Modulation of Detoxification, Immune, and Epigenetic Systems by Two Aryl Organophosphorus Flame Retardants During Early Development in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Flame Retardant Solutions
2.2. Animal Rearing Conditions
2.3. Experimental In Vivo Design
2.4. Expression Analysis
2.5. Baseline Activities of B-Esterases: Carboxylesterase (CE) and Acetylcholinesterase (AChE)
2.6. In Vitro Inhibition Tests of CE and AChE by Aryl-OPFRs
2.7. Statistics
3. Results
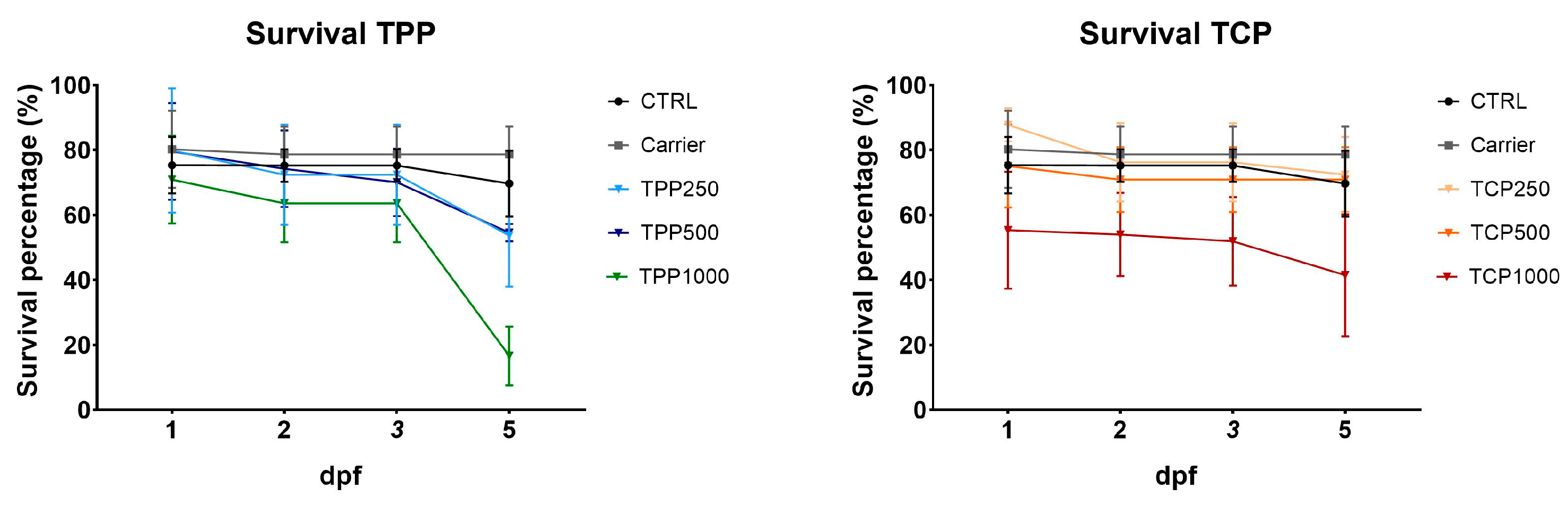
3.1. Survival Rate
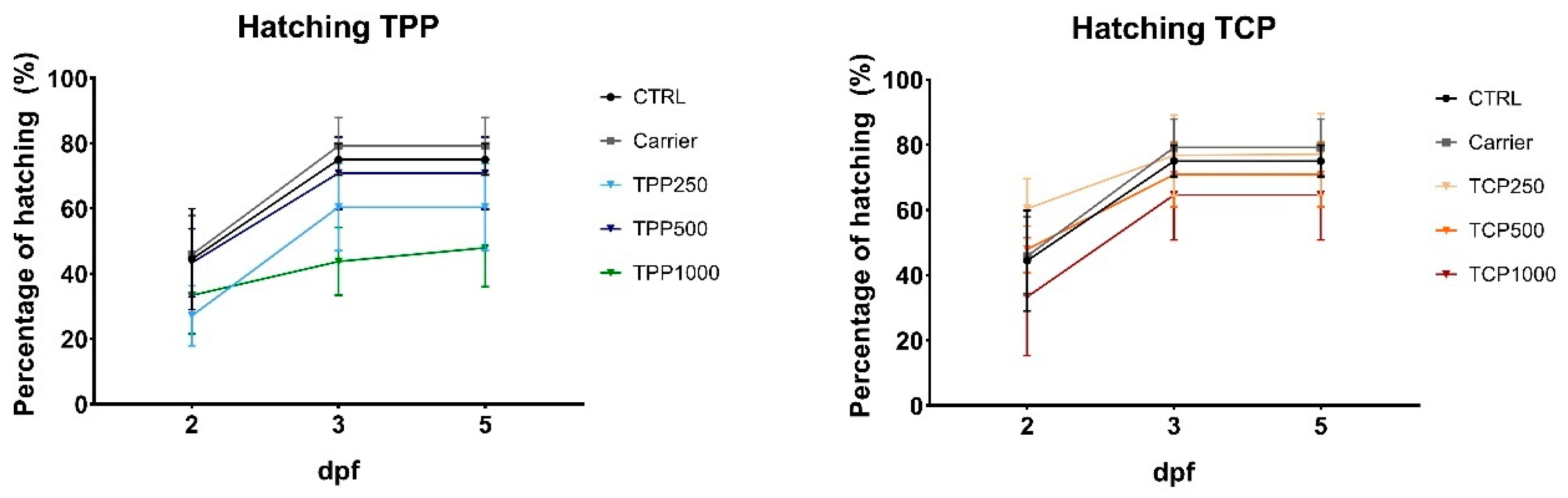
3.2. Cumulative Hatching Rate
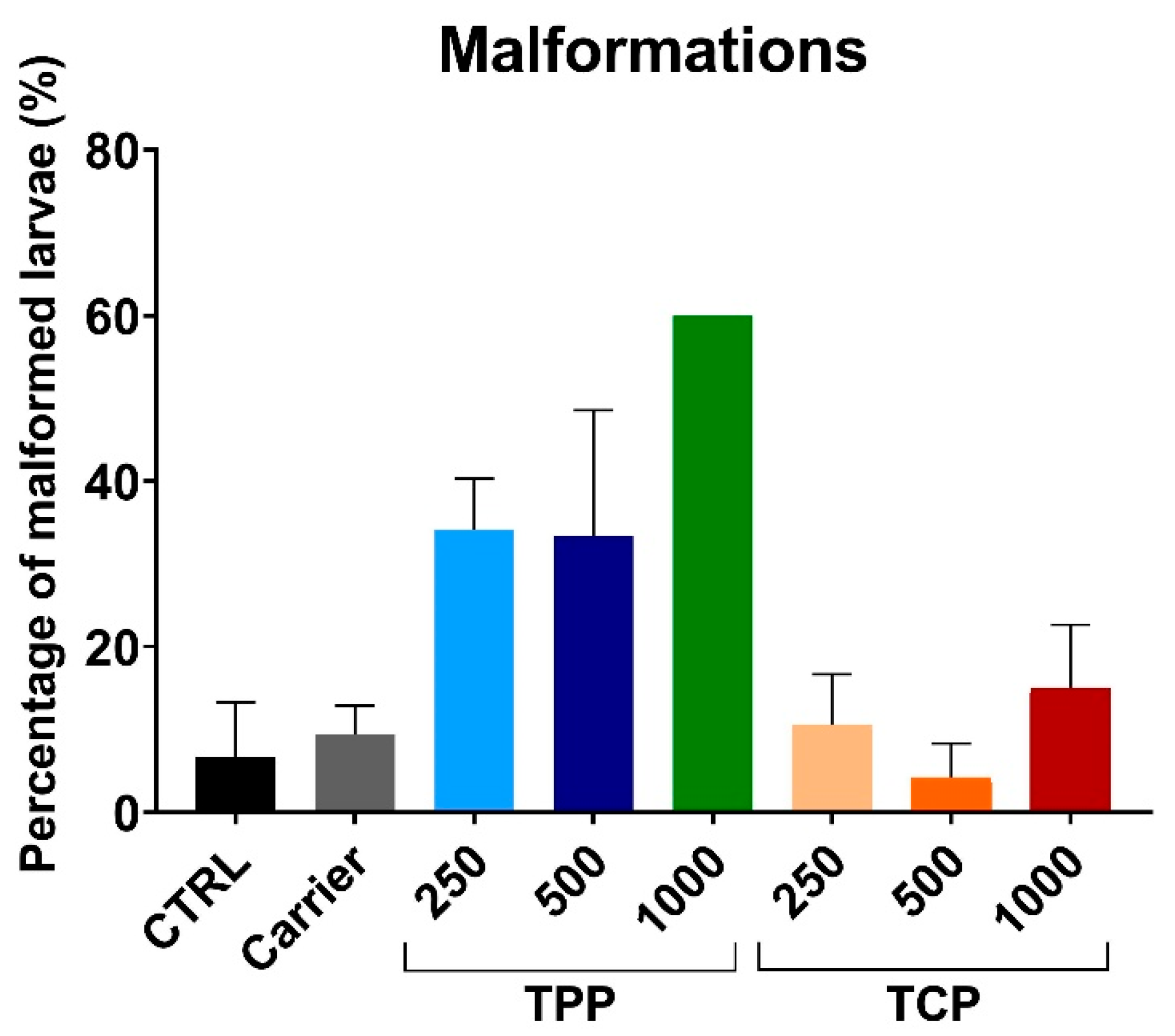
3.3. Developmental Malformations
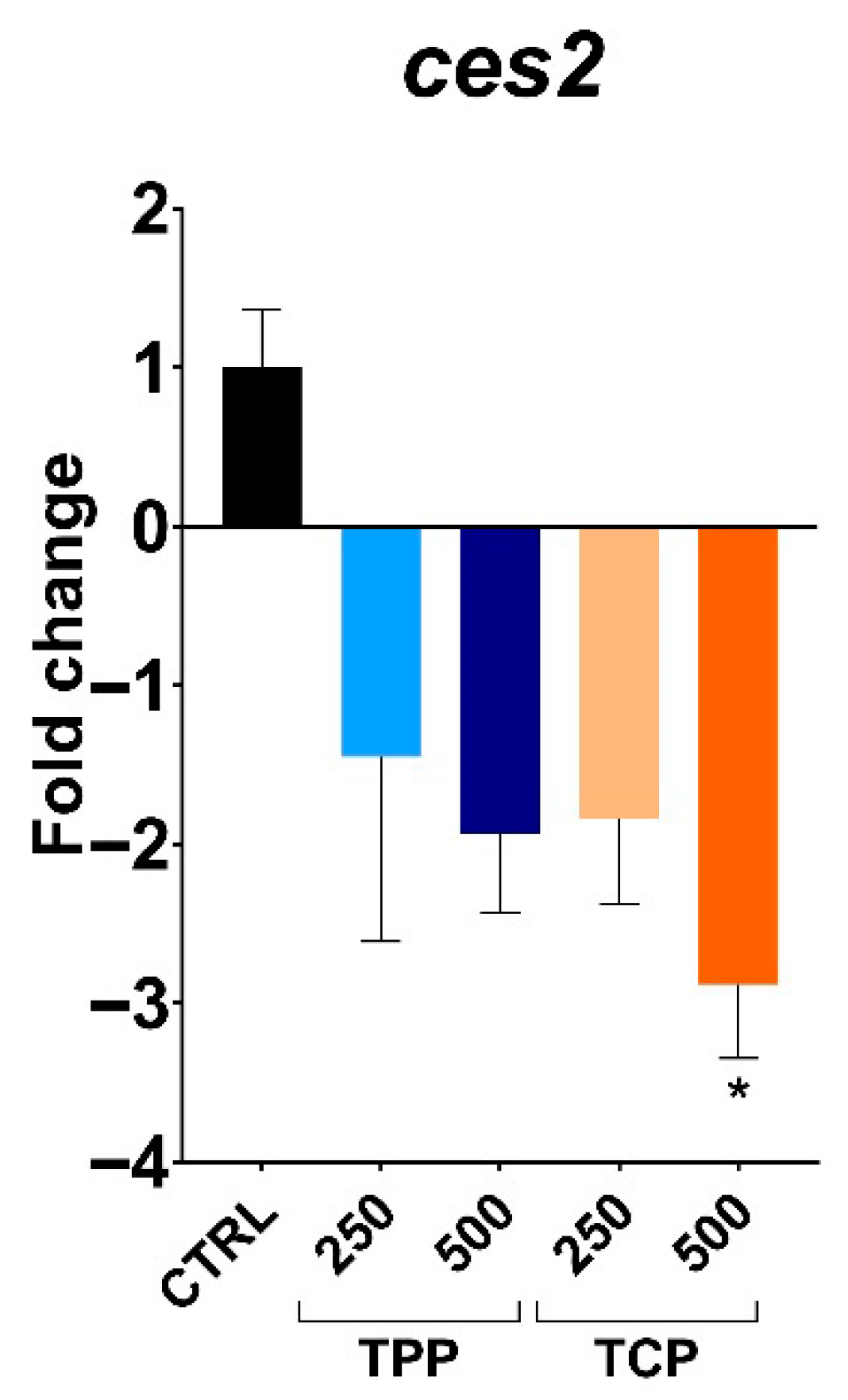
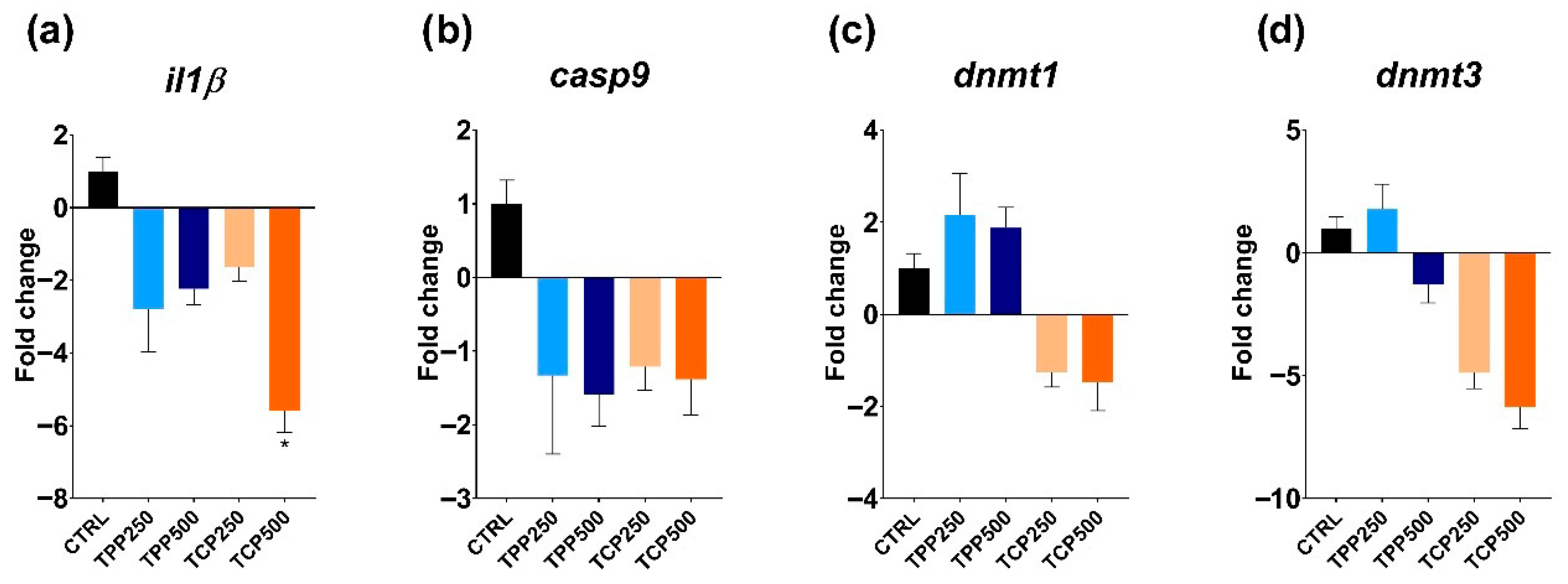
3.4. Gene Expression of Detoxification-, Immune-, and Epigenetic-Related Genes
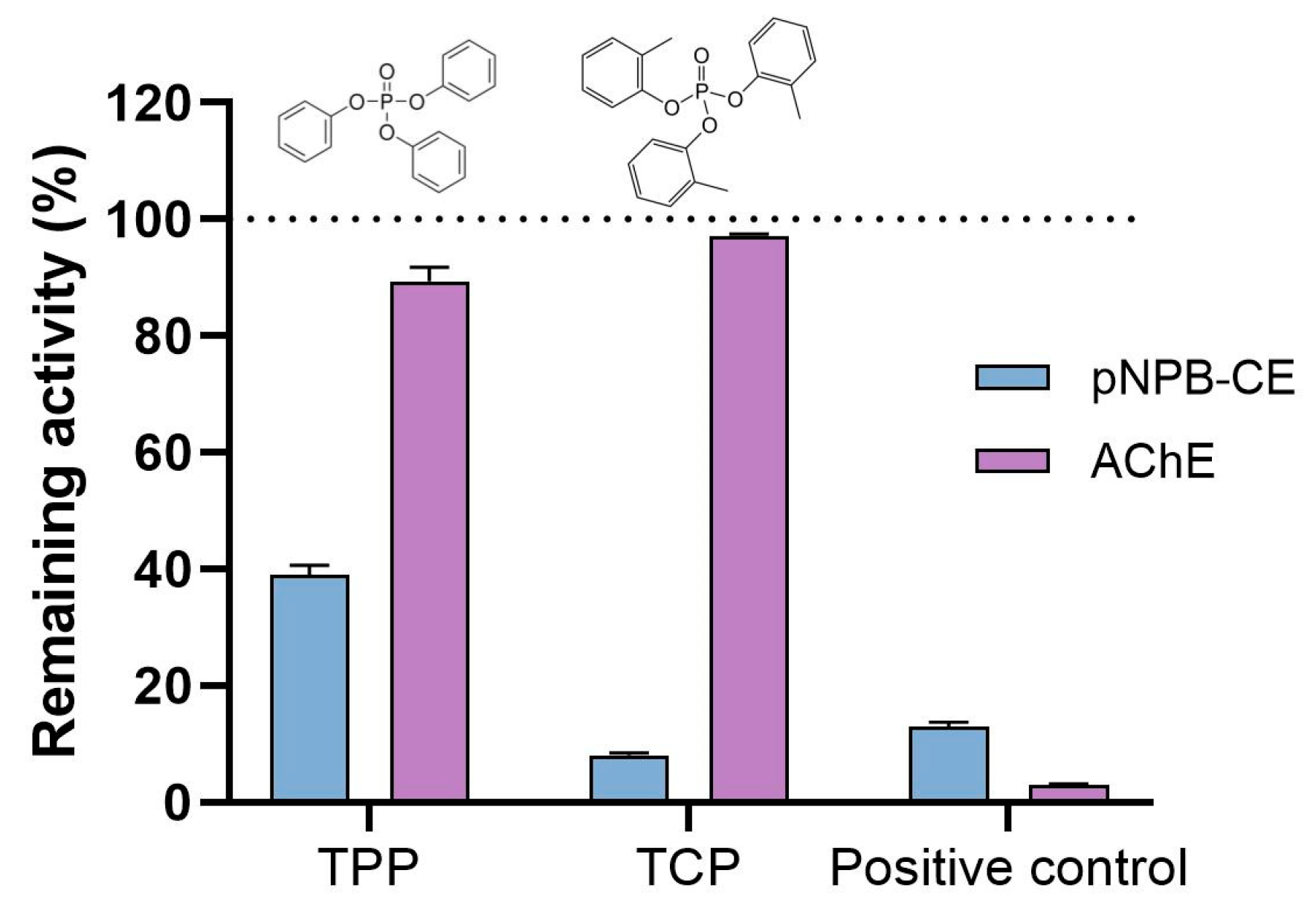
3.5. Basal B-esterase Activities and In Vitro Inhibition by TPP and TCP
4. Discussion
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blum, A.; Behl, M.; Birnbaum, L.S.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef]
- Yao, C.; Yang, H.; Li, Y. A Review on Organophosphate Flame Retardants in the Environment: Occurrence, Accumulation, Metabolism and Toxicity. Sci. Total Environ. 2021, 795, 148837. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Yan, X.; Wang, Y.; Liao, C.; Jiang, G. A Review of Organophosphate Flame Retardants and Plasticizers in the Environment: Analysis, Occurrence and Risk Assessment. Sci. Total Environ. 2020, 731, 139071. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lee, S.; Lee, H.-K.; Moon, H.-B. Organophosphate Flame Retardants and Plasticizers in Sediment and Bivalves along the Korean Coast: Occurrence, Geographical Distribution, and a Potential for Bioaccumulation. Mar. Pollut. Bull. 2020, 156, 111275. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.; Wang, L. A Review on Organophosphate Esters: Physiochemical Properties, Applications, and Toxici-ties as Well as Occurrence and Human Exposure in Dust Environment. J Environ. Manag. 2023, 325, 116601. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, P.; Wang, X.; Castro-Jiménez, J.; Kallenborn, R.; Liao, C.; Mi, W.; Lohmann, R.; Vila-Costa, M.; Dachs, J. Organophosphate Ester Pollution in the Oceans. Nat. Rev. Earth Environ. 2022, 3, 309–322. [Google Scholar] [CrossRef]
- Yan, Z.; Feng, C.; Leung, K.M.Y.; Luo, Y.; Wang, J.; Jin, X.; Wu, F. Insights into the Geographical Distribution, Bioaccumulation Characteristics, and Ecological Risks of Organophosphate Esters. J. Hazard. Mater. 2023, 445, 130517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, Z.; Yu, X.-Y.; Zhang, Y.; Zhu, L. Integration of Nontarget Screening and QSPR Models to Identify Novel Organophosphate Esters of High Priority in Aquatic Environment. Environ. Sci. Technol. 2024, 58, 14506–14517. [Google Scholar] [CrossRef]
- Tsugoshi, Y.; Watanabe, Y.; Tanikawa, Y.; Inoue, C.; Sugihara, K.; Kojima, H.; Kitamura, S. Inhibitory Effects of Organophosphate Esters on Carboxylesterase Activity of Rat Liver Microsomes. Chem. Biol. Interact. 2020, 327, 109148. [Google Scholar] [CrossRef]
- Satoh, T.; Hosokawa, M. Structure, Function and Regulation of Carboxylesterases. Chem. Biol. Interact. 2006, 162, 195–211. [Google Scholar] [CrossRef]
- Wheelock, C.E.; Phillips, B.M.; Anderson, B.S.; Miller, J.L.; Miller, M.J.; Hammock, B.D. Applications of Car-boxylesterase Activity in Environmental Monitoring and Toxicity Identification Evaluations (TIEs). Rev. Environ. Contam. Toxicol. 2008, 195, 117–178. [Google Scholar] [CrossRef] [PubMed]
- Velki, M.; Meyer-Alert, H.; Seiler, T.-B.; Hollert, H. Enzymatic Activity and Gene Expression Changes in Zebrafish Embryos and Larvae Exposed to Pesticides Diazinon and Diuron. Aquat. Toxicol. 2017, 193, 187–200. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.; Mackenzie, S. Fish Immune System. A Crossroads between Innate and Adaptive Responses. Inmunologia 2003, 22, 277–286. [Google Scholar]
- Charles Mc Lean Press; Evensen, Ø. The Morphology of the Immune System in Teleost Fishes. Fish. Shellfish. Immunol. 1999, 9, 309–318. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Bird, S. The Interleukins of Fish. Dev. Comp. Immunol. 2011, 35, 1336–1345. [Google Scholar] [CrossRef]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family. Hum. Genom. 2010, 5, 30. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, New and Emerging Functions of Caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; Li, D.; Chen, Y.; Feng, W.; Zhao, T.; Yang, L.; Mao, G.; Wu, X. A Review of Cumulative Toxic Effects of Environmental Endocrine Disruptors on the Zebrafish Immune System: Characterization Methods, Toxic Effects and Mechanisms. Environ. Res. 2024, 246, 118010. [Google Scholar] [CrossRef]
- Ni, A.; Fang, L.; Xi, M.; Li, J.; Qian, Q.; Wang, Z.; Wang, X.; Wang, H.; Yan, J. Neurotoxic Effects of 2-Ethylhexyl Diphenyl Phosphate Exposure on Zebrafish Larvae: Insight into Inflammation-Driven Changes in Early Motor Behavior. Sci. Total Environ. 2024, 915, 170131. [Google Scholar] [CrossRef]
- Turek-Plewa, J.; Jagodziński, P.P. The Role of Mammalian DNA Methyltransferases in the Regulation of Gene Expression. Cell Mol. Biol. Lett. 2005, 10, 631–647. [Google Scholar] [PubMed]
- Pradhan, S.; Bacolla, A.; Wells, R.D.; Roberts, R.J. Recombinant Human DNA (Cytosine-5) Methyltransferase. J. Biol. Chem. 1999, 274, 33002–33010. [Google Scholar] [CrossRef]
- Aluru, N.; Kuo, E.; Helfrich, L.W.; Karchner, S.I.; Linney, E.A.; Pais, J.E.; Franks, D.G. Developmental Exposure to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Alters DNA Methyltransferase (Dnmt) Expression in Zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2015, 284, 142–151. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Williams, T.D.; Tung, H.; Mirbahai, L.; Sanden, M.; Skjaerven, K.H.; Ellingsen, S. Impacts of TCDD and MeHg on DNA Methylation in Zebrafish (Danio rerio) across Two Generations. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 165, 17–27. [Google Scholar] [CrossRef]
- Cavalieri, V.; Spinelli, G. Environmental Epigenetics in Zebrafish. Epigenetics Chromatin 2017, 10, 46. [Google Scholar] [CrossRef]
- Ribas, L.; Vanezis, K.; Imués, M.A.; Piferrer, F. Treatment with a DNA Methyltransferase Inhibitor Feminizes Zebrafish and Induces Long-Term Expression Changes in the Gonads. Epigenetics Chromatin 2017, 10, 59. [Google Scholar] [CrossRef]
- Piferrer, F.; Ribas, L. The Use of the Zebrafish as a Model in Fish Aquaculture Research. Fish Physiol. 2020, 38, 273–313. [Google Scholar]
- Ribas, L.; Piferrer, F. The Zebrafish (Danio rerio) as a Model Organism, with Emphasis on Applications for Fin-fish Aquaculture Research. Rev. Aquac. 2014, 6, 209–240. [Google Scholar] [CrossRef]
- Nagel, R.; Bresch, H.; Caspers, N.; Hansen, P.D.; Markert, M.; Munk, R.; Scholz, N.; ter Höfte, B.B. Effect of 3,4-Dichloroaniline on the Early Life Stages of the Zebrafish (Brachydanio retio): Results of a Comparative Laboratory Study. Ecotoxicol. Environ. Saf. 1991, 21, 157–164. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- Alzualde, A.; Behl, M.; Sipes, N.S.; Hsieh, J.-H.; Alday, A.; Tice, R.R.; Paules, R.S.; Muriana, A.; Quevedo, C. Toxicity Profiling of Flame Retardants in Zebrafish Embryos Using a Battery of Assays for Developmental Toxicity, Neurotoxicity, Cardiotoxicity and Hepatotoxicity toward Human Relevance. Neurotoxicol. Teratol. 2018, 70, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, K.; Choi, K. Endocrine Disruption Potentials of Organophosphate Flame Retardants and Related Mechanisms in H295R and MVLN Cell Lines and in Zebrafish. Aquat. Toxicol. 2012, 114–115, 173–181. [Google Scholar] [CrossRef]
- Ramesh, M.; Angitha, S.; Haritha, S.; Poopal, R.-K.; Ren, Z.; Umamaheswari, S. Organophosphorus Flame Retardant Induced Hepatotoxicity and Brain AChE Inhibition on Zebrafish (Danio rerio). Neurotoxicol. Teratol. 2020, 82, 106919. [Google Scholar] [CrossRef]
- Shi, Q.; Guo, W.; Shen, Q.; Han, J.; Lei, L.; Chen, L.; Yang, L.; Feng, C.; Zhou, B. In Vitro Biolayer Interferometry Analysis of Acetylcholinesterase as a Potential Target of Aryl-Organophosphorus Flame-Retardants. J. Hazard. Mater. 2021, 409, 124999. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, M.; Shi, F.; Yang, L.; Guo, Y.; Feng, C.; Liu, J.; Zhou, B. Developmental Neurotoxicity of Triphenyl Phosphate in Zebrafish Larvae. Aquat. Toxicol. 2018, 203, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Ribas, L.; Liew, W.C.; Díaz, N.; Sreenivasan, R.; Orbán, L.; Piferrer, F. Heat-Induced Masculinization in Domes-ticated Zebrafish Is Family-Specific and Yields a Set of Different Gonadal Transcriptomes. Proc. Natl. Acad. Sci. USA 2017, 114, E941–E950. [Google Scholar] [CrossRef]
- OECD. Test No. 234: Fish Sexual Development Test; OECD Publishing: Paris, France, 2011. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Tang, R.; Dodd, A.; Lai, D.; McNabb, W.C.; Love, D.R. Validation of Zebrafish (Danio rerio) Reference Genes for Quantitative Real-Time RT-PCR Normalization. Acta Biochim. Biophys. Sin. 2007, 39, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Omedes, S.; Andrade, M.; Escolar, O.; Villanueva, R.; Freitas, R.; Solé, M. B-Esterases Characterisation in the Digestive Tract of the Common Octopus and the European Cuttlefish and Their in Vitro Responses to Contaminants of Environmental Concern. Environ. Res. 2022, 210, 112961. [Google Scholar] [CrossRef]
- Solé, M.; Figueres, E.; Mañanós, E.; Rojo-Solís, C.; García-Párraga, D. Characterisation of Plasmatic B-Esterases in Bottlenose Dolphins (Tursiops truncatus) and Their Potential as Biomarkers of Xenobiotic Chemical Expo-sures. Environ. Pollut. 2022, 313, 120149. [Google Scholar] [CrossRef]
- Solé, M.; Omedes, S.; Brandts, I.; Estévez, J. B-Esterases Activities in Several Tissues of the Marine Fish: Sea Bass and Hake, as Potential Biomarkers of Bisphenol A Derivatives. J. Environ. Expo. Assess. 2024, 3. [Google Scholar] [CrossRef]
- Shimizu, M.; Fukami, T.; Nakajima, M.; Yokoi, T. Screening of Specific Inhibitors for Human Carboxylesterases or Arylacetamide Deacetylase. Drug Metab. Dispos. 2014, 42, 1103–1109. [Google Scholar] [CrossRef]
- Schmandt, B.; Diduff, M.; Smart, G.; Williams, L.M. Environmentally Relevant Concentrations of Triphenyl Phosphate (TPhP) Impact Development in Zebrafish. Toxics 2024, 12, 368. [Google Scholar] [CrossRef]
- Barata, C.; Damasio, J.; López, M.A.; Kuster, M.; de Alda, M.L.; Barceló, D.; Riva, M.C.; Raldúa, D. Combined Use of Biomarkers and in Situ Bioassays in Daphnia Magna to Monitor Environmental Hazards of Pesticides in the Field. Environ. Toxicol. Chem. 2007, 26, 370–379. [Google Scholar] [CrossRef]
- Hou, R.; Xu, Y.; Wang, Z. Review of OPFRs in Animals and Humans: Absorption, Bioaccumulation, Metabolism, and Internal Exposure Research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, Z.; Xia, M.; Chen, S.; Wan, X.; He, A.; Daniel Sheng, G.; Wang, X.; Qian, Q.; Wang, H. Induction of Lipid Metabolism Dysfunction, Oxidative Stress and Inflammation Response by Tris(1-Chloro-2-Propyl)Phosphate in Larval/Adult Zebrafish. Environ. Int. 2022, 160, 107081. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, L.; Dong, F.; Zhang, W.; Hu, F. Effects of Tris (2-Chloroethyl) Phosphate (TCEP) on Survival, Growth, Histological Changes and Gene Expressions in Juvenile Yellow Catfish Pelteobagrus fulvidraco. Environ. Toxicol. Pharmacol. 2021, 87, 103699. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, Y.; Wang, W.; Yang, S.; Gao, Y.; Shi, W.; Hou, H.; Chen, J.; Guo, R. Toxicity of TPhP on the Gills and Intestines of Zebrafish from the Perspectives of Histopathology, Oxidative Stress and Immune Response. Sci. Total Environ. 2024, 908, 168212. [Google Scholar] [CrossRef] [PubMed]
- Negi, C.K.; Bláhová, L.; Phan, A.; Bajard, L.; Blaha, L. Triphenyl Phosphate Alters Methyltransferase Expression and Induces Genome-Wide Aberrant DNA Methylation in Zebrafish Larvae. Chem. Res. Toxicol. 2024, 37, 1549–1561. [Google Scholar] [CrossRef]
- Li, R.; Yang, L.; Han, J.; Zou, Y.; Wang, Y.; Feng, C.; Zhou, B. Early-Life Exposure to Tris (1,3-Dichloro-2-Propyl) Phosphate Caused Multigenerational Neurodevelopmental Toxicity in Zebrafish via Altering Maternal Thy-roid Hormones Transfer and Epigenetic Modifications. Environ. Pollut. 2021, 285, 117471. [Google Scholar] [CrossRef]
- Volz, D.C.; Leet, J.K.; Chen, A.; Stapleton, H.M.; Katiyar, N.; Kaundal, R.; Yu, Y.; Wang, Y. Tris(1,3-Dichloro-2-Propyl)Phosphate Induces Genome-Wide Hypomethylation within Early Zebrafish Em-bryos. Environ. Sci. Technol. 2016, 50, 10255–10263. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, W.; Lei, L.; Zhang, L.; Liu, Y.; Han, J.; Chen, L.; Zhou, B. Early-Life Exposure to the Organophosphorus Flame-Retardant Tris (1,3-Dichloro-2-Propyl) Phosphate Induces Delayed Neurotoxicity Associated with DNA Methylation in Adult Zebrafish. Environ. Int. 2020, 134, 105293. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Thornton, C.; Scheffler, B.E.; Willett, K.L. Benzo[a]Pyrene Decreases Global and Gene Specific DNA Methylation during Zebrafish Development. Environ. Toxicol. Pharmacol. 2013, 36, 40–50. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Wang, H.; Wu, C.; Cao, R.; Zhao, M.; Su, G.; Wang, C. A Comprehensive Evaluation of the Endocrine-Disrupting Effects of Emerging Organophosphate Esters. Environ. Int. 2024, 193, 109120. [Google Scholar] [CrossRef]
- Lian, J.; Nelson, R.; Lehner, R. Carboxylesterases in Lipid Metabolism: From Mouse to Human. Protein Cell 2018, 9, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Howarth, D.L.; Yin, C.; Yeh, K.; Sadler, K.C. Defining Hepatic Dysfunction Parameters in Two Models of Fatty Liver Disease in Zebrafish Larvae. Zebrafish 2013, 10, 199–210. [Google Scholar] [CrossRef]
| Gene Name | Gene Symbol | Accession Number | Forward (5′-3′) | Reverse (3′-5′) | Primer Efficiency (%) |
|---|---|---|---|---|---|
| Carboxylesterases | ces2 | NM_001077252.1 | GTGGAGCTTGCATGTTTAAGG | GCTGATCTCCTGTGCTGAAGTA | 105 |
| DNA (cytosine-5-)-methyltransferase | dnmt1 | NM_131189 | TCTTCAGCACTACAGTTACCAATCCT | CGTGCACATTCCCTGACACT | 96 |
| DNA (cytosine-5-)-methyltransferase 3bb.2 | dnmt3 | NM_131386 | AAGATTTAGGCGTCGGTTTCG | GTGTCACCCCCTTCAATTAACTG | 104 |
| Interleukin 1 β | il1β | NM_212844 | TGGACTTCGCAGCACAAAATG | GTTCACTTCACGCTCTTGGATG | 106 |
| Caspase 9 | casp9 | NM_001007404 | CAACATCGACTGCGACAAGC | CAACATCGACTGCGACAAGC | 99 |
| Eukaryotic translation elongation factor 1 alpha 1 | eef1a1l1 | NM_131263 | CTGGAGGCCAGCTCAAACAT | ATCAAGAAGAGTAGTACCGCTAGCATTAC | 95 |
| Ribosomal protein L13a | rpl13a | NM_212784 | TCTGGAGGACTGTAAGAGGTATGC | AGACGCACAATCTTGAGAGCAG | 97 |
| Chemical | pNPB-hCE1 | ATC-eel AChE |
|---|---|---|
| TPP | 97.7 ± 0.1 | 21.6 ± 8.2 |
| TCP | 93.8 ± 0.1 | 58.9 ± 3.3 |
| BNPP | 96.6 ± 0.1 | NS |
| BW284c51 | NS | 99.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solé, M.; Joly, S.; Omedes, S.; Forner-Piquer, I.; Ribas, L. Modulation of Detoxification, Immune, and Epigenetic Systems by Two Aryl Organophosphorus Flame Retardants During Early Development in Zebrafish. Toxics 2025, 13, 794. https://doi.org/10.3390/toxics13090794
Solé M, Joly S, Omedes S, Forner-Piquer I, Ribas L. Modulation of Detoxification, Immune, and Epigenetic Systems by Two Aryl Organophosphorus Flame Retardants During Early Development in Zebrafish. Toxics. 2025; 13(9):794. https://doi.org/10.3390/toxics13090794
Chicago/Turabian StyleSolé, Montserrat, Sílvia Joly, Sergi Omedes, Isabel Forner-Piquer, and Laia Ribas. 2025. "Modulation of Detoxification, Immune, and Epigenetic Systems by Two Aryl Organophosphorus Flame Retardants During Early Development in Zebrafish" Toxics 13, no. 9: 794. https://doi.org/10.3390/toxics13090794
APA StyleSolé, M., Joly, S., Omedes, S., Forner-Piquer, I., & Ribas, L. (2025). Modulation of Detoxification, Immune, and Epigenetic Systems by Two Aryl Organophosphorus Flame Retardants During Early Development in Zebrafish. Toxics, 13(9), 794. https://doi.org/10.3390/toxics13090794








