Associations Between Indoor Air Pollution and Urinary Volatile Organic Compound Biomarkers in Korean Adults
Highlights
- This nationwide study determined that urinary VOC biomarker levels were significantly associated with specific indoor air pollutants, with disparities by age, sex, socioeconomic status, and smoking status.
- Targeted indoor air quality interventions should be developed for vulnerable groups, and integrating biomonitoring with environmental measurements can guide the implementation of effective public health and housing policies.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. VOC and Indoor Air Pollutant Measurements
2.3. Biomarker Analysis
2.4. Laboratory Procedures and Quality Control
2.5. Covariates
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Participant Characteristics
3.2. Association Between Subjects’ Characteristics and Biomarker Levels
3.2.1. Sex and Biomarker Levels
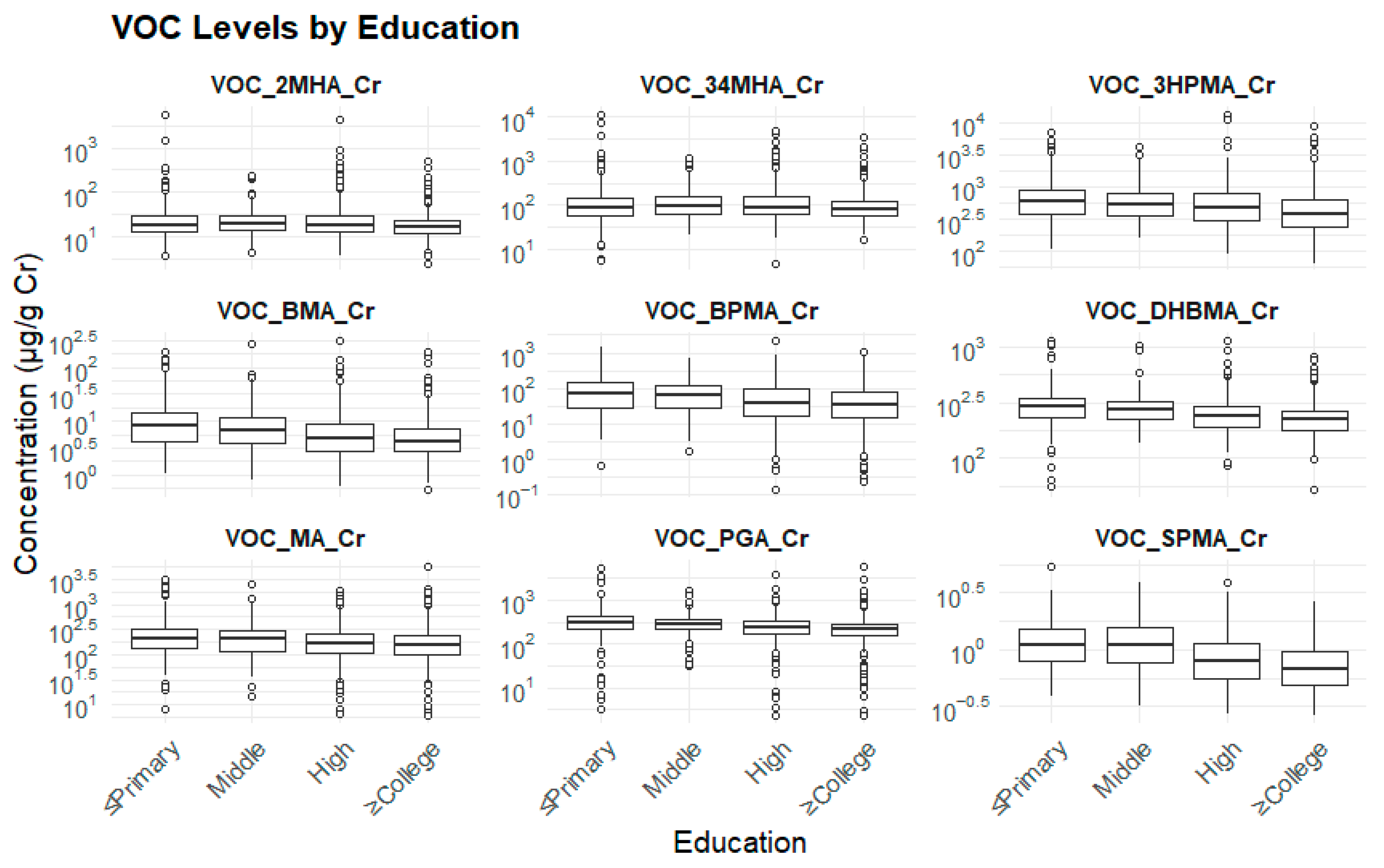
3.2.2. Education Levels and Biomarker Levels
3.2.3. Household Income Levels and Biomarker Levels
3.2.4. Usage of Air Purifier and Biomarker Levels
3.2.5. Home Repairs and Biomarker Levels
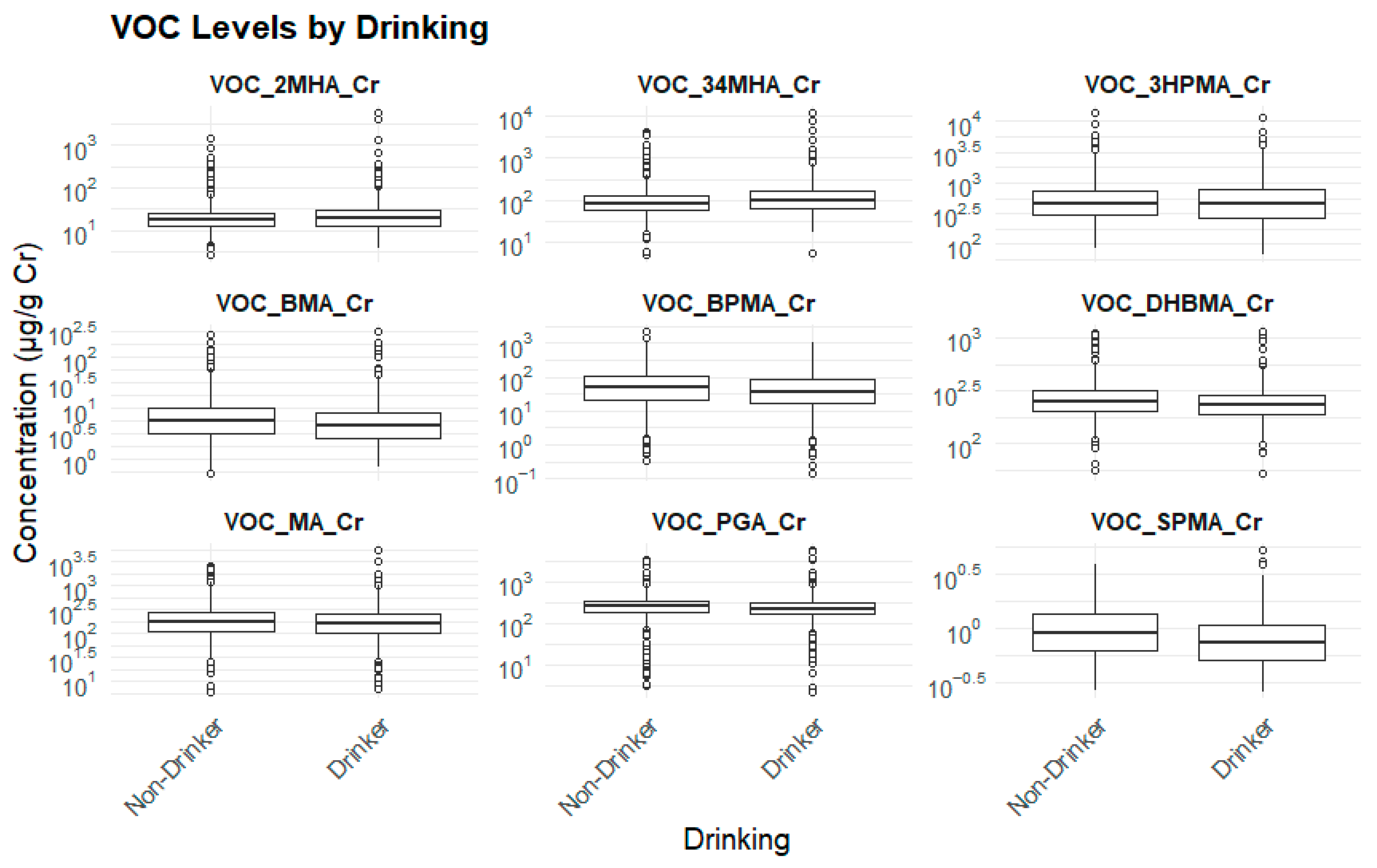
3.2.6. Drinking Status and Biomarker Levels
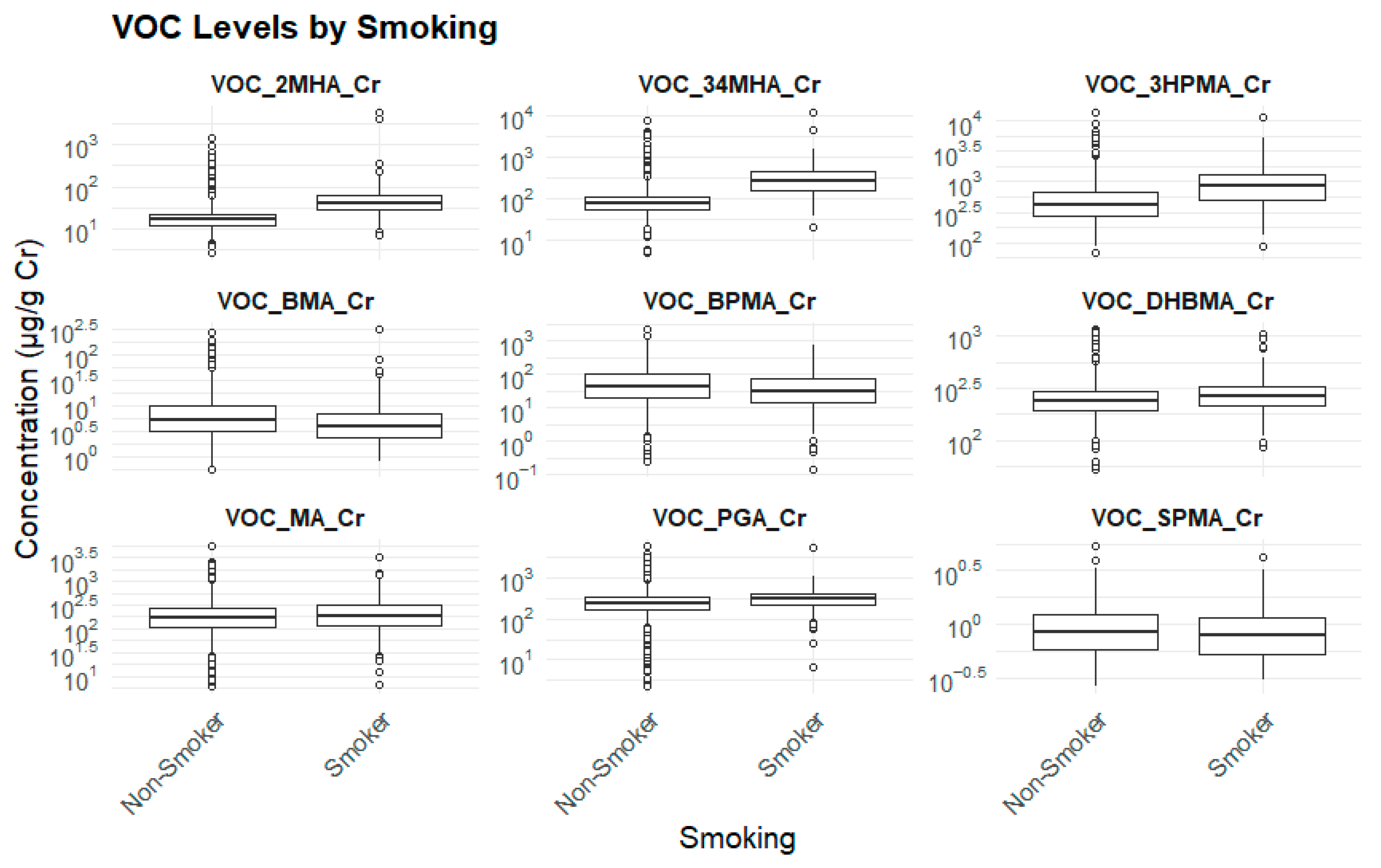
3.2.7. Smoking Status and Biomarker Levels
3.2.8. Age and Biomarker Levels
3.2.9. Time at Home on Weekdays and Biomarker Levels
3.3. Multivariable Regression Analysis for VOC Biomarkers
3.4. Correlation Between Indoor Air Pollutants and Urinary Metabolites
3.5. Correlation Among Indoor Air Pollutants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOC | Volatile organic compounds |
| WHO | World Health Organization |
| PM2.5 | Fine particulate matter |
| CO2 | Carbon dioxide |
| HCHO | Formaldehyde |
| IAQ | Indoor air quality |
| KNHANES | Korea National Health and Nutrition Examination Survey |
Appendix A
| Study Overview | The Family Indoor Air Quality and Environmental Hazardous Substance Biomarker Survey was conducted from 8 July 2020 to 3 August 2021. The indoor air quality component measured concentrations of ultrafine particulate matter (PM2.5), carbon dioxide (CO2), formaldehyde (HCHO), and total volatile organic compounds (TVOCs) in residential environments under typical daily living conditions. The environmental hazardous substance biomarker component analyzed nine urinary biomarkers of volatile organic compounds (VOCs) using biological samples collected from household members aged 19 years and older. |
| Study Design and Sampling Frame | This survey was carried out as part of the National Health and Nutrition Examination Survey (NHANES). The sampling frame was based on the most recent Population and Housing Census available at the time of sample design, enabling the selection of a representative sample of individuals aged ≥ 1 year residing in Korea. Although the Population and Housing Census serves as the primary sampling frame for NHANES, during the 5th survey cycle (2010–2012), when census data had become outdated, the resident registration population data and the Apartment Complex Market Survey were used as alternative sampling sources. In the 7th cycle (2016–2018), public housing price data were additionally incorporated to improve sampling efficiency. A two-stage stratified cluster sampling method was applied, using primary and secondary sampling units. In the 8th cycle (2019–2021), stratification was based on administrative divisions (cities, districts, and towns), housing type (general housing vs. apartments), proportion of inherited residential properties, age of household head, and proportion of single-person households. Each year, 192 survey districts were selected, yielding a total of 576 districts over the 3-year cycle. Within each sampled district, 25 households were randomly selected, excluding institutional facilities such as nursing homes, military bases, prisons, and foreign households. All members aged ≥ 1 year in the selected households who met the eligibility criteria were included as survey participants. In 2020, the second year of the 8th cycle, field operations were affected by the COVID-19 pandemic. Of the 192 planned survey districts, the health interview and health examination surveys were completed in 180 districts (completion rate: 93.8%), and the nutrition survey was completed in 166 districts (completion rate: 86.5%). |
| Survey Contents | The survey comprised four components: household survey, health interview, health examination, and nutrition survey. The household survey serves as the foundational component of NHANES, designed to assess the current status of all dwellings and households within the selected sampling areas and to determine eligible households for participation in the other survey components. Household membership verification allowed updates to be made for any changes in target areas or residential status since the creation of the sampling frame, ensuring accurate identification of survey households at the time of data collection. Information gathered from the household verification process was also used to coordinate visits for mobile health examination units (nutrition surveys were conducted via household visits), calculate extraction and response rates, and determine sampling weights based on survey outcomes and household characteristics. |
| Indoor Air Quality Assessment | A total of approximately 1200 households were selected for the indoor air quality survey. Seasonal variation, geographic region, and housing type were considered in the design to capture diverse environmental conditions. After obtaining informed consent, survey teams conducted home visits to measure and analyze four indoor air pollutants—PM2.5, CO2, HCHO, and TVOCs—in accordance with national indoor air quality testing standards. Measurement methods and duration:
|
| Seasonal ventilation assessment | Daily ventilation frequency and duration were calculated for each measurement season using participants’ self-reported data on the average number of ventilations per day and the average duration per ventilation session. When the total calculated ventilation time exceeded 24 h in a day (observed in 38 households), the value was retained for analysis and group-level ventilation volume calculations, without truncation. |
| Rounding rules for results: |
|
| Data Matching and Time Interval Considerations | Indoor air quality survey results were recorded at the household level, while biomarker results were recorded at the individual level for household members aged ≥19 years who participated in the indoor air quality survey. In some cases, an indoor air quality survey was conducted for a household, but no biological samples (urine) were collected from eligible members; consequently, no biomarker data were available for those households. To maximize comparability, biological samples were generally collected within four weeks after indoor air quality measurement. However, due to the suspension of metropolitan-area surveys during the COVID-19 pandemic (August 2021), there were instances where the time difference exceeded one month (n = 81; range: 46–61 days). While this gap does not affect the validity of biomarker data, analyses requiring matched household indoor air quality data should account for this interval. A separate variable (IAQ_VOC_ETC) was created to classify cases as “1 = within one month” or “2 = more than one month.” This variable should be included in raw data releases, and statistical results should be calculated using all available data with disclosure of the measurement interval. |
| Test Items | Official Test Method | Measurement Time | Measurement and Analysis Method, Equipment | Method Detection Limits (ug/mL) |
|---|---|---|---|---|
| Particulate Matter (PM2.5) | Gravimetric Method-ES 02302.1c | 24 h |
| |
| Carbon Dioxide (CO2) | Non-dispersive Infrared Sensor-ES 02905.1a | 1 h |
| |
| Formaldehyde (HCHO) | 2,4 DNPH Cartridge and Liquid Chromatography Method-ES 02601.1b | 30 min, 2 times |
| 0.0004 |
| Total Volatile Organic Compounds (TVOC) Benzene Toluene Ethylbenzene Xylene Styrene | Solid Adsorption Tube and Gas Chromatography-MS/FID Method-ES 02602.1b | 30 min, 2 times |
| Benzene: 0.000489 Toluene: 0.00095 Ethylbenzene: 0.00095 Xylene: 0.001736 Styrene: 0.001083 |
| Substance | Biomarker (Metabolite) | Test Method | Equipment (Manufacturer/Country) | Standard Manufacturer | LOD (μg/L) |
|---|---|---|---|---|---|
| Benzene | N-Acetyl-S-(phenyl)-L-cysteine (SPMA) | Quantitative simultaneous measurement of VOC metabolites in urine using LC-MS/MS | HPLC: Nexera XR LC-20AD System (Shimadzu/Kyoto, Japan) Mass Spectrometer: Triple Quad API 5500 (Sciex/Framingham, USA) | Sigma Aldrich | 0.251 |
| Toluene | N-Acetyl-S-(benzyl)-L-cysteine (BMA) | TRC | 0.01 | ||
| Ethylbenzene and Styrene | Phenylglyoxylic acid (PGA) | Sigma Aldrich | 0.985 | ||
| Xylene | 2-Methylhippuric acid (2-MHA) | Sigma Aldrich | 1.078 | ||
| Xylene | 3- and 4-Methylhippuric acid (3-MHA + 4-MHA) | Sigma Aldrich | 0.252 | ||
| Styrene | Mandelic acid (MA) | Sigma Aldrich | 5.337 | ||
| Acrolein | N-Acetyl-S-(3-hydroxypropyl)-L-cysteine (3-HPMA) | Sigma Aldrich | 0.128 | ||
| 1-Bromopropane | N-Acetyl-S-(n-propyl)-L-cysteine (BPMA) | Clearsynth | 0.103 | ||
| 1,3-Butadiene | N-Acetyl-S-(3,4-dihydroxybutyl)-L-cysteine (DHBMA) | TRC | 0.179 |
| Parameter | Result | Acceptance Criteria |
|---|---|---|
| Limit of Detection (LOD) Range | ~0.5–15 ng/mL | ≤20 ng/mL |
| Linearity (R2) | ≥0.99 | ≥0.98 |
| QC Coefficient of Variation (CV, %) | <15% | ≤15% |
| Recovery (%) | 85–105% | 80–120% |
| Precision (Relative Standard Deviation, %) | <10% | ≤15% |
References
- Vilcins, D.; Christofferson, R.C.; Yoon, J.H.; Nazli, S.N.; Sly, P.D.; Cormier, S.A.; Shen, G. Updates in air pollution: Current research and future challenges. Ann. Glob. Health 2024, 90, 9. [Google Scholar] [CrossRef]
- Apte, K.; Salvi, S. Household air pollution and its effects on health. F1000Research 2016, 5, F1000 Faculty Rev-2593. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, G. Indoor Air Pollution was Nonnegligible during COVID-19 Lockdown. Aerosol Air Qual. Res. 2020, 20, 1851–1855. [Google Scholar] [CrossRef]
- Abouleish, M.Y.Z. Indoor air quality and COVID-19. Public Health 2021, 191, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Bing, R.; Kiang, J.; Bashir, S.; Spath, N. Adverse health effects associated with household air pollution: A systematic review, meta-analysis, and burden estimation study. Lancet Glob. Health 2020, 8, e1427–e1434. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Household Air Pollution. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/household-air-pollution (accessed on 23 June 2025).
- Tsai, W.T. An overview of health hazards of volatile organic compounds regulated as indoor air pollutants. Rev. Environ. Health 2019, 34, 81–89. [Google Scholar] [CrossRef]
- Mannan, M.; Al-Ghamdi, S.G. Indoor air quality in buildings: A comprehensive review on the factors influencing air pollution in residential and commercial structure. Int. J. Environ. Res. Public Health 2021, 18, 3276. [Google Scholar] [CrossRef]
- Senerat, A.M.; Manemann, S.M.; Clements, N.S.; Brook, R.D.; Hassett, L.C.; Roger, V.L. Biomarkers and indoor air quality: A translational research review. J. Clin. Transl. Sci. 2020, 4, e39. [Google Scholar] [CrossRef]
- Nakai, J.S.; Chu, I.; Li-Muller, A.; Aucoin, R. Effect of environmental conditions on the penetration of benzene through human skin. J. Toxicol. Environ. Health 1997, 51, 447–462. [Google Scholar] [CrossRef]
- Marchand, A.; Aranda-Rodriguez, R.; Tardif, R.; Nong, A.; Haddad, S. Evaluation and modeling of the impact of coexposures to VOC mixtures on urinary biomarkers. Inhal. Toxicol. 2016, 28, 260–273. [Google Scholar] [CrossRef]
- Rappaport, S.M.; Kupper, L.L. Variability of environmental exposures to volatile organic compounds. J. Expo. Sci. Environ. Epidemiology 2004, 14, 92–107. [Google Scholar] [CrossRef]
- Adgate, J.L.; Church, T.R.; Ryan, A.D. Outdoor, indoor, and personal exposure to VOCs in children. Environ. Health Perspect. 2004, 112, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Satish, U.; Mendell, M.J.; Shekhar, K. Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance. Environ. Health Perspect. 2012, 120, 1671–1677. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs Volume 120: Benzene. Available online: https://www.iarc.who.int/news-events/iarc-monographs-volume-120-benzene/ (accessed on 5 June 2025).
- Simoni, M.; Annesi-Maesano, I.; Sigsgaard, T.; Norback, D.; Wieslander, G.; Nystad, W.; Canciani, M. School air quality related to dry cough, rhinitis and nasal patency in children. Eur. Respir. J. 2010, 35, 742–749. [Google Scholar] [CrossRef]
- Uzoigwe, J.; Prum, T.; Bresnahan, E.; Garelnabi, M. The emerging role of outdoor and indoor air pollution in cardiovascular disease. N. Am. J. Med Sci. 2013, 5, 445–453. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines for Indoor Air Quality. Available online: https://www.who.int/publications/i/item/9789289002134 (accessed on 10 August 2025).
- Ferguson, L.; Taylor, J.; Davies, M.; Shrubsole, C.; Symonds, P.; Dimitroulopoulou, S. Exposure to indoor air pollution across socio-economic groups in high-income countries: A scoping review of the literature and a modelling methodology. Environ. Int. 2020, 143, 105748. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Sohn, J. The effect of environmental and structural factors on indoor air quality of apartments in Korea. Build Environ. 2009, 44, 1794–1802. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.H.; Sohn, J.R. Indoor air quality assessment in child care and medical facilities in Korea. Environ. Monit. Assess 2012, 184, 6395–6409. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review of diseases associated with household air pollution due to the use of biomass fuels. J. Hazard. Mater. 2011, 192, 425–431. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Air Quality, Energy and Health. Health Risks. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-energy-and-health/sectoral-interventions/household-air-pollution/health-risks (accessed on 14 August 2025).
- Abbah, A.; Xu, S.; Johannessen, A. Long-term health effects of outdoor air pollution on asthma and respiratory symptoms among adults in low-and middle-income countries (LMICs): A systematic review and meta-analysis. Front Environ. Health 2024, 3, 1352786. [Google Scholar] [CrossRef]
- Raju, S.; Siddharthan, T.; McCormack, M.C. Indoor Air Pollution and Respiratory Health. Clin. Chest Med. 2020, 41, 825–843. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Hwang, Y.; Oh, K.; Park, J.; Lee, K. Development of the indoor air quality monitoring model based on Korea national health and nutrition examination survey (KNHANES). Public Health Wkly. Rep. 2021, 14, 245–247. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Environmental Public Health Tracking: Biomonitoring: Population Exposures 2023. Available online: https://www.cdc.gov/environmental-health-tracking/php/data-research/biomonitoring.html (accessed on 10 August 2025).
- International Program on Chemical Safety; World Health Organization; Työterveyslaitos. Biological Monitoring of Chemical Exposure in the Workplace: Guidelines; World Health Organization: Geneva, Switzerland, 1996.
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. National Center for Health Statistics. Vital Health Stat. 2013, 2, 161. [Google Scholar]
- Tong, X.; Wang, B.; Dai, W.T. Indoor air pollutant exposure and determinant factors controlling household air quality for elderly people in Hong Kong. Air Qual. Atmos. Health 2018, 11, 695–704. [Google Scholar] [CrossRef]
- Dehghani, S.; Yousefi, S.; Oskoei, V. Ecological study on household air pollution exposure and prevalent chronic disease in the elderly. Sci. Rep. 2023, 13, 11763. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Han, D.; Eom, S.; Cho, Y. Levels of exposure markers among residents in environmentally vulnerable areas in Korea, the general population in Korea, and Asians in the United States. Epidemiol. Health 2025, 47, e2025007. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, S. Efficacy of Ventilation Air Purifiers in Improving Classroom Air Quality: A Case Study in South Korea. Atmosphere 2025, 16, 448. [Google Scholar] [CrossRef]
- Hu, Y.; Niu, Z.; Cao, C.; Gao, J.; Pan, M.; Cai, Y.; Zhao, Z. Volatile organic compounds (VOC) metabolites in urine are associated with increased systemic inflammation levels, and smokers are identified as a vulnerable population. Ecotoxicol. Environ. Saf. 2024, 288, 117398. [Google Scholar] [CrossRef]
- Ferdous, T.; Siddiqi, K.; Semple, S.; Fairhurst, C.; Dobson, R.; Mdege, N.; Marshall, A. Smoking behaviours and indoor air quality: A comparative analysis of smoking-permitted versus smoke-free homes in Dhaka, Bangladesh. Tob. Control. 2022, 31, 444–451. [Google Scholar] [CrossRef]
- Cheek, E.; Guercio1, V.; Shrubsole, C.; Dimitroulopoulou, S. Portable air purification: Review of impacts on indoor air quality and health. Sci. Total Environ. 2021, 766, 142585. [Google Scholar] [CrossRef]
- Schulz, C.; Conrad, A.; Becker, K.; Kolossa-Gehring, M.; Seiwert, M.; Seifert, B. Twenty years of the German Environmental Survey (GerES): Human biomonitoring–temporal and spatial (West Germany/East Germany) differences in population exposure. Int. J. Hyg. Environ. Health 2007, 210, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Govarts, E.; Gilles, L.; Rodriguez Martin, L. Harmonized human biomonitoring in European children, teenagers and adults: EU-wide exposure data of 11 chemical substance groups from the HBM4EU Aligned Studies (2014–2021). Int. J. Hyg. Environ. Health 2023, 249, 114119. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Park, D.; Lee, Y. Indoor Air Pollution, Related Human Diseases, and Recent Trends in the Control and Improvement of Indoor Air Quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef] [PubMed]





| Parent Compound | Metabolite (Biomarker) | Abbreviation | LOD (μg/L) |
|---|---|---|---|
| Benzene | N-Acetyl-S-(phenyl)-L-cysteine | SPMA | 0.251 |
| Toluene | N-Acetyl-S-(benzyl)-L-cysteine | BMA | 0.010 |
| Ethylbenzene and Styrene | Phenylglyoxylic acid | PGA | 0.985 |
| Styrene | Mandelic acid | MA | 5.337 |
| Xylene | 2-Methylhippuric acid | 2-MHA | 1.078 |
| 3- and 4-Methylhippuric acid | 3-MHA + 4-MHA | 0.252 | |
| Acrolein | N-Acetyl-S-(3-hydroxypropyl)-L-cysteine | 3-HPMA | 0.128 |
| 1-Bromopropane | N-Acetyl-S-(n-propyl)-L-cysteine | BPMA | 0.103 |
| 1,3-Butadiene | N-Acetyl-S-(3,4-dihydroxybutyl)-L-cysteine | DHBMA | 0.179 |
| Characteristic | Male 846 (45.3%) | Female 1034 (54.7%) | p-Value | |
|---|---|---|---|---|
| Age (years), mean ± SE (IQR) | 52.70 ± 0.786 (40–68) | 53.35 ± 0.712 (40–69) | 0.000 *** | |
| Education, n (%) | ≤Elementary School | 98 (11.7) | 254 (21.7) | 0.000 *** |
| Middle School | 78 (7.7) | 106 (11.1) | ||
| High School | 306 (39.1) | 287 (29.8) | ||
| ≥College | 364 (41.5) | 387 (37.4) | ||
| Household Income, n (%) | Low | 151 (16.7) | 229 (18.9) | 0.200 |
| Low-Middle | 219 (25.6) | 278 (27.3) | ||
| Middle-High | 234 (25.8) | 259 (24.2) | ||
| High | 287 (32.0) | 316 (29.6) | ||
| Drinking Status, n (%) | No | 307 (33.1) | 680 (61.7) | 0.000 *** |
| Yes | 583 (66.9) | 404 (38.3) | ||
| Smoking Status, n (%) | No | 634 (69.8) | 1050 (96.7) | 0.000 *** |
| Yes | 256 (30.2) | 34 (3.3) | ||
| Time at home on weekdays(hours), mean ± SE (IQR) | 15.34 ± 0.228 (12–20) | 17.87 ± 0.180 (14–22) | 0.000 *** | |
| Housing Repair within 6 months, n (%) | No | 818 (91.4) | 999 (91.1) | 0.754 |
| Yes | 75 (8.6) | 88 (8.9) | ||
| Air Purifier, n (%) | No | 529 (58.1) | 607 (54.7) | 0.054 |
| Yes | 364 (41.9) | 480 (45.3) | ||
| Variable | Mean ± SE | Variable | Mean ± SE |
|---|---|---|---|
| PM2.5 (μg/m3) | 17.32 ± 0.758 | 2MHA (μg/g cr.) | 36.75 ± 4.730 |
| CO2 (ppm) | 791.87 ± 17.983 | 3HPMA (μg/g cr.) | 634.87 ± 21.211 |
| HCHO (μg/ m3) | 27.79 ± 0.981 | BPMA (μg/g cr.) | 79.70 ± 3.307 |
| TVOC (μg/ m3) | 257.26 ± 26.600 | DHBMA (μg/g cr.) | 263.06 ± 3.337 |
| Benzene (μg/ m3) | 5.21 ± 0.978 | SPMA (μg/g cr.) | 0.97 ± 0.020 |
| Toluene (μg/ m3) | 24.61 ± 2.338 | BMA (μg/g cr.) | 9.23 ± 0.433 |
| Ethylbenzene (μg/ m3) | 4.56 ± 0.393 | PGA (μg/g cr.) | 285.06 ± 7.698 |
| Xylene (μg/ m3) | 8.94 ± 0.909 | 3,4MHA (μg/g cr.) | 172.74 ± 11.505 |
| Styrene (μg/ m3) | 3.73 ± 0.606 | MA (μg/g cr.) | 228.27 ± 9.164 |
| Biomarker (μg/g cr.) | Male (Mean ± SE) | Female (Mean ± SE) | p-Value |
|---|---|---|---|
| 3HPMA | 686.1 ± 55.6 | 723.5 ± 54.2 | 0.407 |
| PGA | 275.9 ± 33.6 | 308.2 ± 35.1 | 0.035 * |
| MA | 212.3 ± 17.9 | 242.2 ± 21.9 | 0.038 * |
| SPMA | 0.86 ± 0.04 | 1.10 ± 0.04 | 0.000 *** |
| BPMA | 56.9 ± 11.8 | 78.3 ± 12.0 | 0.000 *** |
| DHBMA | 250.2 ± 12.9 | 281.7 ± 11.9 | 0.000 *** |
| 2MHA | 55.38 ± 11.91 | 51.70 ± 10.46 | 0.359 |
| BMA | 5.05 ± 1.21 | 7.93 ± 1.27 | 0.000 *** |
| 3,4MHA | 258.9 ± 24.4 | 256.1 ± 23.9 | 0.888 |
| Biomarker (μg/g cr.) | ≤Elementary School a (Mean ± SE) | Middle School b (Mean ± SE) | High School c (Mean ± SE) | ≥College d (Mean ± SE) | p-Value | Bonferroni |
|---|---|---|---|---|---|---|
| 3HPMA | 876.8 ± 67.1 | 698.9 ± 64.3 | 648.6 ± 53.9 | 618.9 ± 52.5 | 0.000 *** | a > b, a > c, a > d, b > d |
| PGA | 415.8 ± 23.4 | 335.2 ± 16.8 | 315.3 ± 15.8 | 281.1 ± 17.5 | 0.000 *** | a > b, a > c, a > d, b > c, b > d, c > d |
| MA | 273.4 ± 28.5 | 229.1 ± 21.7 | 196.6 ± 19.4 | 210.6 ± 24.1 | 0.008 ** | a > b, a > c, a > d, b > c, c > d |
| SPMA | 1.21 ± 0.041 | 1.26 ± 0.050 | 0.94 ± 0.031 | 0.84 ± 0.021 | 0.000 *** | a > d, a < b, a > c, b > c, b > d, c > d |
| BPMA | 98.7 ± 10.3 | 68.5 ± 12.2 | 57.4 ± 10.4 | 49.9 ± 9.5 | 0.000 *** | a > b, a > c, a > d, b > d |
| DHBMA | 289.9 ± 12.9 | 262.3 ± 16.1 | 264.3 ± 12.9 | 247.3 ± 13.1 | 0.001 ** | a > b, a > c, a > d, b > d, c > d |
| 2MHA | 37.21 ± 6.13 | 40.87 ± 12.28 | 38.93 ± 9.27 | 25.19 ± 1.66 | 0.453 | |
| BMA | 9.76 ± 1.37 | 9.92 ± 3.21 | 4.42 ± 0.83 | 4.16 ± 0.84 | 0.000 *** | a > c, a > d, b > c, b > d |
| 3,4MHA | 299.3 ± 72.4 | 231.8 ± 64.4 | 276.3 ± 65.1 | 219.4 ± 64.3 | 0.002 ** | a > b, a > d |
| Biomarker (μg/g cr.) | Low Income a (Mean ± SE) | Low-Middle Income b (Mean ± SE) | Middle-High Income c (Mean ± SE) | High Income d (Mean ± SE) | p-Value | Bonferroni |
|---|---|---|---|---|---|---|
| 3HPMA | 733.8 ± 96.1 | 667.0 ± 59.0 | 656.3 ± 71.9 | 611.6 ± 57.7 | 0.042 * | a > d |
| PGA | 327.6 ± 39.2 | 326.3 ± 19.9 | 320.3 ± 21.5 | 292.2 ± 18.4 | 0.316 | |
| MA | 223.8 ± 32.0 | 190.1 ± 24.1 | 206.3 ± 19.8 | 216.6 ± 19.7 | 0.666 | |
| SPMA | 1.20 ± 0.043 | 1.07 ± 0.032 | 1.02 ± 0.028 | 0.95 ± 0.028 | 0.000 *** | a > b, a > c, a > d, b > c, b > d, c > d |
| BPMA | 71.70 ± 14.39 | 77.05 ± 10.39 | 74.17 ± 9.89 | 62.21 ± 7.87 | 0.372 | |
| DHBMA | 291.8 ± 16.1 | 270.4 ± 12.0 | 267.4 ± 11.6 | 248.7 ± 11.6 | 0.003 * | a > b, a > c, a > d, b > d, c > d |
| 2MHA | 37.21 ± 6.13 | 40.87 ± 12.28 | 38.93 ± 9.27 | 25.19 ± 1.66 | 0.453 | |
| BMA | 7.14 ± 2.423 | 5.48 ± 1.264 | 7.26 ± 1.408 | 5.98 ± 1.490 | 0.684 | |
| 3,4MHA | 317.2 ± 85.9 | 243.7 ± 26.5 | 307.4 ± 48.9 | 242.1 ± 41.2 | 0.460 |
| Biomarker (μg/g cr.) | Use (Mean ± SE) | No-Use (Mean ± SE) | p-Value |
|---|---|---|---|
| 3HPMA | 535.75 ± 23.68 | 714.87 ± 31.326 | 0.000 *** |
| PGA | 264.02 ± 10.90 | 302.06 ± 10.59 | 0.014 * |
| MA | 208.12 ± 11.98 | 244.53 ± 11.82 | 0.020 * |
| SPMA | 0.86 ± 0.021 | 1.06 ± 0.028 | 0.000 *** |
| BPMA | 72.94 ± 4.751 | 85.15 ± 4.227 | 0.045 * |
| DHBMA | 242.16 ± 3.864 | 279.92 ± 4.289 | 0.000 *** |
| 2MHA | 55.72 ± 13.99 | 51.35 ± 10.04 | 0.359 |
| BMA | 7.13 ± 0.358 | 10.92 ± 0.739 | 0.000 *** |
| 3,4MHA | 170.98 ± 20.21 | 174.17 ± 12.939 | 0.895 |
| Biomarker (μg/g cr.) | No (Mean ± SE) | Yes (Mean ± SE) | p-Value |
|---|---|---|---|
| 3HPMA | 654.4 ± 58.8 | 767.2 ± 51.9 | 0.016 * |
| PGA | 326.7 ± 18.0 | 345.2 ± 13.4 | 0.217 |
| MA | 226.6 ± 26.9 | 228.3 ± 16.7 | 0.949 |
| SPMA | 0.99 ± 0.06 | 1.03 ± 0.03 | 0.554 |
| BPMA | 62.1 ± 9.9 | 75.1 ± 9.3 | 0.076 |
| DHBMA | 262.9 ± 10.0 | 278.4 ± 8.9 | 0.043 * |
| 2MHA | 48.62 ± 8.76 | 58.46 ± 14.24 | 0.249 |
| BMA | 6.76 ± 0.91 | 7.36 ± 0.88 | 0.430 |
| 3,4MHA | 247.4 ± 25.3 | 262.2 ± 26.6 | 0.558 |
| Biomarker (μg/g cr.) | Non-Drinker (Mean ± SE) | Drinker (Mean ± SE) | p-Value |
|---|---|---|---|
| 3HPMA | 637.02 ± 25.950 | 631.14 ± 29.005 | 0.867 |
| PGA | 287.94 ± 9.231 | 282.69 ± 10.942 | 0.691 |
| MA | 239.77 ± 13.461 | 217.43 ± 10.743 | 0.163 |
| SPMA | 1.07 ± 0.030 | 0.88 ± 0.023 | 0.000 *** |
| BPMA | 90.64 ± 5.127 | 69.47 ± 3.333 | 0.000 *** |
| DHBMA | 275.58 ± 5.025 | 250.98 ± 3.879 | 0.000 *** |
| 2MHA | 52.06 ± 9.67 | 55.02 ± 12.71 | 0.541 |
| BMA | 10.53 ± 0.628 | 8.03 ± 0.464 | 0.000 *** |
| 3,4MHA | 143.84 ± 10.342 | 199.57 ± 18.171 | 0.004 ** |
| Biomarker (μg/g cr.) | Non-Smoker (Mean ± SE) | Smoker (Mean ± SE) | p-Value |
|---|---|---|---|
| 3HPMA | 510.7 ± 45.1 | 910.9 ± 68.1 | 0.000 *** |
| PGA | 303.7 ± 12.7 | 368.2 ± 19.1 | 0.000 *** |
| MA | 212.4 ± 16.9 | 242.5 ± 22.5 | 0.086 |
| SPMA | 1.00 ± 0.04 | 1.02 ± 0.04 | 0.639 |
| BPMA | 74.7 ± 8.6 | 62.3 ± 10.2 | 0.050 |
| DHBMA | 251.0 ± 7.7 | 291.1 ± 11.8 | 0.000 *** |
| 2MHA | 23.55 ± 3.62 | 83.53 ± 24.02 | 0.024 * |
| BMA | 7.73 ± 0.85 | 6.90 ± 0.91 | 0.280 |
| 3,4MHA | 148.97 ± 21.5 | 368.83 ± 32.09 | 0.000 *** |
| Biomarker (μg/g cr.) | Estimate | Std. Error | p-Value |
|---|---|---|---|
| 3HPMA | −4.4015 × 10−4 | 0.001 | 0.965 |
| PGA | 0.007 | 0.003 | 0.007 ** |
| MA | −0.001 | 0.002 | 0.781 |
| SPMA | 9.377 | 1.062 | 0.000 *** |
| BPMA | 0.008 | 0.005 | 0.086 |
| DHBMA | 0.025 | 0.005 | 0.000 *** |
| 2MHA | 0.009 | 0.005 | 0.077 |
| BMA | 0.103 | 0.023 | 0.000 *** |
| 3,4MHA | −0.007 | 0.003 | 0.007 ** |
| Biomarker (μg/g cr.) | Estimate | Std. Error | p-Value |
|---|---|---|---|
| 3HPMA | 0.000 | 0.001 | 0.654 |
| PGA | 0.001 | 0.001 | 0.080 |
| MA | −3.014 × 10−5 | 0.001 | 0.965 |
| SPMA | 1.574 | 0.272 | 0.000 *** |
| BPMA | 0.002 | 0.001 | 0.089 |
| DHBMA | 0.005 | 0.001 | 0.000 *** |
| 2MHA | 0.003 | 0.002 | 0.184 |
| BMA | 0.007 | 0.006 | 0.235 |
| 3,4MHA | −0.002 | 0.001 | 0.012 * |
| Biomarker | Variable | Estimate | Std. Error | p-Value |
|---|---|---|---|---|
| 3HPMA (μg/g cr.) Adj. R2 = 0.157 | Age | 4.684 | 1.616 | 0.005 ** |
| Smoking | 402.342 | 89.940 | 0.000 *** | |
| Air purifier | −136.877 | 54.355 | 0.013 * | |
| Ethylbenzene | 2.044 | 0.626 | 0.001 ** | |
| Styrene | 5.154 | 2.310 | 0.028 * | |
| PGA (μg/g cr.) Adj. R2 = 0.481 | Age | 2.384 | 0.610 | 0.000 *** |
| Sex | −35.961 | 16.605 | 0.033 * | |
| Smoking | 83.698 | 24.180 | 0.001 ** | |
| BMI | −4.915 | 1.982 | 0.015 * | |
| Air Purifier | −56.536 | 15.038 | 0.001 ** | |
| CO2 | −0.065 | 0.027 | 0.016 * | |
| Benzene | 6.317 | 0.972 | 0.000 *** | |
| Ethylbenzene | 1.837 | 0.553 | 0.001 ** | |
| MA (μg/g cr.) Adj. R2 = 0.050 | Age | 1.390 | 0.634 | 0.031 * |
| 2MHA (μg/g cr.) Adj. R2 = 0.058 | Benzene | 0.841 | 0.245 | 0.001 ** |
| SPMA (μg/g cr.) Adj. R2 = 0.246 | Age | 0.008 | 0.002 | 0.000 *** |
| Sex | −0.221 | 0.048 | 0.000 *** | |
| Education | −0.200 | 0.063 | 0.016 * | |
| Air purifier | −0.180 | 0.054 | 0.001 ** | |
| Xylene | −0.001 | 0.000 | 0.020 * | |
| BPMA (μg/g cr.) Adj. R2 = 0.088 | Age | 0.904 | 0.317 | 0.000 *** |
| Smoking | 18.223 | 8.383 | 0.032 * | |
| CO2 | −0.021 | 0.010 | 0.034 * | |
| Ethylbenzene | 0.468 | 0.089 | 0.000 *** | |
| DHBMA (μg/g cr.) Adj. R2 = 0.210 | Age | 1.230 | 0.486 | 0.013 * |
| Sex | −47.791 | 8.288 | 0.000 *** | |
| Education | −23.509 | 9.662 | 0.016 * | |
| Income | −18.677 | 7.773 | 0.018 * | |
| Smoking | 56.789 | 14.455 | 0.000 *** | |
| Air purifier | −20.787 | 9.780 | 0.032 * | |
| CO2 | −0.029 | 0.012 | 0.019 * | |
| Xylene | 0.307 | 0.136 | 0.029 * | |
| BMA (μg/g cr.) Adj. R2 = 0.108 | Age | 0.171 | 0.047 | 0.000 *** |
| Drinking | −3.398 | 1.113 | 0.003 ** | |
| Smoking | 2.714 | 1.067 | 0.012 * | |
| 3.4MHA (μg/g cr.) Adj. R2 = 0.171 | Smoking | 270.180 | 70.437 | 0.000 *** |
| BMI | −8.545 | 4.148 | 0.044 * | |
| HCHO | 1.520 | 0.660 | 0.023 * | |
| Benzene | 3.378 | 1.029 | 0.000 *** | |
| Ethylbenzene | 2.910 | 0.784 | 0.000 *** |
| PM2.5 | CO2 | HCHO | TVOC | Benzene | Toluene | Ethylbenzene | Xylene | Styrene | |
|---|---|---|---|---|---|---|---|---|---|
| BMA | −0.006 | −0.059 * | −0.0569 | −0.006 | 0.003 | 0.008 | 0.004 | 0.023 | 0.026 |
| 2MHA | 0.003 | 0.078 ** | 0.004 | 0.047 * | 0.076 * | 0.029 | 0.061 * | 0.079 ** | 0.023 |
| 3,4MHA | 0.040 | 0.051 * | 0.012 | 0.090 ** | 0.151 ** | 0.092 ** | 0.205 ** | 0.211 ** | 0.069 * |
| PGA | 0.017 | 0.004 | 0.039 | 0.126 ** | 0.302 ** | 0.123 ** | 0.245 ** | 0.277 ** | 0.085 * |
| MA | 0.044 | 0.028 | −0.005 | 0.022 | 0.010 | −0.020 | 0.039 | 0.039 | −0.005 |
| SPMA | 0.042 | −0.049 * | −0.083 ** | 0.005 | −0.039 | −0.050 * | 0.007 | −0.001 | 0.015 |
| 3HPMA | 0.078 ** | −0.019 | −0.053 * | 0.013 | −0.022 | −0.030 | 0.041 | 0.029 | 0.054 |
| BPMA | −0.031 | −0.036 | −0.008 | 0.003 | −0.027 | 0.017 | 0.086 ** | 0.056 * | −0.028 |
| DHBMA | 0.080 ** | −0.089 ** | −0.066 ** | 0.009 | 0.006 | −0.020 | 0.023 | 0.060 * | 0.004 |
| PM2.5 | CO2 | HCHO | TVOC | Benzene | Toluene | Ethylbenzene | Xylene | Styrene | |
|---|---|---|---|---|---|---|---|---|---|
| PM2.5 | |||||||||
| CO2 | 0.014 | ||||||||
| HCHO | −0.040 | 0.450 ** | |||||||
| TVOC | 0.034 | 0.325 ** | 0.241 ** | ||||||
| Benzene | −0.043 | 0.161 ** | 0.084 * | 0.446 ** | |||||
| Toluene | −0.002 | 0.201 ** | 0.175 ** | 0.420 ** | 0.490 ** | ||||
| Ethylbenzene | 0.004 | 0.065 ** | 0.102 ** | 0.242 ** | 0.143 ** | 0.240 ** | |||
| Xylene | 0.002 | 0.084 ** | 0.064 * | 0.374 ** | 0.448 ** | 0.306 ** | 0.748 ** | ||
| Styrene | 0.030 | 0.171 ** | 0.146 ** | 0.480 ** | 0.185 ** | 0.196 ** | 0.151 ** | 0.374 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, B.-J.; Kim, S.-R. Associations Between Indoor Air Pollution and Urinary Volatile Organic Compound Biomarkers in Korean Adults. Toxics 2025, 13, 692. https://doi.org/10.3390/toxics13080692
Cho B-J, Kim S-R. Associations Between Indoor Air Pollution and Urinary Volatile Organic Compound Biomarkers in Korean Adults. Toxics. 2025; 13(8):692. https://doi.org/10.3390/toxics13080692
Chicago/Turabian StyleCho, Byung-Jun, and Seon-Rye Kim. 2025. "Associations Between Indoor Air Pollution and Urinary Volatile Organic Compound Biomarkers in Korean Adults" Toxics 13, no. 8: 692. https://doi.org/10.3390/toxics13080692
APA StyleCho, B.-J., & Kim, S.-R. (2025). Associations Between Indoor Air Pollution and Urinary Volatile Organic Compound Biomarkers in Korean Adults. Toxics, 13(8), 692. https://doi.org/10.3390/toxics13080692







