Abstract
Conventional health risk assessments do not adequately reflect short-term exposure characteristics following chemical accidents. We aimed to evaluate the efficacy of existing assessment methods and propose a more suitable risk assessment approach for short-term exposure to hazardous chemicals. We analyzed foundational studies used to derive reference concentration (RfC), reference dose (RfD), and minimal risk level (MRL) values and applied these health guidance values (HGVs) to a hypothetical chemical accident scenario. An analysis of the studies underlying each HGV revealed that, except for the RfC for formaldehyde and the RfD for toluene, all values were derived under research conditions comparable to their respective exposure durations. Given the differing toxicity mechanisms between acute and chronic exposures, MRLs that were aligned with the corresponding exposure durations supported more appropriate risk management decisions. The health risk assessment results showed that RfC/RfD-based hazard quotients (HQs) were consistently higher than MRL-based HQs across all age groups and both substances, indicating that RfC/RfD values tend to yield more conservative risk estimates. For formaldehyde, the use of RfC instead of MRL resulted in an additional 208 tiles (2.08 km2) being classified as areas of potential concern (HQ > 1) relative to the MRL-based evaluation. These findings highlighted that the selection of HGVs can significantly influence the spatial extent of areas of potential concern, potentially altering health risk determinations for large population groups. This study provides a scientific basis for improving exposure and risk assessment frameworks under short-term exposure conditions. It also serves as valuable foundational data for developing effective and rational risk management strategies during actual chemical accidents. To the best of our knowledge, this is the first study to apply MRLs to a short-term chemical accident scenario and directly compare them with traditional reference values.
1. Introduction
With growing economic development and industrialization in modern society, the volume of chemical substances required for certain industrial processes has significantly increased, leading to the continued occurrence of chemical accidents [1]. These incidents cause localized damage at the accident site and often affect the surrounding environment [2], causing extensive human and material losses, environmental contamination, and harm to local communities [3]. Consequently, considerable efforts have been made to develop tools to assess the health risks associated with exposure to hazardous chemicals released during accidents. For example, Park et al. [4] proposed a health risk assessment methodology to evaluate the potential long-term health effects of hazardous chemical exposure among residents living near chemical accident sites, aiming to support both damage prevention and post-accident management. This methodology adheres to the four-step health risk assessment framework devised by the United States National Research Council and the United States National Academy of Sciences, as adopted by the United States Environmental Protection Agency (US EPA) [5]. To reflect the fate and transport characteristics of hazardous chemicals released during an accident, the exposure assessment component incorporates multimedia transport modeling, accounting for intermedia transfer and degradation half-lives to estimate the time until dissipation in each medium. The total exposure dose is then calculated for the period between the time of release and dissipation. The health risk is subsequently assessed using health guidance values (HGVs), which are defined as exposure levels deemed sufficiently protective of human health for a given duration and exposure route; specifically, the reference concentration (RfC) and reference dose (RfD) values determined by the US EPA. However, RfC and RfD are estimates of exposure levels at which no adverse effects are expected over a lifetime of continuous exposure [6,7]. As such, applying these chronic values to hazardous substances with short-term exposure characteristics, such as those involved in chemical accidents, may introduce limitations in accurately characterizing health risks.
Although the US EPA is developing the acute reference exposure (ARE) methodology to establish HGVs for exposures ≤24 h, no guidance currently exists for exposures lasting >1 d, i.e., several days [7]. In contrast, the Agency for Toxic Substances and Disease Registry (ATSDR) has developed minimal risk levels (MRLs), defined as daily human exposure estimates below which adverse non-cancer health effects are not expected to occur. MRLs are categorized by exposure duration (acute [1–14 d], intermediate [15–364 d], and chronic [≥1 year]), and are provided for both inhalation and oral exposure routes [8]. Despite the availability of MRLs, most health risk assessments in practice continue to rely on RfC/RfD values, with limited application of MRLs. Chronic values such as RfC and RfD are often applied to short-term exposure scenarios, creating a potential mismatch between actual exposure characteristics and the applied HGVs. This mismatch may result in either underestimating or overestimating health risks, underscoring the need for a systematic comparison between the two HGV types to evaluate how their application may influence risk outcomes.
Therefore, this study aimed to analyze the foundational studies used to derive RfC/RfD and MRL values and to conduct a health risk assessment based on a hypothetical chemical accident scenario using both HGV types. By comparing and analyzing the differences in application between these two approaches, we aimed to evaluate the efficacy of conventional assessment methods and propose a more valid risk assessment strategy for short-term exposures to hazardous chemicals resulting from chemical accidents. To the best of our knowledge, this study is the first to empirically apply and compare RfC/RfD- and MRL-based health risk assessments in the context of a short-term chemical accident scenario.
2. Materials and Methods
2.1. Chemicals
To select target substances for the health risk assessment, we reviewed the 97 substances designated as accident preparedness chemicals under the Chemical Substances Control Act managed by the Ministry of Environment of Korea [9]. Accident preparedness chemicals are substances with high acute toxicity, explosiveness, or other hazardous properties, which make them more likely to cause chemical accidents or lead to large scale damage in the event of an incident. Therefore, these substances require special preparedness and management. From these, we identified substances for which both RfC/RfD and MRL values were available. We selected one substance with low persistence and another with high persistence to represent chemicals with varying environmental persistence. Consequently, toluene and formaldehyde were selected as the target substances for assessment. Toluene is characterized by high volatility and a short residence time in the environment [10], whereas formaldehyde can persist for a relatively long time in the atmosphere, with a half-life of up to 114 d, as photochemical reactions are limited under certain environmental conditions, delaying its degradation [11]. Additionally, these two substances have a high frequency of occurrence in actual chemical accidents. According to chemical accident statistics in the Korea from 2014 to July 2025, formaldehyde ranked 8th and toluene 16th [12]. These rankings underscore their representativeness as hazardous chemicals involved in chemical accidents.
2.2. Health Guidance Values
We reviewed the respective HGVs and the foundational studies for the selected substances (toluene and formaldehyde) used to derive these values. RfC and RfD values were sourced from the Integrated Risk Information System (IRIS) of the US EPA, and the corresponding MRLs were obtained from the ATSDR. Some acute, intermediate, or chronic MRL values were not available, as the ATSDR has not established them for the respective exposure durations. We also examined the exposure durations, test species, and experimental conditions used in the foundational studies for each HGV in order to compare their differences and evaluate their suitability for short-term health risk assessments.
2.3. Multimedia Environmental Dynamics Model
The multimedia environmental dynamics model developed by Lee et al. [13] is a FORTRAN-based model designed to simulate the environmental behavior of chemicals across air, soil, and water media following a chemical accident [4,14,15]. Operating under non-steady-state conditions, the model incorporates meteorological data (e.g., wind direction, wind speed, and precipitation) sourced from the Korea Meteorological Administration for the duration of the simulated accident. The modeled domain was set to a 15 km × 15 km area centered on the accident site, with a nested grid resolution of 100 m × 100 m.
The virtual accident scenario was based on the region with the highest frequency of chemical release incidents in Korea between January 2016 and April 2025, which, in this case, was Gyeonggi Province [12]. Among the 123 documented chemical accidents in this period, the scenario was modeled after the incident with the largest recorded release quantity (11 tons).
The endpoint of chemical dispersion was defined as the point at which the concentration of the released substance decreased to below its background level, i.e., 8.36 µg/m3 for toluene [16] and 4.80 µg/m3 for formaldehyde [17].
The final accident scenario was established as follows. At 12:00 p.m. on 3 July 2019, an accident occurred at a chemical plant in Ansan, Gyeonggi Province, resulting in the complete release of 11 tons of a chemical substance over the course of 1 h. It was assumed that the exposed population remained in the affected area throughout the entire persistence period of the chemical.
2.4. Non-Carcinogenic Health Risk Assessment
Based on the persistence durations estimated using the multimedia environmental dynamics model, toluene remained in the environment for 9 d, and formaldehyde persisted for 69 d. Accordingly, the acute MRL was applied to toluene, and the intermediate MRL was applied to formaldehyde (Table 1). The concept of the toxicity reference value (TRV) was introduced to facilitate a comprehensive comparison and evaluation of different HGVs. TRVs are categorized into inhalation exposure (TRVinhal) and oral exposure (TRVoral), defined as follows: TRVinhal refers to a toxicity benchmark for inhalation exposure and is derived by converting RfC and MRLinhal values using Equation (1); TRVoral refers to the toxicity benchmark for oral exposure and is defined as either RfD or MRLoral.
Here, TRVinhal is the toxicity reference value for inhalation exposure (mg/kg/d), RfC is the reference concentration (mg/m3), and MRLinhal is the minimal risk level for inhalation exposure (mg/m3).

Table 1.
Health guidance values (HGVs) of target chemicals analyzed in this study.
Table 1.
Health guidance values (HGVs) of target chemicals analyzed in this study.
| Substance | Exposure Pathway | HGV | Duration | Value | Reference |
|---|---|---|---|---|---|
| Toluene | Inhalation | RfC a | Chronic (lifespan) | 5.00 mg/m3 | [18] |
| MRL b | Acute (1–14 d) | 7.54 mg/m3 | [10] | ||
| Oral | RfD c | Chronic (lifespan) | 0.08 mg/kg/d | [18] | |
| MRL | Acute (1–14 d) | 0.8 mg/kg/d | [10] | ||
| Formaldehyde | Inhalation | RfC | Chronic (lifespan) | 0.007 mg/m3 | [19] |
| MRL | Intermediate (15–364 d) | 0.037 mg/m3 | [20] | ||
| Oral | RfD | Chronic (lifespan) | 0.2 mg/kg/d | [21] | |
| MRL | Intermediate (15–364 d) | 0.3 mg/kg/d | [20] |
a Reference concentration. b Minimal risk level. c Reference dose.
The assessment was stratified by the age groups 0–9 years, 10–18 years, 19–64 years, and ≥65 years. It was assumed that individuals engaged in typical daily activities and that exposure routes were evaluated through inhalation and incidental soil ingestion. This is due to chemicals released during accidents persisting in air or soil and being re-emitted, resulting in prolonged environmental contamination and ongoing human exposure [4,22]. Exposure factors used in the assessment are summarized in Table 2.

Table 2.
Exposure factors used in this study.
Inhalation exposure to the target chemicals was assessed for both outdoor and indoor environments. Outdoor exposure levels were determined based on the results of the multimedia environmental dynamics model. It was assumed that the released chemical substances infiltrated indoor environments for indoor exposure. Indoor concentrations were estimated using the indoor concentration prediction model developed by Park et al. [26] through meta-analysis, as expressed in Equation (2):
where Cn indoor is the concentration of indoor target chemical n days after accident occurrence (mg/m3) and Cn outdoor is the concentration of outdoor target chemical n days after accident occurrence (mg/m3).
The total inhalation exposure dose (incorporating both indoor and outdoor concentrations) was calculated as the average daily dose (ADD; mg/kg/d) using Equation (3), under the assumption of 100% absorption of the inhaled chemical:
where CLT is the period until extinction of the target chemical in the environment (d), IHR is the inhalation rate (m3/d), OET is the outdoor exposure time (d), IET is the indoor exposure time (d), BW is body weight (kg), and AT is the average exposure time (d)
Oral exposure via soil ingestion was assessed by calculating the ADD using Equation (4), which also assumed a 100% absorption of the ingested chemical:
where Cn soil is the concentration of the target chemical in soil n days after accident occurrence (mg/kg) and ITRsoil is the soil intake rate (kg/d).
The non-carcinogenic risk was assessed by calculating the hazard quotient (HQ) for each exposure route, as defined in Equation (5):
by dividing the route-specific ADD by the corresponding TRV; an HQ > 1 indicates potential for adverse health effects associated with that particular exposure route [27].
3. Results and Discussion
3.1. Health Guidance Values
A review of the foundational studies used to derive inhalation-based RfC and MRL values for toluene revealed the following findings (Table 3). Both the RfC and chronic MRL were derived from occupational studies involving long-term repeated exposures for toluene, and the acute MRL was based on experimental results from a single acute exposure study. When comparing chronic values, the RfC for toluene was approximately 1.3 times higher than the chronic MRL.
The RfC for formaldehyde was derived from three short-term human exposure studies (≤2 weeks). On the other hand, acute, intermediate, and chronic MRLs were based on exposure durations of 2 h, 26 weeks, and 10.4 years, respectively (Table 4). Notably, the RfC for formaldehyde was approximately 0.7 times lower than its chronic MRL, indicating a more conservative RfC.

Table 3.
Summary of key studies used to derive inhalation reference concentration (RfC) and minimal risk level (MRL) values for toluene.
Table 3.
Summary of key studies used to derive inhalation reference concentration (RfC) and minimal risk level (MRL) values for toluene.
| Category | RfC | Acute MRL | Chronic MRL |
|---|---|---|---|
| Critical study | Multiple human studies (n = 10) | Little et al. [28] | Multiple human studies (n = 6) |
| Test subjects | Workers | Sensitive group | Workers |
| Exposure duration | ≥1 year | 20 min | 13.5 years |
| Critical effect | Neurological effects | Neurological effects | Neurological effects |
| Point of departure | NOAEL (ADJ) a, 12.21 ppm | LOAEL b 15 ppm | NOAEL (ADJ) c, 10 ppm |
| Uncertainty factors | 10 (intraspecies variation) | 3 (LOAEL to NOAEL) 3 (intraspecies variation) | 10 (intraspecies variation) |
| Value | 5.00 mg/m3 | 7.54 mg/m3 | 3.77 mg/m3 |
| Reference | [18] | [10] | [10] |
a Arithmetic mean of NOAELs (No-observed-adverse-effect levels) from 10 studies was adjusted from occupational exposure scenario to continuous exposure scenario. b Lowest-observed-adverse-effect level. c NOAEL was adjusted from occupational exposure scenario to continuous exposure scenario.

Table 4.
Summary of key studies used to derive inhalation reference concentration (RfC) and minimal risk level (MRL) values for formaldehyde.
Table 4.
Summary of key studies used to derive inhalation reference concentration (RfC) and minimal risk level (MRL) values for formaldehyde.
| Category | RfC | Acute MRL | Intermediate MRL | Chronic MRL |
|---|---|---|---|---|
| Critical study | Krzyzanowski et al. [29]; Venn et al. [30]; Annesi-Maesano et al. [31] | Pazdrak et al. [32] | Rusch et al. [33] | Holmström et al. [34] |
| Test subjects | Children | Human | Cynomolgus monkey | Workers |
| Exposure duration | ≤2 weeks | 2 h | 26 weeks | 10.4 years |
| Critical effect | Pulmonary function decreases; Asthma prevalence or control; Allergic conditions | Nasal and eye irritation | Nasopharyngeal irritation; Nasal epithelial lesions | Nasal epithelium damage; Eyes and upper respiratory tract irritation |
| Point of departure | osRfC a, 0.006–0.008 mg/m3 (midpoint = 0.007 mg/m3) | LOAEL b, 0.4 ppm | NOAEL c, 0.98 ppm | LOAEL, 0.24 ppm |
| Uncertainty factors | 3 or 10 (intraspecies variation) | 3 (LOAEL to NOAEL) 3 (intraspecies variation) | 3 (interspecies variation) 10 (intraspecies variation) | 3 (LOAEL to NOAEL) 10 (intraspecies variation) |
| Value | 0.007 mg/m3 | 0.049 mg/m3 | 0.037 mg/m3 | 0.010 mg/m3 |
| Reference | [19] | [20] | [20] | [20] |
a RfC derived from evidence of effects on specific organ or physiological system. b Lowest-observed-adverse-effect level. c No-observed-adverse-effect level.
For oral exposure, the RfD for toluene was derived from a 13-week sub-chronic repeated dose study due to the absence of adequate chronic data. An uncertainty factor (UF) of 10 was applied to extrapolate the findings to a chronic exposure context (Table 5). Acute and intermediate MRLs for toluene were based on exposure durations of 45 min and 28 d, respectively.

Table 5.
Summary of key studies used to derive oral reference dose (RfD) and minimal risk level (MRL) values for toluene.
In the case of formaldehyde, both the RfD and the chronic MRL were derived from the same chronic data source and were assigned identical values, and the intermediate MRL was derived from a 4-week study (Table 6).

Table 6.
Summary of key studies used to derive oral reference dose (RfD) and minimal risk level (MRL) values for formaldehyde.
The analysis of the exposure durations used in the foundational studies for each HGV revealed that, except for the RfC for formaldehyde and the RfD for toluene, all HGVs were derived under research conditions comparable to their respective exposure durations. This alignment served to minimize uncertainty. The rationale for this may be that certain toxic effects may manifest during low-dose chronic exposure but not during high-dose acute exposure [42]. Therefore, selecting reference studies with comparable exposure durations is essential to avoid underestimating or overestimating risk. The toxicity from single exposures differs significantly from that of repeated exposures for several chemicals. A single exposure can produce severe effects in some cases, whereas the same cumulative dose distributed over time may produce no effects at all [43]. In line with this, the RfC and chronic MRL for toluene and the RfD for formaldehyde were based on studies involving >1 year of chronic exposure. The RfD for toluene was derived from a 13-week sub-chronic study, with a UF of 10 applied in order to extrapolate chronic exposure conditions. Notably, the RfC for formaldehyde, although classified as a chronic reference value, was based on three human studies with exposure durations of <2 weeks. As clarified by the US EPA [19], acute data may be prioritized over chronic data when exposure elicits stronger effects or when sensitive populations are disproportionately affected. With this exception, RfC and RfD values are, in principle, based on chronic exposure data. Given the differing toxicity mechanisms between acute and chronic exposures, applying RfC/RfD values to short-term exposure scenarios may be inappropriate. In contrast, MRLs are clearly categorized by exposure duration (i.e., acute [1–14 d], intermediate [15–364 d], and chronic [≥1 year]), making them more appropriate for short-term scenarios such as chemical accidents. Acute MRLs were derived from acute exposure studies (e.g., 20 min, 2 h, and 45 min), and intermediate MRLs were based on studies of intermediate duration (e.g., 26 weeks, 28 d, and 4 weeks). Therefore, the use of acute and intermediate MRLs in such contexts is considered a more valid approach to risk assessment. Additionally, the similarity in magnitude between RfC and chronic MRL values for both substances (1.3- and 0.7-fold variations for toluene and formaldehyde, respectively) suggested consistency between the two HGVs. In particular, the fact that the RfD and chronic MRL for formaldehyde were identical (being derived from the same dataset) supported the methodological validity and scientific credibility of MRL derivation when sufficient toxicological data were available.
3.2. Non-Carcinogenic Health Risk Assessment
To examine the differences in HQs resulting from the application of each HGV, we derived age-specific HQs for inhalation and ingestion exposures based on both RfC/RfD and MRL values (Table 7). The comparative analysis of RfC/RfD- and MRL-based HQs enabled an age-stratified assessment of potential health risks. For inhalation exposure, both RfC- and MRL-based HQs for toluene and formaldehyde were > 1 at their respective maximums, indicating a potential health concern. In terms of oral exposure, although RfD-based HQs were higher than MRL-based HQs for both chemicals, all values remained <1 across all age groups, suggesting no significant health risk via ingestion for either substance. These results confirm that RfC/RfD-based HQs were consistently higher than MRL-based HQs for all age groups and both substances. This implied that RfC/RfD values tended to yield more conservative risk estimates. Therefore, sole reliance on RfC/RfD, as is common in several studies, may result in overly conservative assessments and could lead to unnecessary or excessive regulatory actions in situations where the actual health risk is low.
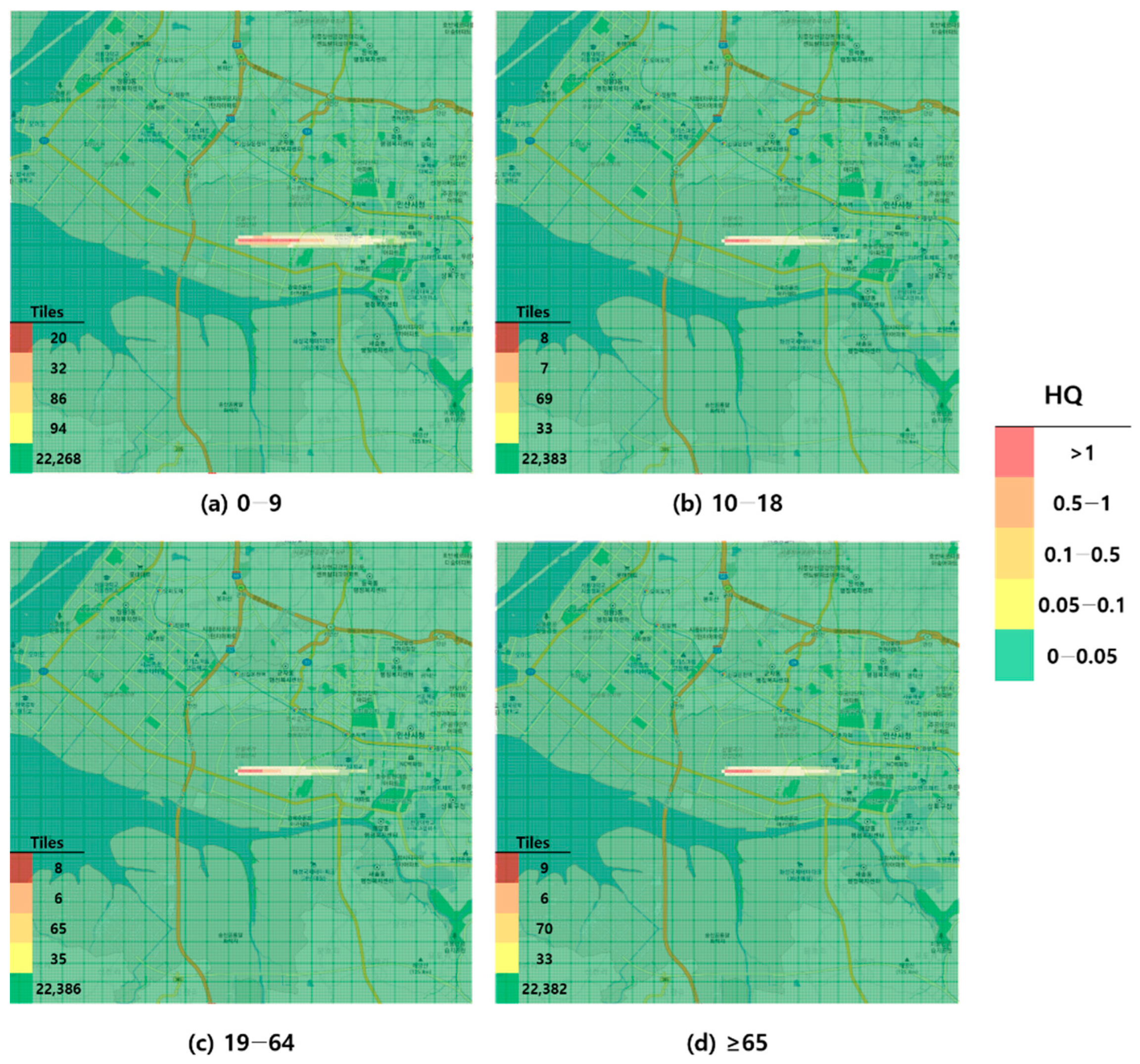
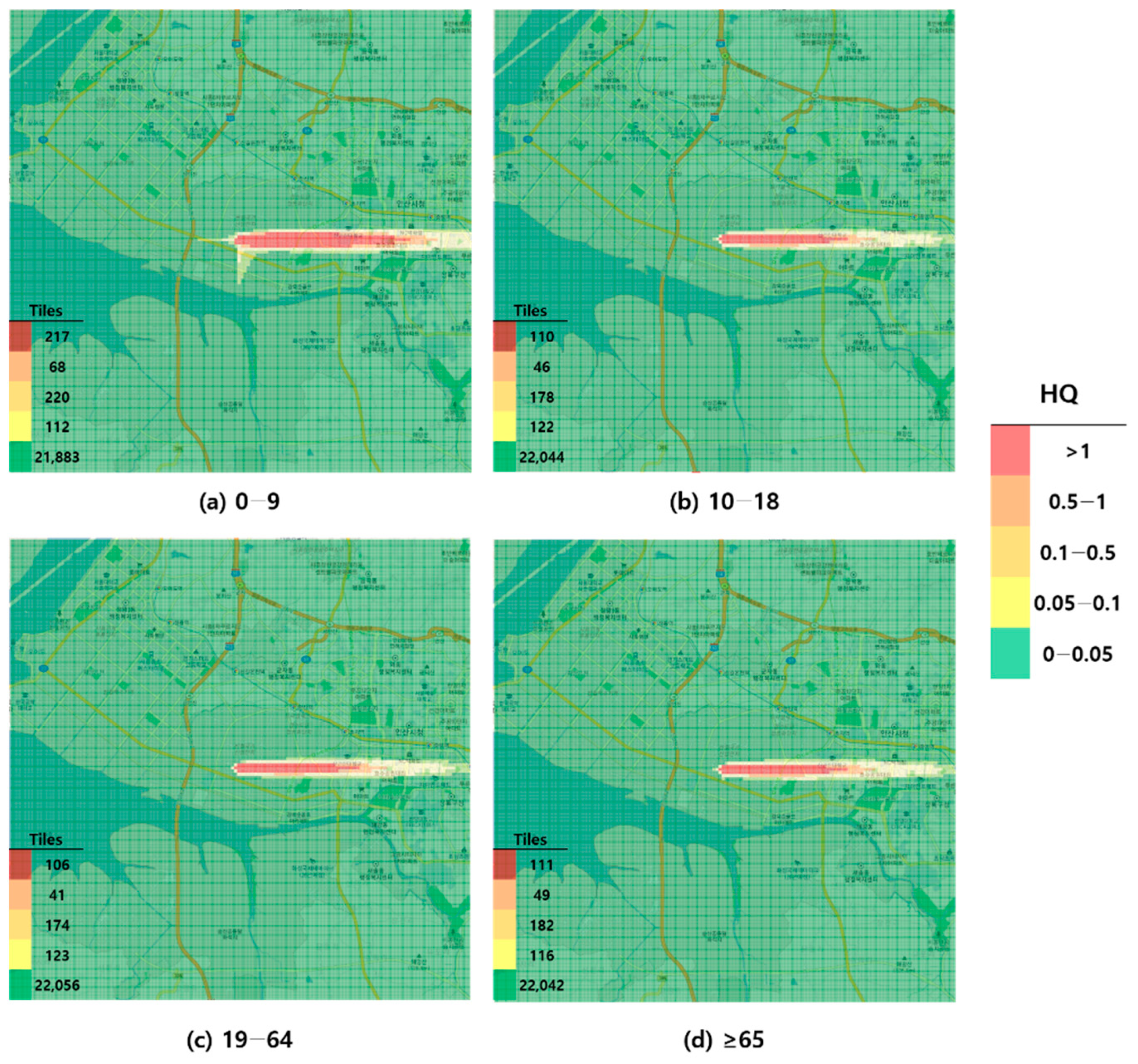
When visualized through hazard maps illustrating the spatial distribution of HQ values for inhalation exposure, it was evident that RfC-based assessments identified more areas of potential concern (HQ > 1) than MRL-based assessments (Figure 1, Figure 2, Figure 3 and Figure 4). The greatest discrepancy in the number of tiles (grid size: 100 m × 100 m) exceeding an HQ of 1 was observed in the 0–9 years age group. Due to the higher HQ values calculated using RfCs, an additional five tiles (0.05 km2) for toluene and 208 tiles (2.08 km2) for formaldehyde were classified as areas of potential concern relative to the MRL-based evaluation. The 2.08 km2 of additional area corresponds to approximately 41% of the average administrative district area in Korea (5.10 km2), where the average population per district is 19,716 [44]. These findings highlighted that the selection of HGVs can significantly influence the extent of areas of potential concern under identical exposure conditions, potentially altering health risk determinations for large population groups. This underscores the critical importance of appropriate HGV selection in risk assessment frameworks.

Table 7.
Hazard quotient (HQ) results for each exposure route by age group.
Table 7.
Hazard quotient (HQ) results for each exposure route by age group.
| Substance | TRV a | Age | Inhalation | Ingestion | ||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Median | Min | Max | Median | |||
| Toluene | RfC | 0–9 | 8.86 × 10−12 | 1.37 × 101 | 1.25 × 10−7 | 5.47 × 10−19 | 8.46 × 10−7 | 7.73 × 10−15 |
| 10–18 | 2.91 × 10−12 | 4.49 × 100 | 4.11 × 10−8 | 3.79 × 10−20 | 5.86 × 10−8 | 5.35 × 10−16 | ||
| 19–64 | 2.68 × 10−12 | 4.13 × 100 | 3.78 × 10−8 | 3.16 × 10−20 | 4.89 × 10−8 | 4.47 × 10−16 | ||
| ≥65 | 3.00 × 10−12 | 4.62 × 100 | 4.23 × 10−8 | 3.51 × 10−20 | 5.43 × 10−8 | 4.96 × 10−16 | ||
| Acute MRL | 0–9 | 5.88 × 10−12 | 9.07 × 100 | 8.30 × 10−8 | 5.47 × 10−20 | 8.46 × 10−8 | 7.73 × 10−16 | |
| 10–18 | 1.93 × 10−12 | 2.98 × 100 | 2.73 × 10−8 | 3.79 × 10−21 | 5.86 × 10−9 | 5.35 × 10−17 | ||
| 19–64 | 1.77 × 10−12 | 2.74 × 100 | 2.51 × 10−8 | 3.16 × 10−21 | 4.89 × 10−9 | 4.47 × 10−17 | ||
| ≥65 | 1.99 × 10−12 | 3.07 × 100 | 2.81 × 10−8 | 3.51 × 10−21 | 5.43 × 10−9 | 4.96 × 10−17 | ||
| Formaldehyde | RfC | 0–9 | 1.74 × 10−5 | 1.28 × 103 | 3.90 × 10−3 | 6.02 × 10−16 | 4.41 × 10−8 | 1.35 × 10−13 |
| 10–18 | 5.72 × 10−6 | 4.19 × 102 | 1.28 × 10−3 | 4.17 × 10−17 | 3.05 × 10−9 | 9.35 × 10−15 | ||
| 19–64 | 5.26 × 10−6 | 3.85 × 102 | 1.18 × 10−3 | 3.48 × 10−17 | 2.55 × 10−9 | 7.81 × 10−15 | ||
| ≥65 | 5.89 × 10−6 | 4.31 × 102 | 1.32 × 10−3 | 3.86 × 10−17 | 2.83 × 10−9 | 8.66 × 10−15 | ||
| Intermediate MRL | 0–9 | 2.48 × 10−6 | 2.43 × 102 | 7.42 × 10−4 | 4.01 × 10−16 | 2.94 × 10−8 | 9.00 × 10−14 | |
| 10–18 | 8.16 × 10−7 | 7.96 × 101 | 2.43 × 10−4 | 2.78 × 10−17 | 2.04 × 10−9 | 6.23 × 10−15 | ||
| 19–64 | 7.50 × 10−7 | 7.32 × 101 | 2.24 × 10−4 | 2.32 × 10−17 | 1.70 × 10−9 | 5.21 × 10−15 | ||
| ≥65 | 1.12 × 10−6 | 8.20 × 101 | 2.51 × 10−4 | 2.57 × 10−17 | 1.89 × 10−9 | 5.78 × 10−15 | ||
a Toxicity reference value.
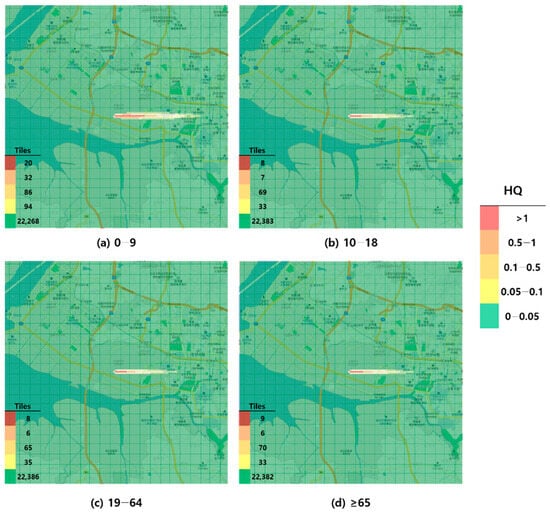
Figure 1.
Hazard map of toluene inhalation exposure based on reference concentration (RfC)-derived hazard quotient (HQ) values by age group; (a) 0–9 years; (b) 10–18 years; (c) 19–64 years; and (d) ≥65 years.
Figure 1.
Hazard map of toluene inhalation exposure based on reference concentration (RfC)-derived hazard quotient (HQ) values by age group; (a) 0–9 years; (b) 10–18 years; (c) 19–64 years; and (d) ≥65 years.
Accordingly, this study provides empirical evidence that MRLs reflecting exposure duration are more appropriate than the chronically oriented RfC/RfD values in short-term chemical accident scenarios. To the best of our knowledge, this is the first study to apply MRLs in short-term chemical accident scenarios and directly compare them with RfC/RfD values. It emphasizes the influence of HGV selection on risk characterization and provides a scientific foundation for improving short-term exposure assessment frameworks. These findings are expected to support the estimation of damage following chemical accidents and the development of appropriate compensation and response measures.
However, this study has two limitations. First, the results were derived from an assessment of only two substances: toluene and formaldehyde. In reality, a wide range of hazardous chemicals can be released during chemical accidents, and it is difficult to generalize conclusions drawn from a limited number of substances to all chemical types. Therefore, further evaluations involving chemicals with diverse toxicological mechanisms and physicochemical properties are necessary to generalize and refine the framework for health risk assessment under short-term exposure conditions. Second, the analysis was based on a single hypothetical accident scenario. As such, the study may not fully capture the variability in chemical dispersion patterns and risk assessment outcomes under different geographical, meteorological, and release conditions. Thus, further research incorporating a variety of scenarios is needed to enhance the applicability of the findings.
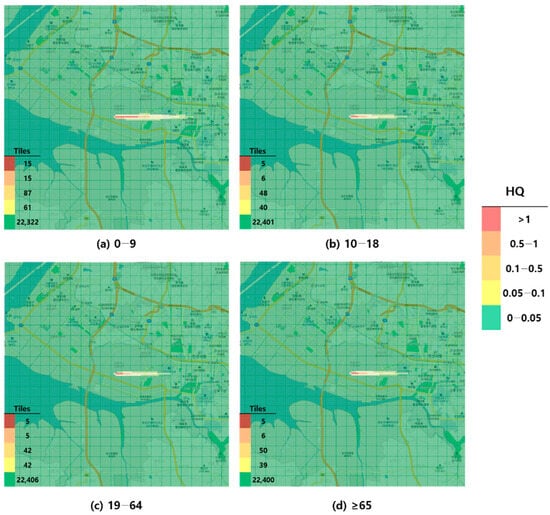
Figure 2.
Hazard map of toluene inhalation exposure based on acute minimal risk level (MRL)-derived hazard quotient (HQ) values by age group; (a) 0–9 years; (b) 10–18 years; (c) 19–64 years; and (d) ≥65 years.
Figure 2.
Hazard map of toluene inhalation exposure based on acute minimal risk level (MRL)-derived hazard quotient (HQ) values by age group; (a) 0–9 years; (b) 10–18 years; (c) 19–64 years; and (d) ≥65 years.
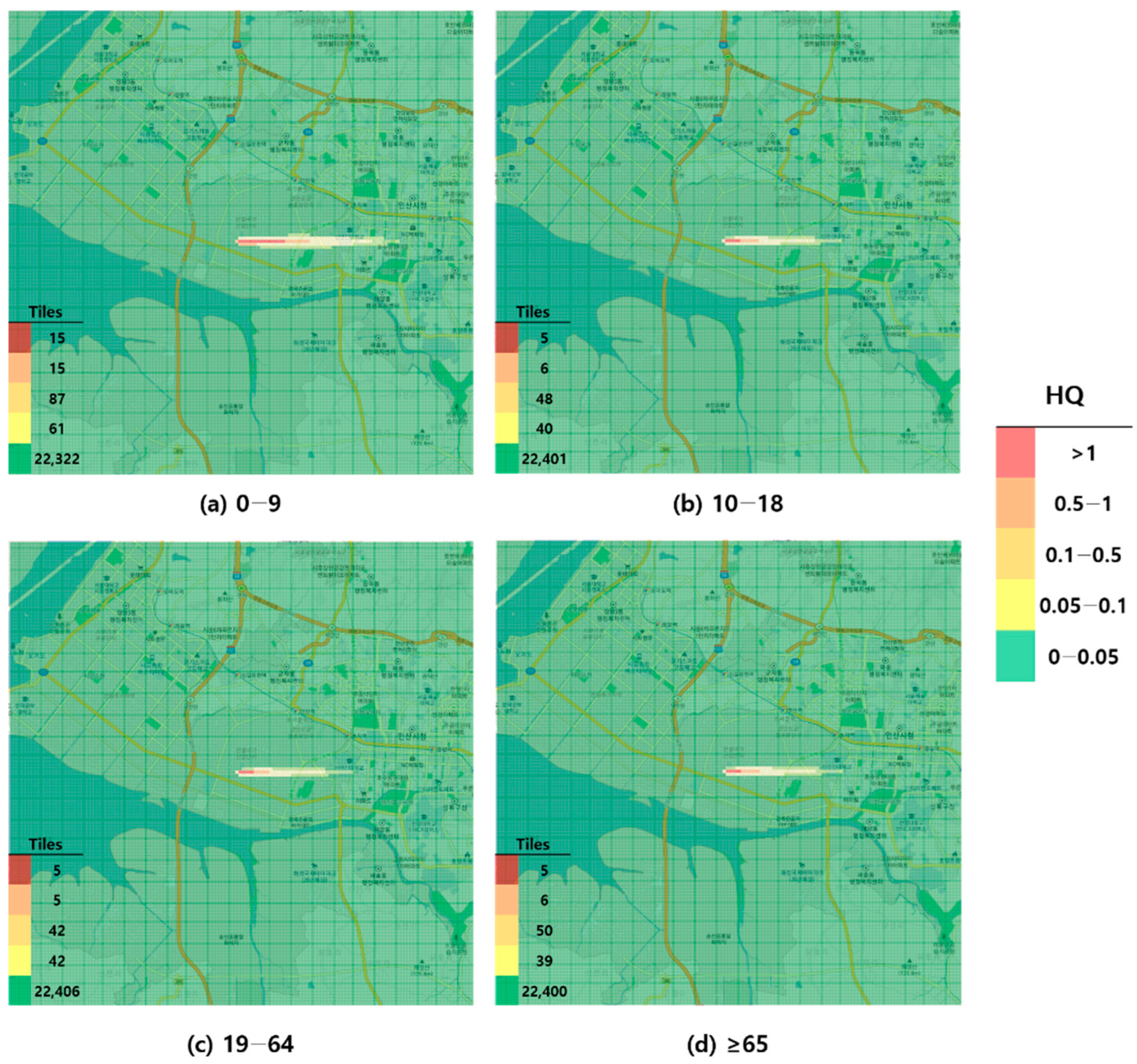
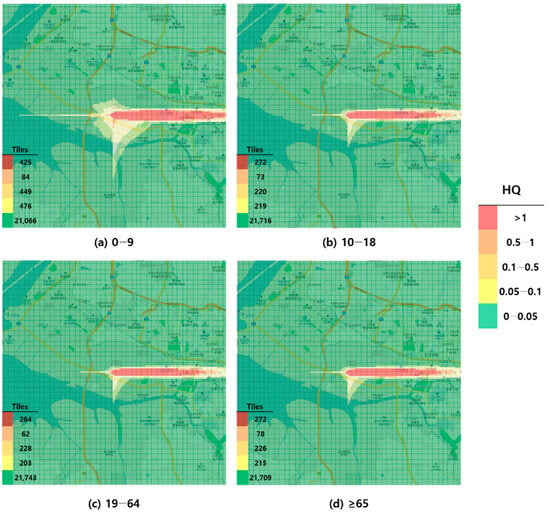
Figure 3.
Hazard map of formaldehyde inhalation exposure based on reference concentration (RFC)-derived hazard quotient (HQ) values by age group; (a) 0–9 years; (b) 10–18 years; (c) 19–64 years; and (d) ≥65 years.
Figure 3.
Hazard map of formaldehyde inhalation exposure based on reference concentration (RFC)-derived hazard quotient (HQ) values by age group; (a) 0–9 years; (b) 10–18 years; (c) 19–64 years; and (d) ≥65 years.
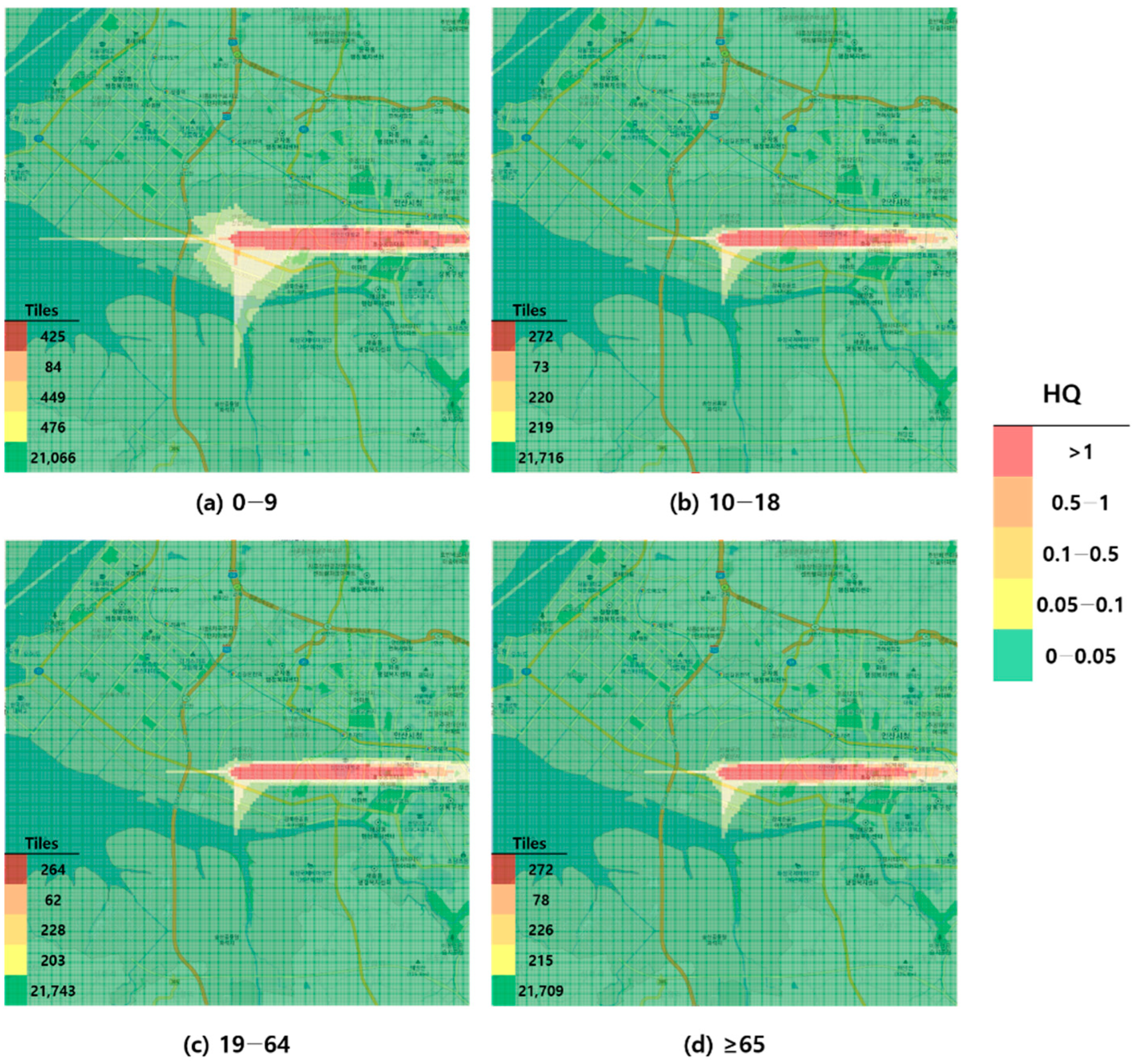
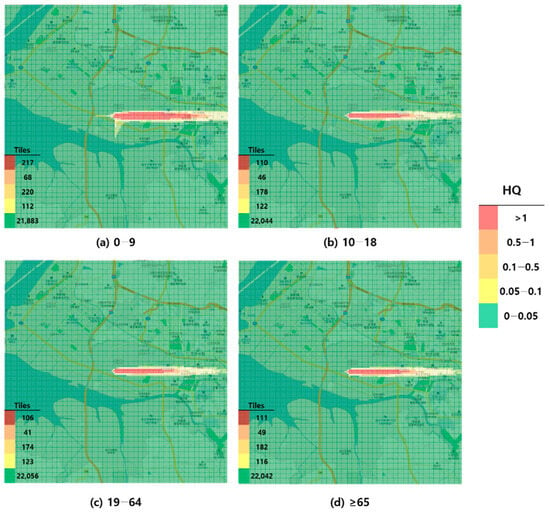
Figure 4.
Hazard map of formaldehyde inhalation exposure based on intermediate minimal risk level (MRL)-derived hazard quotient (HQ) values by age group; (a) 0–9 years; (b) 10–18 years; (c) 19–64 years; and (d) ≥65 years.
Figure 4.
Hazard map of formaldehyde inhalation exposure based on intermediate minimal risk level (MRL)-derived hazard quotient (HQ) values by age group; (a) 0–9 years; (b) 10–18 years; (c) 19–64 years; and (d) ≥65 years.
4. Conclusions
To date, health risk assessments for hazardous chemical exposures resulting from chemical accidents have commonly relied on chronic-based benchmarks such as the RfC and RfD, even in short-term exposure scenarios. However, this approach has inherent limitations, as it does not adequately reflect the unique characteristics of short-term exposure. To address these limitations, this study aimed to evaluate the efficacy of existing assessment methods and propose a more suitable risk assessment approach for short-term exposure to hazardous chemicals.
Examination of exposure durations in the foundational studies revealed that most HGVs were based on studies closely aligned with their designated exposure durations. RfC/RfD values are fundamentally derived from chronic data. Therefore, they have inherent limitations when applied directly to short-term exposure scenarios, as they may not sufficiently reflect the toxicological mechanism involved in short-term exposures. In contrast, MRLs are stratified by exposure duration, making them more suitable benchmarks for short-term risk assessments.
As a result of comparing the HQs, the MRL-based HQs were lower than the RfC/RfD-based HQs across all exposure routes (inhalation and oral) for both toluene and formaldehyde. This suggests that the RfC/RfD-based HQs provide a relatively conservative assessment. In particular, for formaldehyde, the use of RfC instead of MRL resulted in the identification of an additional 2.08 km2 of land (equivalent to 41% of the average area of an administrative district in Korea) as a potential concern (HQ > 1). This discrepancy underscores how the selection of HGVs can significantly affect risk determinations and potentially impact numerous residents.
These findings provide empirical evidence that the application of MRLs is more appropriate than RfC/RfD values in short-term health risk assessments and establish a scientific foundation for improving short-term risk assessment frameworks and developing rational risk management strategies. To the best of our knowledge, this study is the first to empirically apply and compare MRL- and RfC/RfD-based health risk assessments in the context of a short-term chemical accident scenario. In addition, the findings of this study are expected to contribute to estimating the scale of damage following chemical accidents as well as to the development of compensation and response measures. However, as this study was based on a limited number of substances and a single hypothetical accident scenario, further research that incorporates a wider range of chemicals and exposure conditions is necessary.
Author Contributions
Conceptualization, J.-E.M. and C.-M.L.; methodology, J.-E.M., S.-H.P., and J.-Y.J.; formal analysis, J.-E.M.; investigation, J.-E.M., Y.-H.K., and H.J.; data curation, J.-E.M., Y.-H.K., and H.J.; writing—original draft preparation, J.-E.M.; writing—review and editing, S.-H.P. and C.-M.L.; visualization, J.-E.M.; project administration, supervision, writing—review and editing, S.-W.Y.; project administration, C.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study received support from the Ministry of Environment’s Chemical Accident Prediction and Prevention Advancement Technology Development Project (2022003620002) and the Graduate School Support Program for Chemical Substance Specialization as part of the 2025 Initiative for Training Professionals in Chemical Safety Management, a project undertaken under the auspices of the Ministry of Environment.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADD | Average daily dose |
| ARE | Acute reference exposure |
| AT | Average exposure time |
| ATSDR | Agency for Toxic Substances and Disease Registry |
| BMDL | A lower one-sided confidence limit on the benchmark dose |
| BW | Body weight |
| C | Concentration |
| CLT | Period until extinction of the target chemical in the environment |
| HGV | Health guidance value |
| HQ | Hazard quotient |
| IET | Indoor exposure time |
| IHR | Inhalation rate |
| IRIS | Integrated Risk Information System |
| ITR | Intake rate |
| LOAEL | Lowest-observed-adverse-effect level |
| MoE | Ministry of the Environment |
| MOIS | Ministry of Interior and Safety |
| MRL | Minimal risk level |
| NICS | National Institute of Chemical Safety |
| NIER | National Institute of Environmental Research |
| NOAEL | No-observed-adverse-effect level |
| OET | outdoor exposure time |
| RfC | Reference concentration |
| RfD | Reference dose |
| TRV | Toxicity reference value |
| UF | Uncertainty factor |
| US EPA | United States Environmental Protection Agency |
References
- Choi, J.U. Re-evaluation of the Impact Range Considering Decomposition Products in the Event of Chemical Accidents Involving Nitric Acid Leakage. Master’s Thesis, Graduate School of Industry Kumoh National Institute of Technology, Gyeongbuk, Republic of Korea, 2020. [Google Scholar]
- Jeon, B.H.; Kim, H.S. Improvement on accident statistic analysis and response of hazardous chemical transport vehicle. J. Soc. Disaster Inf. 2018, 14, 59–64. [Google Scholar]
- Chun, K.S.; Kim, S.B.; Ahn, S.R.; Park, Y.S.; Park, C.H. Chemical accident response information systems improvement risk assessment research, Korean. J. Hazard. Mater. 2013, 1, 69–74. [Google Scholar]
- Park, S.H.; Lim, H.B.; Hong, H.J.; Kim, H.S.; Yoon, D.K.; Lee, H.W.; Kong, H.K.; Jeon, J.I.; Choi, J.W.; Cho, E.M.; et al. Health risk assessment for multimedia exposure of formaldehyde emitted by chemical accident. Environ. Sci. Pollut. Res. Int. 2021, 28, 9712–9722. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Risk Assessment in the Federal Government: Managing the Process; National Academies Press: Washington, DC, USA, 1983. [CrossRef]
- United States Environmental Protection Agency (US EPA). Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry; US EPA: Durham, NC, USA, 1994.
- United States Environmental Protection Agency (US EPA). A Review of the Reference Dose and Reference Concentration Processes; US EPA: Washington, DC, USA, 2002.
- Agency for Toxic Substances and Disease Registry (ATSDR). About Minimal Risk Levels. Available online: https://www.atsdr.cdc.gov/minimal-risk-levels/php/about/?CDC_AAref_Val=https://www.atsdr.cdc.gov/mrls/index.html (accessed on 25 June 2025).
- Ministry of the Environment (MoE). Designation of Substances Requiring Accident Preparedness. Available online: https://www.law.go.kr/%ED%96%89%EC%A0%95%EA%B7%9C%EC%B9%99/%EC%82%AC%EA%B3%A0%EB%8C%80%EB%B9%84%EB%AC%BC%EC%A7%88%EC%9D%98%EC%A7%80%EC%A0%95 (accessed on 25 June 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Toluene; ATSDR: Atlanta, GA, USA, 2017.
- United States Environmental Protection Agency (US EPA). Chemistry, Fate, and Transport Assessment for Formaldehyde CASRN 50-00-0; US EPA: Washington, DC, USA, 2024.
- National Institute of Chemical Safety (NICS). Current Status and Cases of Chemical Accidents. Available online: https://icis.me.go.kr/search/searchType2.do (accessed on 25 June 2025).
- Lee, Y.A.; Kim, H.S.; Lee, D.S. Evaluation Using A Multimedia Fate and Transport Model of Surface Water Pollution Potential of Organic Chemicals Accidentally Release into Air During A Rainfall Event; SETAC: Helsinki, Finland, 2019. [Google Scholar]
- Jung, J.Y.; Park, S.H.; Moon, J.E.; Yoon, J.H.; Yoon, S.W.; Lee, C.M. Aggregate risk assessment for multi-route exposure to hazardous chemicals caused by chemical accidents, with a focus on toluene. Asian J. Atmos. Environ. 2024, 18, 17. [Google Scholar] [CrossRef]
- Hong, H.J.; Park, S.H.; Lim, H.B.; Lee, C.M. Development on health risk assessment method for multi-media exposure of hazardous chemical by chemical accident. Int. J. Environ. Res. Public Health 2020, 17, 3385. [Google Scholar] [CrossRef]
- National Institute of Environmental Research (NIER). Multi-Media and Multi-Pathway Aggregate Risk Aassessment (I)-Toluene-; Ministry of Environment: Sejong City, Republic of Korea, 2011. [Google Scholar]
- National Institute of Environmental Research (NIER). Multi-Media and Multi-Pathway Aggregate Risk Assessment (Ⅱ)-Formaldehyde-; Ministry of Environment: Sejong City, Republic of Korea, 2012. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Toxicological Review of Toluene (CAS No. 108-88-3) in Support of Summary Information on the Integrated Risk Information System (IRIS); US EPA: Washington, DC, USA, 2005.
- United States Environmental Protection Agency (US EPA). IRIS Toxicological Review of Formaldehyde (Inhalation) CASRN 50-00-0; US Environmental Protection Agency, Office of Research and Development; US EPA: Washington, DC, USA, 2024.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Formaldehyde; ATSDR: Atlanta, GA, USA, 1999.
- United States Environmental Protection Agency (US EPA). IRIS Summary of Formaldehyde (Noncancer, 1991); US EPA: Washington, DC, USA, 1991.
- Hong, H.J.; Kong, H.K.; Park, S.H.; Yoon, D.K.; Lim, H.B.; Namgoung, S.J.; Lee, C.M. Health Risk Assessment of Hazardous Chemicals from Soil Ingestion in Chemical Accident. In Proceedings of the Korea Environmental Sciences Society Conference, JESI, Gyeongju City, Repulic of Korea, 7–9 November 2019. [Google Scholar]
- National Institute of Environmental Research (NIER). Korean Exposure Factors Handbook for Children; Ministry of Environment: Sejong City, Republic of Korea, 2019. [Google Scholar]
- National Institute of Environmental Research (NIER). Korean Exposure Factors Handbook; Ministry of Environment: Sejong City, Republic of Korea, 2019. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Update for Chapter 5 of the Exposure Factors Handbook-Soil and Dust Ingestion); US EPA: Washington, DC, USA, 2017.
- Park, S.H.; Yoon, D.K.; Park, T.H.; Hong, H.J.; Lee, E.S. A study on the prediction of hazardous substance concentration in the indoor space by indoor inflow of chemical accident material. Proc. Environ. Sci. Soc. 2018, 27, 95. [Google Scholar]
- National Institute of Environmental Research (NIER). Regulation on Specific Methods of Chemical Risk Assessment. Available online: https://www.law.go.kr/%ED%96%89%EC%A0%95%EA%B7%9C%EC%B9%99/%ED%99%94%ED%95%99%EB%AC%BC%EC%A7%88%EC%9C%84%ED%95%B4%EC%84%B1%ED%8F%89%EA%B0%80%EC%9D%98%EA%B5%AC%EC%B2%B4%EC%A0%81%EB%B0%A9%EB%B2%95%EB%93%B1%EC%97%90%EA%B4%80%ED%95%9C%EA%B7%9C%EC%A0%95 (accessed on 25 June 2025).
- Little, C.H.; Georgiou, G.M.; Shelton, M.J.; Simpson, F.; Cone, R.E. Clinical and immunological responses in subjects sensitive to solvents. Arch. Environ. Health 1999, 54, 6–14. [Google Scholar] [CrossRef]
- Krzyzanowski, M.; Quackenboss, J.J.; Lebowitz, M.D. Chronic respiratory effects of indoor formaldehyde exposure. Environ. Res. 1990, 52, 117–125. [Google Scholar] [CrossRef]
- Venn, A.J.; Cooper, M.; Antoniak, M.; Laughlin, C.; Britton, J.; Lewis, S.A. Effects of volatile organic compounds, damp, and other environmental exposures in the home on wheezing illness in children. Thorax 2003, 58, 955–960. [Google Scholar] [CrossRef]
- Annesi-Maesano, I.; Hulin, M.; Lavaud, F.; Raherison, C.; Kopferschmitt, C.; de Blay, F.; Charpin, D.A.; Denis, C. Poor air quality in classrooms related to asthma and rhinitis in primary schoolchildren of the French 6 Cities Study. Thorax 2012, 67, 682–688. [Google Scholar] [CrossRef]
- Pazdrak, K.; Górski, P.; Krakowiak, A.; Ruta, U. Changes in nasal lavage fluid due to formaldehyde inhalation. Int. Arch. Occup. Environ. Health 1993, 64, 515–519. [Google Scholar] [CrossRef]
- Rusch, G.M.; Clary, J.J.; Rinehart, W.E.; Bolte, H.F. A 26-week inhalation toxicity study with formaldehyde in the monkey, rat, and hamster. Toxicol. Appl. Pharmacol. 1983, 68, 329–343. [Google Scholar] [CrossRef]
- Holmström, M.; Wilhelmsson, B.; Hellquist, H.; Rosén, G. Histological changes in the nasal mucosa in persons occupationally exposed to formaldehyde alone and in combination with wood dust. Acta OtoLaryngol. 1989, 107, 120–129. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP toxicology and carcinogenesis studies of toluene (CAS No. 108-88-3) in F344/N rats and B6C3F1 mice (inhalation studies). Natl. Toxicol. Program Tech. Rep. Ser. 1990, 371, 1–253. [Google Scholar]
- Dyer, R.S.; Bercegeay, M.S.; Mayo, L.M. Acute exposures to p-xylene and toluene alter visual information processing. Neurotoxicol. Teratol. 1988, 10, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, G.C.; Sharma, R.P.; Parker, R.D.R. Immunotoxicological evaluation of toluene exposure via drinking water in mice. Environ. Res. 1989, 49, 93–103. [Google Scholar] [CrossRef]
- Hsieh, G.C.; Parker, R.D.R.; Sharma, R.P.; Hughes, B.J. Subclinical effects of groundwater contaminants: III. Effects of repeated oral exposure to combinations of benzene and toluene on immunologic responses in mice. Arch. Toxicol. 1990, 64, 320–328. [Google Scholar] [CrossRef]
- Hsieh, G.C.; Sharma, R.P.; Parker, R.D.R. Hypothalamic-pituitary-adrenocortical axis activity and immune function after oral exposure to benzene and toluene. Immunopharmacology 1991, 21, 23–31. [Google Scholar] [CrossRef]
- Til, H.P.; Woutersen, R.A.; Feron, V.J.; Hollanders, V.H.M.; Falke, H.E.; Clary, J.J. Two-year drinking-water study of formaldehyde in rats. Food Chem. Toxicol. 1989, 27, 77–87. [Google Scholar] [CrossRef]
- Til, H.P.; Woutersen, R.A.; Feron, V.J.; Clary, J.J. Evaluation of the oral toxicity of acetaldehyde and formaldehyde in a 4-week drinking-water study in rats. Food Chem. Toxicol. 1988, 26, 447–452. [Google Scholar] [CrossRef]
- Barnes, D.G.; Dourson, M.; Preuss, P.; Barnes, D.G.; Bellin, J.; Derosa, C.; Engler, R.; Erdreich, L.; Farber, T.; Fenner-Crisp, P.; et al. Reference dose (RfD): Description and use in health risk assessments. Regul. Toxicol. Pharmacol. 1988, 8, 471–486. [Google Scholar] [CrossRef]
- Casarett, L.J.; Doull, J.; Klaassen, C.D. Casarett and Doull’s Toxicology: The Basic Science of Poisons; McGraw-Hill: New York, NY, USA, 2008; Volume 7. [Google Scholar]
- Ministry of Interior and Safety (MOIS). Status of Administrative Divisions and Population of Local Governments; MOIS: Sejong City, Republic of Korea, 2024.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
