Polystyrene Microplastic Interferes with Yolk Reserve Utilisation in Early Artemia salina Nauplii
Highlights
- This study reveals, for the first time, that microplastics interfere with yolk resorption during development, as evidenced by the depletion of N-acetyl galactosamine in Artemia salina embryos.
- It was observed that 3 µm polystyrene microbeads exert toxicity primarily through the release of organic solvents (ethylbenzene, xylene, styrene, and benzaldehyde) rather than direct physical interaction.
- Toxic effects occur without particle entry, highlighting a "remote toxicity" mechanism mediated by soluble compounds.
- The disruption of yolk resorption provides a novel biochemical link between microplastic exposure and impaired growth.
- The findings challenge the current understanding of microplastic toxicity as being limited to ingestion or mechanical interference.
- This study underscores the need to include chemical leachates in ecological risk assessments and supports a re-evaluation of environmental regulations related to microplastic pollution.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. MPs and Reagent Characterisation and Analytical Methods
2.2.1. Polystyrene Bead Characterisation
2.2.2. Gas Chromatography Analysis of Naupliar Culture Media
2.2.3. Oxidative Damage in Encysted Embryos and Newborn Nauplii
2.3. Test Organism Handling and Endpoints Measured
2.3.1. Care and Treatment of Artemia salina
2.3.2. Cysts Decapsulation
2.3.3. Hatching Test
2.3.4. Naupliar Growth
2.3.5. Polystyrene Beads Feeding
2.3.6. Histological Investigations
2.4. Analysis of Data
3. Results
3.1. Polystyrene Beads Characterisation
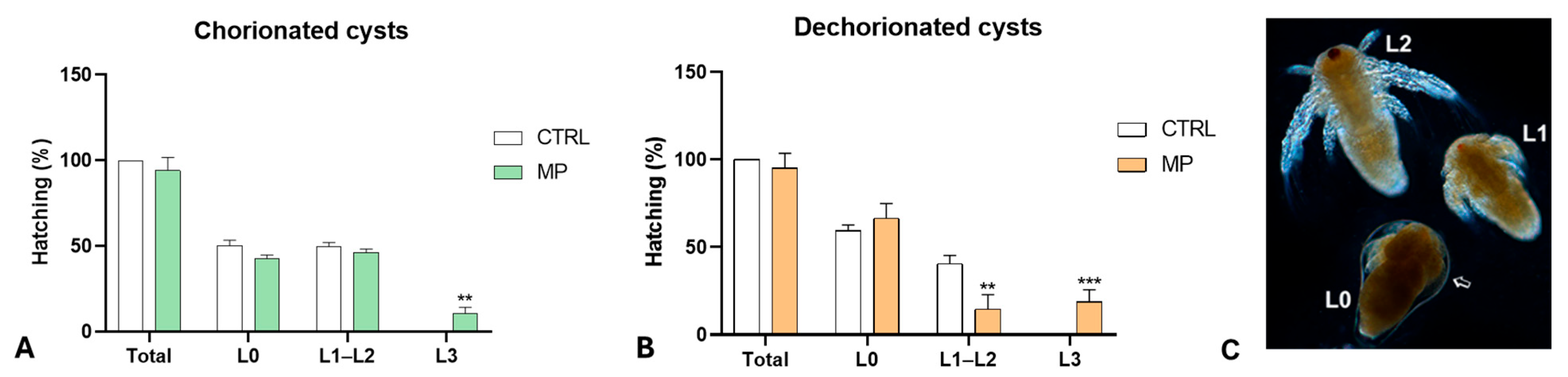
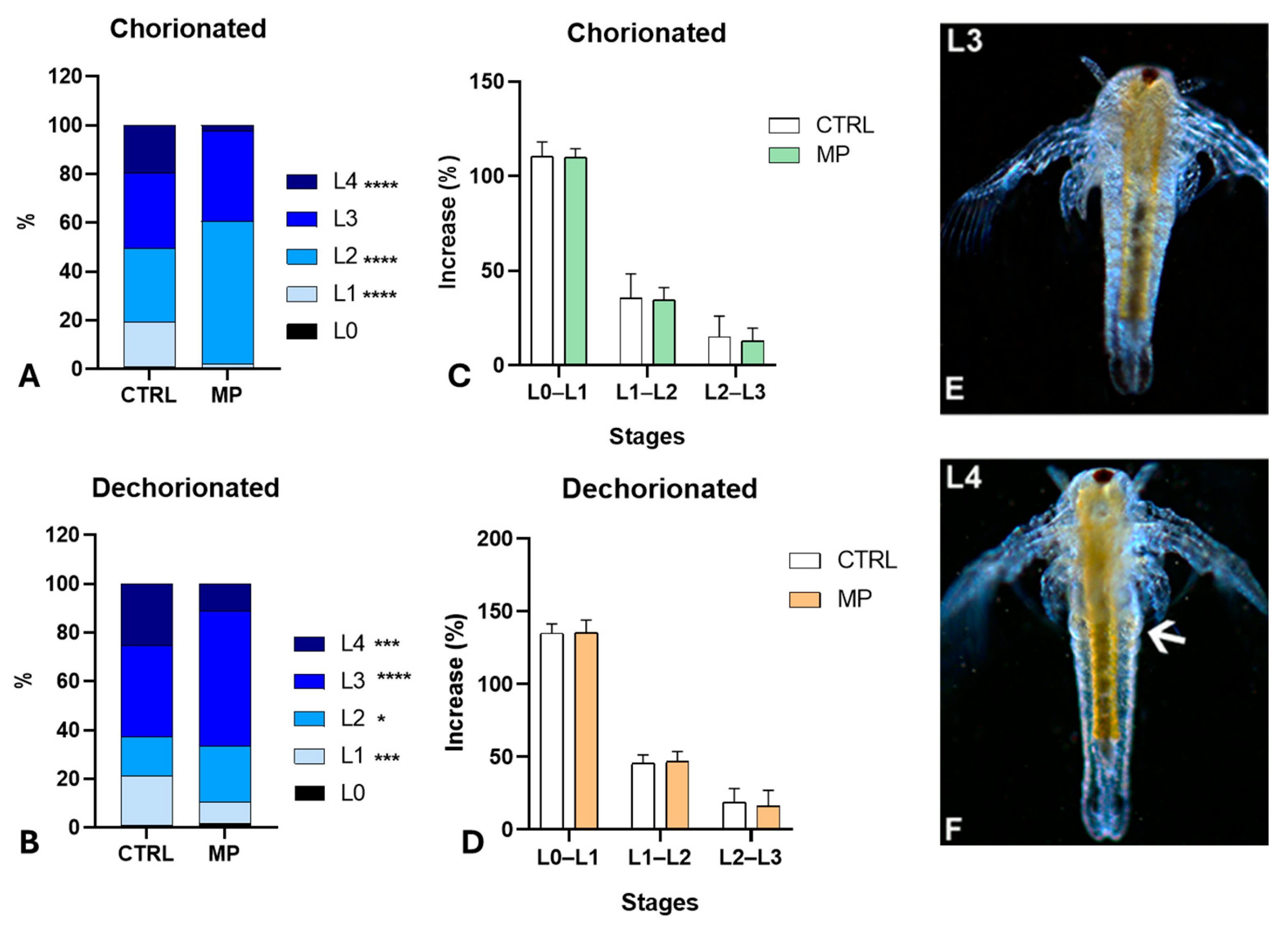
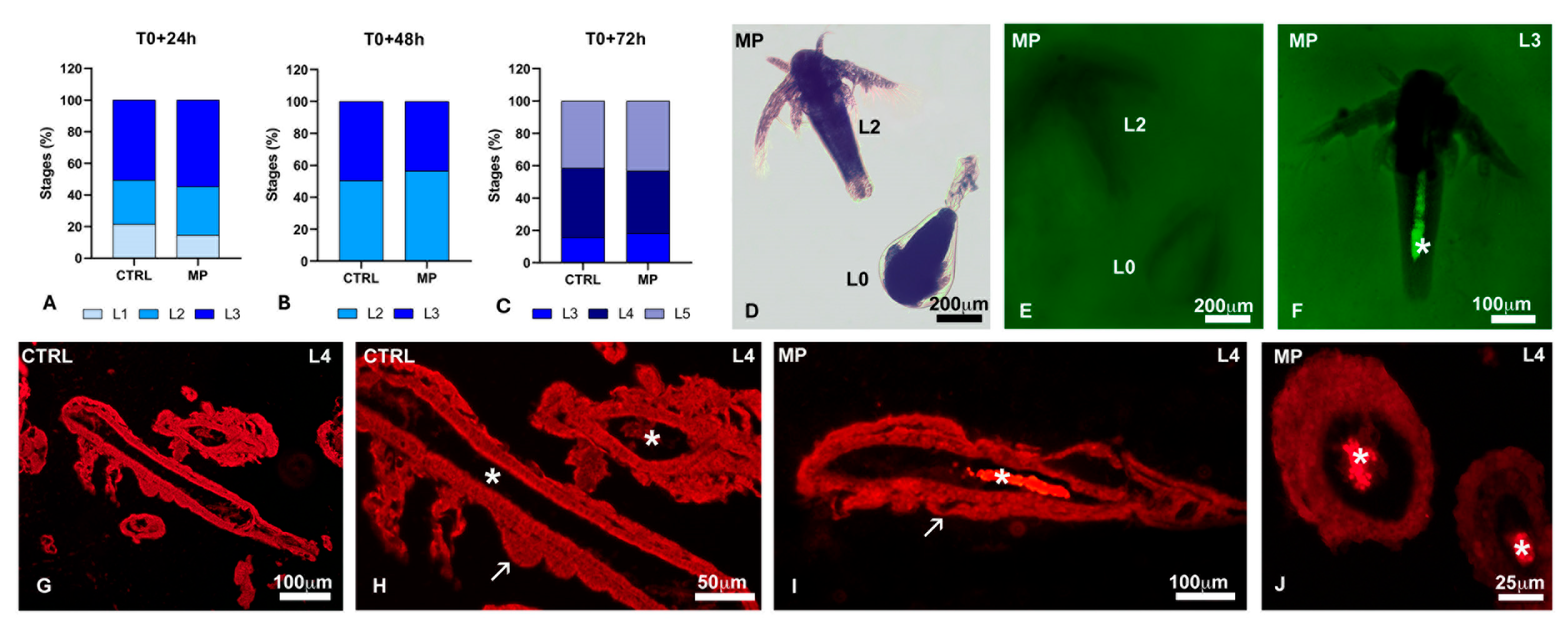
3.2. Effects of MPs on Hatching and Growth
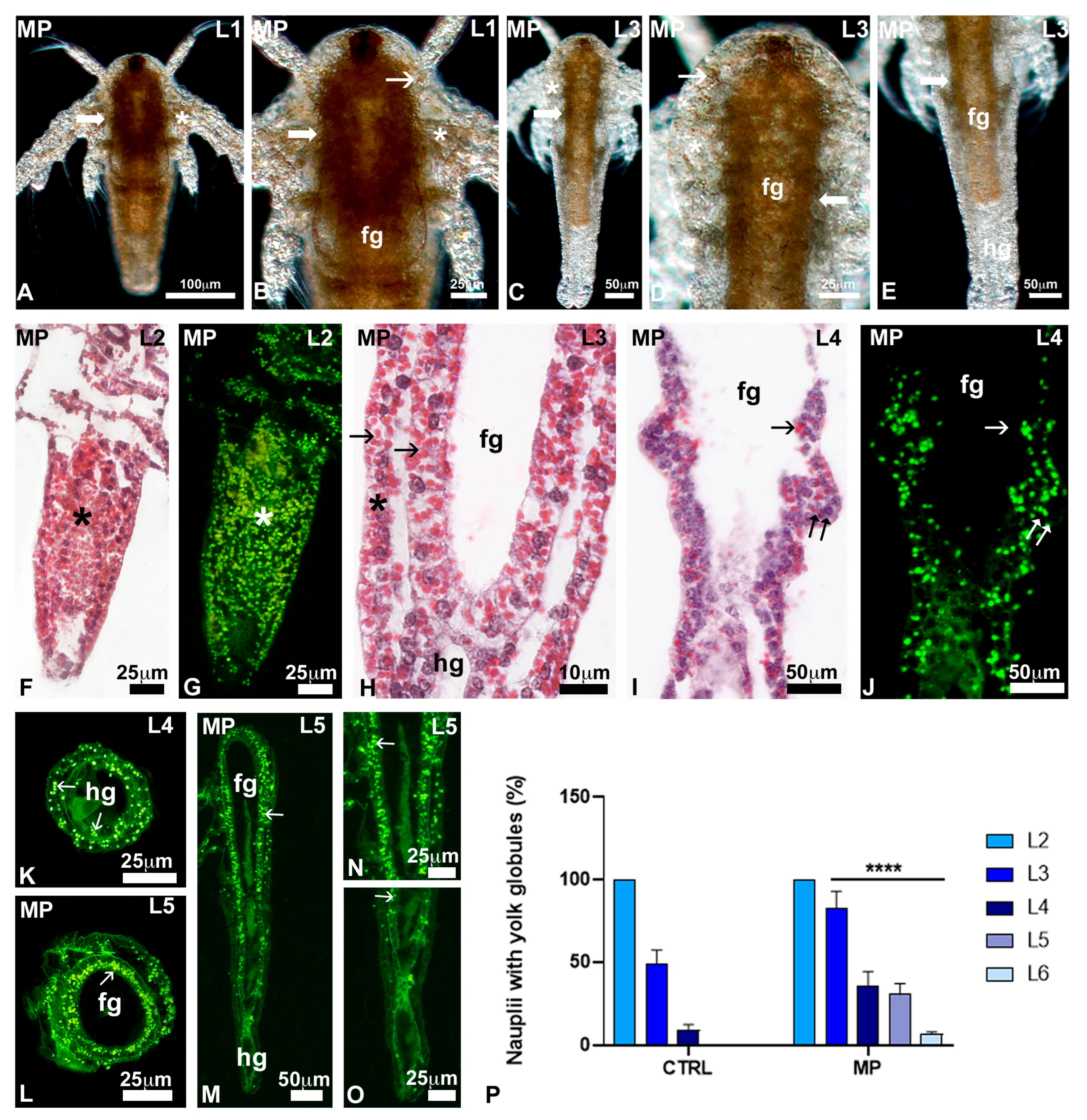
3.3. Effects of Polystyrene on Naupliar Anatomy
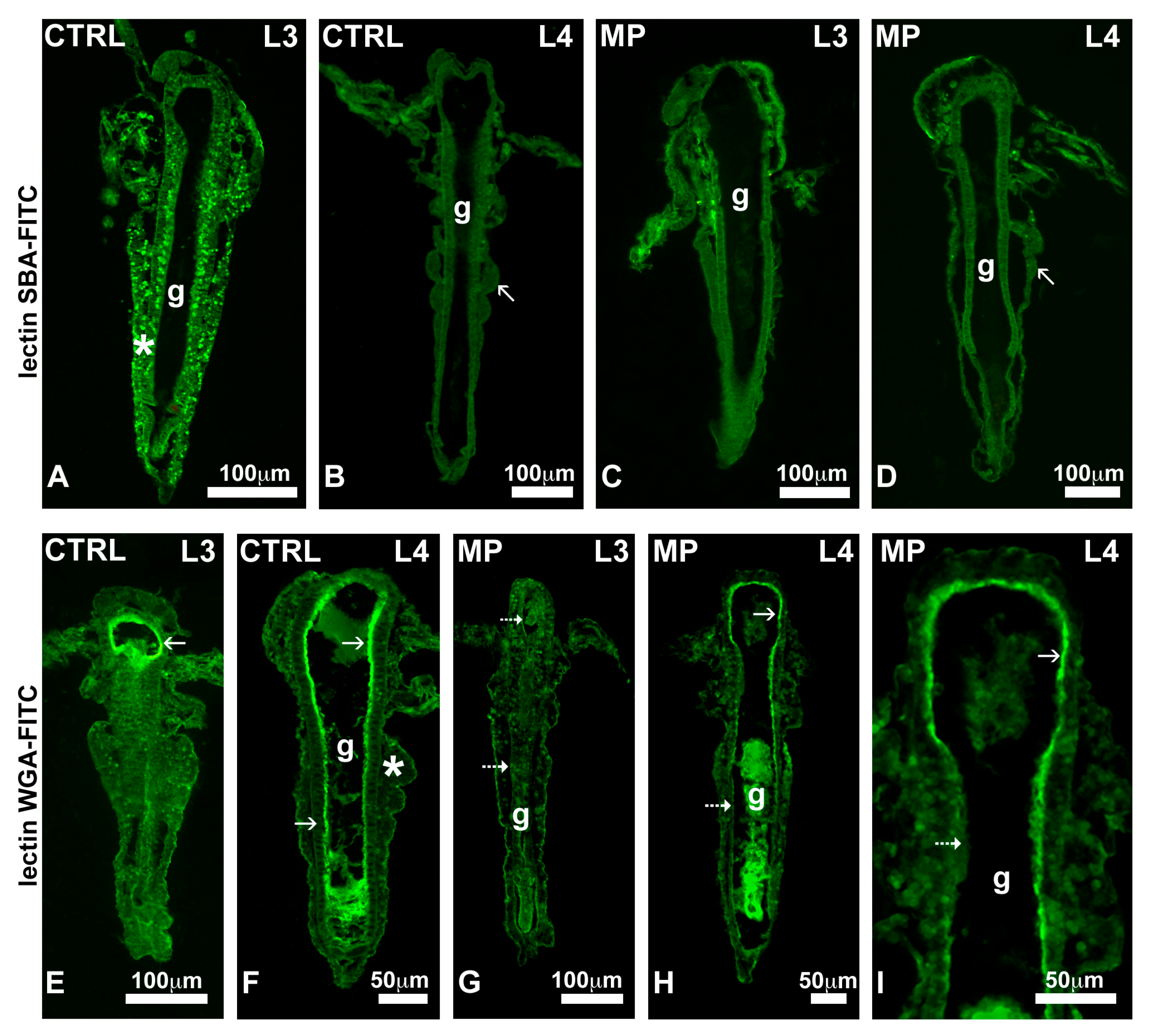
3.4. Effect of MPs on Carbohydrates—Staining with FITC-Conjugated SBA and WGA Lectins
3.5. Effects of Polystyrene Feeding on Growth
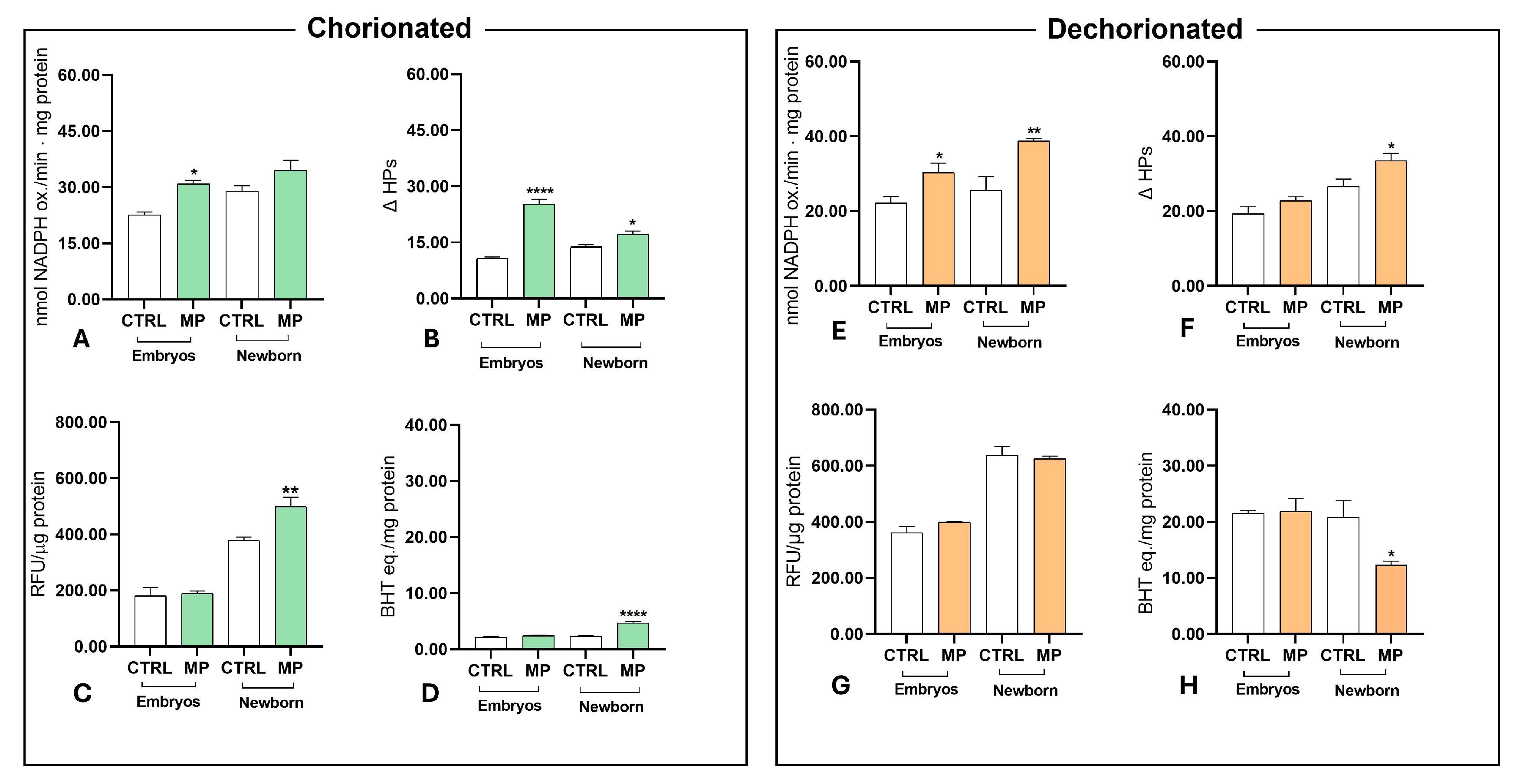
3.6. Effect of MPs on Oxidative Stress
3.7. VOCs in Culture Medium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Besio, G.; Borgogno, F.; Canesi, L.; Canuto, S.; Dara, M.; Enrile, F.; Forioso, I.; Greco, G.; et al. Microplastics in seawater: Sampling strategies, laboratory methodologies, and identification techniques applied to port environment. Environ. Sci. Pollut. Res. Int. 2020, 27, 8938–8952. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Microplastics contamination along the coastal waters of NW Portugal. Case Study Chem. Environ. Eng. 2020, 2, 100056. [Google Scholar] [CrossRef]
- Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment—Biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733. [Google Scholar]
- Wootton, N.; Reis-Santos, P.; Gillanders, B.M. Microplastics in fish—A global synthesis. Rev. Fish Biol. Fish. 2021, 31, 753–771. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.M.; Ivar do Sul, J.A.; Costa, M.F. Uptake and ingestion are the main pathways for microplastics to enter marine benthos: A review. Food Webs 2020, 24, e00150. [Google Scholar] [CrossRef]
- Porter, A.; Barber, D.; Hobbs, C.; Love, J.; Power, A.L.; Bakir, A.; Galloway, T.S.; Lewis, C. Uptake of microplastics by marine worms depends on feeding mode and particle shape but not exposure time. Sci. Total Environ. 2023, 857, 159287. [Google Scholar] [CrossRef]
- Sun, X.; Liang, J.; Zhu, M.; Zhao, Y.; Zhang, B. Microplastics in seawater and zooplankton from the Yellow Sea. Environ. Pollut. 2018, 242, 585–595. [Google Scholar] [CrossRef]
- Lievens, S.; Vervoort, E.; Bruno, D.; Van der Donck, T.; Tettamanti, G.; Seo, J.W.; Poma, G.; Covaci, J.; De Smet, A.; Van Der Borght, M. Ingestion and excretion dynamics of microplastics by black soldier fly larvae and correlation with mouth opening size. Sci. Rep. 2023, 13, 4341. [Google Scholar] [CrossRef]
- Eom, H.J.; Nam, S.E.; Rhee, J.S. Polystyrene microplastics induce mortality through acute cell stress and inhibition of cholinergic activity in a brine shrimp. Mol. Cell. Toxicol. 2020, 16, 233–243. [Google Scholar] [CrossRef]
- Varó, I.; Perini, A.; Torreblanca, A.; Garcia, Y.; Bergami, E.; Vannuccini, M.L.; Corsi, I. Time-dependent effects of polystyrene nanoparticles in brine shrimp Artemia franciscana at physiological, biochemical and molecular levels. Sci. Total Environ. 2019, 675, 570–580. [Google Scholar] [CrossRef]
- Płuciennik, K.; Sicińska, P.; Misztal, W.; Bukowska, B. Important factors affecting induction of cell death, oxidative stress and DNA damage by nano- and microplastic particles in vitro. Cells 2024, 13, 768. [Google Scholar] [CrossRef]
- Tang, K.H.D. Genotoxicity of microplastics on living organisms: Effects on chromosomes, DNA and gene expression. Environments 2025, 12, 10. [Google Scholar] [CrossRef]
- Yang, W.; Jannatun, N.; Zeng, Y.; Liu, T.; Zhang, G.; Chen, C.; Li, Y. Impacts of microplastics on immunity. Front. Toxicol. 2022, 4, 956885. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Bilal, M.; Hassan, H.U.; Taj, M.; Rafiq, N.; Nabi, G.; Ali, A.; Gabol, K.; Ali Shah, M.I.; Gaffar, R.A.; Sohail, M.; et al. Biological magnification of microplastics: A look at the induced reproductive toxicity from simple invertebrates to complex vertebrates. Water 2023, 15, 2831. [Google Scholar] [CrossRef]
- De Felice, B.; Sabatini, V.; Antenucci, S.; Gattoni, G.; Santo, N.; Bacchetta, R.; Ortenzi, M.A.; Parolini, M. Polystyrene microplastics ingestion induced behavioural effects in the cladoceran Daphnia magna. Chemosphere 2019, 231, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, X.; Hou, Q.; Wang, Z. From natural environment to animal tissues: A review of microplastics (nanoplastics) translocation and hazards studies. Sci. Total Environ. 2023, 855, 158686. [Google Scholar] [CrossRef]
- Chemello, G.; Trotta, E.; Notarstefano, V.; Papetti, L.; Di Renzo, L.; Matiddi, M.; Silvestri, S.; Carnevali, O.; Gioacchini, G. Microplastics evidence in yolk and liver of loggerhead sea turtles (Caretta caretta), a pilot study. Environ. Pollut. 2023, 337, 122589. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Morgana, S.; Ferrando, S.; Bramini, M.; Piazza, V.; Costa, E.; Garaventa, F.; Faimali, M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol. Environ. Saf. 2017, 145, 250–257. [Google Scholar] [CrossRef]
- Han, X.; Zheng, Y.; Dai, C.; Duan, H.; Gao, M.; Ali, M.R.; Sui, L. Effect of polystyrene microplastics and temperature on growth, intestinal histology and immune responses of brine shrimp Artemia franciscana. J. Oceanol. Limnol. 2021, 39, 979–988. [Google Scholar] [CrossRef]
- Suman, T.Y.; Jia, P.P.; Li, W.G.; Junaid, M.; Xin, G.Y.; Wang, Y.; Pei, D.S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater. 2020, 400, 123220. [Google Scholar] [CrossRef] [PubMed]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes re-productive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Hua, X.; Dang, Y.; Han, Y.; Yu, Z.; Chen, X.; Ding, P.; Li, H. Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 726, 138679. [Google Scholar] [CrossRef]
- Motta, C.M.; Cerciello, R.; De Bonis, S.; Mazzella, V.; Cirino, P.; Panzuto, R.; Ciaravolo, M.; Simoniello, P.; Toscanesi, M.; Trifuoggi, M.; et al. Potential toxicity of improperly discarded exhausted photovoltaic cells. Environ. Pollut. 2016, 216, 786–792. [Google Scholar] [CrossRef]
- Motta, C.M.; Simoniello, P.; Arena, C.; Capriello, T.; Panzuto, R.; Vitale, E.; Agnisola, C.; Tizzano, M.; Avallone, B.; Ferrandino, I. Effects of four food dyes on the development of three model species, Cucumis sativus, Artemia salina and Danio rerio: Assessment of potential risk for the environment. Environ. Pollut. 2019, 253, 1126–1135. [Google Scholar] [CrossRef]
- de Chaffoy de Courcelles, D.; Kondo, M. Lipovitellin from the crustacean, Artemia salina. Biochemical analysis of lipovitellin complex from the yolk granules. J. Biol. Chem. 1980, 255, 6727–6733. [Google Scholar] [CrossRef]
- Ahmad, M.; Bajahlan, A.S. Leaching of styrene and other aromatic compounds in drinking water from PS bottles. J. Environ. Sci. 2007, 19, 421–426. [Google Scholar] [CrossRef]
- Iizuka, A.; Mizukoshi, A.; Noguchi, M.; Yamasaki, A. Emission fluxes of styrene monomers and other chemicals for products containing expanded polystyrene beads. PLoS ONE 2020, 15, e0239458. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.; Kwon, E.E.; Jeong, S. Quantitative analysis of polystyrene microplastic and styrene monomer released from plastic food containers. Heliyon 2023, 9, e15787. [Google Scholar] [CrossRef]
- Bruck, S.; Ford, A.T. Chronic ingestion of polystyrene microparticles in low doses has no effect on food consumption and growth of the intertidal amphipod Echinogammarus marinus? Environ. Pollut. 2018, 233, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.P.J.; Estrela, F.N.; Rodrigues, A.S.L.; Guimarães, A.T.B.; Rocha, T.L.; Malafaia, G. Behavioural and biochemical consequences of Danio rerio larvae exposure to polylactic acid bioplastic. J. Hazard. Mater. 2021, 404 Pt A, 124152. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic polymer contamination in bottled water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; Gomes, R.L.; Needham, T.; Burson, A. Exploring the efficacy of Nile Red in microplastic quantification: A co-staining approach. Environ. Sci. Technol. Lett. 2019, 6, 606–611. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef]
- Fasciolo, G.; Napolitano, G.; Aprile, M.; Cataldi, S.; Costa, V.; Ciccodicola, A.; Di Meo, S.; Venditti, P. Hepatic insulin resistance in hyperthyroid rat liver: Vitamin E supplementation highlights a possible role of ROS. Antioxidants 2022, 11, 1295. [Google Scholar] [CrossRef]
- Fogliano, C.; Motta, C.M.; Venditti, P.; Fasciolo, G.; Napolitano, G.; Avallone, B.; Carotenuto, R. Environmental concentrations of a delorazepam-based drug impact on embryonic development of non-target Xenopus laevis. Aquat. Toxiccol. 2022, 250, 106244. [Google Scholar] [CrossRef] [PubMed]
- Panico, G.; Fasciolo, G.; Migliaccio, V.; De Matteis, R.; Lionetti, L.; Napolitano, G.; Agnisola, C.; Venditti, P.; Lombardi, A. 1,3-Butanediol administration increases β-hydroxybutyrate plasma levels and affects redox homeostasis, endoplasmic reticulum stress, and adipokine production in rat gonadal adipose tissue. Antioxidants 2023, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, P.; Persoone, G.; Claus, C.; Sorgeloos, P. Proposal for a short-term toxicity test with Artemia nauplii. Ecotox. Environ. Saf. 1981, 5, 382–387. [Google Scholar] [CrossRef]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef]
- Leusch, F.D.; Ziajahromi, S. Converting mg/L to particles/L: Reconciling the occurrence and toxicity literature on microplastics. Environ. Sci. Technol. 2021, 55, 11470–11472. [Google Scholar] [CrossRef]
- Sorgeloos, P.; Bossuyt, E.; Laviña, E.; Baeza-Mesa, M.; Persoone, G. Decapsulation of Artemia cysts: A simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture 1977, 12, 311–315. [Google Scholar] [CrossRef]
- Copf, T.; Rabet, N.; Celniker, S.E.; Averof, M. Posterior patterning genes and the identification of a unique body region in the brine shrimp Artemia franciscana. Development 2003, 130, 5915–5927. [Google Scholar] [CrossRef]
- Avallone, B.; Arena, C.; Simoniello, P.; Di Lorenzo, M.; Vitale, E.; Capriello, T.; Ferrandino, I.; Raggio, A.; Sasso, M.; Napolitano, G.; et al. Comparative toxicity of vegan red, e124, and e120 food dyes on three rapidly proliferating model systems. Environments 2022, 9, 89. [Google Scholar] [CrossRef]
- La Pietra, A.; Fasciolo, G.; Lucariello, D.; Motta, C.M.; Venditti, P.; Ferrandino, I. Polystyrene microplastics effects on zebrafish embryological development: Comparison of two different sizes. Environ. Toxicol. Pharmacol. 2024, 106, 104371. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, C.; Carotenuto, R.; Panzuto, R.; Spennato, V.; De Bonis, S.; Simoniello, P.; Raggio, A.; Avallone, B.; Agnisola, C.; Motta, C.M. Behavioural alterations and gill damage in Mytilus galloprovincialis exposed to an environmental concentration of delorazepam. Environ. Toxicol. Pharmacol. 2023, 97, 104030. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, C.; Carotenuto, R.; Cirino, P.; Panzuto, R.; Ciaravolo, M.; Simoniello, P.; Sgariglia, I.; Motta, C.M.; Avallone, B. Benzodiazepine interference with fertility and embryo development: A preliminary survey in the sea urchin Paracentrotus lividus. Int. J. Mol. Sci. 2024, 25, 1969. [Google Scholar] [CrossRef]
- Dey, P.; Bradley, T.M.; Boymelgreen, A. The impact of selected abiotic factors on Artemia hatching process through real-time observation of oxygen changes in a microfluidic platform. Sci. Rep. 2023, 13, 6370. [Google Scholar] [CrossRef]
- Dey, P.; Bradley, T.M.; Boymelgreen, A. Real-time assessment of the impacts of polystyrene and silver nanoparticles on the hatching process and early-stage development of Artemia using a microfluidic platform. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chaffoy, D.D.; Maeyer-Criel, G.D.; Kondo, M. On the permeability and formation of the embryonic cuticle during development in vivo and in vitro of Artemia salina embryos. Differentiation 1979, 12, 99–109. [Google Scholar] [CrossRef]
- Gunaalan, K.; Fabbri, E.; Capolupo, M. The hidden threat of plastic leachates: A critical review on their impacts on aquatic organisms. Water Res. 2020, 184, 116170. [Google Scholar] [CrossRef] [PubMed]
- Gulizia, A.M.; Patel, K.; Philippa, B.; Motti, C.A.; van Herwerden, L.; Vamvounis, G. Understanding plasticiser leaching from polystyrene microplastics. Sci. Total Environ. 2023, 857 Pt 1, 159099. [Google Scholar] [CrossRef]
- Duan, Z.; Duan, X.; Zhao, S.; Wang, X.; Wang, J.; Liu, Y.; Peng, Y.; Gong, Z.; Wang, L. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard. Mater. 2020, 395, 122621. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Zhou, Q.; Chen, C. Responses of oxidative stress biomarkers and DNA damage on a freshwater snail (Bellamya aeruginosa) stressed by ethylbenzene. Arch. Environ. Contam. Toxicol. 2013, 65, 251–259. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Xie, X.; Lin, D.; Dong, L. Oxidative stress and DNA damage in the earthworm Eisenia fetida induced by toluene, ethylbenzene and xylene. Ecotoxicology 2010, 19, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Ulker, Z.; Alpsoy, L.; Mihmanli, A. Assessment of cytotoxic and apoptotic effects of benzaldehyde using different assays. Hum. Exp. Toxicol. 2013, 32, 858–864. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Serrano, A.; Gallego, M. Direct screening and confirmation of benzene, toluene, ethylbenzene and xylenes in water. J. Chromatog. A 2004, 1045, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.M.; de Andrade, M.V.F.; Marins, R.V.; Oliveira, L.M. Development of a headspace-gas chromatography (HS-GC-PID-FID) method for the determination of VOCs in environmental aqueous matrices: Optimisation, verification and elimination of matrix effect and VOC distribution on the Fortaleza Coast, Brazil. Polar Chem. 2010, 96, 337–343. [Google Scholar] [CrossRef]
- Suaidi, N.A.; Alshawsh, M.A.; Hoe, S.Z.; Mokhtar, M.H.; Md Zin, S.R. Impact of xylene exposure during organogenesis on foeto-placental efficiency and foetal viability: Exploring its association with oxidative stress-induced inflammation and apoptosis in utero. Toxicol. Ind. Health 2024, 40, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.M.; Gallissot, F.; Sabaté, J.P.; Bourges-Abella, N.; Muller, S. Developmental toxic effects of ethylbenzene or toluene alone and in combination with butyl acetate in rats after inhalation exposure. J. Appl. Toxicol. 2007, 27, 32–42. [Google Scholar] [CrossRef]
- Chatterjee, N.; Kim, C.; Im, J.; Kim, S.; Choi, J. Mixture and individual effects of benzene, toluene, and formaldehyde in zebrafish (Danio rerio) development: Metabolomics, epigenetics, and behavioural approaches. Environ. Toxicol. Pharmacol. 2023, 97, 104031. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Wang, F.; Liu, Q. Environmental behaviour and eco-toxicity of xylene in aquatic environments: A review. Ecotoxicol. Environ. Saf. 2017, 145, 324–332. [Google Scholar] [CrossRef]
- Alexander, M. Environmental fate and effects of styrene. Crit. Rev. Environ. Sci. Technol. 1997, 27, 383–410. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Mulligan, C.N. Styrene toxicity: An ecotoxicological assessment. Ecotoxicol. Environ. Saf. 1997, 38, 181–194. [Google Scholar] [CrossRef]
- Richardson, C.R.; Burritt, D.J.; Allan, B.J.; Lamare, M.D. Microplastic ingestion induces asymmetry and oxidative stress in larvae of the sea urchin Pseudechinus huttoni. Mar. Pollut. Bull. 2021, 168, 112369. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoon, H.; Choi, J.S.; Jung, Y.J.; Park, J.W. Chronic effects of nano and microplastics on reproduction and development of the marine copepod Tigriopus japonicus. Ecotoxicol. Environ. Saf. 2022, 243, 113962. [Google Scholar] [CrossRef]
- Frank, Y.A.; Interesova, E.A.; Solovyev, M.M.; Xu, J.; Vorobiev, D.S. Effect of microplastics on the activity of digestive and oxidative-stress-related enzymes in peled whitefish (Coregonus peled Gmelin) larvae. Int. J. Mol. Sci. 2023, 24, 10998. [Google Scholar] [CrossRef]
- Pannetier, P.; Morin, B.; Le Bihanic, F.; Dubreil, L.; Clérandeau, C.; Chouvellon, F.; Van Arkel, K.; Danion, M.; Cachot, J. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ. Int. 2020, 134, 105047. [Google Scholar] [CrossRef]
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mat. 2020, 385, 121560. [Google Scholar] [CrossRef]
- Mazur, A.A.; Chelomin, V.P.; Zhuravel, E.V.; Kukla, S.P.; Slobodskova, V.V.; Dovzhenko, N.V. Genotoxicity of polystyrene (PS) microspheres in short-term exposure to gametes of the sand dollar Scaphechinus mirabilis (Agassiz, 1864; Echinodermata, Echinoidea). J. Mar. Sci. Eng. 2021, 9, 1088. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic properties of polystyrene (PS) microspheres in the filter-feeder mollusc Mytilus trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- Kim, G.W.; Hong, J.Y.; Yu, S.Y.; Ahn, J.J.; Kim, Y.; Son, S.W.; Park, J.T.; Hwang, S.Y. Integrative analyses of differential gene expression and DNA methylation of ethylbenzene-exposed workers. BioChip J. 2015, 9, 259–267. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hong, J.Y.; Yu, S.Y.; Kim, G.W.; Ahn, J.J.; Kim, Y.; Son, S.W.; Park, J.T.; Hwang, S.Y. Identification of potential biomarkers for xylene exposure by microarray analyses of gene expression and methylation. Mol. Cell. Toxicol. 2016, 12, 15–20. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, R.; Tussellino, M.; Ronca, R.; Benvenuto, G.; Fogliano, C.; Fusco, S.; Netti, P.A. Toxic effects of SiO2NPs in early embryogenesis of Xenopus laevis. Chemosphere 2022, 289, 133233. [Google Scholar] [CrossRef]
- Hao, Y.; Sun, Y.; Li, M.; Fang, X.; Wang, Z.; Zuo, J.; Zhang, C. Adverse effects of polystyrene micro-plastics in the freshwater commercial fish, grass carp (Ctenopharyngodon idella): Emphasis on physiological response and intestinal microbiome. Sci. Total Environ. 2023, 856 Pt 2, 159270. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene microplastics alter the behaviour, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef]
- Bartolomaeus, T.; Quast, B.; Koch, M. Nephridial development and body cavity formation in Artemia salina (Crustacea: Branchiopoda): No evidence for any transitory coelom. Zoomorphology 2009, 128, 247–262. [Google Scholar] [CrossRef]
- Peterson, R.H.; Metcalfe, J.L.; Ray, S. Effects of cadmium on yolk utilisation, growth, and survival of Atlantic salmon alevins and newly feeding fry. Arch. Environ. Contam. Toxicol. 1983, 12, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Han, R.; Ren, Z.; Ai, N.; Yu, S.; Lee, S.M.Y.; Ge, W.; Chen, Y.; Zheng, Y. Flavour compound geraniol induces inhibited nutrient utilisation and developmental toxicity on embryonic–larval zebrafish. Food Front. 2025, 6, 374–387. [Google Scholar] [CrossRef]
- Varshney, S.; Hegstad-Pettersen, M.M.; Siriyappagouder, P.; Olsvik, P.A. Enhanced neurotoxic effect of PCB-153 when co-exposed with polystyrene nanoplastics in zebrafish larvae. Chemosphere 2024, 355, 141783. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Suazo, D.; Guillén-Chable, F.; Escobedo-Hinojosa, W.I.; Galindo-Sánchez, C.E.; Rosas, C. Insights into Octopus maya cathepsins from metatranscriptome and genome: Structure, evolutionary relationships and functional role prediction in digestive processes. Biol. Open 2025, 14, bio061778. [Google Scholar] [CrossRef]
- Roth, Z.; Parnes, S.; Wiel, S.; Sagi, A.; Zmora, N.; Chung, J.S.; Khalaila, I. N-glycan moieties of the crustacean egg yolk protein and their glycosylation sites. Glycoconj. J. 2010, 27, 159–169. [Google Scholar] [CrossRef]


| VOC | Seawater with Beads (ng/L) |
|---|---|
| Benzene | <1 |
| Toluene | <1 |
| Ethylbenzene | 400 ± 10 |
| Xylenes | 300 ± 80 |
| Styrene | 1.500 ± 400 |
| Methyl styrene | 8 ± 2 |
| Ethyl styrene | <1 |
| Benzaldehyde | 10 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, C.M.; Fogliano, C.; Trifuoggi, M.; Toscanesi, M.; Raggio, A.; Di Marino, S.; Venditti, P.; Fasciolo, G.; Avallone, B.; Carotenuto, R. Polystyrene Microplastic Interferes with Yolk Reserve Utilisation in Early Artemia salina Nauplii. Toxics 2025, 13, 700. https://doi.org/10.3390/toxics13080700
Motta CM, Fogliano C, Trifuoggi M, Toscanesi M, Raggio A, Di Marino S, Venditti P, Fasciolo G, Avallone B, Carotenuto R. Polystyrene Microplastic Interferes with Yolk Reserve Utilisation in Early Artemia salina Nauplii. Toxics. 2025; 13(8):700. https://doi.org/10.3390/toxics13080700
Chicago/Turabian StyleMotta, Chiara Maria, Chiara Fogliano, Marco Trifuoggi, Maria Toscanesi, Anja Raggio, Simona Di Marino, Paola Venditti, Gianluca Fasciolo, Bice Avallone, and Rosa Carotenuto. 2025. "Polystyrene Microplastic Interferes with Yolk Reserve Utilisation in Early Artemia salina Nauplii" Toxics 13, no. 8: 700. https://doi.org/10.3390/toxics13080700
APA StyleMotta, C. M., Fogliano, C., Trifuoggi, M., Toscanesi, M., Raggio, A., Di Marino, S., Venditti, P., Fasciolo, G., Avallone, B., & Carotenuto, R. (2025). Polystyrene Microplastic Interferes with Yolk Reserve Utilisation in Early Artemia salina Nauplii. Toxics, 13(8), 700. https://doi.org/10.3390/toxics13080700













