Methamphetamine Exposure Induces Neuronal Programmed Necrosis by Permeabilizing Mitochondria via the RIPK1–RIPK3–MLKL Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Primary Cortical Cell Culture
2.3. Hoechst 33342/Propidium Iodide (PI) Dual Staining
2.4. Lactate Dehydrogenase (LDH) Detection
2.5. Mitochondrial Membrane Potential Detection
2.6. MitoROS Production Assay
2.7. Quantitative RT-PCR
2.8. mtDNA Copy Number
2.9. ATP Detection
2.10. Western Blot Assay
2.11. Immunofluorescence Analysis
2.12. Quantitative Analysis
3. Results
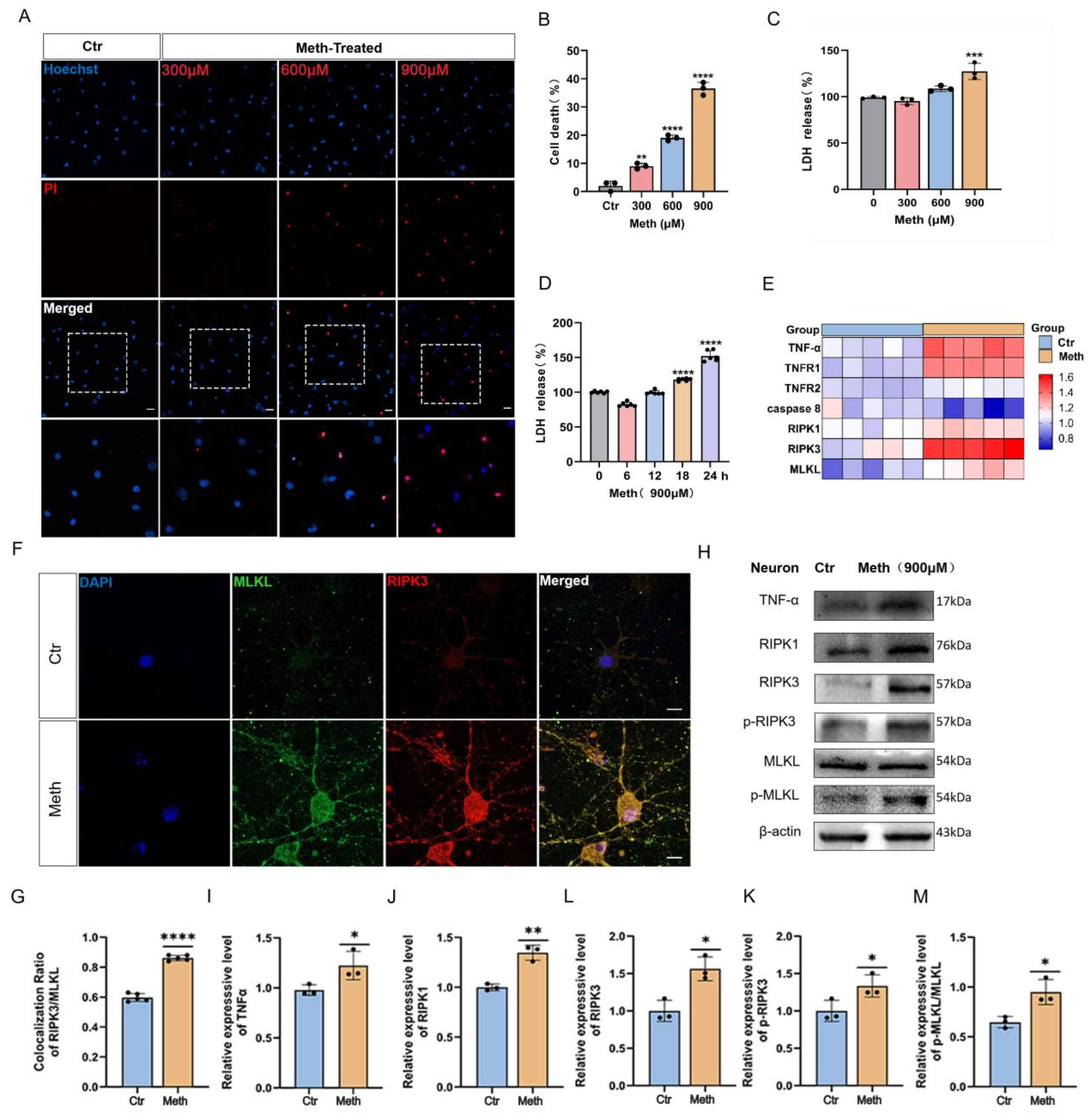
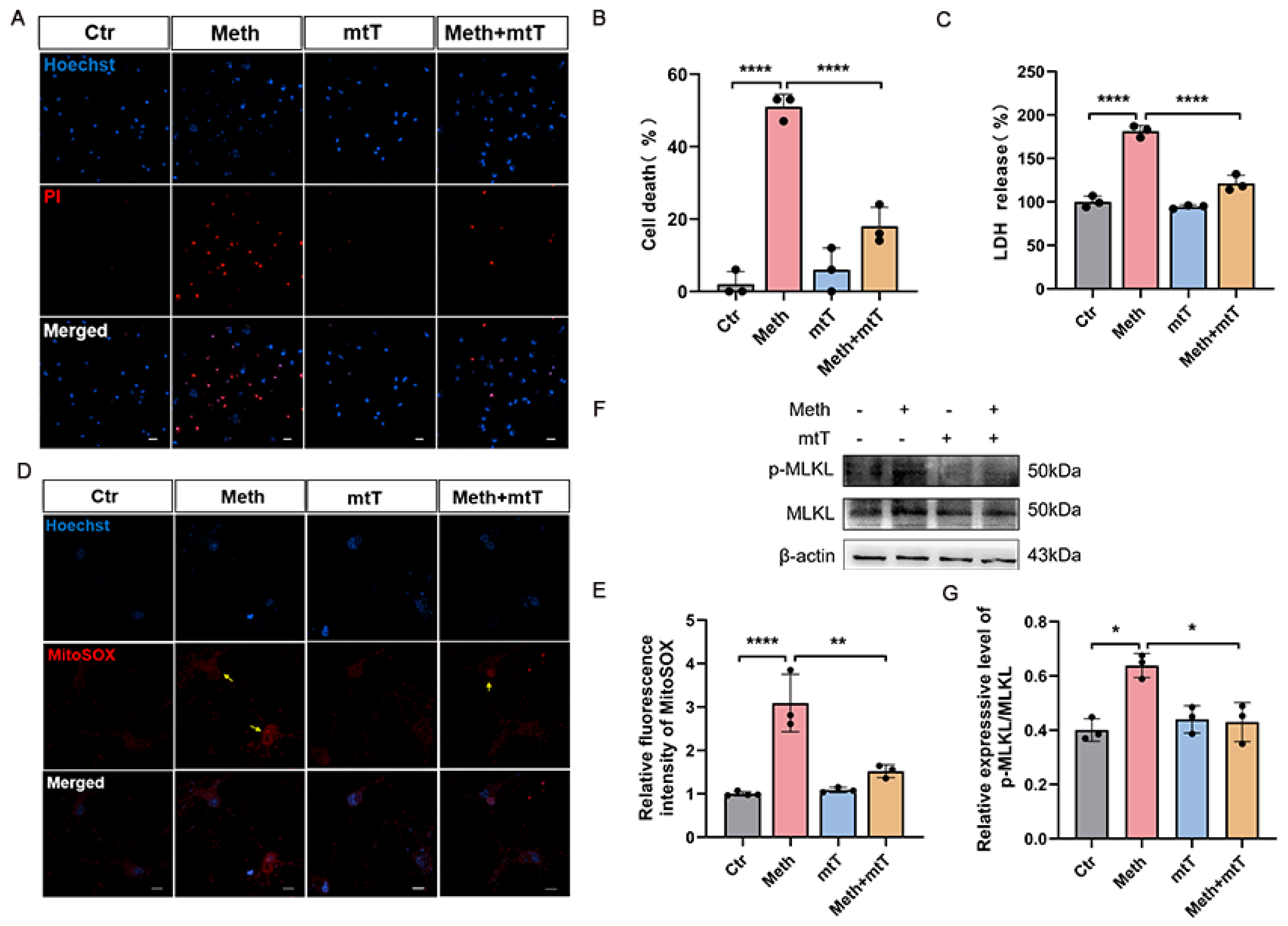
3.1. Methamphetamine Induces Necroptosis in Primary Neurons
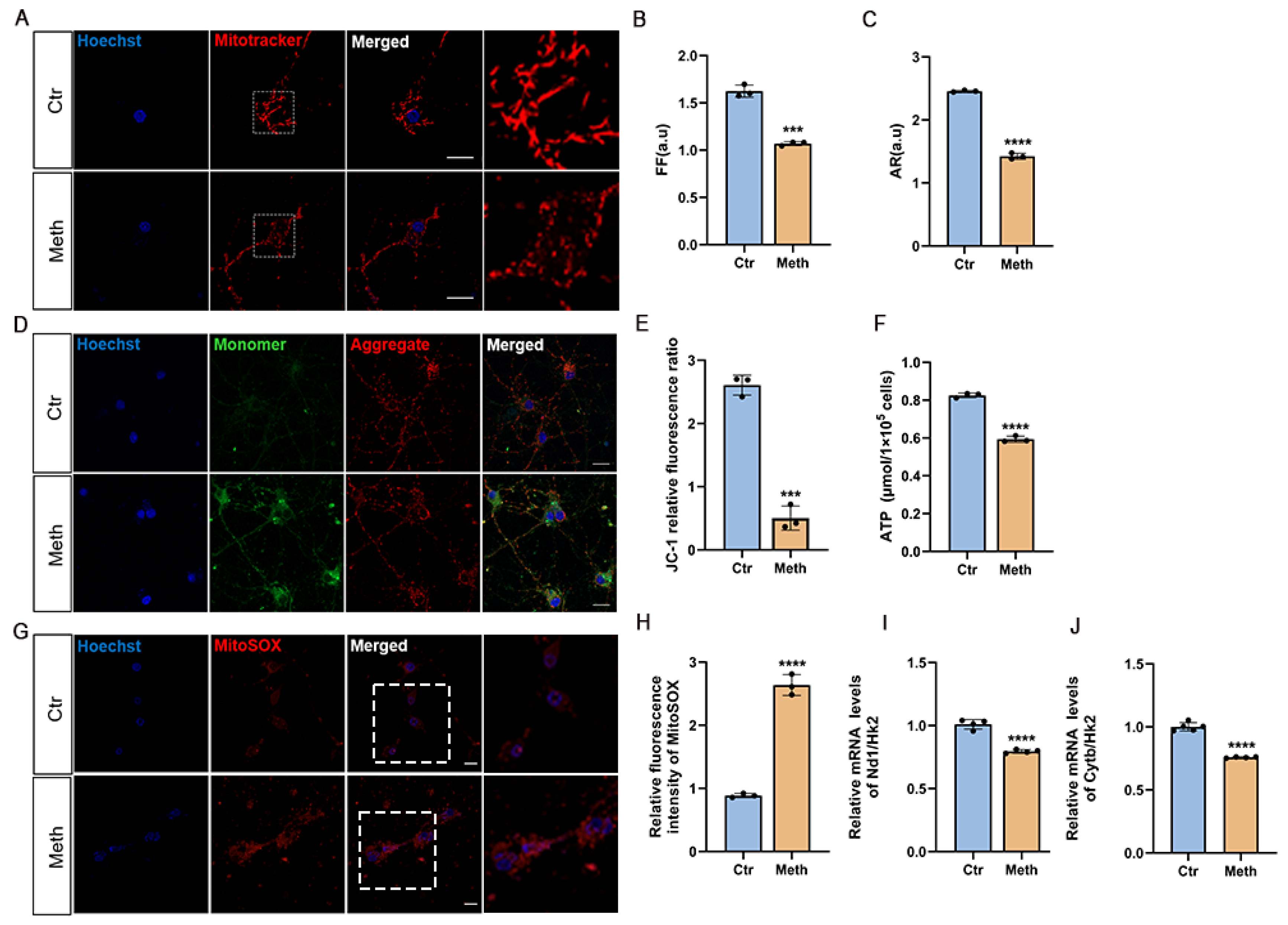
3.2. Methamphetamine Induces Mitochondrial Dysfunction
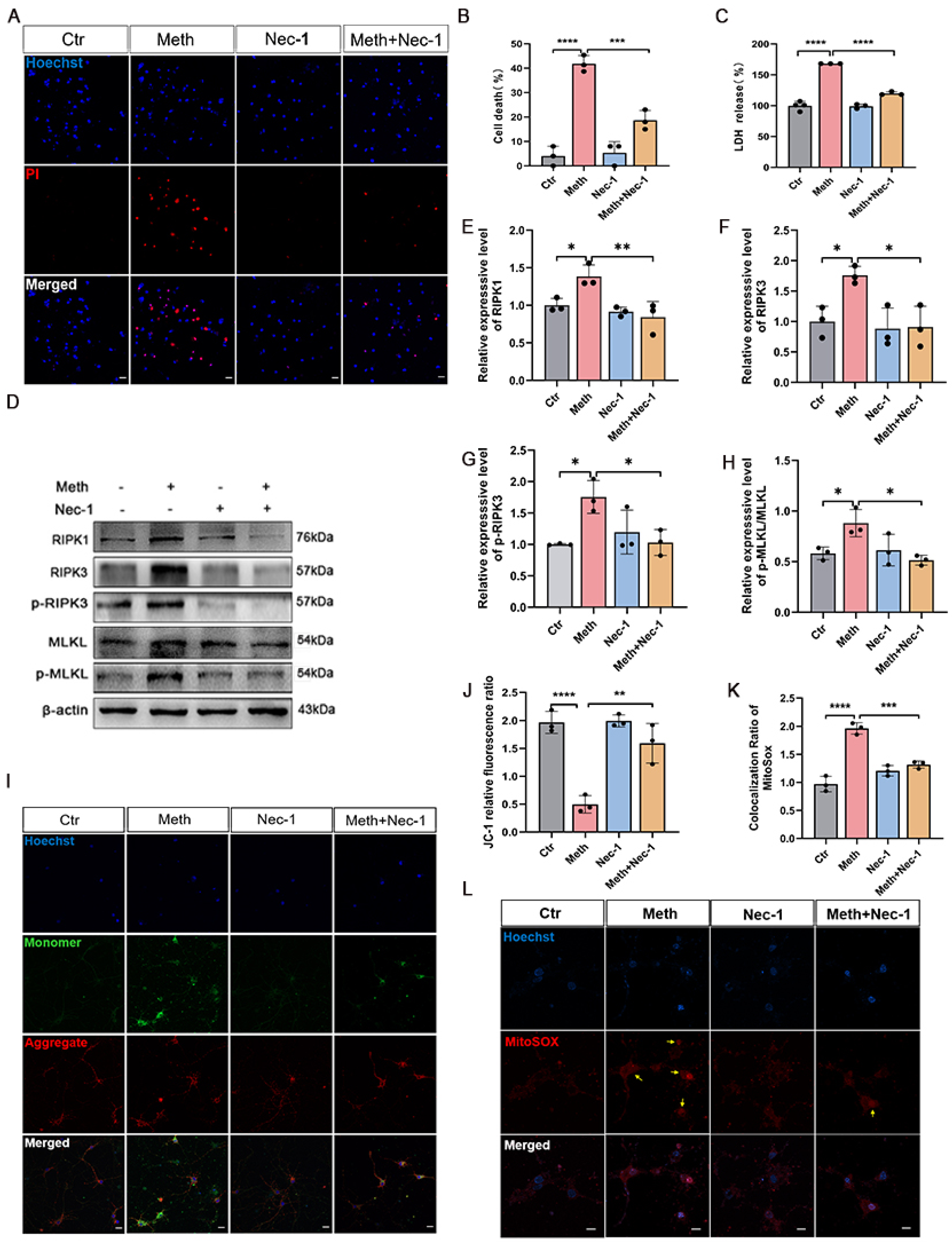
3.3. The RIPK1–RIPK3 Axis Is Involved in Meth-Induced Mitochondrial Damage
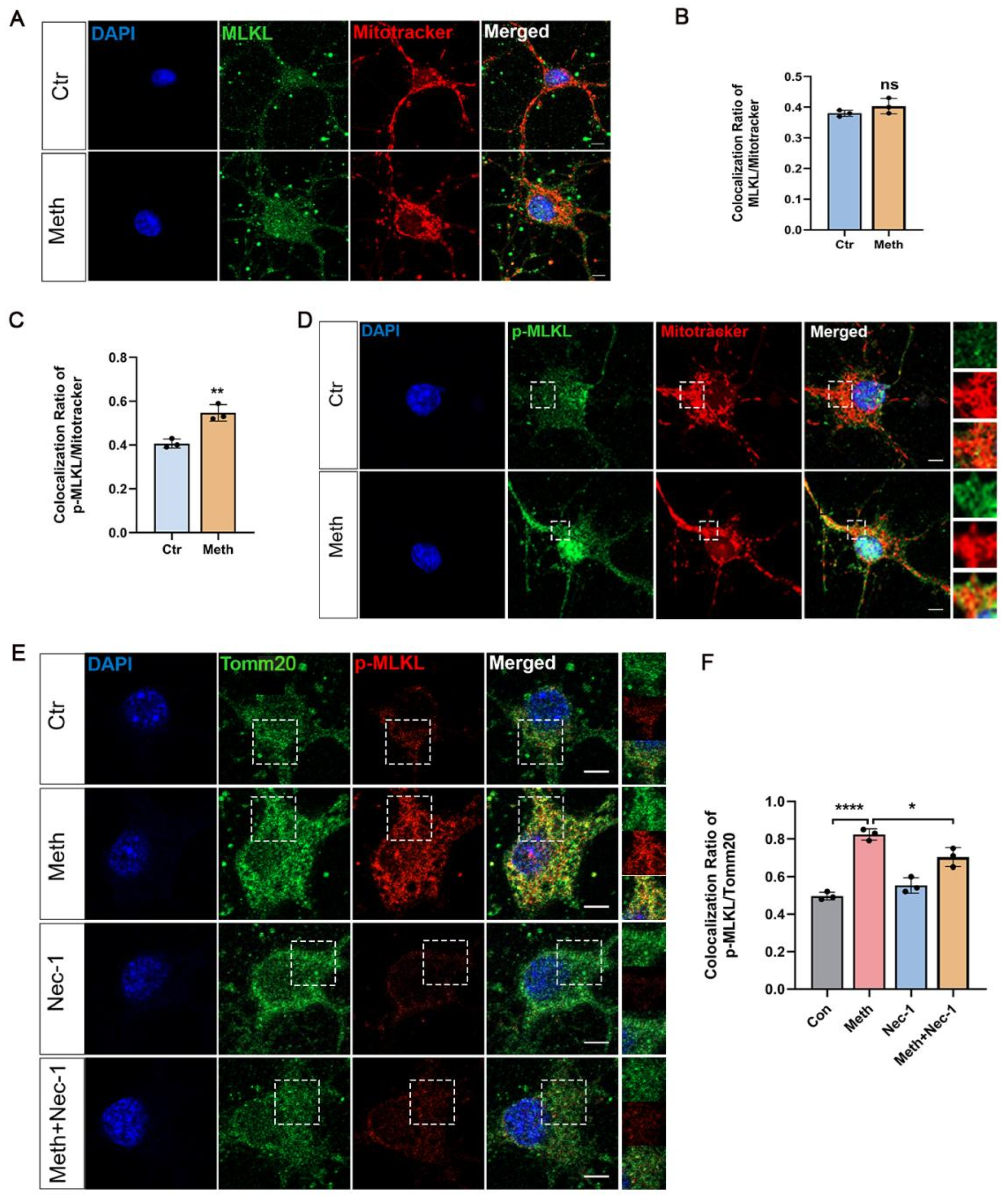
3.4. MLKL Activation and Mitochondrial Membrane Translocation After Meth Treatment
3.5. Mitochondrial ROS Amplifies Neuronal Necroptosis Induced by Meth
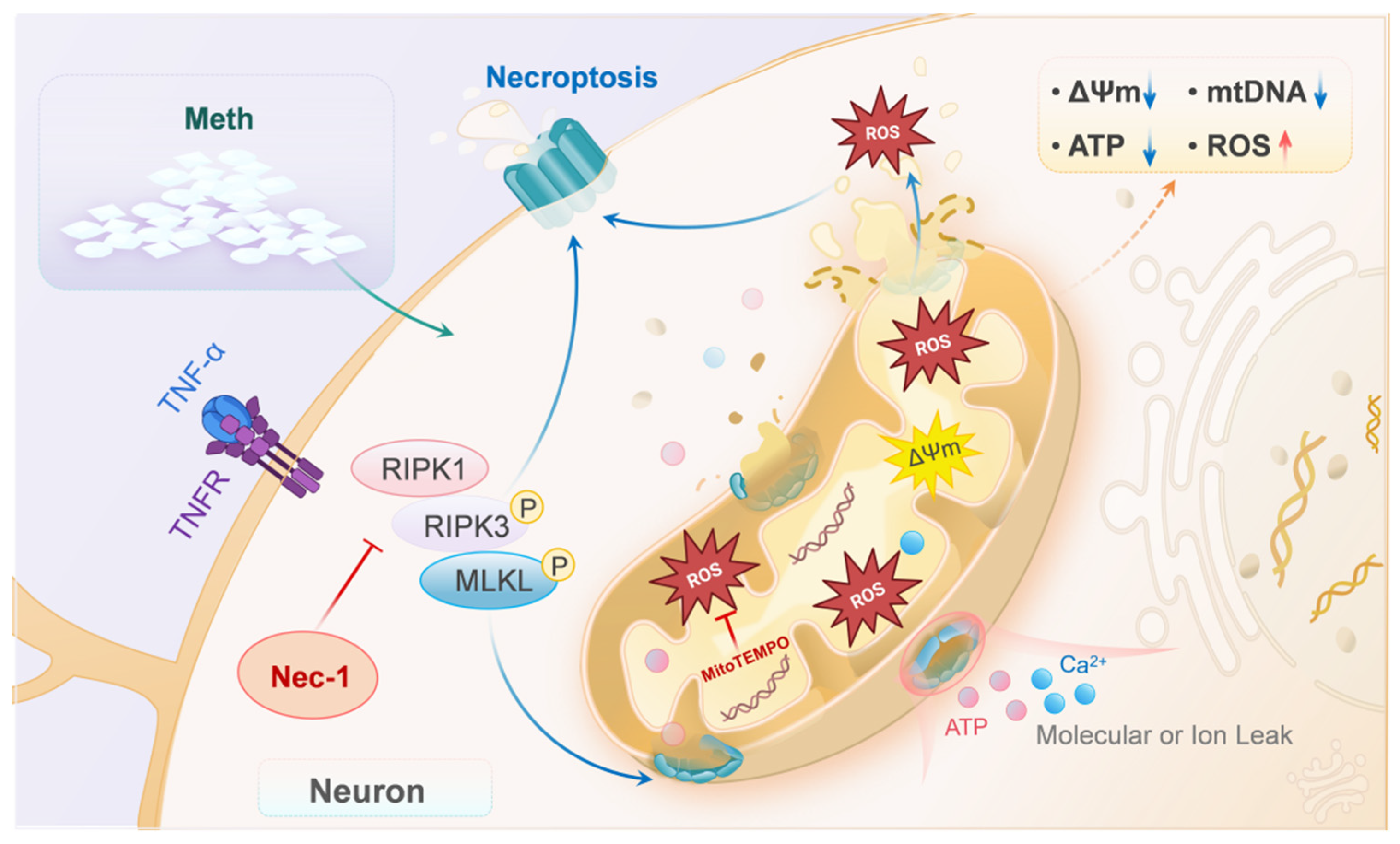
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chacho, N.M.; Adams, E.; Stairs, D.J. Enrichment-induced differences in methamphetamine drug discrimination in male rats. Pharmacol. Biochem. Behav. 2019, 179, 80–88. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Yang, J.; Liu, J.; Li, J.; Liu, Y.; Li, X.; Chen, L.; Hsu, C.; Zeng, J.; et al. Gut microbiota-derived short-chain fatty acids ameliorate methamphetamine-induced depression- and anxiety-like behaviors in a Sigmar-1 receptor-dependent manner. Acta Pharm. Sin. B 2023, 13, 4801–4822. [Google Scholar] [CrossRef]
- Jayanthi, S.; Daiwile, A.P.; Cadet, J.L. Neurotoxicity of methamphetamine: Main effects and mechanisms. Exp. Neurol. 2021, 344, 113795. [Google Scholar] [CrossRef]
- Kevil, C.G.; Goeders, N.E.; Woolard, M.D.; Bhuiyan, M.S.; Dominic, P.; Kolluru, G.K.; Arnold, C.L.; Traylor, J.G.; Orr, A.W. Methamphetamine Use and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1739–1746. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Chen, J.; Lou, X.; Zhang, H.; Li, M.; Cheng, J.; Ma, T.; Xiong, J.; Gao, R.; et al. Key roles of autophagosome/endosome maturation mediated by Syntaxin17 in methamphetamine-induced neuronal damage in mice. Mol. Med. 2024, 30, 4. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Chen, X.; Yang, T.; Wang, X.; Song, X.; Xie, X.; Hu, M.; Jiang, L.; Cheng, J.; et al. Mystery of methamphetamine-induced autophagosome accumulation in hippocampal neurons: Loss of syntaxin 17 in defects of dynein-dynactin driving and autophagosome-late endosome/lysosome fusion. Arch. Toxicol. 2021, 95, 3263–3284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lu, J.; Chen, X.; Gao, Z.; Zhang, C.; Chen, C.; Qiao, D.; Wang, H. Methamphetamine exposure induces neuronal programmed necrosis by activating the receptor-interacting protein kinase 3-related signalling pathway. FASEB J. 2021, 35, e21561. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.M.; Wang, Z.; Li, S.P.; Wang, M.; Yan, W.T.; Liu, F.X.; Wang, C.D.; Zhang, X.D.; Chen, D.; Yan, J.; et al. RIP3/MLKL-mediated neuronal necroptosis induced by methamphetamine at 39 °C. Neural Regen. Res. 2020, 15, 865–874. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Huang, J.; Yao, Z.; Yu, J.; Zhang, W.; Zhang, L.; Wang, Z.; Zhuang, C. Discovery of bardoxolone derivatives as novel orally active necroptosis inhibitors. Eur. J. Med. Chem. 2021, 212, 113030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, M.-B.; Luo, H.-Y.; Shi, C.-H.; Xu, Y.-M. Necroptosis in neurodegenerative diseases: A potential therapeutic target. Cell Death Dis. 2017, 8, e2905. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- Thadathil, N.; Nicklas, E.H.; Mohammed, S.; Lewis, T.L., Jr.; Richardson, A.; Deepa, S.S. Necroptosis increases with age in the brain and contributes to age-related neuroinflammation. Geroscience 2021, 43, 2345–2361. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, H.; Li, X.; Bian, K.; Zheng, Y.; Dai, M.; Feng, X.; Sun, Y.; He, Y.; Yu, B.; et al. Hyperphosphorylated tau mediates neuronal death by inducing necroptosis and inflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 205. [Google Scholar] [CrossRef]
- Oñate, M.; Catenaccio, A.; Salvadores, N.; Saquel, C.; Martinez, A.; Moreno-Gonzalez, I.; Gamez, N.; Soto, P.; Soto, C.; Hetz, C.; et al. The necroptosis machinery mediates axonal degeneration in a model of Parkinson disease. Cell Death Differ. 2020, 27, 1169–1185. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kroemer, G. Necroptosis: A specialized pathway of programmed necrosis. Cell 2008, 135, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; He, X.; Wang, H.; Lin, Z.; Hou, W.; Lu, Y.; Hu, S.; Li, M. Single-Molecule Monitoring of Membrane Association of the Necroptosis Executioner MLKL with Discernible Anchoring and Insertion Dynamics. Nano Lett. 2023, 23, 4770–4777. [Google Scholar] [CrossRef]
- Wu, Z.; Henning, L.; Steinhäuser, C.; Bedner, P. Targeting necroptosis protects against astrocyte death and hippocampal sclerosis in experimental temporal lobe epilepsy. J. Physiol. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef]
- Petrie, E.J.; Sandow, J.J.; Jacobsen, A.V.; Smith, B.J.; Griffin, M.D.W.; Lucet, I.S.; Dai, W.; Young, S.N.; Tanzer, M.C.; Wardak, A.; et al. Conformational switching of the pseudokinase domain promotes human MLKL tetramerization and cell death by necroptosis. Nat. Commun. 2018, 9, 2422. [Google Scholar] [CrossRef]
- Iwata, R.; Casimir, P.; Erkol, E.; Boubakar, L.; Planque, M.; Gallego López, I.M.; Ditkowska, M.; Gaspariunaite, V.; Beckers, S.; Remans, D.; et al. Mitochondria metabolism sets the species-specific tempo of neuronal development. Science 2023, 379, eabn4705. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.M.; Weinberg, S.E.; Chandel, N.S. Mitochondrial control of immunity: Beyond ATP. Nat. Rev. Immunol. 2017, 17, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Jiang, C.; Chang, W.Y.; Zhang, H.; An, J.; Ho, F.; Chen, P.; Zhang, H.; Junqueira, C.; Amgalan, D.; et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 2023, 56, 2523–2541.e8. [Google Scholar] [CrossRef] [PubMed]
- Deragon, M.A.; McCaig, W.D.; Truong, P.V.; Metz, K.R.; Carron, K.A.; Hughes, K.J.; Knapp, A.R.; Dougherty, M.J.; LaRocca, T.J. Mitochondrial Trafficking of MLKL, Bak/Bax, and Drp1 Is Mediated by RIP1 and ROS which Leads to Decreased Mitochondrial Membrane Integrity during the Hyperglycemic Shift to Necroptosis. Int. J. Mol. Sci. 2023, 24, 8609. [Google Scholar] [CrossRef] [PubMed]
- Parameyong, A.; Charngkaew, K.; Govitrapong, P.; Chetsawang, B. Melatonin attenuates methamphetamine-induced disturbances in mitochondrial dynamics and degeneration in neuroblastoma SH-SY5Y cells. J. Pineal Res. 2013, 55, 313–323. [Google Scholar] [CrossRef]
- Teodorof-Diedrich, C.; Spector, S.A. Human Immunodeficiency Virus Type 1 and Methamphetamine-Mediated Mitochondrial Damage and Neuronal Degeneration in Human Neurons. J. Virol. 2020, 94, e00924-20. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Quinton, M.S.; Yamamoto, B.K. Methamphetamine-induced inhibition of mitochondrial complex II: Roles of glutamate and peroxynitrite. J. Neurochem. 2005, 95, 429–436. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, Y.H.; Jang, J.H.; Jeong, C.H.; Lee, S.; Park, B. Asiatic acid attenuates methamphetamine-induced neuroinflammation and neurotoxicity through blocking of NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway. J. Neuroinflamm. 2017, 14, 240. [Google Scholar] [CrossRef]
- Hosseini, M.J.; Arabiyan, A.; Mobassem, S.; Ghavimi, H. Metformin attenuates depressive-like behaviour of methamphetamine withdrawal in mice: A mechanistic approach. World J. Biol. Psychiatry 2023, 24, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Polvat, T.; Prasertporn, T.; Na Nakorn, P.; Pannengpetch, S.; Suwanjang, W.; Panmanee, J.; Ngampramuan, S.; Cornish, J.L.; Chetsawang, B. Proteomic Analysis Reveals the Neurotoxic Effects of Chronic Methamphetamine Self-Administration-Induced Cognitive Impairments and the Role of Melatonin-Enhanced Restorative Process during Methamphetamine Withdrawal. J. Proteome Res. 2023, 22, 3348–3359. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zhang, Y.; He, X.; Zhong, C.Q.; Ni, H.; Chen, X.; Liang, Y.; Wu, J.; Zhao, S.; et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 2018, 20, 186–197. [Google Scholar] [CrossRef]
- Irrinki, K.M.; Mallilankaraman, K.; Thapa, R.J.; Chandramoorthy, H.C.; Smith, F.J.; Jog, N.R.; Gandhirajan, R.K.; Kelsen, S.G.; Houser, S.R.; May, M.J.; et al. Requirement of FADD, NEMO, and BAX/BAK for aberrant mitochondrial function in tumor necrosis factor alpha-induced necrosis. Mol. Cell Biol. 2011, 31, 3745–3758. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: Twenty-something years on. Nature Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Chieffi Baccari, G.; Falvo, S.; Di Fiore, M.M.; Cioffi, F.; Giacco, A.; Santillo, A. High-fat diet affects autophagy and mitochondrial compartment in rat Harderian gland. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 1025–1038. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, D.; Gao, J.; Xie, B.; Yu, H.; Shen, Q.; Zhang, J.; Jing, W.; Cong, B.; Ma, C. Methamphetamine induces GSDME-dependent cell death in hippocampal neuronal cells through the endoplasmic reticulum stress pathway. Brain Res. Bull. 2020, 162, 73–83. [Google Scholar] [CrossRef]
- Coelho-Santos, V.; Gonçalves, J.; Fontes-Ribeiro, C.; Silva, A.P. Prevention of methamphetamine-induced microglial cell death by TNF-α and IL-6 through activation of the JAK-STAT pathway. J. Neuroinflamm. 2012, 9, 103. [Google Scholar] [CrossRef]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. MLKL: Functions beyond serving as the Executioner of Necroptosis. Theranostics 2021, 11, 4759–4769. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Sun, Y. The relationship between childhood abuse and depression in a sample of Chinese people who use methamphetamine. Int. J. Clin. Health Psychol. 2019, 19, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Huang, H.; Guan, T.; Liu, C.; Qu, D.; Xu, Y.; Yang, J.; Yan, L.; Xiong, Y.; Liang, T.; et al. Involvement of C/EBPβ-related signaling pathway in methamphetamine-induced neuronal autophagy and apoptosis. Toxicol. Lett. 2019, 312, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, M.; Wu, W.; Lou, X.; Gao, R.; Ma, T.; Dheen, S.T.; Cheng, J.; Xiong, J.; Chen, X.; et al. Indole derivatives ameliorated the methamphetamine-induced depression and anxiety via aryl hydrocarbon receptor along “microbiota-brain” axis. Gut Microbes 2025, 17, 2470386. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Jayanthi, S.; Deng, X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotox. Res. 2005, 8, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Krasnova, I.N.; Jayanthi, S.; Lyles, J. Neurotoxicity of substituted amphetamines: Molecular and cellular mechanisms. Neurotox. Res. 2007, 11, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Huang, E.; Luo, B.; Yang, Y.; Zhang, F.; Liu, C.; Lin, Z.; Xie, W.B.; Wang, H. Nupr1/Chop signal axis is involved in mitochondrion-related endothelial cell apoptosis induced by methamphetamine. Cell Death Dis. 2016, 7, e2161. [Google Scholar] [CrossRef]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef]
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef]
- Xu, C.; Wu, J.; Wu, Y.; Ren, Z.; Yao, Y.; Chen, G.; Fang, E.F.; Noh, J.H.; Liu, Y.U.; Wei, L.; et al. TNF-α-dependent neuronal necroptosis regulated in Alzheimer’s disease by coordination of RIPK1-p62 complex with autophagic UVRAG. Theranostics 2021, 11, 9452–9469. [Google Scholar] [CrossRef]
- Apaijai, N.; Jinawong, K.; Singhanat, K.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, S.C.; Chattipakorn, N. Necrostatin-1 reduces cardiac and mitochondrial dysfunction in prediabetic rats. J. Endocrinol. 2021, 251, 27–39. [Google Scholar] [CrossRef]
- Majdi, A.; Aoudjehane, L.; Ratziu, V.; Islam, T.; Afonso, M.B.; Conti, F.; Mestiri, T.; Lagouge, M.; Foufelle, F.; Ballenghien, F.; et al. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J. Hepatol. 2020, 72, 627–635. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wen, L.; Armstrong, J.A.; Chvanov, M.; Latawiec, D.; Cai, W.; Awais, M.; Mukherjee, R.; Huang, W.; Gough, P.J.; et al. Protective Effects of Necrostatin-1 in Acute Pancreatitis: Partial Involvement of Receptor Interacting Protein Kinase 1. Cells 2021, 10, 1035. [Google Scholar] [CrossRef]
- Baik, J.Y.; Liu, Z.; Jiao, D.; Kwon, H.-J.; Yan, J.; Kadigamuwa, C.; Choe, M.; Lake, R.; Kruhlak, M.; Tandon, M.; et al. ZBP1 not RIPK1 mediates tumor necroptosis in breast cancer. Nat. Commun. 2021, 12, 2666. [Google Scholar] [CrossRef]
- Jayanthi, S.; Deng, X.; Bordelon, M.; McCoy, M.T.; Cadet, J.L. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J. 2001, 15, 1745–1752. [Google Scholar] [CrossRef]
- Liu, S.; Perez, P.; Sun, X.; Chen, K.; Fatirkhorani, R.; Mammadova, J.; Wang, Z. MLKL polymerization-induced lysosomal membrane permeabilization promotes necroptosis. Cell Death Differ. 2024, 31, 40–52. [Google Scholar] [CrossRef]
- Permpoonputtana, K.; Govitrapong, P. The anti-inflammatory effect of melatonin on methamphetamine-induced proinflammatory mediators in human neuroblastoma dopamine SH-SY5Y cell lines. Neurotox. Res. 2013, 23, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Schenk, B.; Fulda, S. Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death. Oncogene 2015, 34, 5796–5806. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.S.; Zhao, S.; Yang, Z.; Zhong, C.Q.; Chen, X.; Cai, Q.; Yang, Z.H.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8, 14329. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Zhang, C.; Zhou, P.; Wang, Y.; Meng, S.; Chen, Y.; Xu, W.; Xuan, J.; Xiong, J.; Cheng, J.; et al. Cleaving PINK1 or PGAM5? Involvement of PARL in Methamphetamine-Induced Excessive Mitophagy and Neuronal Necroptosis. CNS Neurosci. Ther. 2025, 31, e70293. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.Y.; Xia, W.H.; Sun, M.C.; Wan, X.M.; Zhou, X.L.; Wang, L. Cadmium targeting MLKL-Drp1 axis to trigger mitochondrial oxidative stress contributes to necroinflammation in rat kidney. J. Adv. Res. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Robichaux, D.J.; Harata, M.; Murphy, E.; Karch, J. Mitochondrial permeability transition pore-dependent necrosis. J. Mol. Cell. Cardiol. 2023, 174, 47–55. [Google Scholar] [CrossRef]
- Weindel, C.G.; Martinez, E.L.; Zhao, X.; Mabry, C.J.; Bell, S.L.; Vail, K.J.; Coleman, A.K.; VanPortfliet, J.J.; Zhao, B.; Wagner, A.R.; et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 2022, 185, 3214–3231.e23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Xuan, J.; Xu, W.; An, D.; Meng, S.; Zhang, H.; Hu, M.; Hui, W.; Wang, Y.; Cheng, J.; et al. Methamphetamine Exposure Induces Neuronal Programmed Necrosis by Permeabilizing Mitochondria via the RIPK1–RIPK3–MLKL Axis. Toxics 2025, 13, 736. https://doi.org/10.3390/toxics13090736
Zhou P, Xuan J, Xu W, An D, Meng S, Zhang H, Hu M, Hui W, Wang Y, Cheng J, et al. Methamphetamine Exposure Induces Neuronal Programmed Necrosis by Permeabilizing Mitochondria via the RIPK1–RIPK3–MLKL Axis. Toxics. 2025; 13(9):736. https://doi.org/10.3390/toxics13090736
Chicago/Turabian StyleZhou, Peng, Jiankang Xuan, Weixiao Xu, Di An, Sining Meng, Hongchao Zhang, Miaoyang Hu, Wanqingyang Hui, Yifei Wang, Jie Cheng, and et al. 2025. "Methamphetamine Exposure Induces Neuronal Programmed Necrosis by Permeabilizing Mitochondria via the RIPK1–RIPK3–MLKL Axis" Toxics 13, no. 9: 736. https://doi.org/10.3390/toxics13090736
APA StyleZhou, P., Xuan, J., Xu, W., An, D., Meng, S., Zhang, H., Hu, M., Hui, W., Wang, Y., Cheng, J., Xiong, J., Wang, J., & Chen, X. (2025). Methamphetamine Exposure Induces Neuronal Programmed Necrosis by Permeabilizing Mitochondria via the RIPK1–RIPK3–MLKL Axis. Toxics, 13(9), 736. https://doi.org/10.3390/toxics13090736






