Toxicological Mechanisms of Uranium-Induced Apoptosis in HK-2 Cells: A Proteomics and Metabolomics Study
Highlights
- Exposure to uranium exposure predominately induces apoptosis in HK-2 cells through intrinsic pathways.
- Uranium can trigger apoptosis in cells through extrinsic signaling pathways.
- It can also induce apoptosis in cells through endoplasmic reticulum stress.
- The results of this study provide a new basis for examining the toxicological mechanism of uranium-induced apoptosis.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells Culture and Reagents Preparation
2.2. Cytotoxicity Assessment of HK-2 Cells
2.3. Flow Cytometry
2.4. AO/PI Staining
2.5. Comet Assay
2.6. DAPI Staining
2.7. Metabolomic Sequencing and Analysis
2.8. Proteomics Sequencing and Analysis
2.9. Statistical Analysis
3. Results
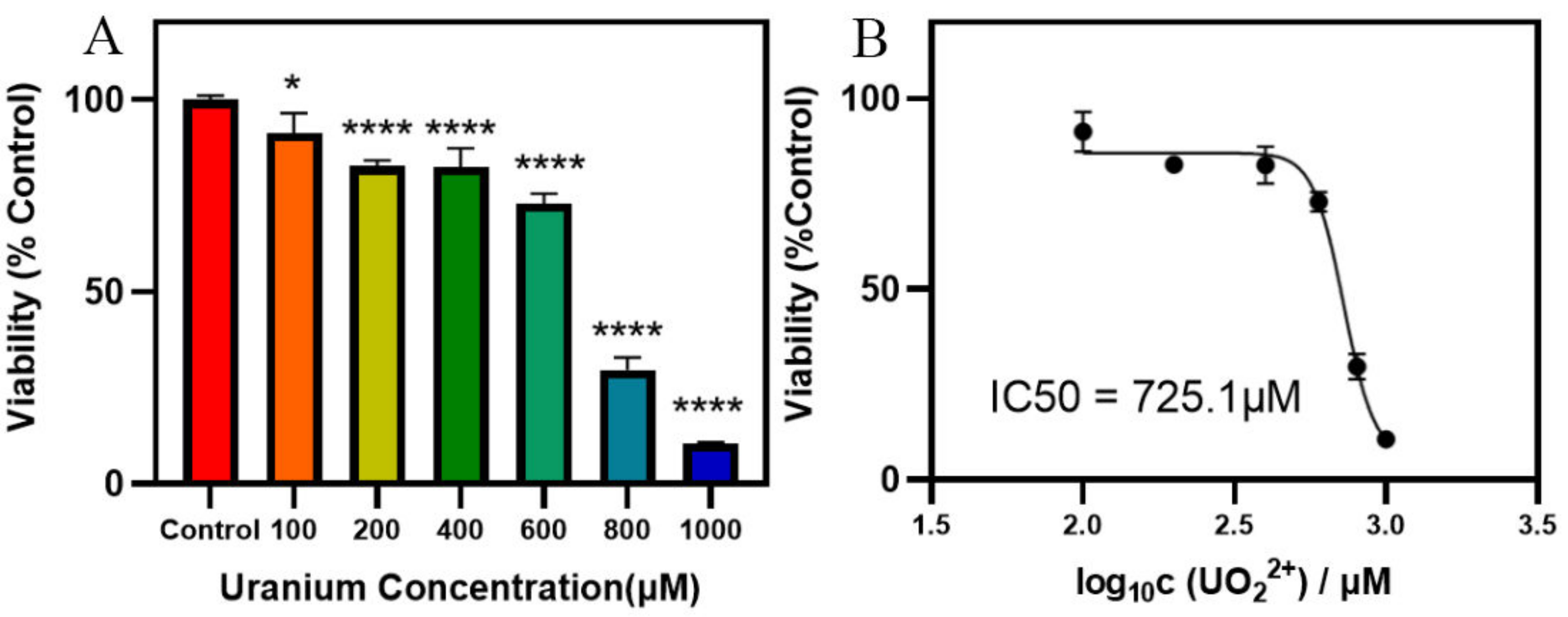
3.1. Cytotoxicity Evaluation
3.2. Uranyl Nitrate Caused Apoptosis in HK-2 Cells
3.3. Genetic Damage Induced by Uranium in HK-2 Cells
3.4. Differentially Expressed Proteins in Proteomics
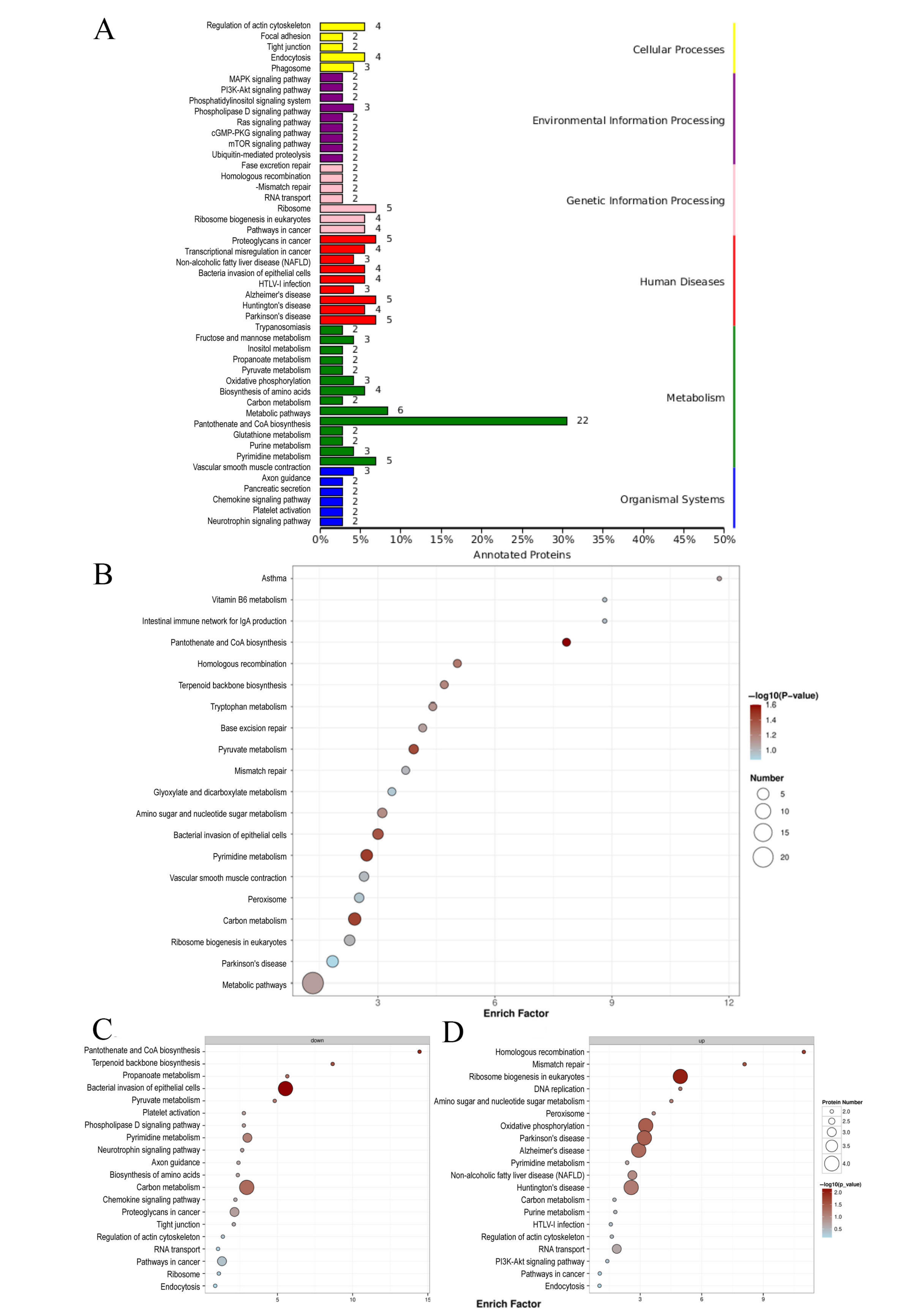
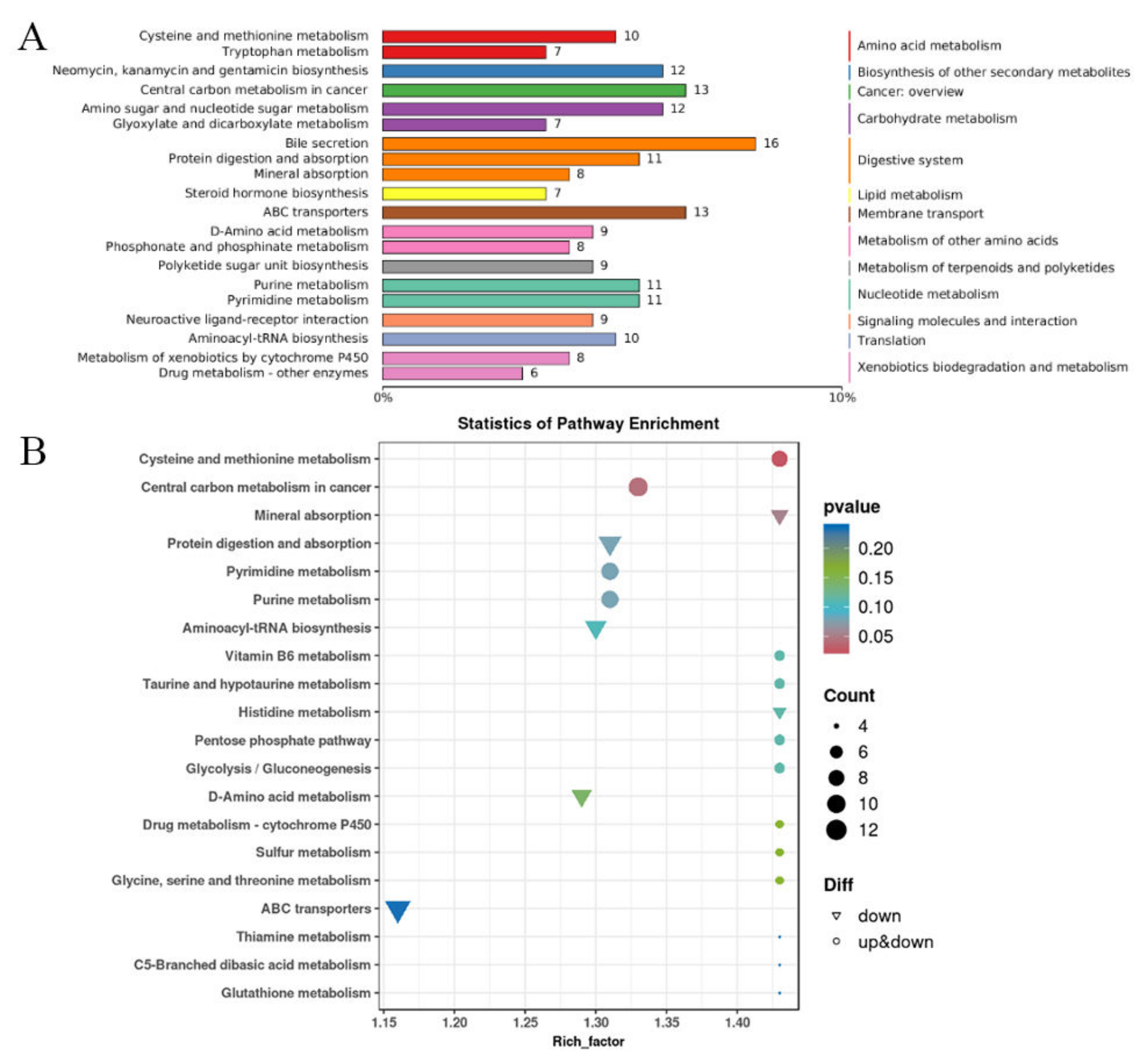
3.5. KEGG Enrichment Analysis in Proteomics
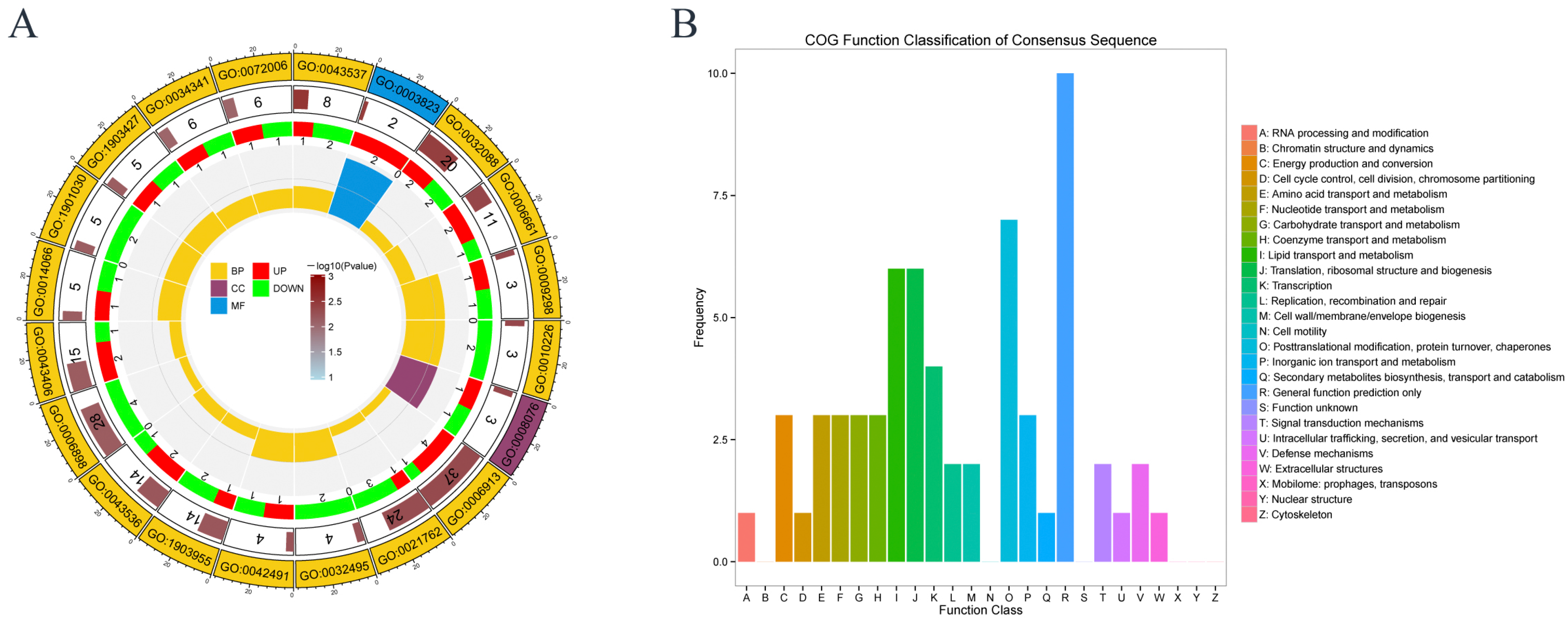
3.6. GO and COG Analysis in Proteomics
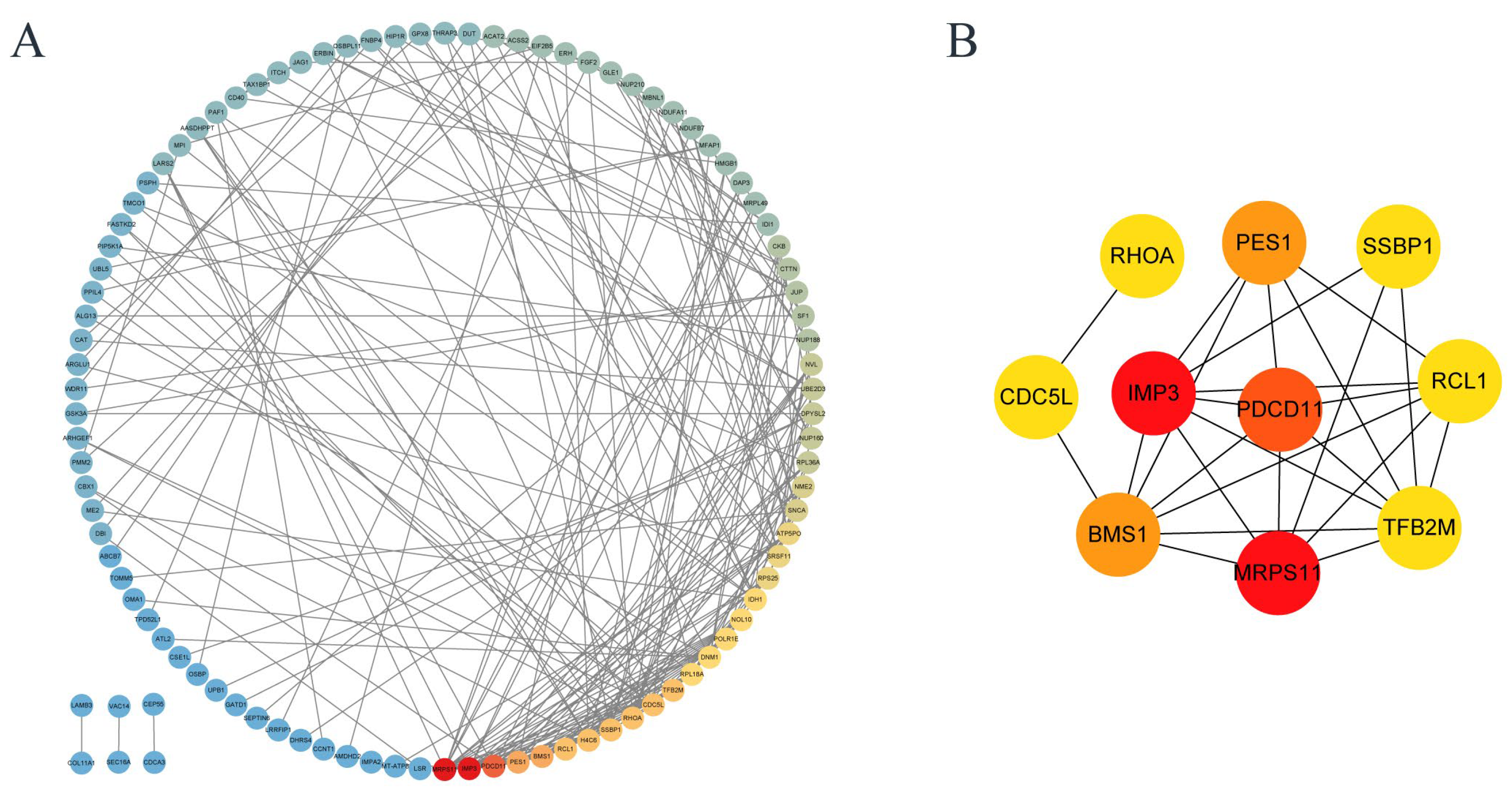
3.7. Protein–Protein Interaction (PPI) Network of Differentially Expressed Proteins
3.8. KEGG Enrichment Analysis of Differentially Changed Metabolites in Metabolomics
4. Discussion
4.1. Uranium Induces Intrinsic Apoptosis in Cells
4.2. Uranium Causes Endoplasmic Reticulum Stress Leading to Cell Apoptosis
4.3. Uranium May Induce Extrinsic Apoptosis in Cells
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appleton, J.D. Radon: Sources, health risks, and hazard mapping. Ambio 2007, 36, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, R.; Xu, L.; Xu, M.; Liu, S. Emerging health risks and underlying toxicological mechanisms of uranium contamination: Lessons from the past two decades. Environ. Int. 2020, 145, 106107. [Google Scholar] [CrossRef] [PubMed]
- Wufuer, R.; Wei, Y.; Lin, Q.; Wang, H.; Song, W.; Liu, W.; Zhang, D.; Pan, X.; Gadd, G.M. Uranium bioreduction and biomineralization. Adv. Appl. Microbiol. 2017, 101, 137–168. [Google Scholar] [CrossRef] [PubMed]
- Babich, R.; Craig, E.; Muscat, A.; Disney, J.; Farrell, A.; Silka, L.; Jayasundara, N. Defining drinking water metal contaminant mixture risk by coupling zebrafish behavioral analysis with citizen science. Sci. Rep. 2021, 11, 17303. [Google Scholar] [CrossRef]
- Arzuaga, X.; Rieth, S.H.; Bathija, A.; Cooper, G.S. Renal effects of exposure to natural and depleted uranium: A review of the epidemiologic and experimental data. J. Toxicol. Environ. Health Part B Crit. Rev. 2010, 13, 527–545. [Google Scholar] [CrossRef]
- Wang, S.; Ran, Y.; Lu, B.; Li, J.; Kuang, H.; Gong, L.; Hao, Y. A review of uranium-induced reproductive toxicity. Biol. Trace Elem. Res. 2020, 196, 204–213. [Google Scholar] [CrossRef]
- Domingo, J.L. Reproductive and developmental toxicity of natural and depleted uranium: A review. Reprod. Toxicol. 2001, 15, 603–609. [Google Scholar] [CrossRef]
- Schnug, E.; Lottermoser, B.G. Fertilizer-derived uranium and its threat to human health. Environ. Sci. Technol. 2013, 47, 2433–2434. [Google Scholar] [CrossRef]
- Wang, L.; Song, H.; Yuan, L.; Li, Z.; Zhang, Y.; Gibson, J.K.; Zheng, L.; Chai, Z.; Shi, W. Efficient U(VI) reduction and sequestration by Ti2CTx MXene. Environ. Sci. Technol. 2018, 52, 10748–10756. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, C.; Huang, J.; Gu, Y.; Li, H.; Yang, Z.; Liu, J.; Wang, W.; Li, R. Ghrelin protects against depleted uranium-induced apoptosis of MC3T3-E1 cells through oxidative stress-mediated p38-mitogen-activated protein kinase pathway. Toxicol. Appl. Pharmacol. 2016, 290, 116–125. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Kitahara, K.; Suzuki, K.; Blyth, B.J.; Suya, N.; Konishi, T.; Terada, Y.; Shimada, Y. Cellular localization of uranium in the renal proximal tubules during acute renal uranium toxicity. J. Appl. Toxicol. 2015, 35, 1594–1600. [Google Scholar] [CrossRef]
- Thiébault, C.; Carrière, M.; Milgram, S.; Simon, A.; Avoscan, L.; Gouget, B. Uranium induces apoptosis and is genotoxic to normal rat kidney (NRK-52E) proximal cells. Toxicol. Sci. 2007, 98, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, W.; Chu, J.; Chen, C.; Li, X.; Tang, W.; Xia, B.; Xiong, Z. Uranium inhibits mammalian mitochondrial cytochrome c oxidase and ATP synthase. Environ. Pollut. 2021, 271, 116377. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, G.; Xu, W.; Li, S.; Qin, X.; An, Q.; Wang, Z.; Li, J. Uranium uptake is mediated markedly by clathrin-mediated endocytosis and induce dose-dependent toxicity in HK-2 cells. Environ. Toxicol. Pharmacol. 2023, 101, 104171. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ren, J.; Liu, C.; Li, H.; Liu, J.; Yang, Z.; Li, R.; Su, Y. Zinc protects human kidney cells from depleted uranium-induced apoptosis. Basic Clin. Pharmacol. Toxicol. 2014, 114, 271–280. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Lam, M.; Marsters, S.A.; Ashkenazi, A.; Walter, P. Misfolded proteins bind and activate death receptor 5 to trigger apoptosis during unresolved endoplasmic reticulum stress. Elife 2020, 9, e52291. [Google Scholar] [CrossRef]
- Upton, J.P.; Austgen, K.; Nishino, M.; Coakley, K.M.; Hagen, A.; Han, D.; Papa, F.R.; Oakes, S.A. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol. Cell. Biol. 2008, 28, 3943–3951. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zheng, J.; Xu, X.N.; Gu, C.; Li, W. Uranium induces kidney cells apoptosis via reactive oxygen species generation, endoplasmic reticulum stress and inhibition of PI3K/AKT/mTOR signaling in culture. Environ. Toxicol. 2022, 37, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Ying, Y.; Huang, J.; Wu, H.; Wei, C.; Li, L.; Chen, L.; Wu, L. The expression, immune infiltration, prognosis, and experimental validation of OSBPL family genes in liver cancer. BMC Cancer 2023, 23, 244. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wan, S.; Liu, S.; Wang, W.; Tang, M.; Bai, L.; Zhu, Y. LARS2 Regulates Apoptosis via ROS-Mediated Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Ovarian Granulosa Cells. Oxidative Med. Cell. Longev. 2022, 2022, 5501346. [Google Scholar] [CrossRef]
- E, Y.; Yu, Q.; Sun, T.; Xue, H.; Zhao, X.R.; Zheng, H.C. The relationship between pepsinogen C and gastric carcinogenesis: A transgene and population study. BMC Cancer 2023, 23, 520. [Google Scholar] [CrossRef]
- Chu, T.H.; Ko, C.Y.; Tai, P.H.; Chang, Y.C.; Huang, C.C.; Wu, T.Y.; Chan, H.H.; Wu, P.H.; Weng, C.H.; Lin, Y.W.; et al. Leukocyte cell-derived chemotaxin 2 regulates epithelial-mesenchymal transition and cancer stemness in hepatocellular carcinoma. J. Biol. Chem. 2022, 298, 102442. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wang, L.; Cui, Z.; Cheng, F.; Wang, W.; Yang, X. FGF2 Is Protective Towards Cisplatin-Induced KGN Cell Toxicity by Promoting FTO Expression and Autophagy. Front. Endocrinol. 2022, 13, 890623. [Google Scholar] [CrossRef]
- Chen, X.Y.; Lai, J.Y.; Shen, W.J.; Wang, D.; Wei, Z.X. Investigation of risk signatures associated with anoikis in thyroid cancer through integrated transcriptome and Mendelian randomization analysis. Front. Endocrinol. 2024, 15, 1458956. [Google Scholar] [CrossRef]
- Wang, M.; Liu, F.; Fang, B.; Huo, Q.; Yang, Y. Proteome-scale profiling reveals MAFF and MAFG as two novel key transcription factors involved in palmitic acid-induced umbilical vein endothelial cell apoptosis. BMC Cardiovasc. Disord. 2021, 21, 448. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Huang, S.; Liu, J.; Tan, C.; Xu, M.; Wang, D.; Huang, M.; Zhu, Y.; Huang, X.; He, S. Deletion of ARGLU1 causes global defects in alternative splicing in vivo and mouse cortical malformations primarily via apoptosis. Cell Death Dis. 2023, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Jia, J.; Lv, G.; Wang, Y. Knockdown of ABCB7 inhibits esophageal cancer progression by inhibiting the TGF-β/Smad signaling. Arch. Biochem. Biophys. 2023, 742, 109620. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, S.; Zhou, W.; Gao, J.; Yin, J.; Wang, Z.; Li, J. Cellular transport of uranium and its cytotoxicity effects on CHO-k1 cells. Ecotoxicol. Environ. Saf. 2022, 246, 114166. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from forests and human health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef][Green Version]
- Vijayan P, S.; PD, R.; AB, A. Role of PI3K-Akt and MAPK Signaling in Uranyl Nitrate-Induced Nephrotoxicity. Biol. Trace Elem. Res. 2019, 189, 405–411. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Lai, J.L.; Yang, X.; Zhang, Y.; Luo, X.G. Non-targeted metabolomics reveals the stress response of a cellulase-containing penicillium to uranium. J. Environ. Sci. 2022, 120, 9–17. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; San Chin, H.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359, eaao6047. [Google Scholar] [CrossRef] [PubMed]
- Nakhoul, H.; Ke, J.; Zhou, X.; Liao, W.; Zeng, S.X.; Lu, H. Ribosomopathies: Mechanisms of disease. Clin. Med. Insights Blood Disord. 2014, 7, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Turi, Z.; Lacey, M.; Mistrik, M.; Moudry, P. Impaired ribosome biogenesis: Mechanisms and relevance to cancer and aging. Aging 2019, 11, 2512–2540. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Y.; Yu, X.Y.; Pan, X.; Zhang, Y.; Tu, J.; Song, Y.H.; Li, Y. Ribosome biogenesis in disease: New players and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 15. [Google Scholar] [CrossRef]
- Wang, W.; Hawkridge, A.M.; Ma, Y.; Zhang, B.; Mangrum, J.B.; Hassan, Z.H.; He, T.; Blat, S.; Guo, C.; Zhou, H.; et al. Ubiquitin-like protein 5 is a novel player in the UPR-PERK arm and ER stress-induced cell death. J. Biol. Chem. 2023, 299, 104915. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Lisbona, F.; Rojas-Rivera, D.; Hetz, C. When ER stress reaches a dead end. Biochim. Et Biophys. Acta 2013, 1833, 3507–3517. [Google Scholar] [CrossRef]
- Seyrek, K.; Ivanisenko, N.V.; Richter, M.; Hillert, L.K.; König, C.; Lavrik, I.N. Controlling cell death through post-translational modifications of DED proteins. Trends Cell Biol 2020, 30, 354–369. [Google Scholar] [CrossRef]
- Wagner, E.F.; Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]


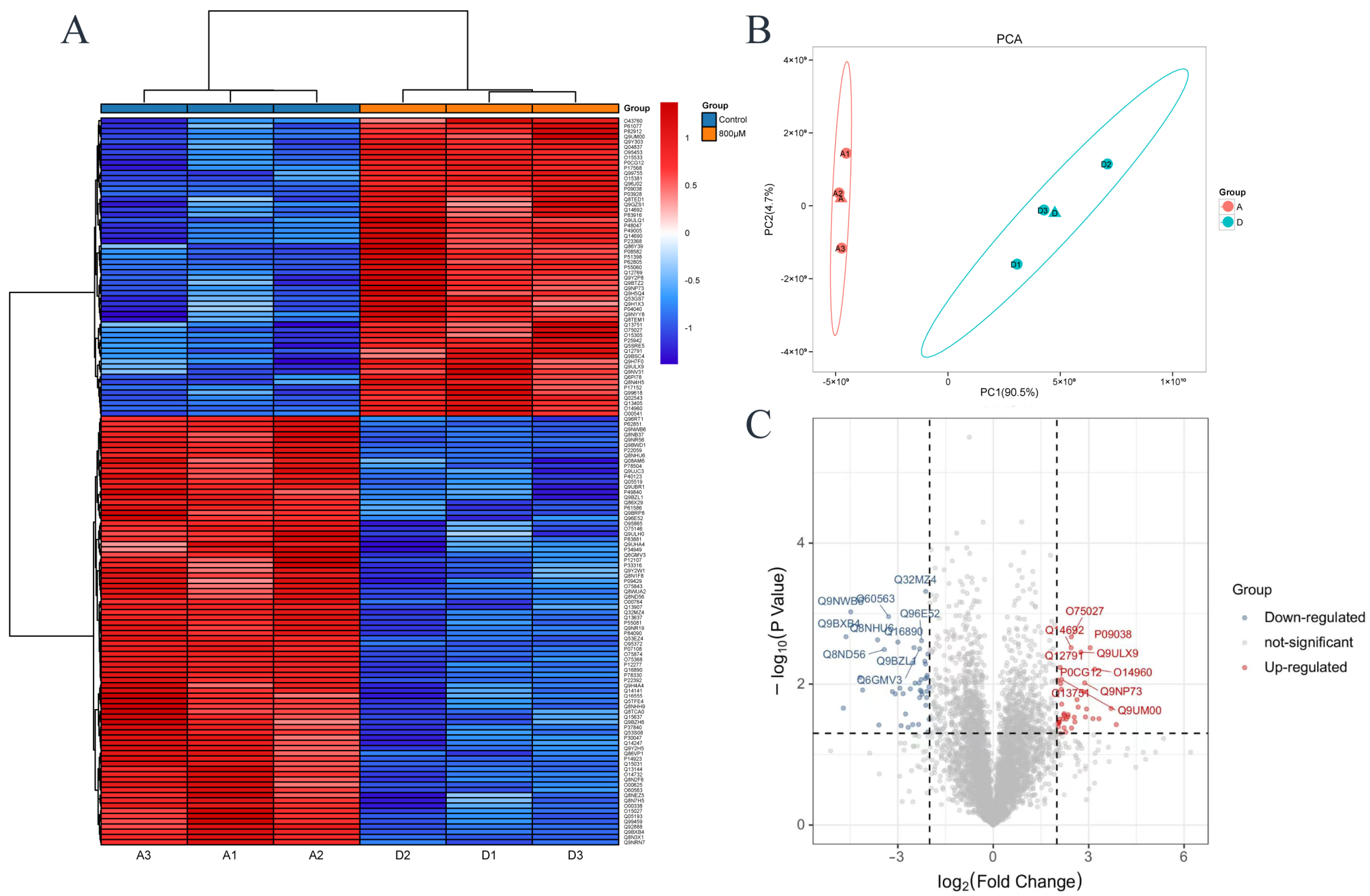
| 800 μM | Gene/Protein Name | log2FC | Up/Down |
|---|---|---|---|
| Q9BXB4 | OSBPL11 (oxysterol-binding protein-related protein 11) | −4.63 | down |
| Q9NWB6 | ARGLU1 (arginine- and glutamate-rich protein 1) | −4.48 | down |
| Q15031 | LARS2 (leucine–tRNA ligase, mitochondrial) | −4.19 | down |
| Q8NHU6 | TDRD7 (Tudor domain-containing protein 7) | −3.64 | down |
| Q8ND56 | LSM14A (protein LSM14 homolog A) | −3.42 | down |
| O60563 | CCNT1 (cyclin-T1) | −3.29 | down |
| O14960 | LECT2 (leukocyte cell-derived chemotaxin-2) | 3.19 | up |
| P09038 | FGF2 (fibroblast growth factor 2) | 3.05 | up |
| Q16890 | TPD52L1 (tumor protein D53) | −3 | down |
| Q9NP73 | ALG13 (UDP-N-acetylglucosamine transferase subunit ALG13) | 2.87 | up |
| Q9ULX9 | MAFF (transcription factor MafF) | 2.76 | up |
| O00338 | SULT1C2 (sulfotransferase 1C2) | −2.54 | down |
| Q9BZL1 | UBL5 (ubiquitin-like protein 5) | −2.49 | down |
| Q8NB37 | GALD1 (glutamine amidotransferase-like class 1 domain-containing protein 1) | −2.46 | down |
| O75027 | ABCB7 (iron–sulfur clusters transporter ABCB7, mitochondrial) | 2.46 | up |
| ID | Name | Up/Down |
|---|---|---|
| Q9NV31 | IMP3 (U3 small nucleolar ribonucleoprotein protein IMP3) | up |
| P82912 | MRPS11 (28S ribosomal protein S11, mitochondrial) | up |
| Q14690 | PDCD11 (protein RRP5 homolog) | up |
| O00541 | PES1 (pescadillo homolog) | up |
| Q14692 | BMS1 (ribosome biogenesis protein BMS1 homolog) | up |
| Q9Y2P8 | RCL1 (RNA 3′-terminal phosphate cyclase-like protein) | up |
| Q9H5Q4 | TFB2M (dimethyladenosine transferase 2, mitochondrial) | up |
| P61586 | RHOA (transforming protein RhoA) | down |
| Q99459 | CDC5L (cell division cycle 5-like protein) | down |
| Q04837 | SSBP1 (single-stranded DNA-binding protein, mitochondrial) | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Huang, Y.; Zhang, Y.; Wu, X.; Yang, Y.; Song, J.; Guo, K.; Wang, M.; Chen, J.; Qiang, S. Toxicological Mechanisms of Uranium-Induced Apoptosis in HK-2 Cells: A Proteomics and Metabolomics Study. Toxics 2025, 13, 699. https://doi.org/10.3390/toxics13080699
Wang Z, Huang Y, Zhang Y, Wu X, Yang Y, Song J, Guo K, Wang M, Chen J, Qiang S. Toxicological Mechanisms of Uranium-Induced Apoptosis in HK-2 Cells: A Proteomics and Metabolomics Study. Toxics. 2025; 13(8):699. https://doi.org/10.3390/toxics13080699
Chicago/Turabian StyleWang, Zihuan, Yongxiang Huang, Yue Zhang, Xuejuan Wu, Yuanyuan Yang, Jiayu Song, Kunling Guo, Mingyuan Wang, Junjie Chen, and Shirong Qiang. 2025. "Toxicological Mechanisms of Uranium-Induced Apoptosis in HK-2 Cells: A Proteomics and Metabolomics Study" Toxics 13, no. 8: 699. https://doi.org/10.3390/toxics13080699
APA StyleWang, Z., Huang, Y., Zhang, Y., Wu, X., Yang, Y., Song, J., Guo, K., Wang, M., Chen, J., & Qiang, S. (2025). Toxicological Mechanisms of Uranium-Induced Apoptosis in HK-2 Cells: A Proteomics and Metabolomics Study. Toxics, 13(8), 699. https://doi.org/10.3390/toxics13080699






