ITF6475, a New Histone Deacetylase 6 Inhibitor, Prevents Painful Neuropathy Induced by Paclitaxel
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Animal Model
2.2. Nerve Conduction Studies
2.3. Pharmacokinetic Assessment
2.4. Dynamic Aesthesiometer Test
2.5. Histopathological Analysis
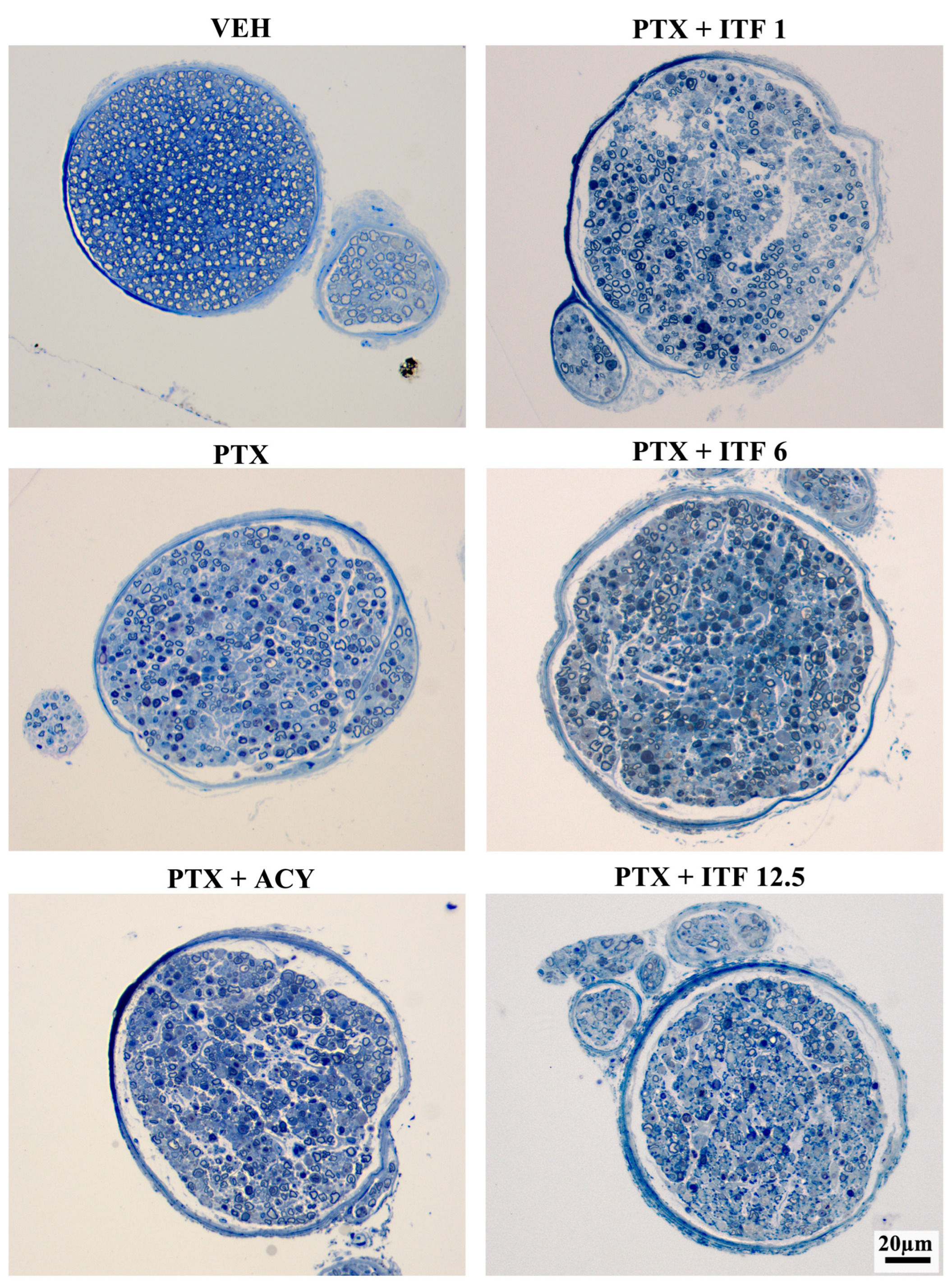
2.6. Neuropathology
2.7. Skin Biopsy
2.8. Serum for NfL Analysis
2.9. Statistical Analysis
3. Results
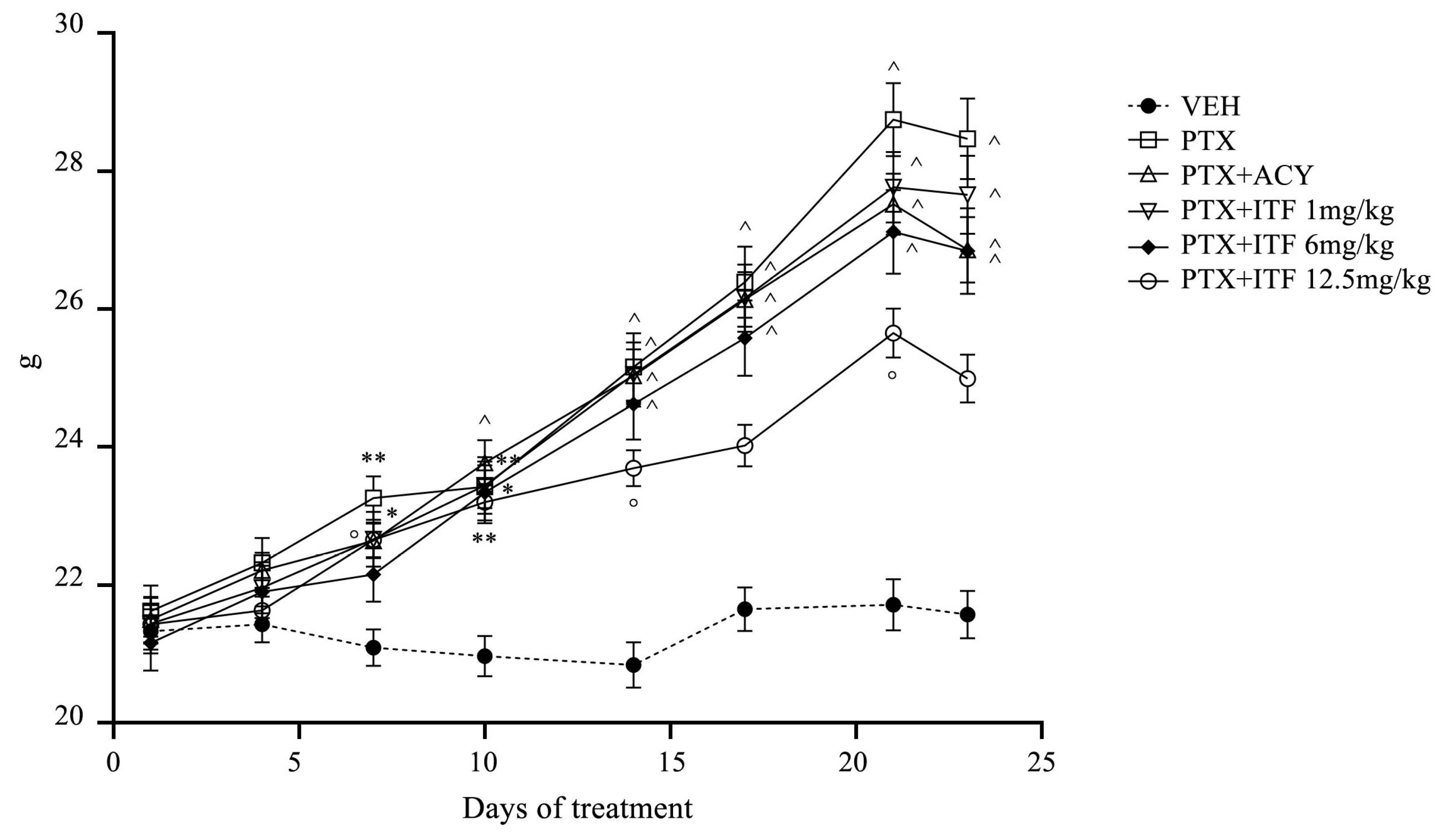
3.1. General Toxicity
3.2. Pharmacokinetic Assessment
3.3. Neurotoxicity Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIPN | chemotherapy-induced neuropathy |
| DMSO | dimethyl sulfoxide |
| EDTA | ethylenediaminetetraacetic acid |
| GTPase | guanosine triphosphate hydrolase |
| HDAC | histone deacetylase |
| IENF | intraepidermal nerve fibers |
| i.v. | intravenous |
| mpk | mg/kg |
| NCV | sensory nerve conduction velocity |
| PEG | polyethylene glycol |
| PGP 9.5 | protein gene product 9.5 |
| PIPN | paclitaxel-induced peripheral neuropathy |
| PLP | paraformaldehyde-lysine and periodate sodium |
| p.o. | oral |
| PTX | paclitaxel |
| SNAP | sensory nerve action potential amplitude |
| VEH | paclitaxel vehicle |
References
- Amarelo, A.; Amarelo, B.; Ferreira, M.C.; Fernandes, C.S. Living with chemotherapy-induced peripheral neuropathy: A qualitative meta-synthesis of patient experiences. Eur. J. Oncol. Nurs. 2025, 77, 102921. [Google Scholar] [CrossRef]
- Su, Y.C.; Shih, Y.H.; Lee, Y.H.; Chang, P.H. Survivors of Non-Hodgkin’s Lymphoma: A Comparative Study on Patients with Vincristine-Induced Neuropathy and Their Quality of Life. J. Nurs. Res. 2025, 33, e393. [Google Scholar] [CrossRef] [PubMed]
- Gehr, N.L.; Timm, S.; Bennedsgaard, K.; Grosen, K.; Jakobsen, E.; Jensen, A.B.; Rønlev, J.D.; Knoop, A.S.; Finnerup, N.B.; Ventzel, L. Chronic chemotherapy-induced peripheral neuropathy and pain following paclitaxel versus docetaxel in breast cancer survivors: A cross-sectional study. Breast 2025, 80, 104424. [Google Scholar] [CrossRef] [PubMed]
- Yarosh, R.A.; Nichols, H.B.; Wang, Q.; Hirschey, R.; Kent, E.E.; Carey, L.A.; Hayes, S.C.; Ogunleye, A.A.; Troester, M.A.; Butler, E.N. Patient-reported persistent lymphedema and peripheral neuropathy among long-term breast cancer survivors in the Carolina Breast Cancer Study. Cancer 2025, 131, e35650. [Google Scholar] [CrossRef]
- Teng, C.; Cohen, J.; Egger, S.; Blinman, P.L.; Vardy, J.L. Systematic review of long-term chemotherapy-induced peripheral neuropathy (CIPN) following adjuvant oxaliplatin for colorectal cancer. Support Care Cancer 2022, 30, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Basal, C.; Seluzicki, C.; Li, S.Q.; Seidman, A.D.; Mao, J.J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 2016, 159, 327–333. [Google Scholar] [CrossRef]
- Rhee, J.Y.; Paulino, M.T.; Finnemore, A.; Tentor, Z.; Cashman, C. Recent Advances in Diagnosis, Management, Treatment, and Prevention of Neuropathies in Cancer Patients. Curr. Neurol. Neurosci. Rep. 2025, 25, 42. [Google Scholar] [CrossRef]
- Loureiro, J.; Costa-Pereira, J.T.; Pozza, D.H.; Tavares, I. The Power of Movement: How Exercise Influences Chemotherapy-Induced Peripheral Neuropathy. Biomedicines 2025, 13, 1103. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, W.; Zhang, H.; Rong, Q.; He, M.; Wu, M.; Zhao, Y.; Yang, P. The efficacy of acupuncture in the treatment of chemotherapy-induced peripheral neuropathy in cancer patients: A systematic review and meta-analysis. J. Cancer Surviv. 2025. [Google Scholar] [CrossRef]
- Mallela, T.; Kaufman, L.; Dulmage, B. Regional limb cooling for the prevention of chemotherapy-induced toxicities: A narrative review. Support. Care Cancer 2025, 33, 678. [Google Scholar] [CrossRef]
- Hu, F.; Wang, F.; Ming, Y.; Long, F. Meta-analysis of compression therapy for prevention of chemotherapy-induced peripheral neuropathy. Support. Care Cancer 2025, 33, 549. [Google Scholar] [CrossRef]
- Prager, K.; Passig, K.; Micke, O.; Zomorodbakhsch, B.; Keinki, C.; Hübner, J. Chemotherapy-induced polyneuropathy in cancer care—The patient perspective. Support. Care Cancer 2023, 31, 235. [Google Scholar] [CrossRef]
- Shin, G.J. Towards a mechanistic understanding of axon transport and endocytic changes underlying paclitaxel-induced peripheral neuropathy. Exp. Neurol. 2023, 359, 114258. [Google Scholar] [CrossRef]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.F.; Yao, T.P. HDAC6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef]
- Chen, S.; Owens, G.C.; Makarenkova, H.; Edelman, D.B. HDAC6 regulates mitochondrial transport in hippocampal neurons. PLoS ONE 2010, 5, e10848. [Google Scholar] [CrossRef]
- Squarzoni, A.; Scuteri, A.; Cavaletti, G. HDACi: The Columbus’ Egg in Improving Cancer Treatment and Reducing Neurotoxicity? Cancers 2022, 14, 5251. [Google Scholar] [CrossRef]
- English, K.; Barton, M.C. HDAC6: A Key Link Between Mitochondria and Development of Peripheral Neuropathy. Front. Mol. Neurosci. 2021, 14, 684714. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.; Choi, Y.I.; Jung, N.; Song, J.Y.; Bae, D.K.; Kim, M.C.; Lee, Y.J.; Song, H.; Kwak, G.; Jeong, S.; et al. A novel histone deacetylase 6 inhibitor improves myelination of Schwann cells in a model of Charcot-Marie-Tooth disease type 1A. Br. J. Pharmacol. 2020, 177, 5096–5113. [Google Scholar] [CrossRef] [PubMed]
- Van Helleputte, L.; Kater, M.; Cook, D.P.; Eykens, C.; Rossaert, E.; Haeck, W.; Jaspers, T.; Geens, N.; Vanden Berghe, P.; Gysemans, C.; et al. Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine-induced peripheral neuropathies and inhibits tumor growth. Neurobiol. Dis. 2018, 111, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Zhao, X.; Liu, H.; Hu, Q.; Chen, X.Q.; Pham, J.; Wei, N.; Liu, Z.; Zhou, J.; Burgess, R.W.; et al. Aberrant GlyRS-HDAC6 interaction linked to axonal transport deficits in Charcot-Marie-Tooth neuropathy. Nat. Commun. 2018, 9, 1007. [Google Scholar] [CrossRef]
- Benoy, V.; Van Helleputte, L.; Prior, R.; d’Ydewalle, C.; Haeck, W.; Geens, N.; Scheveneels, W.; Schevenels, B.; Cader, M.Z.; Talbot, K.; et al. HDAC6 is a therapeutic target in mutant GARS-induced Charcot-Marie-Tooth disease. Brain 2018, 141, 673–687. [Google Scholar] [CrossRef]
- Krukowski, K.; Ma, J.; Golonzhka, O.; Laumet, G.O.; Gutti, T.; Van Duzer, J.H.; Mazitschek, R.; Jarpe, M.B.; Heijnen, C.J.; Kavelaars, A. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain 2017, 158, 1126–1137. [Google Scholar] [CrossRef]
- Benoy, V.; Vanden Berghe, P.; Jarpe, M.; Van Damme, P.; Robberecht, W.; Van Den Bosch, L. Development of Improved HDAC6 Inhibitors as Pharmacological Therapy for Axonal Charcot-Marie-Tooth Disease. Neurotherapeutics 2017, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.; Van Helleputte, L.; Benoy, V.; Van Den Bosch, L. Defective axonal transport: A common pathological mechanism in inherited and acquired peripheral neuropathies. Neurobiol. Dis. 2017, 105, 300–320. [Google Scholar] [CrossRef]
- Shen, S.; Kozikowski, A.P. Why Hydroxamates May Not Be the Best Histone Deacetylase Inhibitors—What Some May Have Forgotten or Would Rather Forget? ChemMedChem 2016, 11, 15–21. [Google Scholar] [CrossRef]
- Cellupica, E.; Caprini, G.; Cordella, P.; Cukier, C.; Fossati, G.; Marchini, M.; Rocchio, I.; Sandrone, G.; Vanoni, M.A.; Vergani, B.; et al. Difluoromethyl-1,3,4-oxadiazoles are slow-binding substrate analog inhibitors of histone deacetylase 6 with unprecedented isotype selectivity. J. Biol. Chem. 2023, 299, 102800. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Alberti, P.; Canta, A.; Carozzi, V.; Cherchi, L.; Chiorazzi, A.; Crippa, L.; Marmiroli, P.; Meregalli, C.; Pozzi, E.; et al. Translation of paclitaxel-induced peripheral neurotoxicity from mice to patients: The importance of model selection. Pain 2024, 165, 2482–2493. [Google Scholar] [CrossRef]
- Monza, L.; Fumagalli, G.; Chiorazzi, A.; Alberti, P. Addressing the Need of a Translational Approach in Peripheral Neuropathy Research: Morphology Meets Function. Brain Sci. 2021, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, C.; Marjanovic, I.; Scali, C.; Monza, L.; Spinoni, N.; Galliani, C.; Brivio, R.; Chiorazzi, A.; Ballarini, E.; Rodriguez-Menendez, V.; et al. High-dose intravenous immunoglobulins reduce nerve macrophage infiltration and the severity of bortezomib-induced peripheral neurotoxicity in rats. J. Neuroinflamm. 2018, 15, 232. [Google Scholar] [CrossRef]
- Cavaletti, G.; Pizzamiglio, C.; Man, A.; Engber, T.M.; Comi, C.; Wilbraham, D. Studies to Assess the Utility of Serum Neurofilament Light Chain as a Biomarker in Chemotherapy-Induced Peripheral Neuropathy. Cancers 2023, 15, 4216. [Google Scholar] [CrossRef]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Carozzi, V.A.; Blennow, K.; Zetterberg, H.; et al. Neurofilament light chain: A specific serum biomarker of axonal damage severity in rat models of Chemotherapy-Induced Peripheral Neurotoxicity. Arch. Toxicol. 2020, 94, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- Pero, M.E.; Chowdhury, F.; Bartolini, F. Role of tubulin post-translational modifications in peripheral neuropathy. Exp. Neurol. 2023, 360, 114274. [Google Scholar] [CrossRef]
- Van Eyll, J.; Prior, R.; Celanire, S.; Van Den Bosch, L.; Rombouts, F. Therapeutic indications for HDAC6 inhibitors in the peripheral and central nervous disorders. Expert. Opin. Ther. Targets 2024, 28, 719–737. [Google Scholar] [CrossRef]
- Ma, J.; Trinh, R.T.; Mahant, I.D.; Peng, B.; Matthias, P.; Heijnen, C.J.; Kavelaars, A. Cell-specific role of histone deacetylase 6 in chemotherapy-induced mechanical allodynia and loss of intraepidermal nerve fibers. Pain 2019, 160, 2877–2890. [Google Scholar] [CrossRef]
- Médard, G.; Sheltzer, J.M. Ricolinostat is not a highly selective HDAC6 inhibitor. Nat. Cancer 2023, 4, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Tamburin, S.; Park, S.B.; Alberti, P.; Demichelis, C.; Schenone, A.; Argyriou, A.A. Taxane and epothilone-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. 2), S40–S51. [Google Scholar] [CrossRef]
- Gornstein, E.L.; Schwarz, T.L. Neurotoxic mechanisms of paclitaxel are local to the distal axon and independent of transport defects. Exp. Neurol. 2017, 288, 153–166. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Fukuda, Y.; Li, Y.; Segal, R.A. A Mechanistic Understanding of Axon Degeneration in Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2017, 11, 481. [Google Scholar] [CrossRef]
- Sajic, M.; Ida, K.K.; Canning, R.; Gregson, N.A.; Duchen, M.R.; Smith, K.J. Mitochondrial damage and “plugging” of transport selectively in myelinated, small-diameter axons are major early events in peripheral neuroinflammation. J. Neuroinflamm. 2018, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Bobylev, I.; Joshi, A.R.; Barham, M.; Ritter, C.; Neiss, W.F.; Höke, A.; Lehmann, H.C. Paclitaxel inhibits mRNA transport in axons. Neurobiol. Dis. 2015, 82, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xiao, W.H.; Bennett, G.J. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp. Neurol. 2011, 232, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ligon, L.A.; Steward, O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J. Comp. Neurol. 2000, 427, 351–361. [Google Scholar] [CrossRef] [PubMed]




| Ricolinostat | ||
|---|---|---|
| Dose | AUC0–8h0–8h00 (ng·h/mL) | Cmax (ng/mL) |
| 25 mpk | 124 | 33.7 |
| 100 mpk | 171 | 29.8 |
| 200 mpk | 234 | 33.9 |
| ITF6475 | ||
| Dose | AUC0–24h0–8h00 (ng·h/mL) | Cmax (ng/mL) |
| 25 mpk | 28,958 | 4328 |
| 100 mpk | 157,797 | 14,631 |
| 200 mpk | 271,434 | 22,485 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaletti, G.; Canta, A.; Chiorazzi, A.; Pozzi, E.; Carozzi, V.; Meregalli, C.; Alberti, P.; Marmiroli, P.; Scuteri, A.; Crippa, L.; et al. ITF6475, a New Histone Deacetylase 6 Inhibitor, Prevents Painful Neuropathy Induced by Paclitaxel. Toxics 2025, 13, 767. https://doi.org/10.3390/toxics13090767
Cavaletti G, Canta A, Chiorazzi A, Pozzi E, Carozzi V, Meregalli C, Alberti P, Marmiroli P, Scuteri A, Crippa L, et al. ITF6475, a New Histone Deacetylase 6 Inhibitor, Prevents Painful Neuropathy Induced by Paclitaxel. Toxics. 2025; 13(9):767. https://doi.org/10.3390/toxics13090767
Chicago/Turabian StyleCavaletti, Guido, Annalisa Canta, Alessia Chiorazzi, Eleonora Pozzi, Valentina Carozzi, Cristina Meregalli, Paola Alberti, Paola Marmiroli, Arianna Scuteri, Luca Crippa, and et al. 2025. "ITF6475, a New Histone Deacetylase 6 Inhibitor, Prevents Painful Neuropathy Induced by Paclitaxel" Toxics 13, no. 9: 767. https://doi.org/10.3390/toxics13090767
APA StyleCavaletti, G., Canta, A., Chiorazzi, A., Pozzi, E., Carozzi, V., Meregalli, C., Alberti, P., Marmiroli, P., Scuteri, A., Crippa, L., Fermi, S., Segmani, I., Vergani, B., Steinkühler, C., & Licandro, S. A. (2025). ITF6475, a New Histone Deacetylase 6 Inhibitor, Prevents Painful Neuropathy Induced by Paclitaxel. Toxics, 13(9), 767. https://doi.org/10.3390/toxics13090767










