Effects of Environmental Cadmium Exposure Sufficient to Induce Renal Tubular Dysfunction on Bone Mineral Density Among Female Farmers in Cadmium-Polluted Areas in Northern Japan
Highlights
- Among female farmers in cadmium-polluted areas in Akita Prefecture, Japan, older postmenopausal subjects were exposed to a high level of cadmium.
- They exhibited a deterioration of renal tubular function, but there was no relationship between cadmium and bone mineral density.
- These findings indicate that cadmium exposure itself does not directly affect bones, and they support the long-standing theory that cadmium induces bone injury secondarily from renal tubular dysfunction.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas and Populations
2.2. Procedures for Health Examinations
2.3. Analyses of Blood and Urine Samples
2.4. Measurement of Cd Concentrations in Whole Blood and Urine
2.5. Statistical Analysis
3. Results
3.1. Grouping of Subjects
3.2. Cd Levels in Peripheral Blood and Urine
3.3. Urinary α1MG and ß2MG Levels and eGFR
3.4. Body Weight and Grip Strength
3.5. BMD and YAM%
3.6. Bone Metabolism
3.7. Age- and Urinary Cd-Classified Analyses
3.8. Multiple Regression Analyses of Renal Tubular Function and BMD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordberg, G.F.; Nogawa, K.; Nordberg, M. Cadmium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Burlington, MA, USA, 2015; pp. 667–716. [Google Scholar]
- Aoshima, K. Recent clinical and epidemiological studies of itai-itai disease (cadmium-Induced renal tubular osteomalacia) and cadmium nephropathy in the Jinzu River basin in Toyama prefecture, Japan. In Cadmium Toxicity; Himeno, S., Aoshima, K., Eds.; Springer: Singapore, 2019; pp. 23–37. [Google Scholar]
- Aoshima, K.; Kato, T.; Teranishi, H.; Horiguchi, H.; Kasuya, M. Abnormalities of calcium, phosphorus and vitamin D metabolism with proximal renal tubular dysfunction in subjects environmentally exposed to cadmium. Jpn. J. Hyg. 1993, 47, 1009–1020. (In Japanese) [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Dietary exposure to cadmium at close to the current provisional tolerable weekly intake does not affect renal function among female Japanese farmers. Environ. Res. 2004, 95, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Environmental exposure to cadmium at a level insufficient to induce renal tubular dysfunction does not affect bone density among female Japanese farmers. Environ. Res. 2005, 97, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Tsuritani, I.; Noborisaka, Y.; Suzuki, H.; Ishizaki, M.; Yamada, Y. Urinary cadmium excretion is correlated with calcaneal bone mass in Japanese women living in an urban area. Environ. Res. 2003, 91, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Schutte, R.; Nawrot, T.S.; Richart, T.; Thijs, L.; Vanderschueren, D.; Kuznetsova, T.; Van Hecke, E.; Roels, H.A.; Staessen, J.A. Bone resorption and environmental exposure to cadmium in women: A population study. Environ. Health Perspect. 2008, 116, 77–783. [Google Scholar] [CrossRef]
- Engström, A.; Michaëlsson, K.; Suwazono, Y.; Wolk, A.; Vahter, M.; Akesson, A. Long-term cadmium exposure and the association with bone mineral density and fr;ctures in a population-based study among women. J. Bone Miner. Res. 2011, 26, 486–495. [Google Scholar] [CrossRef]
- Xie, R.; Liu, Y.; Wang, J.; Zhang, C.; Xiao, M.; Liu, M.; Zhang, Y. Race and Gender Differences in the Associations Between Cadmium Exposure and Bone Mineral Density in US Adults. Biol. Trace Elem. Res. 2023, 201, 4254–4261. [Google Scholar] [CrossRef]
- Kim, E.S.; Shin, S.; Lee, Y.J.; Ha, I.H. Association between blood cadmium levels and the risk of osteopenia and osteoporosis in Korean post-menopausal women. Arch. Osteoporos. 2021, 16, 22. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Okubo, H.; Murakami, K.; Miyamoto, K.; Hosoi, Y.; Murata, K.; Kayama, F. Age-relevant renal effects of cadmium exposure through consumption of home-harvested rice in female Japanese farmers. Environ. Int. 2013, 56, 1–9. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Ono, A.; Kayama, F. Exposure Assessment of Cadmium in Female Farmers in Cadmium-Polluted Areas in Northern Japan. Toxics 2020, 8, 44. [Google Scholar] [CrossRef]
- Donaldson, M.D.; Chambers, R.E.; Woolridge, M.W.; Whicher, J.T. Stability of α1-microglobulin, β2-microglobulin and retinol binding protein in urine. Clin. Chim. Acta 1989, 179, 73–77. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- Bernard, A.; Buchet, J.P.; Roels, H.; Masson, P.; Lauwerys, R. Renal excretion of proteins and enzymes in workers exposed to cadmium. Eur. J. Clin. Invest. 1979, 9, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, M. Recent epidemiological studies on itai-itai disease as a chronic cadmium poisoning in Japan. Water Sci. Technol. 2000, 42, 147–154. [Google Scholar] [CrossRef]
- Horiguchi, H.; Aoshima, K.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Katoh, T.; Kayama, F. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int. Arch. Occup. Environ. Health 2010, 83, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Wallin, E.; Rylander, L.; Jönssson, B.A.G.; Lundh, T.; Isaksson, A.; Hagmar, L. Exposure to CB-153 and p,p’-DDE and bone mineral density and bone metabolism markers in middle-aged and elderly men and women. Osteoporos. Int. 2005, 16, 2085–2094. [Google Scholar] [CrossRef]
- Rignell-Hydbom, A.; Skerfving, S.; Lundh, T.; Lindh, C.H.; Elmståhl, S.; Bjellerup, P.; Jünsson, B.A.G.; Strümberg, U.; Akesson, A. Exposure to cadmium and persistent organochlorine pollutants and its association with bone mineral density and markers of bone metabolism on postmenopausal women. Environ. Res. 2009, 109, 991–996. [Google Scholar] [CrossRef]
- Trzcinka-Ochocka, M.; Jakubowski, M.; Szymczak, W.; Janasik, B.; Brodzka, R. The effects of low environmental cadmium exposure on bone density. Environ. Res. 2010, 110, 286–293. [Google Scholar] [CrossRef]
- Lv, Y.J.; Song, J.; Xiong, L.L.; Huang, R.; Zhu, P.; Wang, P.; Liang, X.X.; Tan, J.B.; Wang, J.; Wu, S.X.; et al. Association of environmental cadmium exposure and bone remodeling in women over 50 years of age. Ecotoxicol. Environ. Saf. 2021, 211, 111897. [Google Scholar] [CrossRef]
- Cauley, J.A.; Chalhoub, B.; Kassem, A.M.; Fuleihan Gel, H. Geographic and ethnic disparities in osteoporotic fractures. Nat. Rev. Endocrinol. 2014, 10, 338–351. [Google Scholar] [CrossRef]
- Armitage, P.; Berry, G. Statistical inference. In Statistical Methods in Medical Research, 3rd ed.; Blackwell Science: Oxford, UK, 1994; pp. 93–153. [Google Scholar]
- Akesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Environ. Health Perspect. 2006, 114, 830–834. [Google Scholar] [CrossRef]
- Li, H.; Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Ohlsson, C.; Mellström, D.; Andersson, E.M. Smoking-induced risk of osteoporosis is partly mediated by cadmium from tobacco smoke: The MrOS Sweden study. J. Bone Miner. Res. 2020, 35, 1424–1429. [Google Scholar] [CrossRef]
- Kägi, J.H. Overview of metallothionein. Methods Enzymol. 1991, 205, 613–626. [Google Scholar]
- Wang, M.; Liu, J.; Zhu, G.; Chen, X. Low levels of cadmium exposure affect bone by inhibiting Lgr4 expression in osteoblasts and osteoclasts. J. Trace Elem. Med. Biol. 2022, 73, 127025. [Google Scholar] [CrossRef]


| Area A | Area B | Area C | Total | |
|---|---|---|---|---|
| All ages | ||||
| Number | 220 | 655 | 392 | 1267 |
| Age | 61.8 ± 7.7 | 57.6 ± 10.7 * | 57.6 ± 9.0 * | 58.3 ± 9.8 |
| Age range | 33–79 | 20–78 | 34–82 | 20–82 |
| Serum LH | 17.3 (11.9–21.9) | 21.0 * (13.4–30.4) | 22.1 * (13.5–30.3) | 19.9 (13.0–28.8) |
| 20–32 years | ||||
| Number | 0 | 22 | 0 | 22 |
| Age | - | 27.3 ± 3.8 | - | 27.3 ± 3.8 |
| Serum LH | - | 4.7 (2.5–18.6) | - | 4.7 (2.5–18.6) |
| 33–48 years (premenopause) | ||||
| Number | 13 | 99 | 63 | 175 |
| Age | 44.5 ± 3.8 | 43.6 ± 3.9 | 43.8 ± 3.7 | 43.7 ± 3.8 |
| Serum LH | 2.9 (1.7–5.4) | 5.0 (3.2–9.5) | 4.6 (2.8–8.9) | 4.6 (2.8–8.5) |
| 49–55 years (perimenopause) | ||||
| Number | 32 | 136 | 101 | 269 |
| Age | 52.3 ± 2.1 | 52.3 ± 1.9 | 52.2 ± 2.1 | 52.3 ± 2.0 |
| Serum LH | 19.5 (10.9–26.3) # | 27.2 (18.4–37.1) # | 22.4 (13.7–37.0) # | 24.5 (15.7–36.6) # |
| 56–65 years (younger postmenopause) | ||||
| Number | 97 | 228 | 149 | 474 |
| Age | 61.2 ± 2.9 | 61.1 ± 2.8 | 60.5 ± 2.6 | 60.9 ± 2.8 |
| Serum LH | 18.0 (13.7–22.4) # | 23.9 (18.1–32.9) *# | 26.7 (19.1–34.1) *# | 23.2 (17.3–31.1) # |
| 66–82 years (older postmenopause) | ||||
| Number | 78 | 170 | 79 | 327 |
| Age | 69.3 ± 3.0 | 69.1 ± 3.0 | 69.9 ± 3.5 | 69.4 ± 3.1 |
| Serum LH | 17.1 (12.1–19.9) # | 20.7 (16.1–27.2) *# | 22.1 (16.9–26.3) *# | 19.6 (15.2–2 5.9) # |
| Area A | Area B | Area C | |
|---|---|---|---|
| Number | 220 | 633 | 392 |
| Blood cadmium (µg/L) | |||
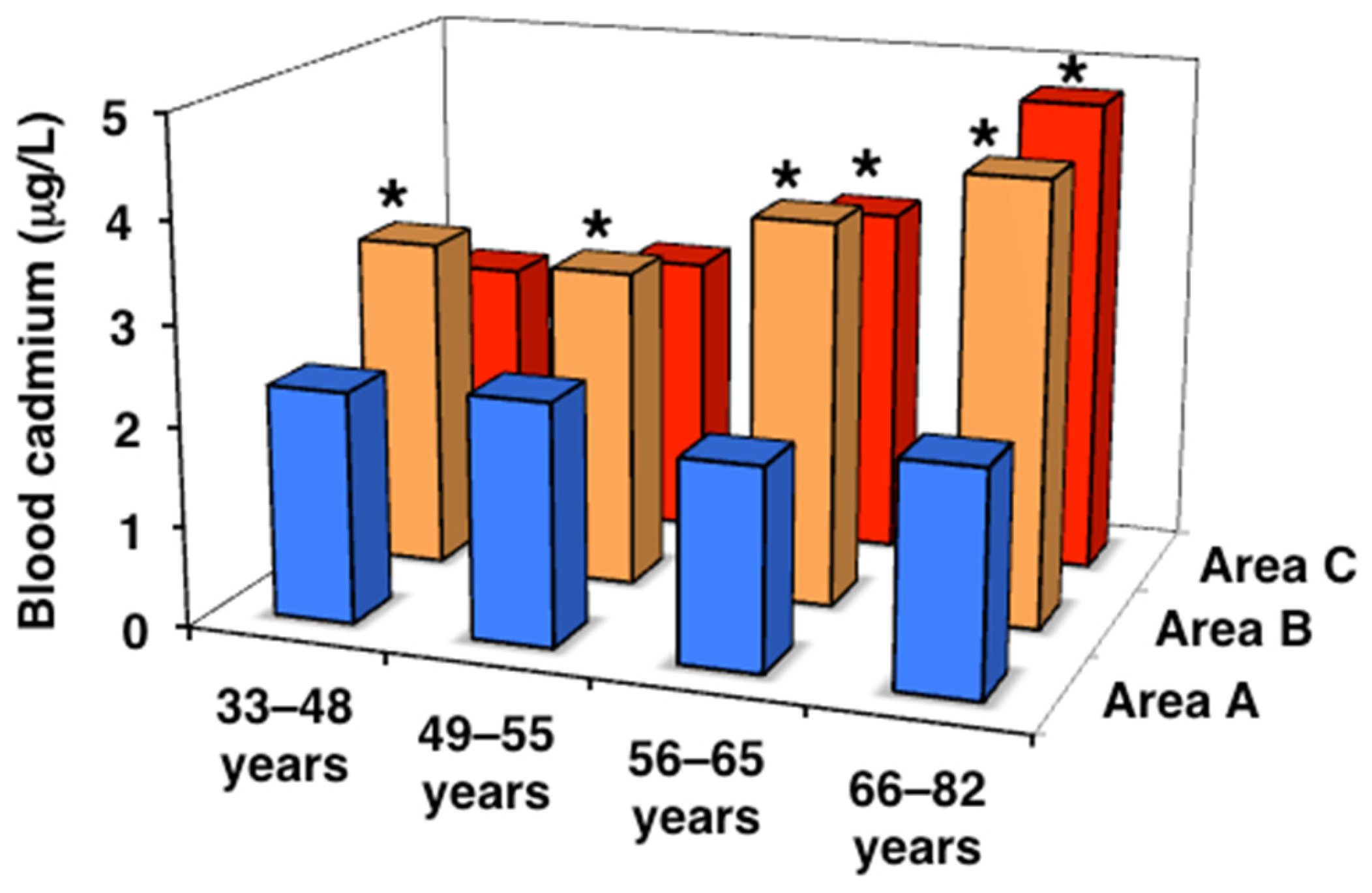
| All ages | 2.10 (1.60–2.88) | 3.78 (2.71–5.09) * | 3.39 (2.38–5.14) * |
| Range 0.76–6.90 | Range 0.55–13.1 | Range 0.74–31.2 | |
| 33–48 years | 2.30 (1.25–3.20) | 3.29 (2.20–4.89) | 2.57 (1.79–4.13) |
| 49–55 years | 2.40 (1.73–3.10) | 3.16 (2.39–4.62) * | 2.80 (2.07–4.20) |
| 56–65 years | 2.00 (1.50–2.70) | 3.82 (2.84–4.97) * | 3.47 (2.50–5.16) * |
| 66–82 years | 2.20 (1.80–2.80) | 4.39 (3.35–5.45) *# | 4.73 (3.35–6.66) *# |
| J-T test | p = 0.560 | p < 0.001 | p < 0.001 |
| Urinary cadmium (µg/g cr.) | |||
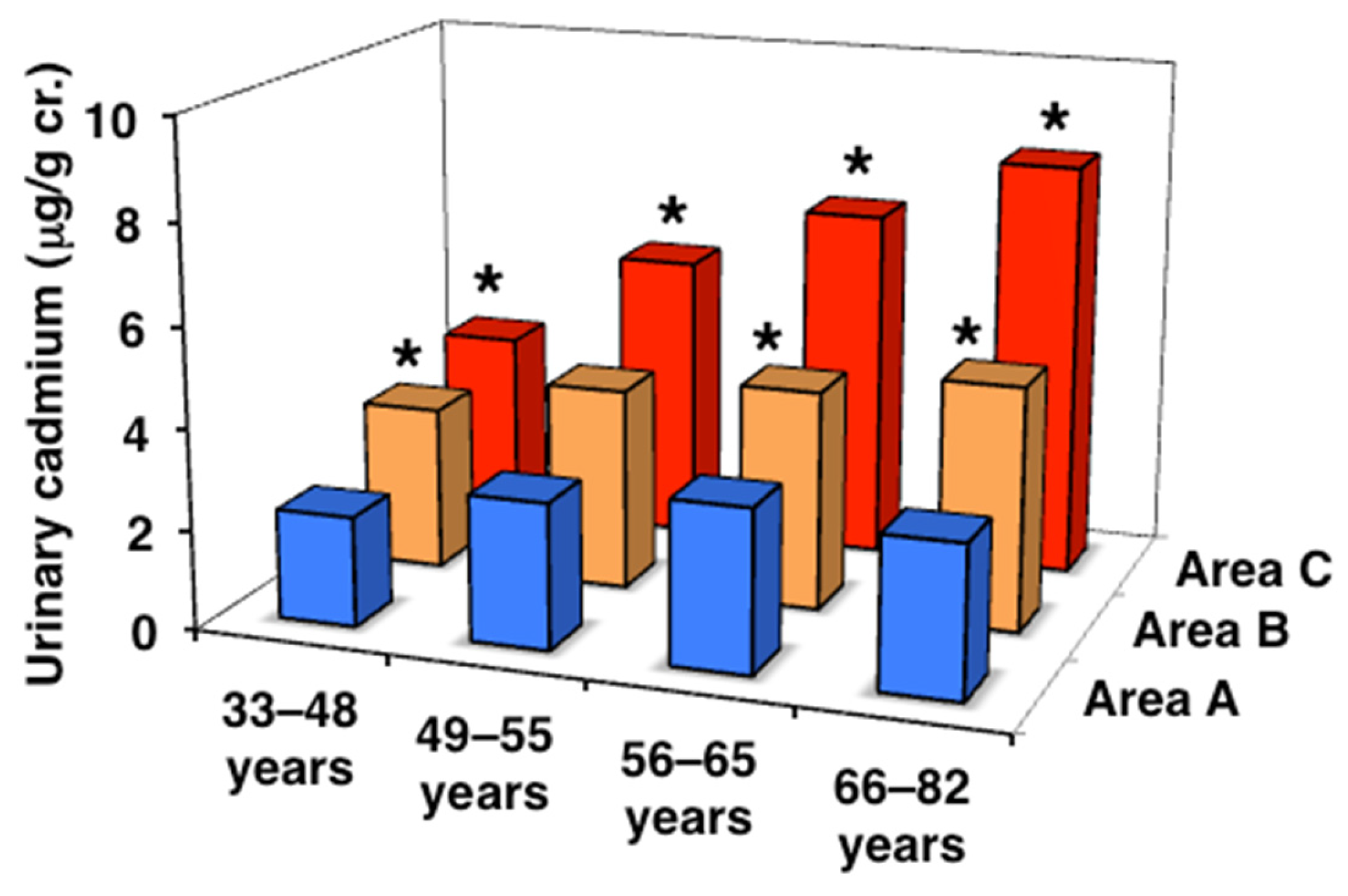
| All ages | 3.02 (2.35–4.32) | 4.29 (3.00–6.03) * | 6.15 (4.29–8.76) *† |
| Range 0.90–16.7 | Range 0.51–27.3 | Range 0.35–29.7 | |
| 33–48 years | 2.19 (1.48–2.68) | 3.26 (2.35–4.85) * | 3.75 (2.37–5.05) * |
| 49–55 years | 2.90 (2.05–4.86) | 4.02 (2.84–5.96) | 5.72 (3.99–7.24) *†# |
| 56–65 years | 3.24 (2.48–4.38) # | 4.37 (3.29–6.18) *# | 6.99 (4.90–9.16) *†# |
| 66–82 years | 3.01 (2.41–4.33) # | 4.85 (3.53–6.61) *# | 8.27 (6.14–10.5) *†# |
| J-T test | p = 0.358 | p < 0.001 | p < 0.001 |
| Area A | Area B | Area C | |
|---|---|---|---|
| Number | 220 | 633 | 392 |
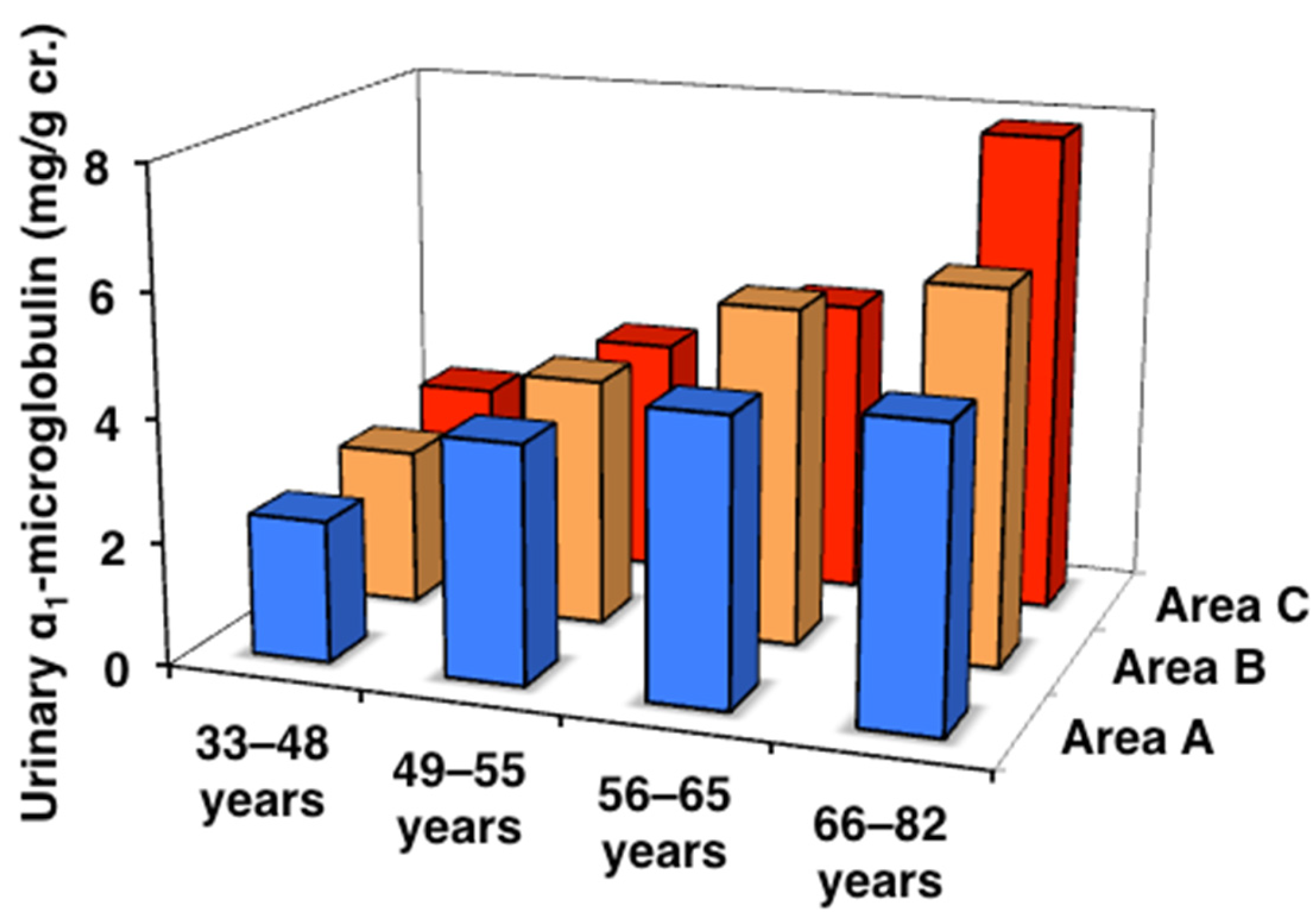
| Urinary α1-microglobulin (mg/g cr.) | |||
| All ages | 4.43 (2.74–7.13) | 4.72 (3.00–8.05) | 4.51 (2.63–7.39) |
| Range ND–24.1 | Range ND–56.0 | Range ND–48.6 | |
| 33–48 years | 2.30 (1.41–3.24) | 2.54 (1.84–4.18) | 2.80 (2.05–5.20) |
| 49–55 years | 3.87 (2.51–6.22) | 4.04 (2.57–6.89) # | 3.87 (2.34–5.60) |
| 56–65 years | 4.63 (2.72–6.86) | 5.49 (3.76–8.74) # | 4.81 (2.85–7.47) # |
| 66–82 years | 4.82 (3.44–9.43) # | 6.08 (3.94–10.9) # | 7.85 (4.51–12.5) # |
| J-T test | p = 0.001 | p < 0.001 | p < 0.001 |
| Urinary β2-microglobulin (μg/g cr.) | |||
| All ages | 133 (83–210) | 146 (94–249) | 146 (95–278) |
| Range ND–1220 | Range ND–5690 | Range ND–15,300 | |
| 33–48 years | 102 (94–118) | 100 (75–143) | 109 (70–155) |
| 49–55 years | 100 (78–168) | 134 (95–228) # | 132 (80–202) |
| 56–65 years | 144 (81–213) | 150 (107–230) # | 151 (97–273) # |
| 66–82 years | 157 (83–275) | 200 (119–408) # | 273 (139–539) *# |
| J-T test | p = 0.016 | p < 0.001 | p < 0.001 |
| eGFR (mL/min/1.73 m2) | |||
| All ages | 79.7 ± 14.8 | 82.0 ± 14.7 | 78.5 ± 14.2 † |
| Range 30.6–130.0 | Range 43.5–143.5 | Range 28.2–133.0 | |
| 33–48 years | 90.0 ± 13.0 | 88.3 ± 14.8 | 86.3 ± 14.6 |
| 49–55 years | 82.3 ± 13.1 | 84.4 ± 13.5 | 81.3 ± 13.3 |
| 56–65 years | 80.9 ± 14.4 | 82.5 ± 13.8 # | 76.9 ± 12.8 # |
| 66–82 years | 75.3 ± 15.0 # | 75.7 ± 14.4 # | 72.0 ± 14.2 # |
| J-T test | p < 0.001 | p < 0.001 | p < 0.001 |
| Area A | Area B | Area C | |
|---|---|---|---|
| Number | 220 | 633 | 392 |
| Body weight (kg) | |||
| All ages | 56.9 ± 8.1 | 55.6 ± 8.2 | 56.4 ± 8.9 |
| Range 36.7–83.4 | Range 34.0–92.6 | Range 34.2–100.3 | |
| 33–48 years | 58.0 ± 7.1 | 58.0 ± 8.9 | 57.3 ± 9.4 |
| 49–55 years | 59.1 ± 7.4 | 57.0 ± 8.0 | 58.8 ± 8.9 |
| 56–65 years | 57.6 ± 8.3 | 55.3 ± 7.8 | 56.6 ± 8.6 |
| 66–82 years | 54.8 ± 8.0 | 53.6 ± 7.9 # | 52.3 ± 8.1 # |
| BMI | |||
| All ages | 24.4 ± 3.2 | 24.2 ± 3.2 | 24.1 ± 3.4 |
| Range 17.8–35.4 | Range 16.5–37.2 | Range 16.3–43.0 | |
| 33–48 years | 23.4 ± 2.8 | 23.8 ± 3.4 | 23.3 ± 4.0 |
| 49–55 years | 24.1 ± 2.5 | 24.1 ± 3.1 | 24.6 ± 3.2 |
| 56–65 years | 24.7 ± 3.2 | 24.4 ± 3.1 | 24.5 ± 3.4 |
| 66–82 years | 24.5 ± 3.4 | 24.3 ± 3.2 | 23.5 ± 2.8 |
| Grip strength (kg) | |||
| All ages | 25.5 ± 4.1 | 24.8 ± 4.8 | 26.0 ± 4.6 † |
| Range 14.5–36.0 | Range 6.0–39.5 | Range 10.0–38.5 | |
| 33–48 years | 28.3 ± 2.8 | 27.6 ± 4.3 | 29.9 ± 4.7 † |
| 49–55 years | 27.8 ± 3.9 | 26.2 ± 4.1 | 26.9 ± 3.8 # |
| 56–65 years | 25.7 ± 4.0 | 24.8 ± 4.6 # | 25.4 ± 3.9 # |
| 66–82 years | 23.8 ± 3.7 # | 22.0 ± 4.4 *# | 23.0 ± 4.5 # |
| Area A | Area B | Area C | |
|---|---|---|---|
| Number | 220 | 633 | 392 |
| Bone mineral density (g/cm2) | |||
| All ages | 0.397 ± 0.082 | 0.400 ± 0.087 | 0.418 ± 0.089 *† |
| Range 0.141–0.624 | Range 0.185–0.680 | Range 0.207–0.641 | |
| 33–48 years | 0.475 ± 0.044 | 0.483 ± 0.055 | 0.487 ± 0.058 |
| 49–55 years | 0.466 ± 0.067 | 0.455 ± 0.071 # | 0.462 ± 0.066 |
| 56–65 years | 0.397 ± 0.073 # | 0.381 ± 0.070 # | 0.406 ± 0.078 †# |
| 66–82 years | 0.355 ± 0.074 # | 0.332 ± 0.065 # | 0.328 ± 0.071 # |
| J-T test | p < 0.001 | p < 0.001 | p < 0.001 |
| YAM% (%) | |||
| All ages | 82.5 ± 17.0 | 83.4 ± 18.5 | 86.9 ± 18.5 *† |
| Range 29.2–129.7 | Range 39.0–188.0 | Range 43.1–133.2 | |
| 33–48 years | 98.8 ± 9.1 | 100.6 ± 11.5 | 101.3 ± 12.0 |
| 49–55 years | 96.9 ± 14.0 | 95.4 ± 16.6 | 96.0 ± 13.7 |
| 56–65 years | 82.6 ± 15.2 # | 79.4 ± 14.5 # | 84.7 ± 16.2 †# |
| 66–82 years | 73.8 ± 15.3 # | 69.1 ± 13.5 # | 68.2 ± 14.7 # |
| J-T test | p < 0.001 | p < 0.001 | p < 0.001 |
| Area A | Area B | Area C | |
|---|---|---|---|
| Number | 220 | 633 | 392 |
| Serum calcium (mg/dL) | |||
| All ages | 9.6 ± 0.3 | 9.4 ± 0.3 * | 9.5 ± 0.3 * |
| Range 8.9–10.6 | Range 8.6–11.4 | Range 8.5–10.5 | |
| 33–48 years | 9.5 ± 0.4 | 9.2 ± 0.3 | 9.4 ± 0.4 |
| 49–55 years | 9.7 ± 0.3 | 9.5 ± 0.3 *# | 9.5 ± 0.3 * |
| 56–65 years | 9.6 ± 0.3 | 9.5 ± 0.3 # | 9.5 ± 0.4 # |
| 66–82 years | 9.6 ± 0.3 | 9.5 ± 0.3 # | 9.5 ± 0.3 * |
| Serum phosphate (mg/dL) | |||
| All ages | 3.8 ± 0.4 | 3.4 ± 0.4 * | 3.6 ± 0.4 *† |
| Range 2.8–5.1 | Range 2.2–4.6 | Range 2.3–4.9 | |
| 33–48 years | 3.6 ± 0.4 | 3.2 ± 0.3 * | 3.4 ± 0.4 † |
| 49–55 years | 3.8 ± 0.5 | 3.5 ± 0.4 *# | 3.7 ± 0.4 †# |
| 56–65 years | 3.8 ± 0.5 | 3.5 ± 0.4 *# | 3.6 ± 0.4 † |
| 66–82 years | 3.7 ± 0.4 | 3.5 ± 0.4 *# | 3.6 ± 0.5 |
| Urinary calcium (mg/g cr.) | |||
| All ages | 141.5 (100.4–222) | 140.1 (95.2–205.8) | 142.8 (100.3–204.4) |
| 20.2–549.1 | 9.7–471.4 | 22.9–500.4 | |
| 33–48 years | 99.8 (67.6–125.5) | 100.1 (66.8–146.5) | 100.2 (74.9–145.5) |
| 49–55 years | 150.0 (126.8–216.5) | 161.4 (110.1–206.9) # | 153.3 (106.5–204.0) # |
| 56–65 years | 149.2 (99.8–217.7) | 153.5 (103.5–218.2) # | 148.6 (108.5–218.5) # |
| 66–82 years | 140.2 (104.3–243.6) | 156.5 (101.9–221.6) # | 157.6 (114.1–236.5) # |
| Area A | Area B | Area C | |
|---|---|---|---|
| Number | 220 | 633 | 392 |
| Bone alkaline phosphatase (U/L) | |||
| All ages | 32.8 (24.9–40.4) | 30.0 (22.8–37.1) * | 32.3 (23.1–42.1) † |
| Range 10.3–86.7 | Range 9.1–81.1 | Range 10.1–103 | |
| 33–48 years | 18.9 (16.3–25.5) | 19.2 (15.7–23.5) | 19.8 (15.9–25.7) |
| 49–55 years | 30.9 (22.5–38.9) # | 29.4 (22.9–36.2) # | 33.7 (24.0–46.8) # |
| 56–65 years | 36.2 (27.1–43.4) # | 31.5 (25.8–38.0) # | 35.9 (28.1–44.0) # |
| 66–82 years | 32.3 (24.9–40.1) # | 35.7 (27.7–42.9) # | 32.3 (23.6–41.3) # |
| Osteocalcin (ng/mL) | |||
| All ages | 8.6 (6.5–10.3) | 8.4 (6.3–10.8) | 7.8 (5.9–10.2) |
| Range 2.6–21.4 | Range 1.5–22.8 | Range 2.1–20.7 | |
| 33–48 years | 5.4 (4.6–6.2) | 5.6 (4.3–6.6) | 4.9 (4.0–5.9) |
| 49–55 years | 7.5 (5.6–9.3) | 8.6 (6.6–10.8) # | 9.0 (5.5–10.7) # |
| 56–65 years | 8.9 (7.2–10.7) # | 9.1 (7.4–11.2) # | 8.4 (6.7–10.3) # |
| 66–82 years | 9.0 (7.0–10.4) # | 9.3 (7.3–11.3) # | 8.7 (7.0–10.8) # |
| Urinary NTx (nmol/mmol cr.) | |||
| All ages | 50.8 (37.6–66.3) | 62.7 (43.1–82.0) * | 49.0 (32.6–73.4) † |
| Range 11.4–153 | Range 13.1–220 | Range 9.7–270 | |
| 33–48 years | 29.5 (22.9–43.9) | 33.7 (26.9–44.8) | 33.0 (23.4–44.6) |
| 49–55 years | 52.3 (31.9–64.8) | 70.2 (44.5–84.3) *# | 52.0 (30.8–74.3) †# |
| 56–65 years | 53.3 (42.6–67.3) # | 68.3 (52.5–86.1) *# | 50.5 (37.5–78.5) †# |
| 66–82 years | 51.8 (36.0–68.5) # | 68.5 (51.0–85.4) *# | 64.7 (40.9–86.4) # |
| Urinary deoxypyridinoline (nmol/mmol cr.) | |||
| All ages | 7.0 (6.0–8.2) | 6.6 (5.1–8.1) * | 6.8 (5.5–8.0) |
| Range 2.8–13.5 | Range 1.3–23.5 | Range 3.0–29.0 | |
| 33–48 years | 6.0 (4.6–6.7) | 5.8 (4.7–6.8) | 5.7 (4.6–6.6) |
| 49–55 years | 7.1 (6.5–8.4) | 7.1 (5.3–8.8) # | 7.5 (5.9–8.7) # |
| 56–65 years | 7.1 (6.1–8.3) | 7.0 (5.1–8.1) # | 6.8 (5.5–7.9) # |
| 66–82 years | 7.0 (6.0–8.1) | 6.6 (5.4–8.4) # | 7.3 (6.2–8.5) # |
| Urinary Cadmium (µg/g cr.) | J-T Test | ||||
|---|---|---|---|---|---|
| <3.0 | ≥3.0, <4.5 | ≥4.5, <6.5 | ≥6.5 | ||
| Number | |||||
| All ages | 313 | 308 | 301 | 323 | |
| 33–48 years | 78 | 45 | 33 | 19 | |
| 49–55 years | 69 | 61 | 73 | 66 | |
| 56–65 years | 98 | 129 | 108 | 139 | |
| 66–82 years | 68 | 73 | 87 | 99 | |
| Age | |||||
| All ages | 56.1 ± 10.1 | 58.8 ± 8.7 * | 59.4 ± 8.5 * | 61.0 ± 7.8 * | |
| Range 33–77 | Range 36–79 | Range 36–82 | Range 34–77 | ||
| 33–48 years | 43.1 ± 4.2 | 44.0 ± 3.3 | 44.4 ± 3.4 | 44.6 ± 3.9 | |
| 49–55 years | 51.8 ± 2.0 | 52.4 ± 2.0 | 52.6 ± 1.8 | 52.3 ± 2.2 | |
| 56–65 years | 60.2 ± 2.7 | 61.2 ± 2.8 | 60.8 ± 2.9 | 61.3 ± 2.7 | |
| 66–82 years | 69.7 ± 3.2 | 69.2 ± 3.2 | 69.2 ± 3.1 | 69.4 ± 3.2 | |
| Blood cadmium (µg/L) | 2.61 (1.64–2.30) | 3.22 (2.10–2.95) * | 3.78 (2.58–3.50) * | 5.53 (3.51–4.90) * | p < 0.001 |
| All ages | Range 0.74–9.86 | Range 0.55–9.48 | Range 1.08–15.0 | Range 1.20–31.2 | |
| 2.19 (1.51–3.21) | 3.08 (2.09–4.10) | 4.06 (3.13–5.16) | 6.50 (4.73–7.56) | ||
| 33–48 years | 2.40 (1.70–3.15) | 2.72 (1.98–3.70) | 2.88 (2.38–4.10) * | 4.31 (2.99–6.11) * | p < 0.001 |
| 49–55 years | 2.31 (1.50–3.23) | 3.02 (2.12–3.88) | 3.29 (2.47–4.39) * | 4.83 (3.34–6.47) * | p < 0.001 |
| 56–65 years | 2.30 (1.80–3.59) | 2.97 (2.15–4.47) * | 3.90 (2.98–5.22) * | 5.27 (3.95–7.85) * | p < 0.001 |
| 66–82 years | 2.61 (1.64–2.30) | 3.22 (2.10–2.95) | 3.78 (2.58–3.50) * | 5.53 (3.51–4.90) * | p < 0.001 |
| J-T test | p = 0.191 | p = 0.270 | p = 0.058 | p = 0.055 | |
| Urinary cadmium (µg/g cr.) | 2.35 (1.89–2.67) | 3.81 (3.39–4.14) * | 5.36 (4.92–5.94) * | 8.71 (7.40–10.32) * | p < 0.001 |
| All ages | Range 0.35–3.00 | Range 3.01–4.50 | Range 4.50–6.50 | Range 6.50–29.7 | |
| 2.21 (1.82–2.55) | 3.65 (3.28–4.20) | 5.12 (4.75–5.73) | 8.11 (7.21–9.34) | ||
| 33–48 years | 2.40 (1.72–2.70) | 3.86 (3.39–4.04) * | 5.36 (4.94–5.91) * | 7.92 (7.11–9.20) * | p < 0.001 |
| 49–55 years | 2.36 (1.91–2.69) | 3.93 (3.42–4.19) * | 5.42 (4.92–5.87) * | 8.91 (7.44–10.4) * | p < 0.001 |
| 56–65 years | 2.41 (2.11–2.74) | 3.73 (3.47–4.15) * | 5.46 (4.93–5.99) * | 9.00 (7.51–10.7) * | p < 0.001 |
| 66–82 years | 2.35 (1.89–2.67) | 3.81 (3.39–4.14) * | 5.36 (4.92–5.94) * | 8.71 (7.40–10.32) * | p < 0.001 |
| J-T test | p = 0.012 | p = 0.487 | p = 0.041 | p = 0.011 | |
| Urinary Cadmium (µg/g cr.) | J-T Test | ||||
|---|---|---|---|---|---|
| <3.0 | ≥3.0, <4.5 | ≥4.5, <6.5 | ≥6.5 | ||
| Urinary α1-microglobulin (mg/g cr.) | |||||
| All ages | 3.87 (2.41–6.40) | 4.62 (2.77–7.32) * | 4.76 (2.99–7.63) * | 5.62 (3.36–8.87) * | p < 0.001 |
| Range ND–30.7 | Range ND–28.1 | Range ND–28.9 | Range ND–56.0 | ||
| 33–48 years | 2.66 (1.76–3.79) | 2.67 (2.03–4.72) | 2.92 (2.05–5.87) | 2.22 (1.70–3.86) | p = 0.344 |
| 49–55 years | 3.48 (2.27–5.42) | 3.87 (2.26–6.24) | 4.02 (3.09–6.05) | 4.62 (2.62–7.98) | p = 0.013 |
| 56–65 years | 4.48 (3.00–7.10) # | 5.15 (3.52–7.65) # | 4.79 (2.76–7.92) | 5.73 (3.55–8.17) # | p = 0.135 |
| 66–82 years | 5.09 (3.57–10.1) # | 5.72 (3.49–11.7) # | 5.86 (4.06–9.60) # | 7.21 (4.36–11.9) # | p = 0.039 |
| J-T test | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Urinary β2-microglobulin (μg/g cr.) | |||||
| All ages | 121.5 (84.3–193.8) | 137.8 (83.3–206.1) | 147.3 (93.0–250.5) * | 180.0 (112.6–335.8) * | p < 0.001 |
| Range ND–1236 | Range ND–2808 | Range ND–9087 | Range ND–15330 | ||
| 33–48 years | 100.6 (80.8–132.1) | 103.6 (67.9–147.6) | 115.1 (65.0–201.7) | 101.3 (55.6–146.4) | p = 0.925 |
| 49–55 years | 119.1 (83.1–185.6) | 124.1 (75.4–161.8) | 127.5 (87.5–220.7) | 164.9 (91.3–295.5) | p = 0.013 |
| 56–65 years | 142.6 (91.9–195.8) # | 143.8 (89.6–212.3) | 147.4 (90.5–232.1) | 160.3 (112.0–286.9) # | p = 0.005 |
| 66–82 years | 160.4 (89.7–301.5) # | 166.2 (89.7–346.8) | 200.7 (121.1–318.6) # | 272.6 (137.0–650.3) # | p < 0.001 |
| J-T test | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Bone mineral density (g/cm2) | |||||
| All ages | 0.426 ± 0.086 | 0.406 ± 0.084 * | 0.402 ± 0.084 * | 0.386 ± 0.090 * | p < 0.001 |
| Range 0.141–0.631 | Range 0.200–0.615 | Range 0.231–0.680 | Range 0.207–0.641 | ||
| 33–48 years | 0.484 ± 0.058 | 0.483 ± 0.050 | 0.476 ± 0.046 | 0.459 ± 0.071 | p = 0.679 |
| 49–55 years | 0.474 ± 0.061 | 0.460 ± 0.067 | 0.467 ± 0.073 | 0.465 ± 0.072 | p = 0.647 |
| 56–65 years | 0.386 ±0.081 # | 0.380 ± 0.073 # | 0.396 ± 0.062 # | 0.380 ± 0.077 # | p = 0.932 |
| 66–82 years | 0.360 ± 0.067 # | 0.339 ± 0.074 # | 0.332 ± 0.066 # | 0.324 ± 0.068 # | p = 0.002 |
| J-T test | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| YAM% | |||||
| All ages | 88.7 ± 17.8 | 84.4 ± 17.5 * | 83.7 ± 17.5 * | 80.7 ± 19.5 * | p < 0.001 |
| Range 29.2–131.2 | Range 42.0–128.0 | Range 48.0–142.0 | Range 43.1–188.0 | ||
| 33–48 years | 101.0 ± 12.1 | 100.4 ± 10.5 | 99.0 ± 9.4 | 95.5 ± 14.8 | p = 0.655 |
| 49–55 years | 99.0 ± 12.8 | 95.6 ± 13.9 | 97.0 ± 15.2 | 97.0 ± 18.5 | p = 0.644 |
| 56–65 years | 80.2 ± 16.9 # | 79.0 ± 15.3 # | 82.4 ± 12.9 # | 79.0 ± 16.1 # | p = 0.865 |
| 66–82 years | 74.9 ± 13.9 # | 70.9 ± 15.4 # | 69.0 ± 13.8 # | 67.3 ± 14.1 # | p = 0.002 |
| J-T test | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Dependent Variables | Independent Variables | Regression Coefficients | β | p-Values | VIF |
|---|---|---|---|---|---|
| log urinary α1-microglobulin | |||||
| Model 1 | Age | 0.011 | 0.315 | <0.001 | 1.1 |
| (R′ = 0.340) | log urinary creatinine | 0.761 | 0.546 | <0.001 | 1.0 |
| log blood cadmium | 0.112 | 0.074 | 0.002 | 1.1 | |
| Model 2 | Age | 0.011 | 0.311 | <0.001 | 1.1 |
| (R′ = 0.342) | log urinary creatinine | 0.659 | 0.474 | <0.001 | 1.9 |
| log urinary cadmium | 0.119 | 0.109 | <0.001 | 1.8 | |
| log urinary β2-microglobulin | |||||
| Model 1 | Age | 0.008 | 0.216 | <0.001 | 1.1 |
| (R′ = 0.215). | log urinary creatinine | 0.591 | 0.406 | <0.001 | 1.0 |
| log blood cadmium | 0.262 | 0.166 | <0.001 | 1.1 | |
| Model 2 | Age | 0.009 | 0.224 | <0.001 | 1.1 |
| (R′ = 0.202) | log urinary creatinine | 0.441 | 0.303 | <0.001 | 1.9 |
| log urinary cadmium | 0.178 | 0.156 | <0.001 | 1.8 |
| Dependent Variables | Independent Variables | Regression Coefficients | β | p-Values | VIF |
|---|---|---|---|---|---|
| BMD, Model 1 | Age | −0.004 | −0.461 | <0.001 | 1.4 |
| (R′ = 0.472). | Body weight | 0.003 | 0.318 | <0.001 | 1.2 |
| Grip strength | 0.002 | 0.116 | <0.001 | 1.3 | |
| log urinary creatinine | 0.009 | 0.025 | 0.311 | 1.5 | |
| log urinary α1-microglobulin | −0.014 | −0.058 | 0.021 | 1.5 | |
| log blood cadmium | −0.007 | −0.018 | 0.386 | 1.1 | |
| Model 2 | Age | −0.004 | −0.469 | <0.001 | 1.4 |
| (R′ = 0.472). | Body weight | 0.003 | 0.321 | <0.001 | 1.2 |
| Grip strength | 0.002 | 0.117 | <0.001 | 1.3 | |
| log urinary creatinine | 0.001 | 0.004 | 0.893 | 2.2 | |
| log urinary α1-microglobulin | −0.015 | −0.063 | 0.013 | 1.5 | |
| log urinary cadmium | 0.009 | 0.035 | 0.213 | 1.8 | |
| Model 3 | Age | −0.004 | −0.463 | <0.001 | 1.3 |
| (R′ = 0.474) | Body weight | 0.003 | 0.320 | <0.001 | 1.2 |
| Grip strength | 0.002 | 0.115 | <0.001 | 1.3 | |
| log urinary creatinine | 0.008 | 0.024 | 0.297 | 1.3 | |
| log urinary β2-microglobulin | −0.017 | −0.075 | 0.001 | 1.3 | |
| log blood cadmium | −0.004 | −0.010 | 0.635 | 1.1 | |
| Model 4 | Age | −0.004 | −0.471 | <0.001 | 1.3 |
| (R′ = 0.475) | Body weight | 0.003 | 0.323 | <0.001 | 1.2 |
| Grip strength | 0.002 | 0.115 | <0.001 | 1.3 | |
| log urinary creatinine | −0.001 | −0.001 | 0.959 | 2.0 | |
| log urinary β2-microglobulin | −0.019 | −0.081 | <0.001 | 1.3 | |
| log urinary cadmium | 0.011 | 0.041 | 0.145 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiguchi, H.; Oguma, E.; Miyamoto, K.; Hosoi, Y.; Kayama, F. Effects of Environmental Cadmium Exposure Sufficient to Induce Renal Tubular Dysfunction on Bone Mineral Density Among Female Farmers in Cadmium-Polluted Areas in Northern Japan. Toxics 2025, 13, 688. https://doi.org/10.3390/toxics13080688
Horiguchi H, Oguma E, Miyamoto K, Hosoi Y, Kayama F. Effects of Environmental Cadmium Exposure Sufficient to Induce Renal Tubular Dysfunction on Bone Mineral Density Among Female Farmers in Cadmium-Polluted Areas in Northern Japan. Toxics. 2025; 13(8):688. https://doi.org/10.3390/toxics13080688
Chicago/Turabian StyleHoriguchi, Hyogo, Etsuko Oguma, Kayoko Miyamoto, Yoko Hosoi, and Fujio Kayama. 2025. "Effects of Environmental Cadmium Exposure Sufficient to Induce Renal Tubular Dysfunction on Bone Mineral Density Among Female Farmers in Cadmium-Polluted Areas in Northern Japan" Toxics 13, no. 8: 688. https://doi.org/10.3390/toxics13080688
APA StyleHoriguchi, H., Oguma, E., Miyamoto, K., Hosoi, Y., & Kayama, F. (2025). Effects of Environmental Cadmium Exposure Sufficient to Induce Renal Tubular Dysfunction on Bone Mineral Density Among Female Farmers in Cadmium-Polluted Areas in Northern Japan. Toxics, 13(8), 688. https://doi.org/10.3390/toxics13080688







