1. Introduction
Heavy metal contamination in soils has been one of the major environmental problems leading to several detrimental concerns regarding the environment and human health [
1,
2,
3]. Heavy metals are released into the environment from the dissolution of primary sources such as minerals and rocks, or they may be leached from soils and sediments from sorbed metal ions, which are called a secondary source. The released metal ions are toxic and may pose a human health risk after prolonged exposure. Health risks arising from heavy metal exposure can be intensified in areas with industrial operations [
4], dumpsites [
4], and mining activities, including mining operations, smelting activity, mine waste, and tailings disposal [
5,
6,
7]. Heavy metal pollution in agricultural soils has been widely reported across various regions. In African contexts, particularly within the six geopolitical zones of Nigeria, the literature indicates that the primary sources of heavy metals such as Cd, Cu, Ni, Pb, Zn, Co, Cr, Fe, and As include urban and industrial effluents, deteriorating sewage infrastructure, sewage sludge, fertilizers, and pesticides [
4]. The concentrations of these metals span a wide range, with maximum levels frequently exceeding the WHO’s permissible limits [
4]. Their presence in agricultural soils poses serious environmental and public health concerns, as evidenced by their accumulation in crops, cow’s milk, and animal organs [
8,
9,
10,
11]. Similarly to Kafr El-Zayat—a major agricultural and industrial city in Egypt—which faces significant soil contamination, particularly with aluminum, arsenic, cadmium, chromium, and nickel [
12], these metals are known carcinogens and can cause damage to the brain, kidneys, liver, and other organs, primarily by inducing oxidative stress through free radical generation [
12,
13,
14]. Furthermore, a review of heavy metal contamination in agricultural soils resulting from mining activities identified 72 affected mining areas in the southern and eastern regions of China [
15]. The results indicated potential non-carcinogenic risks from the ingestion and dermal absorption of As, Cd, Cr, Cu, Ni, Pb, Zn, and Hg, as well as potential carcinogenic risks from As [
15]. It was observed that non-carcinogenic risks were especially associated with lead–zinc, manganese, and tungsten mines, more so than with copper, gold, and iron mines [
15]. These findings highlight that heavy metals in agricultural soils contribute to increasing toxicity levels and elevated public health risks, as evidenced by bioaccumulation and biomagnification.
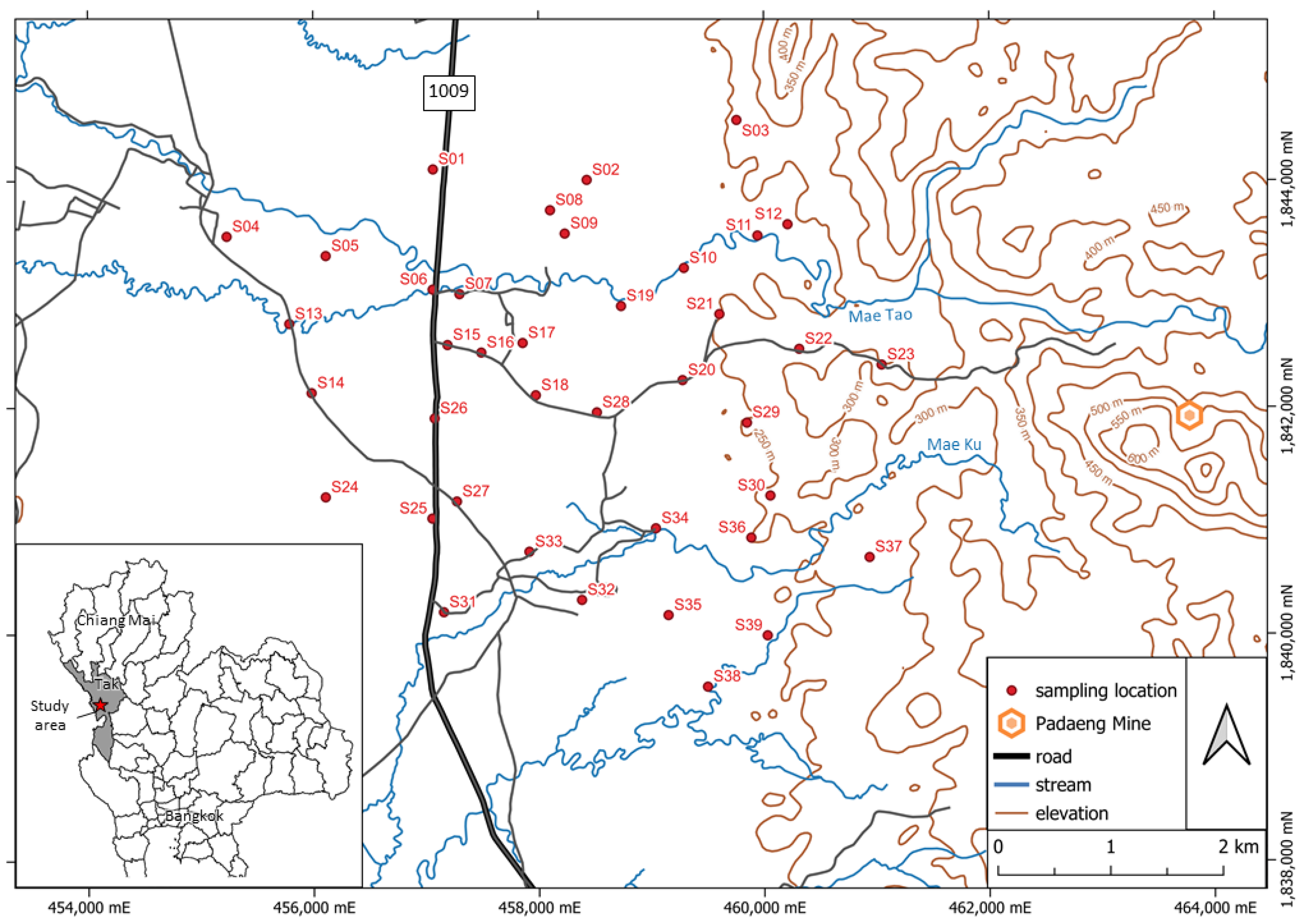
The Pa Daeng zinc mine, located in Mae Tao watershed, Mae Sot district, Tak province, northern Thailand, is the largest zinc mine in Southeast Asia, which has operated for almost four decades. The mining activity continuously feeds cadmium, zinc, and lead contamination into nearby surface water, groundwater, and soils [
16,
17]. The Pa Daeng zinc deposit is characterized as a secondary mineral deposit [
18], which was formed via the infiltration of zinc solution as a result of oxidized primary zinc sulfide and subsequent groundwater transport before it was redeposited along the fault planes, fractures, and pore spaces in the rock [
19]. The redeposited ore was weathered by rain, surface water, and groundwater, thus generating a continuing flux of dissolved heavy metals. The Mae Tao Creek and its tributaries are the major transport route of the sediments and have contributed to the distribution of heavy metals in soils and sediments downstream [
20]. Moreover, the Mae Tao Creek system is the main recharge into the Mae Tao watershed, making it a chief water supply for local people for drinking, consuming, and agriculture [
17]. The Mae Tao watershed, which is close to a mining area and human communities, could cause environmental and health issues.
Over almost two decades, there have been numerous studies on health-related and environmental problems in the Mae Tao watershed. It has been revealed that this area has a wide range contaminants, such as cadmium, zinc, lead, and manganese in soil, water, and plants. The most dangerous situation is that the high mobility of cadmium and zinc in soil–plant systems permits their easy entry into food chains [
21], where they may stimulate both human diseases [
22] and toxic effects on animals, microorganisms, and plants [
23]. The harmful health impacts on cadmium-exposed populations in the Mae Sot district have been reported [
24,
25,
26,
27,
28]. Contamination has led to health risk assessment, where it has been reported that Mae Sot residents have irregularly higher cadmium contamination in their blood, bones, and urine than normal Thai residents, resulting in a 10–86% increase in disability-adjusted life years [
29]. Recently, it was found that Mae Tao’s rice can take up heavy metals, which accumulate in the grain [
30,
31,
32], and that rice consumption causes health impacts. Almost 20% of 159 samples collected in six villages of the Mae Tao sub-district, Mae Sot district, Tak province, were contaminated with a high content of Cd [
30]. Sticky rice contained, on average, 0.1–0.7 mg/kg of Cd, with 2.60 mg/kg as the maximum value, while white rice showed 0.1–0.3 mg/kg, with the maximum at 1.94 mg/kg [
30]. A health risk assessment of rice consumption was conducted and the result identified was that both types were regarded as having a significant hazard quotient (>1) at the maximum Cd concentration; however, only sticky rice poses a health risk when considering the average Cd content [
30]. The stream sediments of the Mae Tao Creek showed hazardous levels of cadmium and zinc, with maxima of 37.11 mg/kg and 1231 mg/kg, and the highest values of suspended solids were found to be 18.27 mg/kg of Cd and 17,767 mg/kg of Zn [
17]. Intense concentrations of Cd and Zn in the soil of the agricultural area receiving water from the Mae Tao Creek were found—5.93 to 30.4 mg/kg and 286 to 594 mg/kg, respectively [
32]. Heavy metal contamination in the Mae Tao watershed has led to a particular focus on health risk assessments of rice consumption, but the persistence of solid concentrations in soil and stream sediments that function as the main heavy metal source for plant uptake, to which humans are directly exposed, have been considered only in concentration and distribution. Therefore, potential health risk assessments of heavy metals in the agricultural soil of the Mae Tao watershed require further study.
Heavy metal contamination can be determined from its intensity and its leaching potential, as well as chemical speciation, since the toxicity of heavy metals depends on the concentrations of species [
33,
34]. Heavy metals will be more harmful when they are leaching and being distributed into biota (bioavailability). John and Leventhal [
35] gave the definition of bioavailability as the ratio of total element concentrations that are available for integration into biota or bioaccumulation [
35]. They also explained that the plant root can take up the heavy metals crossing the membrane of epidermal cells into plants’ cells (bioaccumulation) from the contaminated soil; afterwards, the metal accumulated in plants is consumed by herbivores and humans [
35]. In this case, the trace elements enter through the food chain and accumulate in the higher-order consumer, which causes significant health risks across multiple physiological systems [
36]. For instance, cadmium exposure is extensively linked to a range of adverse outcomes, including respiratory issues like lung diseases, hypertension, and various malignancies such as prostate, bladder, pancreatic, kidney, and breast cancers [
37]. Furthermore, Cd has been implicated in neurological disorders, notably Alzheimer’s and Parkinson’s diseases [
38,
39,
40]. Its ability to cross the placental barrier also raises concerns about its potential impact on fetal development [
41]. Similarly, lead exposure is known to disrupt a wide array of bodily functions, affecting the neurological, skeletal, reproductive, hematopoietic, renal, and cardiovascular systems [
42]. While essential in moderate amounts, excessive zinc intake can lead to toxicity, manifesting as adverse effects on the neuronal, gastrointestinal, or respiratory systems [
43]. Likewise, chronic overexposure to manganese can result in progressive and permanent neurotoxicity, often accompanied by toxicity to the lungs, heart, liver, and reproductive system [
44,
45]. Therefore, the bioavailability of heavy metals that have been identified as having a significant impact on human health will be used for potential health risk assessments.
The primary objectives of this study are to characterize the geochemical properties of native soils and quantify the total concentrations of cadmium (Cd), zinc (Zn), lead (Pb), and manganese (Mn). Soil samples exhibiting elevated levels of these metals will be further evaluated for their leachability under controlled conditions, with a particular focus on pH and ionic strength—two key factors influencing metal bioavailability [
35]. This assessment aims to elucidate the potential for metal mobilization within the environment. In addition, a comprehensive human health risk assessment will be conducted by considering all relevant exposure pathways associated with the use of contaminated agricultural soils.
4. Discussion
4.1. Basic Properties of Soils
The analysis of basic soil properties across samples collected from the Mae Tao watershed revealed dynamic changes in soil chemistry over time. In particular, the pH values of soil–water suspensions demonstrated a notable shift from weakly alkaline to neutral or slightly acidic conditions over a 48 h observation period. The initial alkaline conditions gradually declined with increasing time, suggesting the onset of chemical equilibration. This trend can be attributed to the activity of hydrogen (H
+) and aluminum (Al
3+) ions, which are key contributors to soil acidity. Aluminum ions undergo hydrolysis in the aqueous phase, forming AlOH
2+ and releasing protons (H
+), which in turn reduce pH levels over time [
75,
76]. Each Al
3+ ion can produce up to three H
+ ions, amplifying the acidifying effect in the soil solution during equilibration. Despite this acidification, the mean and median pH values across all samples remained within the neutral range of 6.91–7.91, indicating that the soils tend to stabilize around a neutral pH under natural conditions.
The electrical conductivity (EC) values varied widely among the samples, ranging from 2.60 to 932 µS/cm. This wide distribution indicates heterogeneity in the soluble salt content, likely influenced by agricultural practices such as the use of chemical fertilizers and irrigation water sourced from contaminated streams, particularly the Mae Tao Creek [
77]. Over time, the EC values exhibited a consistent increase, driven by the dissolution of salts and ions present in the soil into the initially deionized water used for extraction. As equilibrium was approached, ion release stabilized, reflecting the chemical dynamics of soil–water interactions.
Oxidation-reduction potential (Eh) measurements showed moderate oxidation conditions across all samples, with values ranging between 214 and 366 mV. None of the samples exhibited reducing conditions (Eh < 100 mV). These values suggest that the soils are aerated and not waterlogged, which is consistent with agricultural usage. The combined data for pH, EC, and Eh further suggest that the soils exhibit considerable variability in physicochemical properties, likely due to the differential impacts of anthropogenic inputs, including fertilizers, pesticides, irrigation, and industrial discharges. This heterogeneity has important implications for the behavior, mobility, and bioavailability of heavy metals in the soil matrix.
4.2. Total Heavy Metal Concentrations and Soil Particle Size Effects
The total concentrations of cadmium (Cd), zinc (Zn), lead (Pb), and manganese (Mn) were determined for both 80-mesh and 200-mesh sieved soil fractions. Notably, the finer 200-mesh particles consistently exhibited higher concentrations of all four metals. For instance, the Cd levels in 200-mesh samples ranged up to 251 mg/kg (mean: 30.00 mg/kg), significantly higher than the 135 mg/kg maximum observed in 80-mesh samples (mean: 23.90 mg/kg). Similar trends were observed for Zn, Pb, and Mn. This size-dependent distribution reflects the greater specific surface area of finer particles, which enhances the sorption capacity and metal retention via surface complexation and ion exchange [
76].
These findings confirm that particle size plays a critical role in determining the total metal concentrations in soil, and that finer particles present a greater risk to metal mobilization and bioavailability. The increased surface area in smaller particles provides more reactive sites for the adsorption of metals, making them more relevant when assessing environmental risk and remediation potential.
4.3. Comparison with Regulatory Standards
When compared with the regulatory limits established by the European Union (EU) [
71] and Thailand’s Pollution Control Department (PCD) [
72], the concentrations of Cd in all samples exceeded the permissible thresholds, with even the minimum values surpassing the guideline levels by a factor of two or more. The Zn and Mn levels were also elevated; the average Zn concentration exceeded the EU limits, and the maximum concentrations exceeded the permissible levels by 10-fold (Zn) and 1.6-fold (Mn). In contrast, the Pb concentrations remained below the regulatory thresholds in all cases.
These results indicate that the Mae Tao watershed is significantly contaminated with Cd, moderately contaminated with Zn and Mn, and not notably impacted by Pb. The elevated Cd levels raise concern due to its high toxicity, persistence, and potential for bioaccumulation in agricultural systems. The excessive Cd concentrations are consistent with previous studies linking contamination in this area to long-term zinc mining and industrial activity upstream of the Mae Tao Creek.
4.4. Heavy Metal Leachability
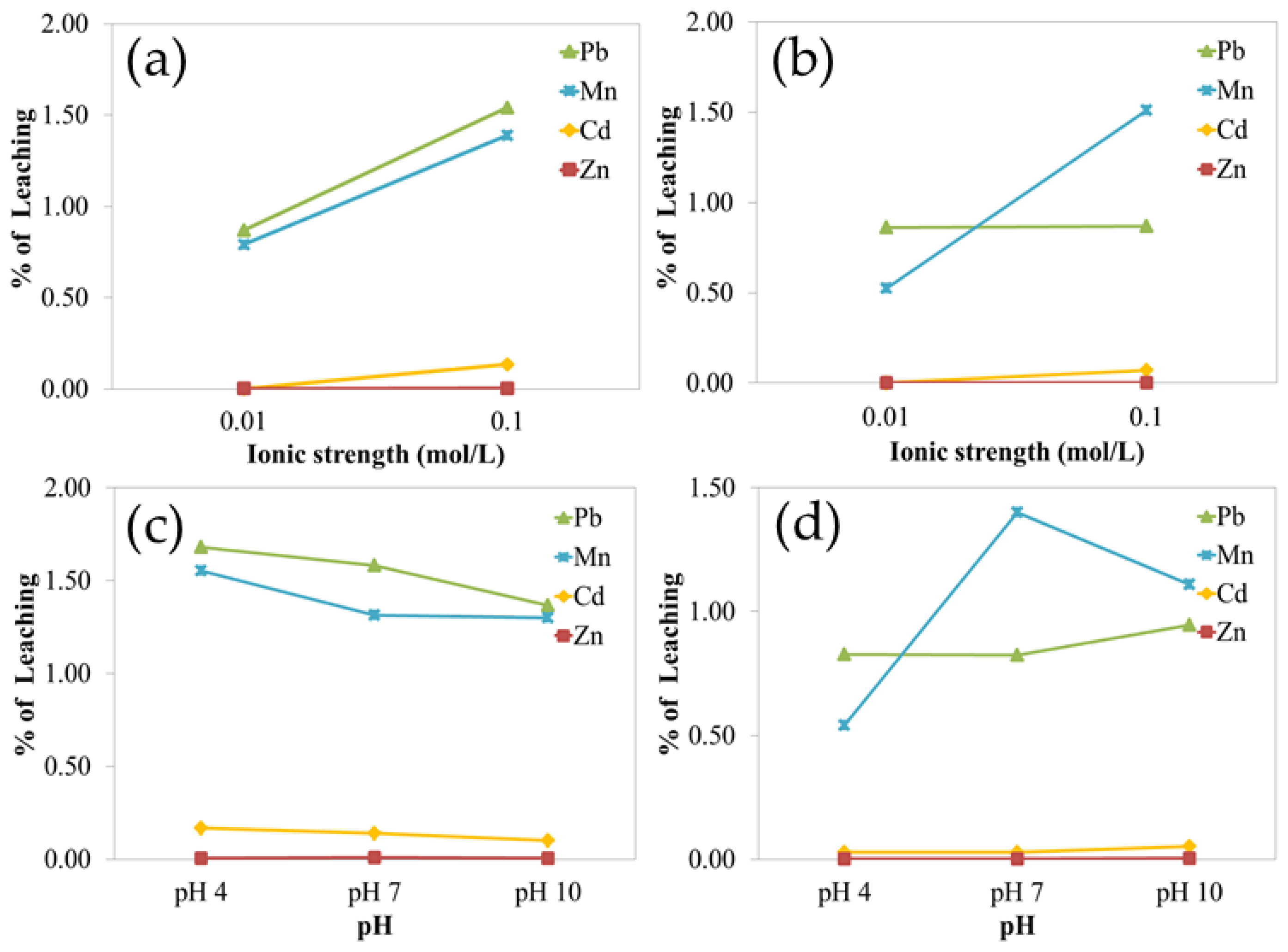
Leaching experiments were conducted on the three most contaminated soil samples (S04, S12, and S19) under varying pH and ionic strength conditions. Results showed that metal leachability was strongly influenced by ionic strength, especially for Cd and Pb. At a higher ionic strength (0.10 M), the electrical double layer surrounding soil particles is compressed, reducing electrostatic repulsion and increasing metal sorption, thus decreasing leachability [
78,
79,
80]. Conversely, lower ionic strength (0.01 M) expands the double layer, allowing for the greater mobility and leaching of Cd and Pb into solution.
In contrast, the leachability of Zn and Mn was not significantly influenced by ionic strength, suggesting that their retention in soil is governed by other factors, such as specific adsorption to mineral surfaces or incorporation into secondary minerals. The influence of pH on leachability was not statistically significant in this study, although its role in surface protonation and the deprotonation of soil minerals is known to affect metal mobility under different conditions [
81,
82]. These results support the conclusion that ionic strength is a dominant factor in Cd and Pb leaching in the study soils, while pH plays a more complex and secondary role.
4.5. Mineralogical Evidence and Metal Speciation
X-ray diffraction (XRD) analysis was conducted on representative samples to evaluate mineralogical compositions. Major phases included quartz (76.65–87.91%), dolomite (5.45–11.91%), magnesium calcite (2.84–4.82%), and minor clay minerals (0–7.87%). Notably, no crystalline phases of cadmium-bearing minerals such as otavite (CdCO3) or greenockite (CdS) were detected, suggesting that Cd exists in the soil primarily in adsorbed or amorphous forms rather than as discrete mineral phases. This supports the interpretation that the observed Cd in leachates originates from loosely bound or exchangeable fractions rather than from the dissolution of cadmium minerals.
4.6. Health Risk Assessment
The human health risk assessment included an evaluation of both non-carcinogenic and carcinogenic risks via the ingestion and dermal exposure pathways. Non-carcinogenic risks were quantified using the hazard quotient () based on the average daily dose (). The values for all metals (Cd, Pb, Zn, and Mn) in both adults and children were below the threshold of 1.0, indicating no significant health risk under the current exposure conditions. Ingestion was the dominant exposure route, with the consistently higher than the across all metals. This aligns with the established exposure patterns for soil contaminants.
Despite earlier studies reporting adverse health outcomes in the local population and concerns about Cd exposure from rice consumption [
27,
31], the present assessment based on soil exposure pathways (ingestion and dermal contact) suggests that non-carcinogenic risk is minimal. One possible explanation for this is that the sampled soils, collected at a depth of 30 cm, may represent a leached zone with lower bioavailable concentrations of contaminants compared to surface soils, where human exposure is more direct.
However, the carcinogenic risk assessment revealed more concerning results. The lifetime cancer risk (
) values for Cd exceeded the critical threshold of 1 × 10
−4 in some scenarios, particularly in ingestion pathways for both adults and children, using both 80-mesh and 200-mesh soil samples. This suggests that Cd in fine soil particles poses a potential carcinogenic risk, especially through the oral pathway. The dermal-based exposure
for Cd exceeded the acceptable limits all of concentrations in both 80-mesh and 200-mesh samples [
55]. In contrast, Pb’s
values were within or below the acceptable range (1 × 10
−6 to 1 × 10
−4 and less than 1 × 10
−6), indicating negligible carcinogenic risk.
Adults exhibited slightly higher vulnerability compared to children, with estimated hazard quotient () and lifetime cancer risk () values approximately one to two times greater across all exposure pathways. This increased susceptibility is primarily attributed to the longer lifespan of adults, which extends the duration of exposure and thereby amplifies both non-carcinogenic and carcinogenic risk accumulations over time, particularly from early life stages onward.
4.7. Implications and Recommendations
The results of this study highlight the complex interplay between soil properties, heavy metal contamination, particle size, leachability, and potential health risks. The Mae Tao watershed remains heavily impacted by cadmium contamination, with measurable risks for long-term human exposure, particularly through the oral pathway with both coarse and fine soil particles. While non-carcinogenic risk appears low under the current exposure assumptions, the carcinogenic potential of Cd warrants continued monitoring, the stricter regulation of agricultural land use, and targeted remediation efforts.
Future work should prioritize surface soil sampling (<15 cm depth), seasonal variations, and food chain transfer, particularly in rice and vegetable crops. Additional studies on metal speciation, bioavailability, and long-term soil–plant–human interactions are essential for developing a comprehensive risk management strategy for the region.
5. Conclusions
This study provides a comprehensive evaluation of heavy metal contamination, leachability behavior, and the associated human health risks in agricultural soils of the Mae Tao watershed, Mae Sot District, Tak Province, Thailand. The findings revealed elevated concentrations of cadmium (Cd), zinc (Zn), lead (Pb), and manganese (Mn) in both surface and subsurface soils, with Cd levels significantly exceeding the national and international regulatory thresholds [
72]. While Zn and Mn were present at moderately elevated levels, Pb concentrations generally remained within the acceptable limits.
Leachability experiments demonstrated that ionic strength is a key factor influencing the mobility of heavy metals, particularly Cd and Pb, in the soil environment. Higher ionic strength was associated with increased metal desorption and mobility, thereby enhancing the potential for bioavailability and environmental transport. In contrast, pH showed no consistent or statistically significant effect on leaching behavior, likely due to its complex interactions with soil mineral surfaces and competing ions. These findings suggest that conventional assessments based solely on total metal concentrations may overestimate or underestimate actual environmental risk unless the influence of soil chemistry is properly accounted for.
Health risk assessments indicated that, under the current exposure scenarios via ingestion and dermal contact, none of the evaluated heavy metals posed a significant non-carcinogenic risk to either adults or children. However, carcinogenic risk from cadmium, particularly through ingestion exposure to both coarse (80-mesh) and fine soil particles (200-mesh), was found to exceed the acceptable threshold (1 × 10−4), signaling potential long-term health concerns. Lead also presented a minor carcinogenic risk, but remained within the acceptable limits.
Overall, this study underscores the importance of understanding the geochemical behavior of heavy metals in agricultural soils. Factors such as particle size, ionic strength, and mineralogical composition play critical roles in determining metal mobility and risk potential. The complex and dynamic nature of soil systems necessitates an integrated approach for effective environmental monitoring and risk management in contaminated agricultural landscapes.