Toxicological Effects of Tartrazine Exposure: A Review of In Vitro and Animal Studies with Human Health Implications
Abstract
1. Introduction
2. Health Implications of Tartrazine Exposure
3. Oxidative Stress Induced by Tartrazine
4. In Vitro Studies on Tartrazine Toxicity
5. Tartrazine Toxicity in Experimental Animal Models
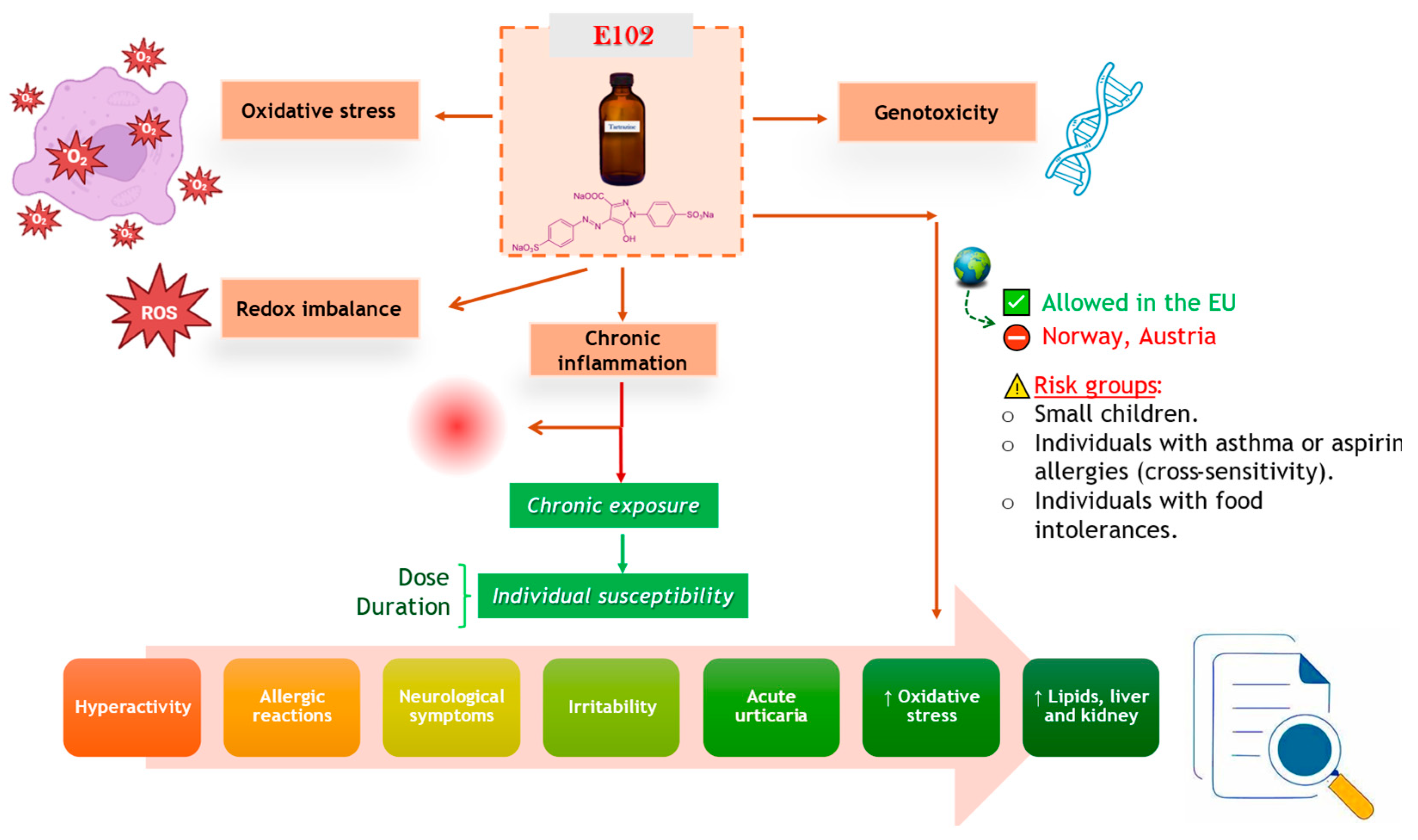
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehman, K.; Ashraf, A.; Azam, F.; Hamid Akash, M.S. Effect of Food Azo-Dye Tartrazine on Physiological Functions of Pancreas and Glucose Homeostasis. Turk. J. Biochem. 2019, 44, 197–206. [Google Scholar] [CrossRef]
- Leulescu, M.; Rotaru, A.; Pălărie, I.; Moanţă, A.; Cioateră, N.; Popescu, M.; Morîntale, E.; Bubulică, M.V.; Florian, G.; Hărăbor, A.; et al. Tartrazine: Physical, Thermal and Biophysical Properties of the Most Widely Employed Synthetic Yellow Food-Colouring Azo Dye. J. Therm. Anal. Calorim. 2018, 134, 209–231. [Google Scholar] [CrossRef]
- Chung, K.T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Ambroziewicz, Z.M.; Siemiątkowski, R.; Łata, M.; Dowgiert, S.; Sikorska, M.; Kamiński, J.; Więcław, K.; Grabowska, H.; Chruściel, J.; Mąsior, G. Long-Term Health Effects of Artificially Colored Foods in Adults and Children: A Review of Scientific Literature on Attention Deficits, Carcinogenicity, and Allergy Risks. J. Educ. Health Sport 2024, 76, 56522. [Google Scholar] [CrossRef]
- Kamat, P.; Shetti, P.P.; Paranjape, R.; Shetti, P.P. Techniques for Detection and Measurement of Tartrazine in Food Products 2008 to 2022: A Review. J. Chem. Health Risks 2024, 14, 721–729. Available online: https://jchr.org/index.php/JCHR/article/view/6813 (accessed on 17 July 2025).
- Mehedi, N.; Ainad-Tabet, S.; Mokrane, N.; Addou, S.; Zaoui, C.; Kheroua, O.; Saidi, D. Reproductive Toxicology of Tartrazine (FD and C Yellow No. 5) in Swiss Albino Mice. Am. J. Pharmacol. Toxicol. 2009, 4, 130–135. [Google Scholar] [CrossRef]
- Barciela, P.; Perez-Vazquez, A.; Prieto, M.A. Azo Dyes in the Food Industry: Features, Classification, Toxicity, Alternatives, and Regulation. Food Chem. Toxicol. 2023, 178, 113935. [Google Scholar] [CrossRef]
- Alshehrei, F. Role of Microorganisms in Biodegradation of Food Additive Azo Dyes: A Review. Afr. J. Biotechnol. 2020, 19, 799–805. [Google Scholar] [CrossRef]
- Aali, R.; Yari, A.R.; Ghafuri, Y.; Behnamipour, S. Health Risk Assessment of Synthetic Tartrazine Dye in Some Food Products in Qom Province (Iran). Curr. Nutr. Food Sci. 2024, 20, 726–733. [Google Scholar] [CrossRef]
- World Health Organization. JECFA Chemical Evaluation: Chemical ID 3885. 2016. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/3885 (accessed on 11 July 2025).
- Vander Leek, T.K. Food Additives and Reactions: Antioxidants, Benzoates, Parabens, Colorings, Flavorings, Natural Protein-Based Additives. Encycl. Food Allergy 2024, 1, 862–881. [Google Scholar] [CrossRef]
- Wróblewska, B. Influence of Food Additives and Contaminants (Nickel and Chromium) on Hypersensitivity and Other Adverse Health Reactions—A Review. Pol. J. Food Nutr. Sci. 2009, 59, 287–294. Available online: https://journal.pan.olsztyn.pl/pdf-98219-30948?filename=30948.pdf (accessed on 11 July 2025).
- Pestana, S.; Moreira, M.; Olej, B. Safety of Ingestion of Yellow Tartrazine by Double-Blind Placebo Controlled Challenge in 26 Atopic Adults. Allergol. Immunopathol. 2010, 38, 142–146. [Google Scholar] [CrossRef]
- Banc, R.; Filip, L.; Cozma-Petruț, A.; Ciobârcă, D.; Miere, D. Yellow and Red Synthetic Food Dyes and Potential Health Hazards: A Mini Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2024, 81, 1–17. [Google Scholar] [CrossRef]
- Bateman, B.J. The Behaviour of Three Year Olds in Relation to Allergy and Exposure to Artificial Additives. Doctoral Thesis, University of Southampton, Southampton, UK, 2004. Available online: https://eprints.soton.ac.uk/465625/ (accessed on 11 July 2025).
- Tuormaa, T.E. The Adverse Effects of Food Additives on Health: A Review of the Literature with Special Emphasis on Childhood Hyperactivity. J. Orthomol. Med. 1994, 9, 225–243. Available online: https://orthomolecular.org/library/jom/1994/articles/1994-v09n04-p225.shtml (accessed on 21 July 2025).
- Feketea, G.; Tsabouri, S. Common Food Colorants and Allergic Reactions in Children: Myth or Reality? Food Chem. 2017, 230, 578–588. [Google Scholar] [CrossRef]
- Arnold, L.E.; Lofthouse, N.; Hurt, E. Artificial Food Colors and Attention-Deficit/Hyperactivity Symptoms: Conclusions to Dye For. Neurotherapeutics 2012, 9, 599–609. [Google Scholar] [CrossRef]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food Colour Additives: A Synoptical Overview on Their Chemical Properties, Applications in Food Products and Health Side Effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef]
- Rowe, K.S.; Rowe, K.J. Synthetic Food Coloring and Behavior: A Dose Response Effect in a Double-Blind, Placebo-Controlled, Repeated-Measures Study. J. Pediatr. 1994, 125, 691–698. [Google Scholar] [CrossRef]
- Visweswaran, B. Oxidative Stress by Tartrazine in the Testis of Wistar Rats. IOSR J. Pharm. Biol. Sci. 2012, 2, 44–49. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Wabaidur, S.M.; Alothman, Z.A.; Habila, M.A. Regulatory Role of Nano-Curcumin against Tartrazine-Induced Oxidative Stress, Apoptosis-Related Genes Expression, and Genotoxicity in Rats. Molecules 2020, 25, 5801. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lv, X.; Zhang, Y.; Xin, Q.; Zou, Y.; Li, X. Tartrazine Exposure Results in Histological Damage, Oxidative Stress, Immune Disorders and Gut Microbiota Dysbiosis in Juvenile Crucian Carp (Carassius carassius). Aquat. Toxicol. 2021, 241, 105998. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Y.; Lv, X.; Chang, X.; Ma, X.; Tian, X.; Shi, X.; Li, X.; Kong, X. Impacts of an Azo Food Dye Tartrazine Uptake on Intestinal Barrier, Oxidative Stress, Inflammatory Response and Intestinal Microbiome in Crucian Carp (Carassius auratus). Ecotoxicol. Environ. Saf. 2021, 223, 112551. [Google Scholar] [CrossRef]
- Elhkim, M.O.; Héraud, F.; Bemrah, N.; Gauchard, F.; Lorino, T.; Lambré, C.; Frémy, J.M.; Poul, J.M. New Con-siderations Regarding the Risk Assessment on Tartrazine. An Update Toxicological Assessment, Intolerance Reactions and Maximum Theoretical Daily Intake in France. Regul. Toxicol. Pharmacol. 2007, 47, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, L.; Essawy, A.; Sorour, J.; Soffar, A. Tartrazine Induces Structural and Functional Aberrations and Genotoxic Effects in Vivo. PeerJ 2017, 5, e3041. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Reproductive and Neurobehavioural Toxicity Study of Tartrazine Administered to Mice in the Diet. Food Chem. Toxicol. 2006, 44, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Mandelker, L. Oxidative Stress, Free Radicals, and Cellular Damage. In Studies in Veterinary Medicine; Humana Press: Totowa, NJ, USA, 2011; pp. 1–17. [Google Scholar] [CrossRef]
- Aldaamy, A.M.Z.; Al-Zubiady, N.M.H. Study on Toxic Effect of Tartrazine Pigment on Oxidative Stress in Male Albino Rats. Biochem. Cell Arch. 2021, 21, 1021–1026. Available online: https://connectjournals.com/03896.2021.21.1021 (accessed on 18 July 2025).
- El-Desoky, G.E.; Abdel-Ghaffar, A.; Al-Othman, Z.A.; Habila, M.A.; Al-Sheikh, Y.A.; Ghneim, H.K.; Giesy, J.P.; Aboul-Soud, M.A.M. Curcumin Protects against Tartrazine-Mediated Oxidative Stress and Hepatotoxicity in Male Rats. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 635–645. Available online: https://pubmed.ncbi.nlm.nih.gov/28239801/ (accessed on 18 July 2025).
- Ramos-Souza, C.; Nass, P.; Jacob-Lopes, E.; Zepka, L.Q.; Braga, A.R.C.; De Rosso, V.V. Changing Despicable Me: Potential Replacement of Azo Dye Yellow Tartrazine for Pequi Carotenoids Employing Ionic Liquids as High-Performance Extractors. Int. Food Res. J. 2023, 174, 113593. [Google Scholar] [CrossRef]
- Amchova, P.; Siska, F.; Ruda-Kucerova, J. Safety of Tartrazine in the Food Industry and Potential Protective Factors. Heliyon 2024, 10, e38111. [Google Scholar] [CrossRef]
- Lee, M.; Fondriest Gentry, A.; Schwartz, R.; Bauman, J. Tartrazine-Containing Drugs. Drug Intell. Clin. Pharm. 1981, 15, 782–788. [Google Scholar] [CrossRef]
- Miller, K. Sensitivity to Tartrazine. Br. Med. J. 1982, 285, 1597–1598. [Google Scholar] [CrossRef][Green Version]
- Pennington, C.R. Diet and Disease. In Therapeutic Nutrition; Springer: Boston, MA, USA, 1988; pp. 226–262. [Google Scholar] [CrossRef]
- Moutinho, I.L.D.; Bertges, L.C.; Assis, R.V.C. Prolonged Use of the Food Dye Tartrazine (FD&C Yellow N° 5) and Its Effects on the Gastric Mucosa of Wistar Rats. Braz. J. Biol. 2007, 67, 141–145. [Google Scholar] [CrossRef]
- Skypala, I. Other Causes of Food Hypersensitivity. In Food Hypersensitivity: Diagnosing and Managing Food Allergies and Intolerance; Blackwell Publisher: Hoboken, NJ, USA, 2009; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781444312119#page=219 (accessed on 18 July 2025).
- Bush, R.K.; Taylor, S.L. Reactions to Food and Drug Additives. In Middleton’s Allergy: Principles and Practice, 8th ed.; Mosby: Maryland Heights, MO, USA, 2014; Volume 2, pp. 1340–1356. [Google Scholar] [CrossRef]
- Breitkreutz, J.; Tuleu, C. Pediatric and Geriatric Pharmaceutics and Formulation. In Modern Pharmaceutics, 5th ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 2, Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/b14445-32/pediatric-geriatric-pharmaceutics-formulation (accessed on 18 July 2025).
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. A Review of Extraction and Analytical Methods for the Determination of Tartrazine (E 102) in Foodstuffs. Crit. Rev. Anal. Chem. 2017, 47, 309–324. [Google Scholar] [CrossRef]
- Sezgin, A.; Ayyildiz, S. Food Additives: Colorants. In Science Within Food: Up-to-Date Advance s on Research and Educational Ideas. Researchgate 2018, 122, 87–94. Available online: https://www.researchgate.net/publication/322404775_Food_Additives_Colorants (accessed on 28 July 2025).
- Sharma, R.; Bamola, S.; Kumar Verma, S. Effects of Acid Yellow 23 Food Dye on Environment and Its Removal on Various Surfaces-A Mini Review. Int. Res. J. Eng. Technol. 2020, 7, 4550–4573. [Google Scholar]
- Koop, B.L.; Maciel, A.G.; Soares, L.S.; Monteiro, A.R.; Valencia, G.A. Natural Colorants. In Natural Additives in Foods; Springer: Cham, Switzerland, 2022; pp. 87–122. Available online: https://link.springer.com/chapter/10.1007/978-3-031-17346-2_4 (accessed on 18 July 2025).
- Athira, N.; Jaya, D.S. Effects of Tartrazine on Growth and Brain Biochemistry of Indian Major Carps on Long-Term Exposure. Int. J. Adv. Biochem. Res. 2022, 6, 17–25. [Google Scholar] [CrossRef]
- Hegazy, A.A.; Awad Hegazy, A.; Haliem, W.A.; Haliem, R.A.; Mamdouh El-Bestawy, E.; Mohammad, G.; Ali, E. Brief Overview about Tartrazine Effects on Health. Eur. Chem. Bull. 2023, 12, 4698–4707. Available online: https://www.academia.edu/106433462/Brief_Overview_about_Tartrazine_Effects_on_Health (accessed on 14 June 2025).
- Food and Drug Administration. Title 21 Code of Federal Regulations. A Point Time in eCFR System. § 74.1705 FD&C Yellow No. 5. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-74/subpart-B/section-74.1705?utm_source (accessed on 1 September 2025).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast) (Text with EEA Relevance). 2009. Available online: https://eur-lex.europa.eu/eli/reg/2009/1223/oj/eng?utm_source (accessed on 1 September 2025).
- Pay, R.; Sharrock, A.V.; Elder, R.; Maré, A.; Bracegirdle, J.; Torres, D.; Malone, N.; Vorster, J.; Kelly, L.; Ryan, A.; et al. Preparation, Analysis and Toxicity Characterisation of the Redox Metabolites of the Azo Food Dye Tartrazine. Food Chem. Toxicol. 2023, 182, 114193. [Google Scholar] [CrossRef]
- Kamal, A.A.; Fawzia, S.E.-S. Toxicological and Safety Assessment of Tartrazine as a Synthetic Food Additive on Health Biomarkers: A Review. Afr. J. Biotechnol. 2018, 17, 139–149. [Google Scholar] [CrossRef]
- dos Santos, J.R.; Soares, L.d.S.; Soares, B.M.; de Gomes Farias, M.; de Oliveira, V.A.; de Sousa, N.A.B.; Negreiros, H.A.; da Silva, F.C.C.; Peron, A.P.; Pacheco, A.C.L.; et al. Cytotoxic and mutagenic effects of the food additive tartrazine on eukaryotic cells. BMC Pharmacol. Toxicol. 2022, 23, 95. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Shen, J.; Yin, H.; An, X.; Jin, H. Effect of Food Azo Dye Tartrazine on Learning and Memory Functions in Mice and Rats, and the Possible Mechanisms Involved. J. Food Sci. 2011, 76, T125–T129. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Nettis, E.; Colanardi, M.C.; Ferrannini, A.; Tursi, A. Suspected Tartrazine-Induced Acute Urticar-ia/Angioedema Is Only Rarely Reproducible by Oral Rechallenge. Clin. Exp. Allergy 2003, 33, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Araújo Caldas, L.; Caldas Marmo, F.; da Costa, P.V.; Viana Jacobson, L.S. Hormesis in Tartrazine Allergic Responses of Atopic Patients: An Overview of Clinical Trials and a Raw Data Revision. Environ. Dis. 2020, 5, 59. [Google Scholar] [CrossRef]
- Soares, B.M.; Araújo, T.M.T.; Ramos, J.A.B.; Pinto, L.C.; Khayat, B.M.; De Oliveira Bahia, M.; Montenegro, R.C.; Burbano, R.M.R.G.; Khayat, A.S. Effects on DNA Repair in Human Lymphocytes Exposed to the Food Dye Tartrazine Yellow. Anticancer. Res. 2015, 35, 1465–1474. Available online: https://pubmed.ncbi.nlm.nih.gov/25750299/ (accessed on 18 July 2025). [PubMed]
- Velioglu, C.; Erdemli, M.E.; Gul, M.; Erdemli, Z.; Zayman, E.; Bag, H.G.; Altinoz, E. Protective Effect of Crocin on Food Azo Dye Tartrazine-Induced Hepatic Damage by Improving Biochemical Parameters and Oxidative Stress Biomarkers in Rats. Gen. Physiol. Biophys. 2019, 38, 73–82. [Google Scholar] [CrossRef]
- Altinoz, E.; Erdemli, M.E.; Gül, M.; Erdemli, Z.; Gül, S.; Turkoz, Y. Prevention of Toxic Effects of Orally Administered Tartrazine by Crocin in Wistar Rats. Toxicol. Environ. Chem. 2021, 103, 184–198. [Google Scholar] [CrossRef]
- Erdemli, M.E.; Gul, M.; Altinoz, E.; Zayman, E.; Aksungur, Z.; Bag, H.G. The Protective Role of Crocin in Tartrazine Induced Nephrotoxicity in Wistar Rats. Biomed. Pharmacother. 2017, 96, 930–935. [Google Scholar] [CrossRef]
- Erdemli, Z.; Altinoz, E.; Erdemli, M.E.; Gul, M.; Bag, H.G.; Gul, S. Ameliorative Effects of Crocin on Tartrazine Dye–Induced Pancreatic Adverse Effects: A Biochemical and Histological Study. Environ. Sci. Pollut. Res. Int. 2021, 28, 2209–2218. [Google Scholar] [CrossRef]
- Aldaamy, A.; Merza Hamza, N.; Masikh Zebalah Al-Daamy, A.; Marza Hamza Al-Zubiady, N. Study of The Toxic Effect of Tartrazine Dye on Some Biochemical Parameters in Male Albino Rats. Sci. J. Med. Res. 2020, 4, 111–117. Available online: https://sjomr.org.in/index.php/SJOMR/article/view/134 (accessed on 18 July 2025).
- Abdelgayed, S.S. Toxicological and Histopathological Studies on the Effect of Tartrazine in Male Albino Rats. Int. J. Agric. Biol. Eng. 2016, 10, 469–474. Available online: https://www.researchgate.net/publication/309791778 (accessed on 7 June 2025).
- El Golli, N.; Bini-Dhouib, I.; Jrad, A.; Boudali, I.; Nasri, B.; Belhadjhmida, N.; El Fazaa, S. Toxicity Induced after Subchronic Administration of the Synthetic Food Dye Tartrazine in Adult Rats, Role of Oxidative Stress. Recent. Adv. Biol. Med. 2016, 2, 20. [Google Scholar] [CrossRef]
- Refai, H.M.; Mahmoud, M.A.A.; Ghuniem, M.M. Elevated Health Risks for Children and Adolescents: Quantification of Artificial Sweeteners and Synthetic Dyes in Powdered Beverages Reveals Regulatory Gaps. J. Food Compos. Anal. 2025, 148, 108239. [Google Scholar] [CrossRef]
- Scientific Opinion on the re-evaluation Tartrazine (E 102). EFSA J. 2009, 7, 1331. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/1331 (accessed on 18 July 2025). [CrossRef]
- Rahnama, H.; Mazloomi, S.M.; Berizi, E.; Abbasi, A.; Gholami, Z. Identification of Tartrazine adulteration and evaluating exposure to synthetic dyes of sunset yellow and Quinoline yellow through consumption of food products among children. Food Sci. Nutr. 2022, 10, 3781–3788. [Google Scholar] [CrossRef]
- Miller, M.D.; Steinmaus, C.; Golub, M.S.; Castorina, R.; Thilakartne, R.; Bradman, A.; Marty, M.A. Potential impacts of synthetic food dyes on activity and attention in children: A review of the human and animal evidence. Environ. Health. 2022, 21, 45. [Google Scholar] [CrossRef]
- Boussada, M.; Lamine, J.A.; Bini, I.; Abidi, N.; Lasrem, M.; El-Fazaa, S.; El-Golli, N. Assessment of a Sub-Chronic Consumption of Tartrazine (E102) on Sperm and Oxidative Stress Features in Wistar Rat. Int. Food Res. J. 2017, 24, 1473–1481. [Google Scholar]
- Amin, K.A.; Abdel Hameid, H.; Abd Elsttar, A.H. Effect of Food Azo Dyes Tartrazine and Carmoisine on Bi-ochemical Parameters Related to Renal, Hepatic Function and Oxidative Stress Biomarkers in Young Male Rats. Food Chem. Toxicol. 2010, 48, 2994–2999. [Google Scholar] [CrossRef]
- Ozougwu, J.C. Physiology of the Liver. Indian J. Res. Pharm. Biotechnol. 2017, 4, 13–24. Available online: https://www.academia.edu/38598619/Physiology_of_the_liver?auto=download (accessed on 18 July 2025).
- Kiran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative Stress and Antioxidants in Health and Disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Albasher, G.; Maashi, N.; Alfarraj, S.; Almeer, R.; Albrahim, T.; Alotibi, F.; Bin-Jumah, M.; Mahmoud, A.M. Perinatal exposure to tartrazine triggers oxidative stress and neurobehavioral alterations in mice offspring. Antioxidants 2020, 9, 53. [Google Scholar] [CrossRef]
- Hosieny, N.A.; Mona, E.; Ahmed, S.M.; Zayed, M. Toxic Effects of Food Azo Dye Tartrazine on the Brain of Young Male Albino Rats: Role of Oxidative Stress. Zagazig J. Forensic Med. 2020, 19, 60–73. [Google Scholar] [CrossRef]
- Wopara, I.; Adebayo, O.G.; Umoren, E.B.; Aduema, W.; Iwueke, A.V.; Etim, O.E.; Pius, E.A.; James, W.B.; Wodo, J. Involvement of Striatal Oxido-Inflammatory, Nitrosative and Decreased Cholinergic Activity in Neurobehavioral Alteration in Adult Rat Model with Oral Co-Exposure to Erythrosine and Tartrazine. Heliyon 2021, 7, e08454. [Google Scholar] [CrossRef] [PubMed]
- Al-Seeni, M.N.; El Rabey, H.A.; Al-Hamed, A.M.; Zamazami, M.A. Nigella sativa Oil Protects against Tartrazine Toxicity in Male Rats. Toxicol. Rep. 2018, 5, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; Moustafa, G.G.; Hashem, M.M.; Ali, H.A.; Abo-EL-Sooud, K.; El-Metwally, A.E. Influence of the Long-Term Exposure to Tartrazine and Chlorophyll on the Fibrogenic Signalling Pathway in Liver and Kidney of Rats: The Expression Patterns of Collagen 1-α, TGFβ-1, Fibronectin, and Caspase-3 Genes. Environ. Sci. Pollut. Res. Int. 2019, 26, 12368–12378. [Google Scholar] [CrossRef] [PubMed]
- Haridevamuthu, B.; Murugan, R.; Seenivasan, B.; Meenatchi, R.; Pachaiappan, R.; Almutairi, B.O.; Arokiyaraj, S.; Kathiravan, M.K.; Arockiaraj, J. Synthetic Azo-Dye, Tartrazine Induces Neurodevelopmental Toxicity via Mitochondria-Mediated Apoptosis in Zebrafish Embryos. J. Hazard. Mater. 2024, 461, 132524. [Google Scholar] [CrossRef]
- Floriano, J.M.; da Rosa, E.; Amaral, Q.D.F.d.; Zuravski, L.; Chaves, P.E.E.; Machado, M.M.; de Oliveir, L.F.S. Is Tartrazine Really Safe? In Silico and Ex Vivo Toxicological Studies in Human Leukocytes: A Question of Dose. Toxicol. Res. 2018, 7, 1128–1134. [Google Scholar] [CrossRef]
- Pasdaran, A.; Azarpira, N.; Heidari, R.; Nourinejad, S.; Zare, M.; Hamedi, A. Effects of Some Cosmetic Dyes and Pigments on the Proliferation of Human Foreskin Fibroblasts and Cellular Oxidative Stress; Potential Cytotoxicity of Chlorophyllin and Indigo Carmine on Fibroblasts. J. Cosmet. Dermatol. 2022, 21, 3979–3985. [Google Scholar] [CrossRef]
- Nasri, A.; Pohjanvirta, R. In Vitro Estrogenic, Cytotoxic, and Genotoxic Profiles of the Xenoestrogens 8-Prenylnaringenine, Genistein and Tartrazine. Environ. Sci. Pollut. Res. 2021, 28, 27988–27997. [Google Scholar] [CrossRef]
- Haverić, A.; Inajetović, D.; Vareškić, A.; Hadžić, M.; Haverić, S. In vitro Analysis of Tartrazine Genotoxicity and Cytotoxicity. Genet. Appl. 2017, 1, 37–43. [Google Scholar] [CrossRef]
- Zand, A.; Macharia, J.M.; Szabó, I.; Gerencsér, G.; Molnár, Á.; Raposa, B.L.; Varjas, T. The Impact of Tartrazine on DNA Methylation, Histone Deacetylation, and Genomic Stability in Human Cell Lines. Nutrients 2025, 17, 913. [Google Scholar] [CrossRef]
- Joshi, V.; Katti, P. Developmental Toxicity Assay for Food Additive Tartrazine Using Zebrafish (Danio rerio) Embryo Cultures. Int. J. Toxicol. 2018, 37, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Li, K.; Yan, D.L.; Yang, M.F.; Ma, L.; Xie, L.Z. Toxicity Assessment of 4 Azo Dyes in Zebrafish Embryos. Int. J. Toxicol. 2020, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Ranjan, S.; Yadav, A.; Verma, B.; Malhotra, K.; Madan, M.; Chopra, O.; Jain, S.; Gupta, S.; Joshi, A.; et al. Toxic Effects of Food Colorants Erythrosine and Tartrazine on Zebrafish Embryo Development. Curr. Res. Nutr. Food Sci. 2019, 7, 876–885. [Google Scholar] [CrossRef]
- Thanh, D.D.; Bich-Ngoc, N.; Paques, C.; Christian, A.; Herkenne, S.; Struman, I.; Muller, M. The Food Dye Tartrazine Disrupts Vascular Formation Both in Zebrafish Larvae and in Human Primary Endothelial Cells. Sci. Rep. 2024, 14, 30367. [Google Scholar] [CrossRef]
- Linskens, A. The Long Term Effects of Tartrazine (FD&C Yellow No. 5) on Learning, Cognitive Flexibility, and Memory of Zebrafish (Danio rerio) Embryos into Adulthood. Available online: https://cpb-us-w2.wpmucdn.com/sites.uwm.edu/dist/8/202/files/2018/06/Linskens_paper_LM_Seymour_2018-1lxiur8.pdf (accessed on 24 July 2025).
- Meena, G.; Meena, B. Evaluation of Possible Toxic Effect of Tartrazine Food dye on Swiss Albino Mice, and Histology of Testis. Int. J. Innov. Res. Multidiscip. Field 2020, 6, 100–105. Available online: https://www.researchgate.net/publication/353750136_Evaluation_of_Possible_Toxic_Effect_of_Tartrazine_Food_dye_on_Swiss_Albino_Mice_and_Histology_of_Testis (accessed on 9 September 2025).
- Arefin, S.; Hossain, M.S.; Neshe, S.A.; Rashid, M.O.; Amin, M.T.; Hussain, S. Tartrazine Induced Changes in Physiological and Biochemical Parameters in Swiss Albino Mice, Mus Musculus. Marmara Pharm. J. 2017, 21, 564–569. [Google Scholar] [CrossRef]
- Kamel, M.M.; El-Lethey, H.S. The Potential Health Hazard of Tartrazine and Levels of Hyperactivity, Anxiety-Like Symptoms, Depression and Anti-Social Behaviour in Rats. Am. J. Sci. 2011, 7, 1211–1218. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3298281 (accessed on 24 July 2025).
- Usman, J.N.; Muhammad, G.A. Sub-acute toxicity study on tartrazine in male albino rats. Dutse J. Pure Appl. Sci. 2022, 8, 97–105. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Wabaidur, S.M.; AlOthman, Z.A.; Habila, M.A. Evaluation of Nano-Curcumin Effects against Tartrazine-Induced Abnormalities in Liver and Kidney Histology and Other Biochemical Parameters. Food Sci. Nutr. 2022, 10, 1344–1356. [Google Scholar] [CrossRef]
- Iroh, G.; Weli, B.O.; Adele, U.A.; Briggs, O.N.; Waribo, H.A.; Elekima, I. Assessment of Atherogenic Indices and Markers of Cardiac Injury in Albino Rats Orally Administered with Tartrazine Azo Dye. J. Adv. Med. Pharm. Sci. 2020, 22, 51–61. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; Hashem, M.M.; El-Metwally, A.E.; Anwar, A.; Abo-EL-Sooud, K.; Moustafa, G.G.; Ali, H.A. Comparative Haemato-Immunotoxic Impacts of Long-Term Exposure to Tartrazine and Chlorophyll in Rats. Int. Immunopharmacol. 2018, 63, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; Sevastre, B.; Mireşan, V.; Taulescu, M.; Raducu, C.; Longodor, A.L.; Marchiş, Z.; Mariş, C.S.; Coroian, A. Protective Effect of Blackthorn Fruits (Prunus spinosa) against Tartrazine Toxicity Development in Albino Wistar Rats. BMC Chem. 2019, 13, 104. [Google Scholar] [CrossRef]
- Himri, I.; Bellahcen, S.; Souna, F.; Belmakki, F.; Aziz, M.; Bnouham, M.; Zoheir, J.; Berkia, Z.; Mekhfi, H.; Saalaoui, E. A 90-Day Oral Toxicity Study of Tartrazine, a Synthetic Food Dye, in Wistar Rats. Int. J. Pharm. Pharm. Sci. 2011, 3, 159–169. Available online: https://www.scirp.org/journal/paperinformation?paperid=20481 (accessed on 15 July 2025).
- El-Sakhawy, M.A.; Mohamed, D.W.; Ahmed, Y.H. Histological and Immunohistochemical Evaluation of the Effect of Tartrazine on the Cerebellum, Submandibular Glands, and Kidneys of Adult Male Albino Rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 9574–9584. [Google Scholar] [CrossRef]
- Abd, B.E.; Naby, E.; Shalaby, R.A.; Fouda, F.M.; Ebiya, R.A. Evaluation of biochemical effects of tartrazine and curcumin in male albino rats. World J. Pharm. Res. 2022, 11, 1472–1486. [Google Scholar] [CrossRef]
- Boroumand, N.; Samarghandian, S.; Isaac Hashemy, S. Immunomodulatory, anti-inflammatory, and antioxidant effects of curcumin. J. Herbmed. Pharmacol. 2018, 7, 211–219. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory Properties of Curcumin, a Major Constituent of Curcuma longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14, 141–153. Available online: https://pubmed.ncbi.nlm.nih.gov/19594223/ (accessed on 15 July 2025).
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-inflammatory effects of curcumin in the inflammatory diseases: Status, limitations and countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary Intake of Carotenoids and Their Antioxidant and Anti-Inflammatory Effects in Cardiovascular Care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, R.; Chen, J.; Yang, N.; Li, K.; Zhang, Z.; Zhang, R. Study of the Anti-Inflammatory Mechanism of β-Carotene Based on Network Pharmacology. Molecules 2023, 28, 7540. [Google Scholar] [CrossRef]
| Category | Product Examples | References |
|---|---|---|
| Food products | TZ is widely used to impart an intense yellow color in various food products such as bread, beverages, cereals, peanuts, candies, jellies, chewing gum, flavored chips, creams, ice cream, yogurts, cakes, instant desserts, soups, sauces, jams, flavored rice, and pasta. Due to potential adverse effects, there is a growing tendency to replace it with natural pigments like annatto or β-carotene. | [2,35,37,40,41,42,43,45] |
| Pharmaceuticals | TZ is used as a coloring excipient in multivitamins, gelatin capsules, tablets, syrups, and pediatric medicines. In sensitive individuals, it may cause allergic reactions or asthma. | [33,36,38,39,40] |
| Non-food products | TZ is also present in non-food products such as soaps, cosmetics, shampoos, hair conditioners, pastels, crayons, and stamp dyes. Skin contact may cause hypersensitivity reactions. | [25,34,44,45] |
| Experimental Organism | n= | Species/Strain | Age/Weight | Dose | Time | Method of Administration | Sample | Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Mice | 60 | Swiss white mice | 25–30 g | 2.5 and 5 mg/kg | day one of pregnancy to day 15 after birth. | oral gavage | Brain tissue | ↑ MDA ↓ GSH ↓ SOD | [71] |
| Rats | 18 | Young male albino rats | 28 days, 60–80 g | 320 mg/kg tartrazine in 1 mL distilled water, daily | 4 weeks | oral gavage | Brain | ↓ GPx ↑ MDA | [72] |
| 50 | Wistar male albino rats | 180 and 200 g | 7.5 mg/kg | 90 days | diets containing dry mass | Liver | ↑ MDA ↓ GSH | [30] | |
| Serum | ↓ SOD ↓ CAT ↓ GPx | ||||||||
| 24 | Male Wistar rats | 10–12 weeks, 180–200 g | 2, 6, 10 mg/kg (erythrosine + TZ 50:50 mix) | 6 weeks | oral gavage | Brain tissue | ↑ MDA ↓ GSH ↓ CAT ↑ ACHe | [73] | |
| 18 | Male albino rats | 175–185 g | 10 mg/kg (+3.75 mg/kg sulfanilic acid) | 8 weeks | oral administration | Serum, liver, and kidney tissue homogenate | ↑ MDA ↓ GSH ↓ SOD ↓ CAT ↓ GR | [74] | |
| 18 | White albino rats | - | Low (10 mg/kg) and high (50 mg/kg) doses | 15 and 30 days | oral administration | Serum | ↓ SOD | [1] | |
| 30 | Sprague–Dawley male albino rats | 150–200 g | 75 mg/kg | 90 days | oral administration by orogastric gavage | Hepatic and renal tissue homogenate | ↑ MDA ↓ GSH ↓ SOD ↓ CAT | [75] | |
| 18 | Male albino rats | 10–15 weeks, 190–250 g | 400 mg/kg | 30 days | oral administration | Serum | ↑ MDA ↓ GSH ↓ SOD ↓ CAT ↓ GPx | [29] | |
| 40 | Female Wistar albino rats | 225–250 g | 500 mg/kg | 21 days | oral gavage | Tissue homogen ates | ↑ MDA ↑ SOD ↑ TOS ↓ GSH ↓ CAT ↓ TAS | [56] | |
| 40 | Adult female Wistar rats | 225–250 g, 8–10 weeks | 500 mg/kg | 3 weeks | oral gavage | Tissue homogenates | ↑ MDA ↑ TOS ↓ GSH ↓ SOD ↓ CAT ↓ TAS | [57] | |
| 30 | Adult male Sprague–Dawley rats | 120–150 g | 200 mg/kg | 60 days | oral administration | Tissue homogenate | ↑ MDA | [61] | |
| 40 | Female Wistar rats | 225–250 g | 500 mg/kg | 3 weeks | oral gavage | Tissue homogenate | ↑ MDA ↑ TOS ↓ GSH ↓ TAS ↓ SOD ↓ CAT | [59] | |
| 20 | Male Wistar rats | 130 ± 40 g | 300 mg | 30 days | oral administration | Tissue homogenate | ↑ MDA ↑ CAT ↑ GST | [62] | |
| 36 | Young male albino rats | 60–80 g | low doses of TZ 15 mg/kg bw | 30 days | oral administration | Liver tissue homogenate | ↑ MDA ↓ CAT ↓ SOD | [68] | |
| high doses were 500 mg/kg bw | ↑ MDA ↓ CAT ↓ SOD ↓ GSH | ||||||||
| 40 | Sprague–Dawley rats | 70 ± 10 g | 0, 175, 350, and 700 mg/kg bw | 30 days | oral gavage | Brain tissue | ↓ GSH ↓ SOD ↑ MDA | [51] |
| Cell Type | Concentration Tested | Tests Performed | Key Findings | Ref. |
|---|---|---|---|---|
| Human lymphocytes | 0.25–64.0 mM | MTT assay, alkaline comet assay | No cytotoxicity; genotoxic at all doses; partial DNA repair. | [55] |
| Human leukocytes | 5–500 μg/mL | Trypan Blue viability, Micronucleus test, Comet assay, Cytogenetics, In silico | No cytotoxicity/mutagenicity; DNA damage at ≥70 μg/mL; supported by in silico models. | [77] |
| Human foreskin fibroblasts | 10, 100, 250, 500, 1000, and 2000 μg/mL for various dyes | MTT assay, ROS, lipid peroxidation, LDH | TZ: no effect; indigo carmine/chlorophyllin cytotoxic at high doses | [78] |
| Yeast assay, MCF-7 breast cancer cells | Not specified (short-term) | Estrogenic activity, LDH release, micronucleus test | 8-PN showed the strongest estrogenic effect, followed by TZ and genistein; all exhibited low cytotoxicity and no genotoxicity. | [79] |
| Human lymphocytes, GR-M melanoma cells | 2.5, 5, and 10 mM | Chromosome aberration, CBMN assay, trypan blue test | No genotoxicity in lymphocytes; low cytotoxicity in lymphocytes; high cytotoxicity in melanoma cells | [80] |
| HaCaT | 20 µM, 40 µM, and 80 µM | qRT-PCR Alkaline Comet Assay | Upregulated DNMT and HDAC genes with increased DNA fragmentation, indicating epigenetic and genotoxic effects. | [81] |
| HepG2 | ||||
| A549 |
| Experimental Model | Number (n=) | Method of Administration | Time | Dose | Analysis | Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Zebrafish | |||||||
| Zebrafish embryo | 280 (20/concentration) | Exposed to E3 medium with varying TZ concentrations in Petri dishes | 24, 48, 72, 96, 120, 144, 168 hpf | 0, 0.1, 1, 2, 3, 4, 5, 10, 20, 30, 40, 50, 75, 100 mM | Developmental anomalies (heart rate, edema, tail distortion, hatching, mortality) observed via bright field microscopy. | Control embryos hatched normally; ≥10 mM caused early hatching with deformities and ≥40 mM increased mortality. | [82] |
| 25 embryos/well | Embryos exposed in 6-well plates with E3 medium supplemented with TZ | 3–4 h post-fertilization to 4 dpf | 0, 5, 10, 20, 50 g/L | Zebrafish embryo toxicity and vascular defects. | Dose-dependent vascular defects: hemorrhage, edema, small eye, vessel abnormalities. | [85] | |
| 20 embryos/well | Exposed in E3 medium | 72 h (hpf) | 5–100 mM (various concentrations) | Developmental and cardiac toxicity parameters | TZ caused dose-dependent drops in survival, hatching, cardiac/yolk sac edema, spinal defects, and heart rate. | [83] | |
| 9 | Exposure via aquatic media | 6 months to a year | 22 μM | Behavioral tests: T-maze test, cognitive flexibility, memory, learning, perseverance, consistency in choices. | Learning, memory, and flexibility impaired task completion and perseverance reduced. | [86] | |
| 100 | Exposed to varying erythrosine and TZ levels in embryo water. | Up to 10 dpf | Erythrosine: 0.001–0.1%; TZ: 0.01–0.5% | Biochemical and genetic analyses | High TZ (≥0.5%) boosted hatching (55% at 48 hpf, 100% at 72 hpf) and triggered SOD1 expression via OS. | [84] | |
| Mice | |||||||
| KunMing mouse (20 ± 2 g) | 40 | Oral gavage | 30 days | 0, 175, 350, and mg/kg body mass | Behavioral (Step-through, Morris maze) and biochemical tests | TZ negatively affects learning and memory in mice, increasing escape time and reducing reaction time in tests. | [51] |
| Male Swiss albino mice (4 weeks) | 15 | Oral administration | 72 days | 100 and 200 mg/kg | Histological analyses | Increased bw, mild deformation of seminiferous tubules, moderate reduction of Leydig and Sertoli cells, fewer spermatozoa | [87] |
| Swiss albino mice (25–30 g) | 15 | Oral administration | 25 days | 200 mg/kg | Physiological and biochemical analyses | Insignificant decrease in the cholesterol level, no significant in triglyceride, significantly increased bilirubin and creatinine | [88] |
| 400 mg/kg | Insignificant decrease in the cholesterol level, significant increase in triglycerides, bilirubin, and creatinine | ||||||
| Rats | |||||||
| Sprague–Dawley rats (70 ± 10 g) | 40 | Oral gavage | 30 days | 175, 350, 700 mg/kg body mass | Behavioral tests: Open-field test. Biochemical analyses | TZ increases activity and anxiety in rats, also causing histopathological changes in the brain. | [51] |
| Male Wistar rats (40–50 g) | 45 | Dissolved in tap drinking water | 16 weeks | 0%, 1% (low dose) and 2.5% (high dose) | Behavioral tests: Open field behaviour test Elevated plus maze test Light-Dark transition task Forced swim test Social interaction test | The study highlights the harmful effects of TZ on anxiety and depression, highlighting the risks of long-term exposure to food dyes on mental health. | [89] |
| Young male albino rats (28 days old, 60–80 g) | 18 | Oral gavage | 4 weeks | 320 mg/kg TZ in 1 mL distilled water, once daily. | Neurobiological and histological analysis: Brains were harvested and analyzed for histological changes. | TZ has a neurotoxic effect, evidenced by histological changes such as neuronal apoptosis and vascular congestion. | [72] |
| White albino rats of either sex | 18 | Oral administration | 15 and 30 days | Low dose: 10 mg/kg High dose: 50 mg/kg | Biochemical, hormonal, and histological analyses | TZ disrupts glucose balance, damages pancreas, alters endocrine function. Increases glucose, lipase decreases insulin, Ca, Mg | [1] |
| Male albino rats | 18 | Oral administration | 8 weeks | 10 mg/kg (+3.75 mg/kg sulfanilic acid) | Biochemical and histological | It caused liver and kidney dysfunction with lesions. Increased cholesterol, triglycerides, LDL, VLDL, ALT, AST, ALP, bilirubin, creatinine, urea, uric acid. Decreased HDL, total protein. | [74] |
| Wistar male albino rats | 50 | Diets containing dry mass | 90 days | 7.5 mg/kg | Biochemical and histological analyses | TZ raised lipids, liver enzymes, kidney function. Increased total cholesterol, triglycerides, LDL, ALT, AST, ALP, LDH. | [30] |
| Female Wistar albino rats (225–250 g) | 40 | Oral gavage | 21 days | 500 mg/kg | Biochemical analyses and histopathological examinations | Increased AST, ALT, ALP indicating liver damage. | [56] |
| Male albino rats (65–80 g) | 12 | Oral administration | 7 weeks | 7.5 and 75 mg/kg | Biochemical and histopathological analyses | Study showed harmful lipids, biochemical changes, and liver/kidney damage. Increased cholesterol, triglycerides, LDL, VLDL, ALT, AST, ALP, creatinine, urea, uric acid. | [90] |
| Male Wistar albino rats (200–250 g) | 40 | Oral administration | 50 days | 7.5 mg/kg | Biochemical and histopathological analyses | TZ impaired liver/kidney, altered histology, lipids, glucose. Increased ALT, AST, ALP, GGT, urea, uric acid, creatinine, protein, cholesterol, triglycerides, LDL decreased HDL. | [91] |
| Adult female Wistar rats (225–250 g, 8–10 weeks old) | 40 | Oral gavage | 3 weeks | 500 mg/kg | Biochemical and histopathological analyses | TZ caused degenerative and metaplastic changes in ileum and colon epithelium. | [57] |
| Wistar albino rats (146–153 g) | 20 | Dissolved in 1 mL of distilled water | 30 days | 7.5 mg/kg bw | Biochemical, histological, and ultrastructural analyses | TZ raised AST, ALT, ALP, uric acid, urea, creatinine, reduced antioxidants, and caused liver and kidney damage. | [26] |
| Male Wistar rats (10–12 weeks old, 180–200 g) | 24 | Oral gavage | 6 weeks | 2, 6, 10 mg/kg (50:50 erythrosine-TZ) | Behavioral (open field test, forced swimming test, tail suspension test), biochemical and enzymatic analyses | Increased nitrite, TNF-α worsened anxiety and depression. | [73] |
| Sprague–Dawley male albino rats (150–200 g) | 30 | Oral administration by orogastric gavage | 90 days | 75 mg/kg | Biochemical, genetic, immunohistochemical, histology analyses | Increased AST, ALT, urea, creatinine liver and kidney damage. | [75] |
| Female Wistar albino rats (225–250 g) | 40 | Oral gavage | 21 days | 500 mg/kg | Biochemical and histopathological analyses | TZ caused kidney glomerular collapse, inflammation, congestion. | [58] |
| Albino rats (~0.2 kg) | 63 | Oral administration | 30 and 60 days | 7.5 mg/kg | Biochemical and histopathological analyses | TZ damages heart, raises nHDL and creatine kinase, increasing cardiovascular risk. | [92] |
| Male rats (10–15 weeks old, 190–250 g) | 18 | Oral administration | 30 days | 400 mg/kg | Biochemical analyses | Increased ALT, AST, ALP, urea, uric acid, creatinine decreased Na, K, Ca. | [60] |
| Adult male Sprague–Dawley albino rats | 30 | Oral administration | 90 days | 1.35 mg/kg | Hematological, immunological, and histopathological analyses | Decreased hemoglobin, RBC, PCV%, platelets increased WBC, neutrophils, lymphocytes, monocytes. | [93] |
| Female Wistar rats (225–250 g) | 40 | Oral gavage | 3 weeks | 500 mg/kg | Biochemical and histopathological analyses | Increased total cholesterol, glucose, triglycerides, LDL, VLDL decreased HDL. | [59] |
| Albino Wistar rats | 20 | Oral administration | 7 weeks | 75 mg/250 mL water 100 mg/250 mL water | Biochemical, hematological, and histopathological analyses | TZ damaged liver, kidneys, spleen no change in cholesterol, triglycerides, ALT. Increased AST, creatinine, WBC, neutrophils, lymphocytes. | [94] |
| Adult male Sprague–Dawley rats (120–150 g) | 30 | Oral administration | 60 days | 200 mg/kg | Biochemical, histological, and physiological analyses | Subchronic TZ affects liver and kidney parameters and induces OS. Increased ALT, AST, urea, total protein. | [61] |
| Male and female Wistar rats (170–200 g) | 30 | Oral administration | 13 weeks | 5 mg/kg | Hematological and histopathological analyses | No effect | [95] |
| 7.5 mg/kg | Decreased platelets increased neutrophils, basophils, and mean platelet volume. | ||||||
| 10 mg/kg | No effect | ||||||
| Adult male albino rats (120–150 g) | 40 | Oral gavage | 30 days | 7.5 mg/kg bw | Histopathological and Immunohistochemical analyses | TZ causes structural damage in cerebellum, glands, kidneys, with edema, congestion, neuron vacuolization, and cell deformation. | [96] |
| 15 mg/kg bw | Edema, dilated perineural spaces, and degenerating Purkinje cells. | ||||||
| 100 mg/kg bw | Severe Purkinje cell degeneration, gray matter vacuolization, edema, nuclear pyknosis, vessel engorgement, increased astrocytes | ||||||
| Male Wistar rats (130 ± 40 g) | 20 | Oral administration | 30 days | 300 mg | Biochemical and histopathological analyses | Increased transaminases, LDH, creatinine, uric acid, kidney proteins decreased total protein, albumin, globulin HDL unchanged. | [62] |
| Young male albino rats (Rattus norvegicus), 60–80 g | 36 | Oral administration | 30 days | Low dose: 15 mg/kg bw | Biochemical analyses | Increased ALT, AST, ALP, total protein, albumin, globulin, creatinine, urea decreased serum cholesterol. | [68] |
| High dose: 500 mg/kg bw | Increased ALP, total protein, albumin, creatinine, urea | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visternicu, M.; Săvucă, A.; Rarinca, V.; Burlui, V.; Plavan, G.; Ionescu, C.; Ciobica, A.; Balmus, I.-M.; Albert, C.; Hogas, M. Toxicological Effects of Tartrazine Exposure: A Review of In Vitro and Animal Studies with Human Health Implications. Toxics 2025, 13, 771. https://doi.org/10.3390/toxics13090771
Visternicu M, Săvucă A, Rarinca V, Burlui V, Plavan G, Ionescu C, Ciobica A, Balmus I-M, Albert C, Hogas M. Toxicological Effects of Tartrazine Exposure: A Review of In Vitro and Animal Studies with Human Health Implications. Toxics. 2025; 13(9):771. https://doi.org/10.3390/toxics13090771
Chicago/Turabian StyleVisternicu, Malina, Alexandra Săvucă, Viorica Rarinca, Vasile Burlui, Gabriel Plavan, Cătălina Ionescu, Alin Ciobica, Ioana-Miruna Balmus, Cristina Albert, and Mihai Hogas. 2025. "Toxicological Effects of Tartrazine Exposure: A Review of In Vitro and Animal Studies with Human Health Implications" Toxics 13, no. 9: 771. https://doi.org/10.3390/toxics13090771
APA StyleVisternicu, M., Săvucă, A., Rarinca, V., Burlui, V., Plavan, G., Ionescu, C., Ciobica, A., Balmus, I.-M., Albert, C., & Hogas, M. (2025). Toxicological Effects of Tartrazine Exposure: A Review of In Vitro and Animal Studies with Human Health Implications. Toxics, 13(9), 771. https://doi.org/10.3390/toxics13090771










