary 1-OHP a (μmol/mol Cre) | CC16 b (ng/mL) | 8-iso-PGF2α c (ng/mL) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Eye irritation | 0.740 (0.550–1.080) | 0.131 | 0.989 (0.959–0.991) | 0.002 ** | 1.049 (1.012–1.091) | 0.01 ** |
| Skin irritation | 0.896 (0.647–1.255) | 0.537 | 0.988 (0.976–1.000) | 0.047 * | 1.025 (0.996–1.055) | 0.093 |
| Respiratory irritation | 0.783 (0.563–1.151) | 0.234 | 0.990 (0.978–1.002) | 0.096 | 1.031 (0.999–1.064) | 0.054 |
| Feeling short of breath | 0.880 (0.636–1.236) | 0.479 | 0.998 (0.987–1.010) | 0.749 | 0.996 (0.969–1.024) | 0.800 |
| Dizzy | 0.999 (0.700–1.426) | 0.995 | 0.990 (0.978–1.003) | 0.137 | 1.007 (0.976–1.039) | 0.647 |
| Fainting | 0.777 (0.401–1.595) | 0.526 | 0.990 (0.968–1.012) | 0.372 | 1.009 (0.958–1.062) | 0.741 |
| Loss of consciousness | 0.839 (0.475–1.527) | 0.59 | 0.981 (0.963–0.999) | 0.043 * | 1.022 (0.979–1.067) | 0.324 |
| Pneumonia | 0.841 (0.593–1.227) | 0.391 | 0.980 (0.967–0.993) | 0.002 ** | 1.039 (1.009–1.071) | 0.012 * |
| Diabetes | 1.157(0.834–1.640) | 0.364 | 1.016 (1.002–1.030) | 0.022 * | 0.965 (0.935–0.997) | 0.033 * |
| Question | CC16 | F2α | 1-OHP | |||
|---|---|---|---|---|---|---|
| Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | |
| 1. I have never had a cough | ||||||
| 0 (87.3%) | 106.4 ± 48.1 | 0.003 * | 53.4 ± 20.8 | 0.202 | 0.81 ± 1.14 | 0.159 |
| 1 (12.7%) | 65.9 ± 30.3 | 45.7 ± 17.1 | 1.27 ± 1.85 | |||
| 2. I have no phlegm in my lungs | ||||||
| 0 (94%) | 103.8 ± 47.9 | 0.152 | 53.2 ± 20.3 | 0.305 | 0.87 ± 1.24 | 0.237 |
| 1 (6%) | 60.4 ± 30.0 | 39.5 ± 23.0 | 0.79 ± 1.30 | |||
| 3. I don’t feel tight in my chest | ||||||
| 0 (96.7%) | 102.4 ± 48.1 | 0.948 | 53.2 ± 20.4 | 0.11 | 0.87 ± 1.26 | 0.925 |
| 1 (3.3%) | 68.5 ± 39.4 | 32.4 ± 14.6 | 0.67 ± 0.63 | |||
| 4. When I walk up a hill or up a flight of stairs, I can still breathe easily | ||||||
| 0 (85.6%) | 106.6 ± 48.7 | 0.001 * | 53.1 ± 21.1 | 0.449 | 0.84 ± 1.22 | 0.612 |
| 1 (14.4%) | 69.9 ± 29.8 | 48.4 ± 16.1 | 0.98 ± 1.38 | |||
| 5. I can do things at home without any restrictions | ||||||
| 0 (78.9%) | 108.5 ± 47.7 | 0.001 * | 53.2 ± 21.5 | 0.163 | 0.87 ± 1.25 | 0.833 |
| 1 (21.1%) | 74.4 ± 35.23 | 49.7 ± 16.1 | 0.85 ± 1.24 | |||
| 6. I have the confidence to go out, even though I have lung problems | ||||||
| 0 (85.6%) | 106.2 ± 49.0 | 0.069 | 53.8 ± 21.0 | 0.546 | 0.90 ± 1.31 | 0.487 |
| 1 (14.4%) | 72.2 ± 28.9 | 44.1 ± 14.7 | 0.61 ± 0.48 | |||
| 7. I sleep very well | ||||||
| 0 (78.9%) | 110.6 ± 47.7 | 0.001 * | 54.3 ± 21.1 | 0.899 | 0.89 ± 1.27 | 0.841 |
| 1 (21.2%) | 66.5 ± 31.2 | 45.4 ± 16.4 | 0.76 ± 1.16 | |||
| 8. I feel very energetic | ||||||
| 0 (77.1%) | 109.9 ± 48.1 | 0.001 * | 53.6 ± 21.6 | 0.295 | 0.89 ± 1.28 | 0.943 |
| 1 (22.9%) | 72.0 ± 35.4 | 48.4 ± 15.9 | 0.76 ± 1.13 | |||
| Study (Author, Year) | Population/Location | Biomarkers Assessed | Main Findings | Comparison with Present Study |
|---|---|---|---|---|
| Current study (2025) | 127 rural residents, Chiang Mai, Thailand | 1-OHP, CC16, 8-iso-PGF2α | CC16 ↑ in smokers; CC16 ↓ in symptomatic groups (eye irritation, pneumonia, lack of energy); 8-iso-PGF2α ↑ in pneumonia and eye irritation; 8-iso-PGF2α ↓ in diabetes | Demonstrates mixed biomarker responses under seasonal haze exposure |
| Rostami et al., 2021 [19] | Smokers, airway epithelial samples | CC16 | Smoking → CC16 ↓ due to club cell depletion | Contrasts with our CC16 ↑ in smokers, possibly reflecting compensatory secretion under haze stress |
| Tang et al., 2024 [17] | Human exposure study | CC16 | Cigarette smoke exposure → CC16 ↓ | Confirms conventional expectation, opposite to our finding |
| Jung et al., 2023 [20] | Biomass smoke exposure model | CC16 | Biomass smoke → CC16 ↑ (acute secretion under oxidative stress) | Supports our interpretation of elevated CC16 in haze-exposed smokers |
| Nauwelaerts et al., 2022 [21] | Controlled PM exposure, general population | CC16 (serum/urine) | Short-term PM2.5 exposure → rapid CC16 ↑ | Consistent with our observation that CC16 responds rapidly to exposure and symptoms |
| Neumann et al., 2024 [22] | Miners, occupational diesel/fire gas exposure | CC16 | Acute rises in serum CC16 after exposure | Parallels our CC16 elevation under haze, but we also observed depletion in symptomatic individuals |
| Laing, 2019 [23] | Longitudinal birth cohort | CC16 + lung function (FEF25–75) | Higher CC16 → better small airway function | Supports our finding of positive correlation between CC16 and respiratory effort (respiratory rate) |
| Mukhtar et al., 2016 [27] | Type 2 diabetes cohort | 8-iso-PGF2α | Diabetes → 8-iso-PGF2α ↑ | Opposite to our finding (↓ in diabetes) |
| Ma et al., 2021 [29] | Clinical cohort, China | 8-iso-PGF2α | Positive correlation with fasting glucose and visceral fat | Contrasts with our negative association in diabetes |
| Lim & Kim, 2024; [24] Sabir et al., 2025 [25] | Traffic-related PM and controlled PM2.5 exposure | 8-iso-PGF2α | PM2.5 exposure → oxidative stress ↑ (lipid peroxidation) | Supports our finding of elevated 8-iso-PGF2α in pneumonia and eye irritation |
| Elfsmark et al., 2018 [26] | Ocular effects of pollutants | Oxidative stress mechanisms | ROS → eye irritation and ocular inflammation | Mechanistic support for our observed link between 8-iso-PGF2α and eye irritation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Yadoung, S.; Tongchai, P.; Wongta, A.; Kulprachakarn, K.; Jeeno, P.; Yana, P.; Jaitham, U.; Li, W.; Zhou, K.; et al. Health Symptoms Related to Polycyclic Aromatic Hydrocarbon (PAH) Exposure in Chiang Mai, Thailand: Associations with Biomarkers of Exposure and Oxidative Stress. Toxics 2025, 13, 796. https://doi.org/10.3390/toxics13090796
Cao X, Yadoung S, Tongchai P, Wongta A, Kulprachakarn K, Jeeno P, Yana P, Jaitham U, Li W, Zhou K, et al. Health Symptoms Related to Polycyclic Aromatic Hydrocarbon (PAH) Exposure in Chiang Mai, Thailand: Associations with Biomarkers of Exposure and Oxidative Stress. Toxics. 2025; 13(9):796. https://doi.org/10.3390/toxics13090796
Chicago/Turabian StyleCao, Xianfeng, Sumed Yadoung, Phannika Tongchai, Anurak Wongta, Kanokwan Kulprachakarn, Peerapong Jeeno, Pichamon Yana, Udomsap Jaitham, Wenting Li, Kai Zhou, and et al. 2025. "Health Symptoms Related to Polycyclic Aromatic Hydrocarbon (PAH) Exposure in Chiang Mai, Thailand: Associations with Biomarkers of Exposure and Oxidative Stress" Toxics 13, no. 9: 796. https://doi.org/10.3390/toxics13090796
APA StyleCao, X., Yadoung, S., Tongchai, P., Wongta, A., Kulprachakarn, K., Jeeno, P., Yana, P., Jaitham, U., Li, W., Zhou, K., Zhang, X., Gong, J., Kosashunhanan, N., & Hongsibsong, S. (2025). Health Symptoms Related to Polycyclic Aromatic Hydrocarbon (PAH) Exposure in Chiang Mai, Thailand: Associations with Biomarkers of Exposure and Oxidative Stress. Toxics, 13(9), 796. https://doi.org/10.3390/toxics13090796







