Mechanisms and Genetic Drivers of Resistance of Insect Pests to Insecticides and Approaches to Its Control
Highlights
- This study examines molecular mechanisms including target-site mutations and metabolic detoxification.
- It identifies emerging mechanisms such as OBP sequestration and RNA regulation.
- It highlights the role of microbiomes and gene transfer in the spread and evolution of resistance genes.
- Cross- and multiple resistance necessitates more sustainable pest control strategies.
- The results reinforce the efficacy of IPM and the use of RNAi and nanoparticles in future resistance management.
- They enable the development of molecular diagnostics and MoA-based insecticide rotation strategies.
Abstract
1. Introduction
2. Key Mechanisms Underlying Insecticide Resistance
2.1. Molecular Mechanisms of Target-Site Resistance
2.1.1. Neurological Targets
Nicotinic Acetylcholine Receptor (nAChR) Mutations
Voltage-Gated Sodium Channel Alterations (Knockdown Resistance, kdr)
2.1.2. Physiological and Metabolic Targets
Aminobenzoic Acid Amide Insecticides (Ryanodine Receptor)
Benzoylurea Insecticides (Chitin Synthase)
2.1.3. Growth and Developmental Targets
Juvenile Hormone Receptors and Ecdysteroid Receptors
2.1.4. Other Novel or Rare Targets
Octopamine Receptors
Midgut Cell Membranes and Oxidative Phosphorylation/Decoupling Agents
2.1.5. GABA Receptor Mutations and Resistance to Dieldrin (Rdl)
2.2. Mechanisms of Metabolic Resistance: Enzymatic Detoxification Pathways
2.2.1. Esterases: Gene Amplification and Detoxification Efficiency
2.2.2. Cytochrome P450 Monooxygenases: The Versatile Detoxifiers
2.2.3. Glutathione-S-Transferases (GSTs): Conjugation- and Sequestration-Based Resistance
2.2.4. UDP-Glucosyltransferases (UGTs): An Overlooked Phase II Component
2.2.5. ABC Transporters: Phase III Toxin Efflux Systems
3. Emerging and Underexplored Mechanisms of Resistance of Insects to Insecticides
3.1. Sequestration Resistance: Repurposing Olfactory Proteins as Insecticide Buffers
3.2. RNA-Mediated Resistance: Noncoding RNAs as Posttranscriptional Regulators
3.3. Role of the Microbiome in Detoxification: A Dynamic Evolutionary Defense
Role of the Microbiome in Insect Detoxification and Resistance: Mechanistic Insights
3.4. Horizontal Gene Transfer (HGT) as a Driver of Novel Resistance Mechanisms
3.5. Future Directions
4. Cross-Resistance and Multiple Resistance in Insect Pests—Mechanisms, Environmental Factors, and Management Strategies
4.1. Resistance Types and Their Management Implications
4.1.1. Cross-Resistance: One Mechanism, Many Failures
4.1.2. Multiple Resistance: Accumulation of Independent Mechanisms
4.1.3. Strategic Implications: Toward Mechanism-Informed IRM
4.2. Resistance Management: A Multitactic Framework for Insecticide Sustainability
4.3. Divergent Strategies for RNAi Resistance to Insecticides Management, a Comparative Framework for Transgenic and Sprayable Applications
4.3.1. Distinct Exposure Dynamics Drive Divergent IRM Needs
4.3.2. Tailored IRM Strategies Reflect Product Format and Biology
4.3.3. Mechanisms of Resistance Inform Risk and Regulatory Design
5. Case Studies
5.1. Mechanisms of Resistance to Insecticides for the Mosquito, Aedes aegypti
5.1.1. Target Site Resistance
V1016I Mutation
F1534C Mutation
V410L Mutation
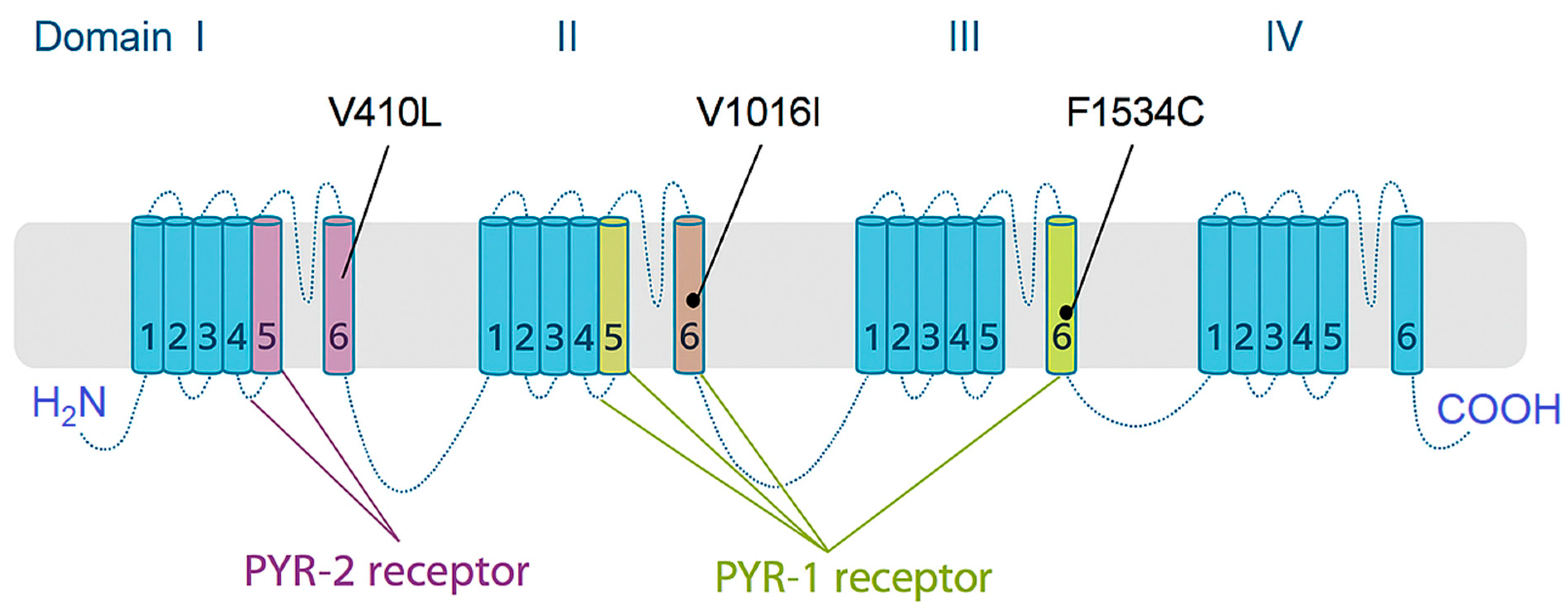
5.1.2. Metabolic Resistance
5.1.3. Resistance to Insecticides and Associated Fitness Costs
5.2. Mechanisms of Resistance to Insecticides in Spodoptera frugiperda
5.2.1. Global Distribution and Severity of Resistance
5.2.2. Integrated Resistance Management Strategies
5.2.3. Future Directions for Resistance Mitigation
6. Strategies for Managing Resistance to Insecticides: Chemical, Biological, and Integrated Approaches
6.1. Conventional Insecticides: Effectiveness and Limitations
6.2. Next-Generation Resistance-Breaking Technologies
6.3. Biopesticides: Eco-Friendly Alternatives
6.4. Biological Control: Natural Regulation of Pest Populations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AI | Artificial Intelligence |
| Bt | Bacillus thuringiensis |
| CESs | Carboxylesterases (commonly abbreviated as CESs or CarEs) |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR associated protein 9 |
| CSPs | Chemosensory Proteins |
| CYPs | Cytochrome P450 Monooxygenases |
| DDT | Dichlorodiphenyltrichloroethane |
| DEM | Diethyl Maleate |
| dsRNA | Double-Stranded RNA |
| FAW | Fall Armyworm (Spodoptera frugiperda) |
| GABA | Gamma-Aminobutyric Acid |
| GPIRM | WHO Global Plan for Insecticide Resistance Management |
| GSTs | Glutathione-S-Transferases |
| HaNPV | Helicoverpa armigera Nucleopolyhedrovirus |
| IPM | Integrated Pest Management |
| IRM | Insecticide Resistance Management |
| kdr | Knockdown Resistance |
| LLINs | Long-Lasting Insecticidal Nets |
| lncRNAs | Long Non-Coding RNAs |
| MACE | Modified Acetylcholinesterase |
| miRNAs | MicroRNAs |
| MoA | Mode of Action |
| nAChRs | Nicotinic Acetylcholine Receptors |
| NPV | Nucleopolyhedrovirus |
| OBPs | Odorant-Binding Proteins |
| OP | Organophosphates |
| PBO | Piperonyl Butoxide |
| PIPs | Plant-Incorporated Protectants |
| qPCR | Quantitative Polymerase Chain Reaction |
| RDL | Resistance to Dieldrin |
| RNAi | RNA Interference |
| RyR | Ryanodine Receptor |
| SliNPV | Spodoptera litura Nucleopolyhedrovirus |
| VGSC | Voltage-Gated Sodium Channel |
| WHO | World Health Organization |
References
- Ádám, B.; Cocco, P.; Godderis, L. Hazardous Effects of Pesticides on Human Health. Toxics 2024, 12, 186. [Google Scholar] [CrossRef]
- Wan, N.-F.; Fu, L.; Dainese, M.; Kiær, L.P.; Hu, Y.-Q.; Xin, F.; Goulson, D.; Woodcock, B.A.; Vanbergen, A.J.; Spurgeon, D.J.; et al. Pesticides have negative effects on non-target organisms. Nat. Commun. 2025, 16, 1360. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Tonnang, H.E.; Sokame, B.M.; Abdel-Rahman, E.M.; Dubois, T. Measuring and modelling crop yield losses due to invasive insect pests under climate change. Curr. Opin. Insect Sci. 2022, 50, 100873. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification—A tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. 2023 Annual Meeting on the Implementation of FAO Global Action (GA) for Fall Armyworm (FAW) Control in Africa—Report; FAO: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/handle/20.500.14283/cd0185en (accessed on 2 August 2025).
- Togola, A.; Beyene, Y.; Bocco, R.; Tepa-Yotto, G.; Gowda, M.; Too, A.; Boddupalli, P. Fall armyworm (Spodoptera frugiperda) in Africa: Insights into biology, ecology and impact on staple crops, food systems and management approaches. Front. Agron. 2025, 7, 1538198. [Google Scholar] [CrossRef]
- Quandahor, P.; Kim, L.; Kim, M.; Lee, K.; Kusi, F.; Jeong, I. Effects of Agricultural Pesticides on Decline in Insect Species and Individual Numbers. Environments 2024, 11, 182. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Database: Insecticides Use Data. 2025. Available online: http://www.fao.org/faostat/en/#data (accessed on 2 August 2025).
- IRAC (Insecticide Resistance Action Committee): Introduction to Resistance. 2025. Available online: https://irac-online.org/training-centre/resistance/ (accessed on 4 August 2025).
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What Can We Learn from Commercial Insecticides? Efficacy, Toxicity, Environmental Impacts, and Future Developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef]
- Stratonovitch, P.; Elias, J.; Denholm, I.; Slater, R.; Semenov, M.A. An Individual-Based Model of the Evolution of Pesticide Resistance in Heterogeneous Environments: Control of Meligethes aeneus Population in Oilseed Rape Crops. PLoS ONE 2014, 9, e115631. [Google Scholar] [CrossRef]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked Evolution: Can We Address the Sociobiological Dilemma of Pesticide Resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Andrews, M.; Cutler, P.; Daniels, M.; Elias, J.; Paul, V.L.; Crossthwaite, A.J.; Denholm, I.; Field, L.M.; et al. Mutation of a Nicotinic Acetylcholine Receptor β Subunit Is Associated with Resistance to Neonicotinoid Insecticides in the Aphid Myzus persicae. BMC Neurosci. 2011, 12, 51. [Google Scholar] [CrossRef]
- Zhou, W.; Arcot, Y.; Medina, R.F.; Bernal, J.; Cisneros-Zevallos, L.; Akbulut, M.E.S. Integrated Pest Management: An Update on the Sustainability Approach to Crop Protection. ACS Omega 2024, 9, 41130–41147. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Slater, R.; Sparks, T.C.; Elbert, A.; Mccaffery, A. IRAC: Insecticide resistance and mode-of-action classification of insecticides. In Modern Crop Protection Compounds, Volume 3: Insecticides, 3rd ed.; Jeschke, P., Witschel, M., Krämer, W., Schirmer, U., Eds.; Wiley-VCH: Weinheim, Germany, 2019; Chapter 28. [Google Scholar] [CrossRef]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide Resistance Management and Industry: The Origins and Evolution of the Insecticide Resistance Action Committee (IRAC) and the Mode of Action Classification Scheme. Pest Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.L.; Govella, N.J.; Azizi, S.; Drakeley, C.J.; Kachur, S.P.; Killeen, G.F. Increased Proportions of Outdoor Feeding among Residual Malaria Vector Populations Following Increased Use of Insecticide-Treated Nets in Rural Tanzania. Malar. J. 2011, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Olson, J.M.; Sanchez-Sedillo, B.; Bradford, B.; Groves, R.L. Changes in emergence phenology, fatty acid composition, and xenobiotic-metabolizing enzyme expression is associated with increased insecticide resistance in the Colorado potato beetle. Arch. Insect Biochem. Physiol. 2020, 103, e21630. [Google Scholar] [CrossRef]
- Sarfraz, M.; Dosdall, L.M.; Keddie, B.A. Evidence for Behavioural Resistance by the Diamondback Moth, Plutella xylostella (L.). J. Appl. Entomol. 2005, 129, 340–341. [Google Scholar] [CrossRef]
- Bass, C.; Jones, C.M. Mosquitoes boost body armor to resist insecticide attack. Proc. Natl. Acad. Sci. USA 2016, 113, 9145–9147. [Google Scholar] [CrossRef]
- Ahmad, M.; Denholm, I.; Bromilow, R.H. Delayed cuticular penetration and enhanced metabolism of deltamethrin in pyrethroid-resistant strains of Helicoverpa armigera from China and Pakistan. Pest Manag. Sci. 2006, 62, 805–810. [Google Scholar] [CrossRef]
- Reddy, M.R.; Godoy, A.; Dion, K.; Matias, A.; Callender, K.; Kiszewski, A.E.; Kleinschmidt, I.; Ridl, F.C.; Powell, J.R.; Caccone, A.; et al. Insecticide resistance allele frequencies in Anopheles gambiae before and after anti-vector interventions in continental Equatorial Guinea. Am. J. Trop. Med. Hyg. 2013, 88, 897–907. [Google Scholar] [CrossRef]
- Djogbénou, L.; Chandre, F.; Berthomieu, A.; Dabiré, R.; Koffi, A.; Alout, H.; Weill, M. Evidence of introgression of the ace-1(R) mutation and of the ace-1 duplication in West African Anopheles gambiae s. s. PLoS ONE 2008, 3, e2172. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Yang, Y.; Li, H.; Wang, X.; Yang, B.; Zhang, J.; Li, C.; Millar, N.S.; Liu, Z. Synergistic and compensatory effects of two point mutations conferring target-site resistance to fipronil in the insect GABA receptor RDL. Sci. Rep. 2016, 6, 32335. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Irving, H.; Ndula, M.; Barnes, K.G.; Ibrahim, S.S.; Paine, M.J.; Wondji, C.S. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 2013, 110, 252–257. [Google Scholar] [CrossRef]
- Poulton, B.C.; Colman, F.; Anthousi, A.; Sattelle, D.B.; Lycett, G.J. Aedes aegypti CCEae3A carboxylase expression confers carbamate, organophosphate and limited pyrethroid resistance in a model transgenic mosquito. PLoS Negl. Trop. Dis. 2024, 18, e0011595. [Google Scholar] [CrossRef]
- Daborn, P.J.; Yen, J.L.; Bogwitz, M.R.; Le Goff, G.; Feil, E.; Jeffers, S.; Tijet, N.; Perry, T.; Heckel, D.; Batterham, P.; et al. A single p450 allele associated with insecticide resistance in Drosophila. Science 2002, 297, 2253–2256. [Google Scholar] [CrossRef]
- Lu, X.; Simma, E.A.; Spanoghe, P.; Van Leeuwen, T.; Dermauw, W. Recombinant expression and characterization of GSTd3 from a resistant population of Anopheles arabiensis and comparison of DDTase activity with GSTe2. Pestic. Biochem. Physiol. 2023, 192, 105397. [Google Scholar] [CrossRef]
- Taillebois, E.; Cartereau, A.; Jones, A.K.; Thany, S.H. Neonicotinoid insecticides mode of action on insect nicotinic acetylcholine receptors using binding studies. Pestic. Biochem. Physiol. 2018, 151, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid Insecticides: Molecular Targets, Resistance, and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ding, Q.; Wang, X.; Wang, R.; Ullah, F.; Gao, X.; Song, D. V101I and R81T mutations in the nicotinic acetylcholine receptor β1 subunit are associated with neonicotinoid resistance in Myzus persicae. Pest Manag. Sci. 2022, 78, 1500–1507. [Google Scholar] [CrossRef]
- Mottet, C.; Fontaine, S.; Caddoux, L.; Brazier, C.; Mahéo, F.; Simon, J.C.; Micoud, A.; Roy, L. Assessment of the Dominance Level of the R81T Target Resistance to Two Neonicotinoid Insecticides in Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 2182–2189. [Google Scholar] [CrossRef]
- Mottet, C.; Caddoux, L.; Fontaine, S.; Plantamp, C.; Bass, C.; Barrès, B. Myzus persicae resistance to neonicotinoids—Unravelling the contribution of different mechanisms to phenotype. Pest Manag. Sci. 2024, 80, 5852–5863. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.E.; Riplinger, C.; Neese, F.; Bistoni, G. Unraveling individual host–guest interactions in molecular recognition from first principles quantum mechanics: Insights into the nature of nicotinic acetylcholine receptor agonist binding. J. Comput. Chem. 2021, 42, 293–302. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Chen, J.; Huang, H.; Liu, K.; Zhang, Y.; Yang, J.; Wu, S. Mutation V65I in the β1 Subunit of the Nicotinic Acetylcholine Receptor Confers Neonicotinoid and Sulfoxaflor Resistance in Insects. J. Agric. Food Chem. 2024, 72, 5671–5681. [Google Scholar] [CrossRef]
- Yin, C.; O’Reilly, A.O.; Liu, S.N.; Du, T.H.; Gong, P.P.; Zhang, C.J.; Wei, X.G.; Yang, J.; Huang, M.J.; Fu, B.L.; et al. Dual mutations in the whitefly nicotinic acetylcholine receptor β1 subunit confer target-site resistance to multiple neonicotinoid insecticides. PLoS Genet. 2024, 20, e1011163. [Google Scholar] [CrossRef]
- Markussen, M.D.; Kristensen, M. Low Expression of Nicotinic Acetylcholine Receptor Subunit Mdα2 in Neonicotinoid-Resistant Strains of Musca domestica L. Pest Manag. Sci. 2010, 66, 1257–1262. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J.; Huang, H.; Wen, H.; Yang, J.; Geng, J.; Wu, S. The Amino Acid Ser223 Acts as a Key Site for the Binding of Thrips palmi α1 Nicotinic Acetylcholine Receptor to Neonicotinoid Insecticides. Pestic. Biochem. Physiol. 2025, 213, 106484. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, C.T.; Garrood, W.T.; Puinean, A.M.; Eckel-Zimmer, M.; Williamson, M.S.; Davies, T.G.; Bass, C. A CRISPR/Cas9 mediated point mutation in the alpha 6 subunit of the nicotinic acetylcholine receptor confers resistance to spinosad in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2016, 73, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.B.; Siebert, M.W.; Wang, N.X.; Loso, M.R.; Sparks, T.C. Sulfoxaflor – A Sulfoximine Insecticide: Review and Analysis of Mode of Action, Resistance and Cross-Resistance. Pestic. Biochem. Physiol. 2021, 178, 104924. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Zhang, B.; Liang, P.; Wang, B.; Gao, X. Overexpression of Multiple Cytochrome P450 Genes Associated with Sulfoxaflor Resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 157, 204–210. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; He, M.; Wu, S.; Shi, Y.; Luo, D.; Zhang, H.; Wang, Z.; Wan, H.; Li, R.; et al. miRNAs modulate altered expression of cytochrome P450s and nicotinic acetylcholine receptor subunits conferring both metabolic and target resistance to sulfoxaflor in Nilaparvata lugens (Stål). Int. J. Biol. Macromol. 2025, 290, 138992. [Google Scholar] [CrossRef]
- Longhurst, C.; Babcock, J.M.; Denholm, I.; Gorman, K.; Thomas, J.D.; Sparks, T.C. Cross-Resistance Relationships of the Sulfoximine Insecticide Sulfoxaflor with Neonicotinoids and Other Insecticides in the Whiteflies Bemisia tabaci and Trialeurodes vaporariorum. Pest Manag. Sci. 2013, 69, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Mezei, I.; Valverde-Garcia, P.; Siebert, M.W.; Gomez, L.E.; Torne, M.; Watson, G.B.; Raquel, A.M.; Fereres, A.; Sparks, T.C. Impact of the nicotinic acetylcholine receptor mutation R81T on the response of European Myzus persicae populations to imidacloprid and sulfoxaflor in laboratory and in the field. Pestic. Biochem. Physiol. 2022, 187, 105187. [Google Scholar] [CrossRef]
- Beckingham, C.; Phillips, J.; Gill, M.; Crossthwaite, A.J. Investigating nicotinic acetylcholine receptor expression in neonicotinoid resistant Myzus persicae FRC. Pestic. Biochem. Physiol. 2013, 107, 293–298. [Google Scholar] [CrossRef]
- Samal, R.R.; Panmei, K.; Lanbiliu, P.; Kumar, S. Metabolic detoxification and ace-1 target site mutations associated with acetamiprid resistance in Aedes aegypti L. Front. Physiol. 2022, 13, 988907. [Google Scholar] [CrossRef]
- Lan, J.; Wang, Z.; Chen, Z.; Zhang, L.; Zhao, J.; Guan, Q.; Liao, C.; Liu, N.; Han, Q. Identification of the Aedes aegypti nAChR gene family and molecular target of spinosad. Pest Manag. Sci. 2021, 77, 1633–1641. [Google Scholar] [CrossRef]
- Fouet, C.; Pinch, M.J.; Ashu, F.A.; Ambadiang, M.M.; Bouaka, C.; Batronie, A.J.; Hernandez, C.A.; Rios, D.E.; Penlap-Beng, V.; Kamdem, C. Field-Evolved Resistance to Neonicotinoids in the Mosquito, Anopheles gambiae, Is Associated with Mutations of Nicotinic Acetylcholine Receptor Subunits Combined with Cytochrome P450-Mediated Detoxification. Pestic. Biochem. Physiol. 2024, 206, 106205. [Google Scholar] [CrossRef] [PubMed]
- Homem, R.A.; Buttery, B.; Richardson, E.; Tan, Y.; Field, L.M.; Williamson, M.S.; Davies, T.G.E. Evolutionary Trade-Offs of Insecticide Resistance—The Fitness Costs Associated with Target-Site Mutations in the nAChR of Drosophila melanogaster. Mol. Ecol. 2020, 29, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Ureña, E.; Guillem-Amat, A.; Couso-Ferrer, F.; Beroiz, B.; Perera, N.; López-Errasquín, E.; Castañera, P.; Ortego, F.; Hernández-Crespo, P. Multiple mutations in the nicotinic acetylcholine receptor Ccα6 gene associated with resistance to spinosad in medfly. Sci. Rep. 2019, 9, 2961. [Google Scholar] [CrossRef] [PubMed]
- Ilias, A.; Lagnel, J.; Kapantaidaki, D.E.; Roditakis, E.; Tsigenopoulos, C.S.; Vontas, J.; Tsagkarakou, A. Transcription analysis of neonicotinoid resistance in Mediterranean (MED) populations of B. tabaci reveal novel cytochrome P450s, but no nAChR mutations associated with the phenotype. BMC Genom. 2015, 16, 939. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.M.; Berger, M.; Bass, C.; Williamson, M.; Moura, D.M.; Ribeiro, L.M.; Siqueira, H.A. Mutation (G275E) of the nicotinic acetylcholine receptor α6 subunit is associated with high levels of resistance to spinosyns in Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Pestic. Biochem. Physiol. 2016, 131, 125432. [Google Scholar] [CrossRef]
- Zuo, Y.; Xue, Y.; Lu, W.; Ma, H.; Chen, M.; Wu, Y.; Yang, Y.; Hu, Z. Functional validation of nicotinic acetylcholine receptor (nAChR) α6 as a target of spinosyns in Spodoptera exigua utilizing the CRISPR/Cas9 system. Pest Manag. Sci. 2020, 76, 2415–2422. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, H.; Hu, M.; Wang, X.; Zhang, L. Discovery of Novel Potential Insecticide-Resistance Mutations in Spodoptera frugiperda. Insects 2024, 15, 186. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Lansdell, S.J.; Zhang, J.; Millar, N.S.; Wu, Y. A three amino acid deletion in the transmembrane domain of the nicotinic acetylcholine receptor α6 subunit confers high-level resistance to spinosad in Plutella xylostella. Insect Biochem. Mol. Biol. 2016, 71, 29–36. [Google Scholar] [CrossRef]
- Mocchetti, A.; Nikoloudi, A.A.; Vontas, J.; De Rouck, S.; Van Leeuwen, T. CRISPR/Cas9 Knock-Out of nAChR α6 Confers Resistance to Spinosyns in Frankliniella occidentalis and Is Associated with a Higher Fitness Cost than Target-Site Mutation G275E. Pestic. Biochem. Physiol. 2025, 212, 106455. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.X.; Narai, Y.; Nakano, A.; Kaneda, T.; Murai, T.; Sonoda, S. Spinosad resistance of melon thrips, Thrips palmi, is conferred by G275E mutation in α6 subunit of nicotinic acetylcholine receptor and cytochrome P450 detoxification. Pestic. Biochem. Physiol. 2014, 112, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Jouraku, A.; Kuwazaki, S.; Kanazawa, J.; Iwasa, T. The R81T mutation in the nicotinic acetylcholine receptor of Aphis gossypii is associated with neonicotinoid insecticide resistance with differential effects for cyano- and nitro-substituted neonicotinoids. Pestic. Biochem. Physiol. 2017, 143, 57–65. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Zhuang, Z.-X.; Zhang, F.; Song, X.-Y.; Ye, W.-N.; Wu, S.-F.; Bass, C.; O’Reilly, A.O.; Gao, C. Contribution of Nilaparvata lugens Nicotinic Acetylcholine Receptor Subunits Toward Triflumezopyrim and Neonicotinoid Susceptibility. Environ. Sci. Technol. 2025, 59, 7054–7065. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular Biology of Insect Sodium Channels and Pyrethroid Resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Rinkevich, F.D.; Su, C.; Lazo, T.A.; Hawthorne, D.J.; Tingey, W.M.; Naimov, S.; Scott, J.G. Multiple Evolutionary Origins of Knockdown Resistance (kdr) in Pyrethroid-Resistant Colorado Potato Beetle, Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2012, 104, 192–200. [Google Scholar] [CrossRef]
- Sun, H.; Tong, K.P.; Kasai, S.; Scott, J.G. Overcoming Super-Knock Down Resistance (Super-Kdr) Mediated Resistance: Multi-Halogenated Benzyl Pyrethroids Are More Toxic to Super-Kdr Than Kdr House Flies. Insect Mol. Biol. 2016, 25, 126–137. [Google Scholar] [CrossRef] [PubMed]
- von Stein, R.T.; Silver, K.S.; Soderlund, D.M. Indoxacarb, Metaflumizone, and Other Sodium Channel Inhibitor Insecticides: Mechanism and Site of Action on Mammalian Voltage-Gated Sodium Channels. Pestic. Biochem. Physiol. 2013, 106, 101–112. [Google Scholar] [CrossRef]
- Roditakis, E.; Mavridis, K.; Riga, M.; Vasakis, E.; Morou, E.; Rison, J.L.; Vontas, J. Identification and Detection of Indoxacarb Resistance Mutations in the Para Sodium Channel of the Tomato Leafminer, Tuta absoluta. Pest Manag. Sci. 2017, 73, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, X.; Li, Z.; Lei, C.; Wang, S. Insecticidal potential and risk assessment of diamide pesticides against Spodoptera frugiperda in maize crops. Ecotoxicol. Environ. Saf. 2024, 282, 116682. [Google Scholar] [CrossRef]
- Du, J.; Fu, Y. Diamide insecticides targeting insect ryanodine receptors: Mechanism and application prospect. Biochem. Biophys. Res. Commun. 2023, 670, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Hasnain, A.; Cheng, Q.-H.; Xia, L.-J.; Cai, Y.-H.; Hu, R.; Gong, C.-W.; Liu, X.-M.; Pu, J.; Zhang, L.; et al. Resistance Monitoring and Mechanism in the Fall Armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) for Chlorantraniliprole from Sichuan Province, China. Front. Physiol. 2023, 14, 1180655. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, K.B.; Tao, L.H.; Li, L.; Li, J.J.; Wu, W.W.; Xiao, C.; Ye, M. Analysis of binding modes and resistance risk between ryanodine receptor of Spodoptera frugiperda and diamide. J. Pestic. Sci. 2021, 23, 856–868. [Google Scholar]
- Ma, R.; Haji-Ghassemi, O.; Ma, D.; Jiang, H.; Lin, L.; Yao, L.; Samurkas, A.; Li, Y.; Wang, Y.; Cao, P. Structural basis for diamide modulation of ryanodine receptor. Nat. Chem. Biol. 2020, 16, 1246–1254. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Yan, K.; Ding, Y.; Gao, X.; Bi, R.; Zhang, H.; Pan, Y.; Shang, Q. Functional Characterization of ABC Transporters Mediates Multiple Neonicotinoid Resistance in a Field Population of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2022, 188, 105264. [Google Scholar] [CrossRef]
- Douris, V.; Steinbach, D.; Panteleri, R.; Livadaras, I.; Pickett, J.A.; Van Leeuwen, T.; Nauen, R.; Vontas, J. Resistance Mutation Conserved Between Insects and Mites Unravels the Benzoylurea Insecticide Mode of Action on Chitin Biosynthesis. Proc. Natl. Acad. Sci. USA 2016, 113, 14692–14697. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shiotsuki, T.; Jouraku, A.; Miura, K.; Minakuchi, C. Benzoylurea Resistance in Western Flower Thrips Frankliniella occidentalis (Thysanoptera: Thripidae): The Presence of a Point Mutation in Chitin Synthase 1. J. Pestic. Sci. 2017, 42, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, E.A.; Mastrantonio, V.; Grigoraki, L.; Porretta, D.; Puggioli, A.; Chaskopoulou, A.; Osório, H.; Weill, M.; Bellini, R.; Urbanelli, S.; et al. Identification and Detection of a Novel Point Mutation in the Chitin Synthase Gene of Culex pipiens Associated with Diflubenzuron Resistance. PLoS Negl. Trop. Dis. 2020, 14, e0008284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Shao, H.; Liu, Z.; Gao, X.; Tian, Z.; Zhang, Y.; Liu, J. Unraveling Key Amino Acid Residues Crucial for PxGSTs1 Conferring Benzoylurea Insecticide Resistance in Plutella xylostella. J. Agric. Food Chem. 2024, 72, 25549–25559. [Google Scholar] [CrossRef]
- Koyama, T.; Saeed, U.; Rewitz, K.; Halberg, K.V. The integrative physiology of hormone signaling: Insights from insect models. Physiology 2025, 40, 343–362. [Google Scholar] [CrossRef]
- Charles, J.P.; Iwema, T.; Epa, V.C.; Takaki, K.; Rynes, J.; Jindra, M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. USA 2011, 108, 21128–21133. [Google Scholar] [CrossRef]
- Ononamadu, C.J.; Abdalla, M.; Ihegboro, G.O.; Li, J.; Owolarafe, T.A.; John, T.D.; Tian, Q. In silico identification and study of potential anti-mosquito juvenile hormone binding protein (MJHBP) compounds as candidates for dengue virus-vector insecticides. Biochem. Biophys. Rep. 2021, 28, 101178. [Google Scholar] [CrossRef]
- Barry, J.; Wang, S.; Wilson, T.G. Overexpression of Methoprene-tolerant, a Drosophila melanogaster gene that is critical for juvenile hormone action and insecticide resistance. Insect Biochem. Mol. Biol. 2008, 38, 346–353. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Sheng, Z.; Liu, H.; Wen, D.; He, Q.; Wang, S.; Shao, W.; Jiang, R.J.; An, S.; Sun, Y.; et al. Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. J. Biol. Chem. 2009, 136, 2015–2025. [Google Scholar] [CrossRef]
- Jindra, M.; Uhlirova, M.; Charles, J.-P.; Smykal, V.; Hill, R.J. Genetic evidence for the function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 2015, 11, e1005394. [Google Scholar] [CrossRef]
- Yang, X.; Wei, X.; Yang, J.; Du, T.; Yin, C.; Fu, B.; Huang, M.; Liang, J.; Gong, P.; Liu, S.; et al. Epitranscriptomic regulation of insecticide resistance. Sci. Adv. 2021, 7, eabe5903. [Google Scholar] [CrossRef] [PubMed]
- Bavithra, C.M.L.; Murugan, M.; Pavithran, S.; Naveena, K. Enthralling genetic regulatory mechanisms meddling insecticide resistance development in insects: Role of transcriptional and post-transcriptional events. Front. Mol. Biosci. 2023, 10, 1257859. [Google Scholar] [CrossRef]
- Mahalle, R.M.; Sun, W.; Posos-Parra, O.A.; Jung, S.; Mota-Sanchez, D.; Pittendrigh, B.R.; Seong, K.M. Identification of differentially expressed miRNAs associated with diamide detoxification pathways in Spodoptera frugiperda. Sci. Rep. 2024, 14, 4308. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.B.; Fujimoto, T.; Potter, D.W.; Deng, Q.; Palli, S.R. A single point mutation in ecdysone receptor leads to increased ligand specificity: Implications for gene switch applications. Proc. Natl. Acad. Sci. USA 2002, 99, 14710–14715. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Hafeez, M.; Desneux, N. RNA interference-mediated silencing of ecdysone receptor (EcR) gene causes lethal and sublethal effects on melon aphid, Aphis gossypii. Entomol. Gen. 2022, 42, 791. [Google Scholar] [CrossRef]
- Saito, K.; Kanno, M.; Tanimoto, H.; Ichinose, T. Ecdysteroid-DopEcR signaling in neuronal and midgut cells mediates toxin avoidance and detoxification in Drosophila. Curr. Biol. 2025, 35, 3418–3428.e4. [Google Scholar] [CrossRef]
- Uchibori-Asano, M.; Uchiyama, T.; Jouraku, A.; Ozawa, A.; Akiduki, G.; Yamamura, K.; Shinoda, T. Tebufenozide resistance in the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae): Establishment of a molecular diagnostic method based on EcR mutation and its application for field-monitoring. Appl. Entomol. Zool. 2019, 54, 223–230. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S.; Jia, Q.; Wu, L.; Yuan, D.; Li, E.Y.; Feng, Q.; Wang, G.; Palli, S.R.; Wang, J.; et al. Juvenile hormone membrane signaling phosphorylates USP and thus potentiates 20-hydroxyecdysone action in Drosophila. Sci. Bull. 2022, 67, 186–197. [Google Scholar] [CrossRef]
- Luo, J.; Ouyang, H.; Liu, S.; Wu, H.; Xiong, F.; Yang, T.; Li, H.; Li, X. Juvenile hormone signaling regulates apoptosis-mediated chlorantraniliprole sensitivity in Spodoptera frugiperda (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2025, 214, 106603. [Google Scholar] [CrossRef]
- Ling, L.; Raikhel, A.S. Cross-talk of insulin-like peptides, juvenile hormone, and 20-hydroxyecdysone in regulation of metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2021, 118, e2023470118. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.Q.; Wang, X.L.; Lyu, X.Y.; Raikhel, A.S.; Zou, Z. Ecdysone-controlled nuclear receptor ERR regulates metabolic homeostasis in the disease vector mosquito Aedes aegypti. PLoS Genet. 2024, 20, e1011196. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.-L.; Wei, J.; Wang, Q.; Yang, Y.-B.; Gao, Y.-N.; Li, L.; Jia, J.; Guo, W.; Zhao, D. RNAi-mediated knockdown of nuclear receptors impairs cuticle formation by disrupting chitin metabolic pathway in Holotrichia oblita. J. Insect Physiol. 2025, 165, 104861. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Pflüger, H.J.; Blenau, W.; Vanden Broeck, J. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 2010, 56, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Roeder, T. The control of metabolic traits by octopamine and tyramine in invertebrates. J. Exp. Biol. 2020, 223, jeb194282. [Google Scholar] [CrossRef]
- Ohta, H.; Ozoe, Y. Molecular signalling, pharmacology, and physiology of octopamine and tyramine receptors as potential insect pest control targets. Adv. Insect Physiol. 2014, 46, 73–166. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. C Toxicol. 2001, 130, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef]
- Ahmed, M.A.I.; Vogel, C.F.; Matsumura, F. Unique biochemical and molecular biological mechanism of synergistic actions of formamidine compounds on selected pyrethroid and neonicotinoid insecticides on the fourth instar larvae of Aedes aegypti (Diptera: Culicidae). Pest Biochem. Physiol. 2015, 120, 57–63. [Google Scholar] [CrossRef]
- Kita, T.; Hayashi, T.; Ohtani, T.; Takao, H.; Takasu, H.; Liu, G.; Ohta, H.; Ozoe, F.; Ozoe, Y. Amitraz and its metabolite differentially activate α- and β-adrenergic-like octopamine receptors. Pest Manag. Sci. 2017, 73, 984–990. [Google Scholar] [CrossRef]
- Monteiro, H.R.; Lemos, M.F.L.; Novais, S.C.; Soares, A.M.V.M.; Pestana, J.L.T. Amitraz toxicity to the midge Chironomus riparius: Life-history and biochemical responses. Chemosphere 2019, 221, 324–332. [Google Scholar] [CrossRef]
- Ahmed, M.I.I.; Vogel, C.F.A. The synergistic effect of octopamine receptor agonists on selected insect growth regulators on Culex quinquefasciatus Say (Diptera: Culicidae) mosquitoes. One Health 2020, 10, 100138. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Z.; Qi, M.; Hu, Z.; Wu, W. Effects of Periplocoside P from Periploca sepium on the Midgut Transmembrane Potential of Mythimna separata Larvae. Sci. Rep. 2016, 6, 36982. [Google Scholar] [CrossRef]
- da Silva Costa, M.; de Paula, S.O.; Martins, G.F.; Zanuncio, J.C.; Santana, A.E.G.; Serrão, J.E. Multiple Modes of Action of the Squamocin in the Midgut Cells of Aedes aegypti Larvae. PLoS ONE 2016, 11, e0160928. [Google Scholar] [CrossRef]
- Vidau, C.; González-Polo, R.A.; Niso-Santano, M.; Gómez-Sánchez, R.; Bravo-San Pedro, J.M.; Pizarro-Estrella, E.; Blasco, R.; Brunet, J.L.; Belzunces, L.P.; Fuentes, J.M. Fipronil is a powerful uncoupler of oxidative phosphorylation that triggers apoptosis in human neuronal cell line SHSY5Y. Neurotoxicology 2011, 32, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Pagadala, V.; Mueller, D.M. Understanding structure, function, and mutations in the mitochondrial ATP synthase. Microb. Cell 2015, 2, 105–125. [Google Scholar] [CrossRef]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial uncoupling: A key controller of biological processes in physiology and diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Che-Mendoza, A.; González-Olvera, G.; Medina-Barreiro, A.; Arisqueta-Chablé, C.; Bibiano-Marin, W.; Correa-Morales, F.; Kirstein, O.D.; Manrique-Saide, P.; Vazquez-Prokopec, G.M. Efficacy of targeted indoor residual spraying with the pyrrole insecticide chlorfenapyr against pyrethroid-resistant Aedes aegypti. PLoS Negl. Trop. Dis. 2021, 15, e0009822. [Google Scholar] [CrossRef]
- Zhou, X.; Lv, H.; Yang, C.; Jiang, D. ROS-Mediated COXI Overexpression by AMPKα Confers Resistance to Dimefluthrin and Cross-Resistance to Indoxacarb in Aedes albopictus. J. Agric. Food Chem. 2025, 73, 16736–16745. [Google Scholar] [CrossRef]
- Remnant, E.J.; Morton, C.J.; Daborn, P.J.; Lumb, C.; Yang, Y.T.; Ng, H.L.; Parker, M.W.; Batterham, P. The Role of Rdl in Resistance to Phenylpyrazoles in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2014, 54, 11–21. [Google Scholar] [CrossRef]
- Gassel, M.; Wolf, C.; Noack, S.; Williams, H.; Ilg, T. The Novel Isoxazoline Ectoparasiticide Fluralaner: Selective Inhibition of Arthropod γ-Aminobutyric Acid- and l-Glutamate-Gated Chloride Channels and Insecticidal/Acaricidal Activity. Insect Biochem. Mol. Biol. 2014, 45, 111–124. [Google Scholar] [CrossRef]
- Nakao, T. Mechanisms of Resistance to Insecticides Targeting RDL GABA Receptors in Planthoppers. Neurotoxicology 2017, 60, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zhou, T.; Zhang, J.; Zhang, L.; Lu, Y.; Huang, J. Functional Validation of A2′N Mutation of the RDL GABA Receptor against Fipronil via Molecular Modeling and Genome Engineering in Drosophila. Pest Manag. Sci. 2024, 80, 1924–1929. [Google Scholar] [CrossRef]
- Xie, N.; Bickley, B.A.; Gross, A.D. GABA-Gated Chloride Channel Mutation (Rdl) Induces Cholinergic Physiological Compensation Resulting in Cross Resistance in Drosophila melanogaster. Pestic. Biochem. Physiol. 2024, 203, 105972. [Google Scholar] [CrossRef]
- Grau-Bové, X.; Tomlinson, S.; O’Reilly, A.O.; Harding, N.J.; Miles, A.; Kwiatkowski, D.; Donnelly, M.J.; Weetman, D.; Anopheles gambiae 1000 Genomes Consortium. Evolution of the Insecticide Target Rdl in African Anopheles Is Driven by Interspecific and Interkaryotypic Introgression. Mol. Biol. Evol. 2020, 37, 2900–2917. [Google Scholar] [CrossRef]
- Xie, N.; Gross, A.D. Muscarinic Acetylcholine Receptor Activation Synergizes the Knockdown and Toxicity of GABA-Gated Chloride Channel Insecticides. Pest Manag. Sci. 2022, 78, 4599–4607. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Bass, C. Does Resistance Really Carry a Fitness Cost? Curr. Opin. Insect Sci. 2017, 21, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gomard, Y.; Alout, H.; Lebon, C.; Latreille, A.; Benlali, A.; Mavingui, P.; Tortosa, P.; Atyame, C. Fitness Costs Associated with a GABA Receptor Mutation Conferring Dieldrin Resistance in Aedes albopictus. Heredity 2022, 129, 273–280. [Google Scholar] [CrossRef]
- Mastrantonio, V.; Porretta, D.; Lucchesi, V.; Güz, N.; Çağatay, N.S.; Bellini, R.; Vontas, J.; Urbanelli, S. Evolution of Adaptive Variation in the Mosquito Culex pipiens: Multiple Independent Origins of Insecticide Resistance Mutations. Insects 2021, 12, 676. [Google Scholar] [CrossRef]
- Hobbs, N.P.; Weetman, D.; Hastings, I.M. Insecticide Resistance Management Strategies for Public Health Control of Mosquitoes Exhibiting Polygenic Resistance: A Comparison of Sequences, Rotations, and Mixtures. Evol. Appl. 2023, 16, 936–959. [Google Scholar] [CrossRef] [PubMed]
- Chamnanya, S.; Kiddela, B.; Saingamsook, J.; Nachaiwieng, W.; Lumjuan, N.; Somboon, P.; Yanola, J. Overexpression of Multiple Cytochrome P450 Genes with and without Knockdown Resistance Mutations Confers High Resistance to Deltamethrin in Culex quinquefasciatus. Infect. Dis. Poverty 2025, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Bryant, R.J. The ABCs of Pesticide Toxicology: Amounts, Biology, and Chemistry. Toxicol. Res. 2017, 6, 755–763. [Google Scholar] [CrossRef]
- Casida, J.E. Pesticide Interactions: Mechanisms, Benefits, and Risks. J. Agric. Food Chem. 2017, 65, 4553–4561. [Google Scholar] [CrossRef] [PubMed]
- Amezian, D.; Nauen, R.; Le Goff, G. Transcriptional Regulation of Xenobiotic Detoxification Genes in Insects—An Overview. Pestic. Biochem. Physiol. 2021, 174, 104822. [Google Scholar] [CrossRef] [PubMed]
- Rivi, M.; Monti, V.; Mazzoni, E.; Cassanelli, S.; Panini, M.; Anaclerio, M.; Cigolini, M.; Corradetti, B.; Bizzaro, D.; Mandrioli, M.; et al. A1-3 Chromosomal Translocations in Italian Populations of the Peach Potato Aphid Myzus persicae (Sulzer) Not Linked to Esterase-Based Insecticide Resistance. Bull. Entomol. Res. 2013, 103, 278–285. [Google Scholar] [CrossRef]
- Lan, W.S.; Cong, J.; Jiang, H.; Jiang, S.R.; Qiao, C.L. Expression and Characterization of Carboxylesterase E4 Gene from Peach–Potato Aphid (Myzus persicae) for Degradation of Carbaryl and Malathion. Biotechnol. Lett. 2005, 27, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.D.; He, W.; Miao, Z.Q.; Tu, Y.Q.; Wang, L.; Dou, W.; Wang, J.J. Characterization of Esterase Genes Involving Malathion Detoxification and Establishment of an RNA Interference Method in Liposcelis bostrychophila. Front. Physiol. 2020, 11, 274. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Xu, K.; Qin, J.; Wang, D.; Xu, L.; Wang, C. Identification and Biochemical Characterization of a Carboxylesterase Gene Associated with β-Cypermethrin Resistance in Dermanyssus gallinae. Poult. Sci. 2024, 103, 103612. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Nayak, B.; Xiong, L.; Xie, C.; Dong, Y.; You, M.; Yuchi, Z.; You, S. The Role of Insect Cytochrome P450s in Mediating Insecticide Resistance. Agriculture 2022, 12, 53. [Google Scholar] [CrossRef]
- Markussen, M.D.K.; Kristensen, M. Cytochrome P450 Monooxygenase-Mediated Neonicotinoid Resistance in the House Fly Musca domestica L. Pestic. Biochem. Physiol. 2010, 98, 50–58. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Battlay, P.; Gledhill-Smith, R.S.; Good, R.T.; Lumb, C.; Fournier-Level, A.; Robin, C. Insights into DDT Resistance from the Drosophila melanogaster Genetic Reference Panel. Genetics 2017, 207, 1181–1193. [Google Scholar] [CrossRef]
- Zimmer, C.T.; Panini, M.; Singh, K.S.; Randall, E.L.; Field, L.M.; Roditakis, E.; Mazzoni, E.; Bass, C. Use of the Synergist Piperonyl Butoxide Can Slow the Development of Alpha-Cypermethrin Resistance in the Whitefly Bemisia tabaci. Insect Mol. Biol. 2017, 26, 152–163. [Google Scholar] [CrossRef]
- Li, P.-R.; Shi, Y.; Ju, D.; Liu, Y.-X.; Wang, W.; He, Y.-S.; Zhang, Y.-Y.; Yang, X.-Q. Metabolic Functional Redundancy of the CYP9A Subfamily Members Leads to P450-Mediated Lambda-Cyhalothrin Resistance in Cydia pomonella. Pest Manag. Sci. 2023, 79, 1452–1466. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, N.; Vontas, J.; Van Leeuwen, T. The Role of Glutathione S-Transferases (GSTs) in Insecticide Resistance in Crop Pests and Disease Vectors. Curr. Opin. Insect Sci. 2018, 27, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Koirala, B.K.S.; Moural, T.; Zhu, F. Functional and Structural Diversity of Insect Glutathione S-Transferases in Xenobiotic Adaptation. Int. J. Biol. Sci. 2022, 18, 5713–5723. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, X.; Deng, M.; Jiang, Y.; Wang, W.; Xu, X.; Li, J.; Zhao, X.; Pan, B.; He, Z.; et al. Unveiling the Functional Contribution of GSTe16 to Pyrethroid Detoxification in Spodoptera litura. Insect Biochem. Mol. Biol. 2025, 182, 104355. [Google Scholar] [CrossRef] [PubMed]
- Kouamo, M.F.M.; Ibrahim, S.S.; Muhammad, A.; Gadji, M.; Hearn, J.; Wondji, C.S. Allelic Variation in a Cluster of Epsilon Glutathione S-Transferase Genes Contributes to DDT and Pyrethroid Resistance in the Major African Malaria Vector Anopheles funestus. BMC Genom. 2025, 26, 452. [Google Scholar] [CrossRef]
- Hu, C.; Liu, J.-Y.; Wang, W.; Mota-Sanchez, D.; He, S.; Shi, Y.; Yang, X.-Q. Glutathione S-Transferase Genes Are Involved in Lambda-Cyhalothrin Resistance in via Sequestration. J. Agric. Food Chem. 2022, 70, 2265–2279. [Google Scholar] [CrossRef]
- Yan, M.-W.; Xing, X.-R.; Wu, F.-A.; Wang, J.; Sheng, S. UDP-Glycosyltransferases Contribute to the Tolerance of Parasitoid Wasps Towards Insecticides. Pestic. Biochem. Physiol. 2021, 174, 104967. [Google Scholar] [CrossRef]
- Du, T.-H.; Yin, C.; Gui, L.-Y.; Liang, J.-J.; Liu, S.-N.; Fu, B.-L.; He, C.; Yang, J.; Wei, X.-G.; Gong, P.-P.; et al. Over-Expression of UDP-Glycosyltransferase UGT353G2 Confers Resistance to Neonicotinoids in Whitefly (Bemisia tabaci). Pestic. Biochem. Physiol. 2023, 196, 105635. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Yang, W.; Zhao, H.; Wang, B.-J.; Shi, Y.; Wang, M.-Y.; Liu, S.-Q.; Liao, X.-L.; Shi, L. Functional Analysis of UDP-Glycosyltransferase Genes Conferring Indoxacarb Resistance in Spodoptera litura. Pestic. Biochem. Physiol. 2023, 196, 105589. [Google Scholar] [CrossRef]
- Al-Yazeedi, T.; Muhammad, A.; Irving, H.; Ahn, S.J.; Hearn, J.; Wondji, C.S. Overexpression and Nonsynonymous Mutations of UDP-Glycosyltransferases Are Potentially Associated with Pyrethroid Resistance in Anopheles funestus. Genomics 2024, 116, 110798. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, X.; Xie, Y.; Zhang, Y.; Huang, Y.; Wu, P.; Lv, J.; Qiu, L. Knocking Out of UDP-Glycosyltransferase Gene UGT2B10 via CRISPR/Cas9 in Helicoverpa armigera Reveals Its Function in Detoxification of Insecticides. J. Agric. Food Chem. 2024, 72, 20862–20871. [Google Scholar] [CrossRef]
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-Binding Cassette (ABC) Transporters: Roles in Xenobiotic Detoxification and Bt Insecticidal Activity. Int. J. Mol. Sci. 2019, 20, 2829. [Google Scholar] [CrossRef]
- Al Khoury, C.; Nemer, N.; Nemer, G. Beauvericin Potentiates the Activity of Pesticides by Neutralizing the ATP-Binding Cassette Transporters in Arthropods. Sci. Rep. 2021, 11, 10865. [Google Scholar] [CrossRef]
- Qiu, Y.-L.; Wu, F.; Zhang, L.; Jiang, H.-Q.; Chen, J.-T.; Pan, Y.-J.; Li, H.-L. A Sublethal Dose of Neonicotinoid Imidacloprid Precisely Sensed and Detoxified by a C-Minus Odorant-Binding Protein 17 Highly Expressed in the Legs of Apis cerana. Sci. Total Environ. 2023, 885, 163762. [Google Scholar] [CrossRef]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond Chemoreception: Diverse Tasks of Soluble Olfactory Proteins in Insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Ingham, V.A.; Anthousi, A.; Douris, V.; Harding, N.J.; Lycett, G.; Morris, M.; Vontas, J.; Ranson, H. A Sensory Appendage Protein Protects Malaria Vectors from Pyrethroids. Nature 2020, 577, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mao, K.; Ren, Z.; Jin, R.; Zhang, Y.; Cai, T.; He, S.; Li, J.; Wan, H. Odorant Binding Protein 3 Is Associated with Nitenpyram and Sulfoxaflor Resistance in Nilaparvata lugens. Int. J. Biol. Macromol. 2022, 209, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liang, Z.; Chen, B. Evidence for the Participation of Chemosensory Proteins in Response to Insecticide Challenge in Conopomorpha sinensis. J. Agric. Food Chem. 2023, 71, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Venthur, H.; Wang, S.; Homem, R.A.; Zhou, J.-J. Evidence for the Involvement of the Chemosensory Protein AgosCSP5 in Resistance to Insecticides in the Cotton Aphid, Aphis gossypii. Insects 2021, 12, 335. [Google Scholar] [CrossRef]
- Pu, J.; Chung, H. New and Emerging Mechanisms of Insecticide Resistance. Curr. Opin. Insect Sci. 2024, 63, 101184. [Google Scholar] [CrossRef]
- Zhu, B.; Li, L.; Wei, R.; Liang, P.; Gao, X. Regulation of GSTu1-Mediated Insecticide Resistance in Plutella xylostella by miRNA and lncRNA. PLoS Genet. 2021, 17, e1009888. [Google Scholar] [CrossRef]
- Kang, J.; Ramirez-Calero, S.; Paula, J.R.; Chen, Y.; Schunter, C. Gene Losses, Parallel Evolution and Heightened Expression Confer Adaptations to Dedicated Cleaning Behaviour. BMC Biol. 2023, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut Symbiont Enhances Insecticide Resistance in a Significant Pest, the Oriental Fruit Fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Soh, L.-S.; Veera Singham, G. Bacterial Symbionts Influence Host Susceptibility to Fenitrothion and Imidacloprid in the Obligate Hematophagous Bed Bug, Cimex hemipterus. Sci. Rep. 2022, 12, 4919. [Google Scholar] [CrossRef]
- Zhao, M.; Lin, X.; Guo, X. The Role of Insect Symbiotic Bacteria in Metabolizing Phytochemicals and Agrochemicals. Insects 2022, 13, 583. [Google Scholar] [CrossRef]
- Sato, Y.; Jang, S.; Takeshita, K.; Itoh, H.; Koike, H.; Tago, K.; Hayatsu, M.; Hori, T.; Kikuchi, Y. Insecticide Resistance by a Host–Symbiont Reciprocal Detoxification. Nat. Commun. 2021, 12, 6432. [Google Scholar] [CrossRef]
- Wei, X.; Peng, H.; Li, Y.; Meng, B.; Wang, S.; Bi, S.; Zhao, X. Pyrethroids Exposure Alters the Community and Function of the Internal Microbiota in Aedes albopictus. Ecotoxicol. Environ. Saf. 2023, 252, 114579. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Huang, Y.Z.; Luan, J.B. Insect–Microbe Symbiosis-Based Strategies Offer a New Avenue for the Management of Insect Pests and Their Transmitted Pathogens. Crop Health 2024, 2, 18. [Google Scholar] [CrossRef]
- Kline, O.; Joshi, N.K. Microbial Symbiont-Based Detoxification of Different Phytotoxins and Synthetic Toxic Chemicals in Insect Pests and Pollinators. J. Xenobiot. 2024, 14, 753–771. [Google Scholar] [CrossRef]
- Liu, X.D.; Guo, H.F. Importance of Endosymbionts Wolbachia and Rickettsia in Insect Resistance Development. Curr. Opin. Insect Sci. 2019, 33, 84–90. [Google Scholar] [CrossRef]
- Ye, Q.-T.; Gong, X.; Liu, H.-H.; Wu, B.-X.; Peng, C.-W.; Hong, X.-Y.; Bing, X.-L. The Symbiont Wolbachia Alleviates Pesticide Susceptibility in the Two-Spotted Spider Mite Tetranychus urticae through Enhanced Host Detoxification Pathways. Insect Sci. 2024, 31, 1822–1837. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, T.; Ren, Z.; Liu, Y.; Yuan, M.; Cai, Y.; Yu, C.; Shu, R.; He, S.; Wong, A.C.N.; et al. Decline in symbiont-dependent host detoxification metabolism contributes to increased insecticide susceptibility of insects under high temperature. ISME J. 2021, 15, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Chen, M.; Yue, L.; Xing, K.; Li, T.; Kang, K.; Liang, Z.; Yuan, L.; Zhang, W. A distinct strain of Arsenophonus symbiont decreases insecticide resistance in its insect host. PLoS Genet. 2018, 14, e1007725. [Google Scholar] [CrossRef] [PubMed]
- Brar, G.; Ngor, L.; McFrederick, Q.S.; Torson, A.S.; Rajamohan, A.; Rinehart, J.; Singh, P.; Bowsher, J.H. High Abundance of Lactobacilli in the Gut Microbiome of Honey Bees During Winter. Sci. Rep. 2025, 15, 7409. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.; Chen, Y.; Wang, S.; Chen, Y.; Hu, F.; Zheng, H. Honey Bee (Apis mellifera) Gut Microbiota Promotes Host Endogenous Detoxification Capability via Regulation of P450 Gene Expression in the Digestive Tract. Microb. Biotechnol. 2020, 13, 1201–1212. [Google Scholar] [CrossRef]
- Zeng, B.; Zhang, F.; Liu, Y.-T.; Wu, S.-F.; Bass, C.; Gao, C.-F. Symbiotic Bacteria Confer Insecticide Resistance by Metabolizing Buprofezin in the Brown Planthopper, Nilaparvata lugens (Stål). PLoS Pathog. 2023, 19, e1011828. [Google Scholar] [CrossRef] [PubMed]
- Fusetto, R.; Denecke, S.; Perry, T.; O’Hair, R.A.J.; Batterham, P. Partitioning the Roles of CYP6G1 and Gut Microbes in the Metabolism of the Insecticide Imidacloprid in Drosophila melanogaster. Sci. Rep. 2017, 7, 11339. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Li, R.; Cheng, S.; Zhang, L.; Liang, P.; Gao, X. The Gut Symbiont Sphingomonas Mediates Imidacloprid Resistance in the Important Agricultural Insect Pest Aphis gossypii Glover. BMC Biol. 2023, 21, 86. [Google Scholar] [CrossRef]
- Zhao, P.; Rensing, C.; Wang, D. Symbiotic Bacteria Modulate Lymantria dispar Immunity by Altering Community Proportions after Infection with LdMNPV. Int. J. Mol. Sci. 2023, 24, 9694. [Google Scholar] [CrossRef]
- Bottino-Rojas, V.; Talyuli, O.A.C.; Carrara, L.; Martins, A.J.; James, A.A.; Oliveira, P.L.; Paiva-Silva, G.O. The redox-sensing gene Nrf2 affects intestinal homeostasis, insecticide resistance, and Zika virus susceptibility in the mosquito Aedes aegypti. J. Biol. Chem. 2018, 293, 9053–9063. [Google Scholar] [CrossRef]
- Feng, H.; Chen, W.; Hussain, S.; Shakir, S.; Tzin, V.; Adegbayi, F.; Ugine, T.; Fei, Z.; Jander, G. Horizontally transferred genes as RNA interference targets for aphid and whitefly control. Plant Biotechnol. J. 2023, 21, 754–768. [Google Scholar] [CrossRef]
- Verster, K.I.; Tarnopol, R.L.; Akalu, S.M.; Whiteman, N.K. Horizontal Transfer of Microbial Toxin Genes to Gall Midge Genomes. Genome Biol. Evol. 2021, 13, evab202. [Google Scholar] [CrossRef]
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W.; et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705.e17. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Jing, W.; Xu, S.; Jin, Y.; Xu, Y.; Wang, H. Horizontally acquired cysteine synthase genes undergo functional divergence in lepidopteran herbivores. Heredity 2021, 127, 21–34. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Chen, Y.; Chen, X.; Huang, J.; Rokas, A.; Shen, X. How Has Horizontal Gene Transfer Shaped the Evolution of Insect Genomes? Environ. Microbiol. 2023, 25, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Suhag, A.; Jaiwal, R.; Chaudhary, D.; Jaiwal, P.K. Current progress and challenges of horizontal gene transfers in whiteflies (Bemisia tabaci) for their sustainable management. J. Asia-Pac. Entomol. 2024, 27, 102216. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, T.; Yuan, M.; Li, Z.; Jin, R.; Ren, Z.; Qin, Y.; Yu, C.; Cai, Y.; Shu, R.; et al. Microbiome variation correlates with the insecticide susceptibility in different geographic strains of a significant agricultural pest, Nilaparvata lugens. npj Biofilms Microbiomes 2023, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.; Furlong, M. Behavior as a Mechanism of Insecticide Resistance: Evaluation of the Evidence. Curr. Opin. Insect Sci. 2017, 21, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Wang, Z.; Chung, H. Climate Change and the Genetics of Insecticide Resistance. Pest Manag. Sci. 2020, 76, 846–852. [Google Scholar] [CrossRef]
- Quintela, V.; Pereira, D.L.; Langa, T.P.; de Oliveira, M.; Siqueira, H.A.A. Field-evolved resistance in Phthorimaea absoluta to abamectin: Genetic foundations, female-linked traits, and cross-resistance pattern. Pest Manag. Sci. 2025, 81, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Thiesen, L.V.; Gonçalves, G.C.; Guidolin, A.S.; Nascimento, A.R.B.; Coutinho, E.F.; Borba, J.P.; Picelli, E.C.M.; Omoto, C. Characterization of cyantraniliprole resistance in Spodoptera frugiperda: Selection, inheritance pattern, and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2025, 81, 4677–4689. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, T.; Chethan, B.R.; Mani, M. Insecticide Resistance and Its Management in the Insect Pests of Horticultural Crops. In Trends in Horticultural Entomology; Mani, M., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Gul, H.; Gadratagi, B.G.; Güncan, A.; Tyagi, S.; Ullah, F.; Desneux, N.; Liu, X. Fitness costs of resistance to insecticides in insects. Front. Physiol. 2023, 14, 1238111. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, H.; Hu, S.; Ren, M.; Wei, Q.; Tian, X.; Elzaki, M.E.A.; Bass, C.; Su, J.; Palli, S.R. Changes in Both Trans- and Cis-Regulatory Elements Mediate Insecticide Resistance in a Lepidopteron Pest, Spodoptera exigua. PLoS Genet. 2021, 17, e1009403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cen, Y.; Liu, Y.; Peng, Y.; Lin, Y.; Feng, Q.; Xiao, Y.; Zheng, S. P450 gene CYP321A8 is responsible for cross-resistance of insecticides in field populations of Spodoptera frugiperda. Insect Sci. 2025, 32, 227–242. [Google Scholar] [CrossRef]
- Zuo, Y.; Pei, Y.; Li, Y.; Wen, S.; Ren, X.; Li, L.; Wu, Y.; Hu, Z. The Synergism Between Metabolic and Target-Site Resistance Enhances the Intensity of Resistance to Pyrethroids in Spodoptera exigua. Insect Biochem. Mol. Biol. 2025, 180, 104313. [Google Scholar] [CrossRef]
- Li, W.; Yang, W.; Shi, Y.; Yang, X.; Liu, S.; Liao, X.; Shi, L. Comprehensive analysis of the overexpressed cytochrome P450-based insecticide resistance mechanism in Spodoptera litura. J. Hazard. Mater. 2024, 461, 132605. [Google Scholar] [CrossRef]
- Qian, C.; Li, J.; Wu, S.; Yang, Y.; Wu, Y.; Wang, X. Cross-Resistance and Genetics of Field-Evolved Resistance to Chlorfenapyr in Plutella xylostella. Insect Sci. 2024, 31, 533–541. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, H.; Chai, R.; Fan, M.; Niu, Z.; Sun, G.; Yan, S.; Jiang, D. Cd exposure confers β-cypermethrin tolerance in Lymantria dispar by activating the ROS/CnCC signaling pathway-mediated P450 detoxification. J. Hazard. Mater. 2024, 478, 135566. [Google Scholar] [CrossRef]
- Yan, S.; Tan, M.; Zhang, A.; Jiang, D. The Exposure Risk of Heavy Metals to Insect Pests and Their Impact on Pests Occurrence and Cross-Tolerance to Insecticides: A Review. Sci. Total Environ. 2024, 916, 170274. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Guo, Z.; Tang, J.; Ling, S.; Zheng, C.; Bass, C.; He, S.; Wan, H.; Li, J.; Ma, K. Upregulation of a cytochrome P450 gene, CYP6B50, confers multi-insecticide resistance in Spodoptera frugiperda. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Kiuru, C.; Constantino, L.; Cole, G.; Karisa, J.; Wanjiku, C.; Okoko, M.; Candrinho, B.; Saute, F.; Rabinovich, N.R.; Chaccour, C.; et al. Multiple insecticide resistance in Anopheles funestus from Mopeia, Central Mozambique. Malar. J. 2025, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Madgwick, P.G.; Slater, R.; Kanitz, R. The evolution of pesticide resistance: A data-driven case study of chlorantraniliprole resistance in Chilo suppressalis and other Lepidopteran pests in China. Evol. Appl. 2025, 18, e70131. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, S.; Liu, C.; Hu, W.K.; Liu, J.W.; Zheng, L.J.; Gao, M.Y.; Guo, F.R.; Qiao, S.T.; Liu, J.L.; et al. Risk assessment, fitness cost, cross-resistance, and mechanism of tetraniliprole resistance in the rice stem borer, Chilo suppressalis. Insect Sci. 2024, 31, 835–846. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Clark, D.A.; Bolgunas, S.P.; Lahm, G.P.; Gutteridge, S.; Rhoades, D.F.; Wu, L.; Sopa, J.S.; Rauh, J.J.; et al. Pyrrole-2 Carboxamides—A Novel Class of Insect Ryanodine Receptor Activators. Pestic. Biochem. Physiol. 2021, 174, 104798. [Google Scholar] [CrossRef]
- Lin, P.-X.; Peng, Y.-X.; Xing, J.-Y.; Liu, Z.-Y.; Guo, F.-R.; Thia, J.A.; Gao, C.-F.; Wu, S.-F. Cis-Regulation of the CYP6CS1 Gene and Its Role in Mediating Cross-Resistance in a Pymetrozine-Resistant Strain of Nilaparvata lugens. Insect Biochem. Mol. Biol. 2025, 177, 104261. [Google Scholar] [CrossRef]
- Lv, M.; Wang, W.; Fang, F.; Fu, X.; Liang, G. The Changes in Cross-Resistance, Fitness, and Feeding Behavior in Aphis gossypii as Their Resistance to Sulfoxaflor Declines. Insects 2024, 15, 920. [Google Scholar] [CrossRef]
- Qu, C.; Yao, J.; Huang, J.; Che, W.; Fang, Y.; Luo, C.; Wang, R. Tetraniliprole Resistance in Field-Collected Populations of Tuta absoluta (Lepidoptera: Gelechiidae) from China: Baseline Susceptibility, Cross-Resistance, Inheritance, and Biochemical Mechanism. Pestic. Biochem. Physiol. 2024, 203, 106019. [Google Scholar] [CrossRef]
- Madgwick, P.G.; Kanitz, R. What Is the Value of Rotations to Insecticide Resistance Management? Pest Manag. Sci. 2024, 80, 1671–1680. [Google Scholar] [CrossRef]
- Hu, B.; Xing, Z.; Dong, H.; Chen, X.; Ren, M.; Liu, K.; Rao, C.; Tan, A.; Su, J. Cytochrome P450 CYP6AE70 Confers Resistance to Multiple Insecticides in a Lepidopteran Pest, Spodoptera exigua. J. Agric. Food Chem. 2024, 72, 23141–23150. [Google Scholar] [CrossRef] [PubMed]
- Narva, K.; Toprak, U.; Alyokhin, A.; Groves, R.; Jurat-Fuentes, J.L.; Moar, W.; Nauen, R.; Whipple, S.; Head, G. Insecticide Resistance Management Scenarios Differ for RNA-Based Sprays and Traits. Insect Mol. Biol. 2025, in press. [Google Scholar] [CrossRef]
- Ullah, F.; Guru-Pirasanna-Pandi, G.; Murtaza, G.; Sarangi, S.; Gul, H.; Li, X.; Chavarín-Gómez, L.E.; Ramírez-Romero, R.; Guedes, R.N.C.; Desneux, N.; et al. Evolving strategies in agroecosystem pest control: Transitioning from chemical to green management. J. Pest Sci. 2025, 1–18. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, M.; Shad, S.A. Genetic Analysis of Chlorantraniliprole Resistance in the Non-Target Bio-Control Agent Trichogramma chilonis. Chemosphere 2025, 370, 143952. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.B.S.; Ijaz, M.; Abbas, N.; Shad, S.A.; Serrão, J.E. Resistance of Lepidopteran Pests to Bacillus thuringiensis Toxins: Evidence of Field and Laboratory Evolved Resistance and Cross-Resistance, Mode of Resistance Inheritance, Fitness Costs, Mechanisms Involved and Management Options. Toxins 2024, 16, 315. [Google Scholar] [CrossRef]
- Anderson, J.A.; Ellsworth, P.C.; Faria, J.C.; Head, G.P.; Owen, M.D.K.; Pilcher, C.D.; Shelton, A.M.; Meissle, M. Genetically Engineered Crops: Importance of Diversified Integrated Pest Management for Agricultural Sustainability. Front. Bioeng. Biotechnol. 2019, 7, 24. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Reisig, D.D. Management of Insect Pests with Bt Crops in the United States. Annu. Rev. Entomol. 2023, 68, 31–49. [Google Scholar] [CrossRef]
- Downes, S.; Kriticos, D.; Parry, H.; Paull, C.; Schellhorn, N.; Zalucki, M.P. A perspective on management of Helicoverpa armigera: Transgenic Bt cotton, IPM, and landscapes. Pest Manag. Sci. 2017, 73, 485–492. [Google Scholar] [CrossRef]
- Suh, P.F.; Elanga-Ndille, E.; Tchouakui, M.; Sandeu, M.M.; Tagne, D.; Wondji, C.; Ndo, C. Impact of Insecticide Resistance on Malaria Vector Competence: A Literature Review. Malar. J. 2023, 22, 19. [Google Scholar] [CrossRef]
- Demissie, D.B.; Fetensa, G.; Desta, T.; Tiyare, F.T. Effectiveness and Efficacy of Long-Lasting Insecticidal Nets for Malaria Control in Africa: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2025, 22, 1045. [Google Scholar] [CrossRef]
- Kouassi, B.L.; Edi, C.; Tia, E.; Konan, L.Y.; Akré, M.A.; Koffi, A.A.; Ouattara, A.F.; Tanoh, A.M.; Zinzindohoue, P.; Kouadio, B.; et al. Susceptibility of Anopheles gambiae from Côte d’Ivoire to insecticides used on insecticide-treated nets: Evaluating the additional entomological impact of piperonyl butoxide and chlorfenapyr. Malar. J. 2020, 19, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Wu, K. Function and Effectiveness of Natural Refuge in IRM Strategies for Bt Crops. Curr. Opin. Insect Sci. 2017, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dively, G.P.; Huang, F.; Oyediran, I.; Burd, T.; Morsello, S. Evaluation of Gene Flow in Structured and Seed Blend Refuge Systems of Non-Bt and Bt Corn. J. Pest Sci. 2020, 93, 439–447. [Google Scholar] [CrossRef]
- Zhu, F.; Lavine, L.; O’Neal, S.; Lavine, M.; Foss, C.; Walsh, D. Insecticide Resistance and Management Strategies in Urban Ecosystems. Insects 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, L.M. Matching Landscapes and Managing Habitats to Enhance Classical Biological Control of Arthropods. Biol. Control 2022, 169, 104896. [Google Scholar] [CrossRef]
- Hatt, S.; Döring, T.F. Designing Pest Suppressive Agroecosystems: Principles for an Integrative Diversification Science. J. Clean. Prod. 2023, 432, 139701. [Google Scholar] [CrossRef]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016. Available online: https://apps.who.int/iris/handle/10665/250677 (accessed on 4 August 2025).
- Chen, L.; Lang, K.; Mei, Y.; Shi, Z.; He, K.; Li, F.; Xiao, H.; Ye, G.; Han, Z. FastD: Fast Detection of Insecticide Target-Site Mutations and Overexpressed Detoxification Genes in Insect Populations from RNA-Seq Data. Ecol. Evol. 2020, 10, 14346–14358. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Zhang, J.; Shen, H.; Wang, X.; Padovan, A.; Walsh, T.K.; Tay, W.T.; Gordon, K.H.J.; James, W.; Czepak, C.; et al. Whole-Genome Sequencing to Detect Mutations Associated with Resistance to Insecticides and Bt Proteins in Spodoptera frugiperda. Insect Sci. 2021, 28, 627–638. [Google Scholar] [CrossRef]
- Mertz, R.W.; Dressel, A.E.; Fisher, C.R.; Moon, R.D.; Donahue, W.A.; Kasai, S.; Scott, J.G. Frequencies and Distribution of Kdr and Ace Alleles that Cause Insecticide Resistance in House Flies in the United States. Pestic. Biochem. Physiol. 2023, 194, 105497. [Google Scholar] [CrossRef]
- Chang, C.-C.; Dai, S.-M.; Chen, C.-Y.; Huang, L.-H.; Chen, Y.-H.; Hsu, J.-C. Insecticide Resistance and Characteristics of Mutations Related to Target Site Insensitivity of Diamondback Moths in Taiwan. Pestic. Biochem. Physiol. 2024, 203, 106001. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, H.C.; Klein, T.A.; Jun, H.; Choi, K.S. Insecticide Resistance Mutations of Anopheles Species in the Republic of Korea. PLoS Negl. Trop. Dis. 2025, 19, e0012748. [Google Scholar] [CrossRef]
- Helps, J.C.; Paveley, N.D.; White, S.; van den Bosch, F. Determinants of Optimal Insecticide Resistance Management Strategies. J. Theor. Biol. 2020, 503, 110383. [Google Scholar] [CrossRef]
- Willow, J.; Cook, S.M.; Veromann, E.; Smagghe, G. Uniting RNAi Technology and Conservation Biocontrol to Promote Global Food Security and Agrobiodiversity. Front. Bioeng. Biotechnol. 2022, 10, 871651. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Zhou, A.; Zhang, J.; Ge, X.; Moussian, B.; Yan, C.; Gao, S.; Wang, Y. Trends and Emerging Hotspots in RNAi-Based Arthropod Pest Control: A Comprehensive Bibliometric Analysis. J. Insect Physiol. 2025, 161, 104754. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, X.; Zhang, J.; Xu, J.; Qin, M.; Cao, M.; Francis, F.; Xia, L. RNAi Technologies for Insect Control in Crop Protection. Crop J. 2025, in press. [Google Scholar] [CrossRef]
- Mnzava, A.P.; Knox, T.B.; Temu, E.A.; Trett, A.; Fornadel, C.; Hemingway, J.; Renshaw, M. Implementation of the Global Plan for Insecticide Resistance Management in Malaria Vectors: Progress, Challenges and the Way Forward. Malar. J. 2015, 14, 173. [Google Scholar] [CrossRef]
- IRAC Group 30 Task Team. Insecticide Resistance Management Guidelines: IRAC Group 30 Insecticides. Version 3.1; Updated 15 December 2021 & 16 February 2022. Available online: https://irac-online.org/documents/irac-moa-group-30-irm-guidelines/ (accessed on 5 August 2025).
- Food and Agriculture Organization of the United Nations. FAO Guidelines on Prevention and Management of Pesticide Resistance; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; Available online: https://openknowledge.fao.org/items/64bb88d3-2a15-4f23-b94e-d25a8777ae5b (accessed on 5 August 2025).
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO Maize against Western Corn Rootworm and Northern Corn Rootworm: Efficacy and Resistance Management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Narva, K.; Otto, E.; Sridharan, K.; Flannagan, R.; Barnes, E.; Mézin, L.; Manley, B. Calantha™: The First Commercialized Sprayable dsRNA Product for Insect Control. In RNA Interference in Agriculture: Basic Science to Applications; Smagghe, G., Palli, S.R., Swevers, L., Eds.; Springer: Cham, Switzerland, 2025; pp. 679–715. [Google Scholar] [CrossRef]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental Fate and Dissipation of Applied dsRNA in Soil, Aquatic Systems, and Plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Merkel, L.; Amari, K. Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment. Int. J. Mol. Sci. 2024, 25, 6530. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, C.; Ivashuta, S.; Wiggins, E.; Flagel, L.; Moar, W.; Pleau, M.; Miller, K.; Zhang, Y.; Ramaseshadri, P.; Jiang, C.; et al. Development and characterization of the first dsRNA-resistant insect population from western corn rootworm, Diabrotica virgifera virgifera LeConte. PLoS ONE 2018, 13, e0197059. [Google Scholar] [CrossRef]
- Mishra, S.; Moar, W.; Jurat-Fuentes, J.L. Larvae of Colorado Potato Beetle (Leptinotarsa decemlineata Say) Resistant to Double-Stranded RNA (dsRNA) Remain Susceptible to Small-Molecule Pesticides. Pest Manag. Sci. 2024, 80, 905–909. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Zhang, J. Characterization and Potential Mechanism of Resistance to Double-Stranded RNA in Willow Leaf Beetle, Plagiodera versicolora. J. Pest Sci. 2024, 97, 2217–2226. [Google Scholar] [CrossRef]
- Pinto, M.M.D.; Ferreira dos Santos, R.; De Bortoli, S.A.; Moar, W.; Jurat-Fuentes, J.L. Lack of Fitness Costs in dsRNA-Resistant Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2023, 116, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- EPA FIFRA Scientific Advisory Panel. Subpanel on Bacillus thuringiensis (Bt) Plant-Pesticides and Resistance Management; U.S. Environmental Protection Agency: Washington, DC, USA, 1998. Available online: https://archive.epa.gov/scipoly/sap/meetings/web/pdf/finalfeb.pdf (accessed on 4 August 2025).
- EPA FIFRA Scientific Advisory Panel. Sets of Scientific Issues Being Considered by the Environmental Protection Agency Regarding Bt Plant-Pesticides Risk and Benefit Assessments; U.S. Environmental Protection Agency: Washington, DC, USA, 2001. Available online: https://archive.epa.gov/scipoly/sap/meetings/web/pdf/octoberfinal.pdf (accessed on 4 August 2025).
- Swevers, L.; Palli, S.R.; Smagghe, G. The Road to RNAi-Based Pest Control: A Historical Perspective. In RNA Interference in Agriculture: Basic Science to Applications; Smagghe, G., Palli, S.R., Swevers, L., Eds.; Springer: Cham, Switzerland, 2025; pp. 1–23. [Google Scholar] [CrossRef]
- Zulfa, R.; Lo, W.-C.; Cheng, P.-C.; Martini, M.; Chuang, T.-W. Updating the Insecticide Resistance Status of Aedes aegypti and Aedes albopictus in Asia: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 306. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.-A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The Evolutionary Origins of Pesticide Resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef]
- Weill, M.; Malcolm, C.; Chandre, F.; Mogensen, K.; Berthomieu, A.; Marquine, M.; Raymond, M. The Unique Mutation in ace-1 Giving High Insecticide Resistance is Easily Detectable in Mosquito Vectors. Insect Mol. Biol. 2004, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Mori, A.; Kozaki, T.; Iwata, Y.; Hidoh, O.; Harada, S.; Kasai, S.; Severson, D.W.; Kono, Y.; Tomita, T. An Amino Acid Substitution Attributable to Insecticide-Insensitivity of Acetylcholinesterase in a Japanese Encephalitis Vector Mosquito, Culex tritaeniorhynchus. Biochem. Biophys. Res. Commun. 2004, 313, 794–801. [Google Scholar] [CrossRef]
- Alout, H.; Berthomieu, A.; Cui, F.; Tan, Y.; Berticat, C.; Qiao, C.; Weill, M. Different Amino-Acid Substitutions Confer Insecticide Resistance through Acetylcholinesterase 1 Insensitivity in Culex vishnui and Culex tritaeniorhynchus (Diptera: Culicidae) from China. J. Med. Entomol. 2007, 44, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Rodriguez, K.; Urdaneta-Marquez, L.; Rajatileka, S.; Moulton, M.; Flores, A.E.; Fernandez-Salas, I.; Bisset, J.; Rodriguez, M.; McCall, P.J.; Donnelly, M.J.; et al. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol. Biol. 2007, 16, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Nomura, Y.; Satar, G.; Hu, Z.; Nauen, R.; He, S.Y.; Zhorov, B.S.; Dong, K. Molecular Evidence for Dual Pyrethroid-Receptor Sites on a Mosquito Sodium Channel. Proc. Natl. Acad. Sci. USA 2013, 110, 11785–11790. [Google Scholar] [CrossRef]
- Yanola, J.; Somboon, P.; Walton, C.; Nachaiwieng, W.; Somwang, P.; Prapanthadara, L. High-Throughput Assays for Detection of the F1534C Mutation in the Voltage-Gated Sodium Channel Gene in Permethrin-Resistant Aedes aegypti and the Distribution of This Mutation throughout Thailand. Trop. Med. Int. Health 2011, 16, 501–509. [Google Scholar] [CrossRef]
- Harris, A.F.; Rajatileka, S.; Ranson, H. Pyrethroid Resistance in Aedes aegypti from Grand Cayman. Am. J. Trop. Med. Hyg. 2010, 83, 277–284. [Google Scholar] [CrossRef]
- Hu, Z.; Du, Y.; Nomura, Y.; Dong, K. A Sodium Channel Mutation Identified in Aedes aegypti Selectively Re-duces Cockroach Sodium Channel Sensitivity to Type I, but Not Type II Pyrethroids. Insect Biochem. Mol. Biol. 2011, 41, 9–13. [Google Scholar] [CrossRef]
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a New Pyrethroid Resistance Mutation (V410L) in the Sodium Channel of Aedes aegypti: A Potential Challenge for Mosquito Control. Sci. Rep. 2017, 7, 46549. [Google Scholar] [CrossRef]
- Saavedra-Rodriguez, K.; Maloof, F.V.; Campbell, C.L.; Garcia-Rejon, J.; Lenhart, A.; Penilla, P.; Rodriguez, A.; Sandoval, A.A.; Flores, A.E.; Ponce, G.; et al. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico. Sci. Rep. 2018, 8, 6747. [Google Scholar] [CrossRef] [PubMed]
- Poupardin, R.; Srisukontarat, W.; Yunta, C.; Ranson, H. Identification of Carboxylesterase Genes Implicated in Temephos Resistance in the Dengue Vector Aedes aegypti. PLoS Negl. Trop. Dis. 2014, 8, e2743. [Google Scholar] [CrossRef]
- Asgarian, T.S.; Vatandoost, H.; Hanafi-Bojd, A.A.; Nikpoor, F. Worldwide Status of Insecticide Resistance of Aedes aegypti and Ae. albopictus, Vectors of Arboviruses of Chikungunya, Dengue, Zika and Yellow Fever. J. Arthropod Borne Dis. 2023, 17, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Nurul-Nastasea, S.; Yu, K.-X.; Ahmad, R.; Mohamed Nor, Z.; Tengku Idzzan Nadzirah, T.-I.; Roza, D.; Masse Rezki, S.; Wan Mohamad Ali, W.N. Insecticide Resistance Status of Aedes aegypti and Aedes albopictus in Malaysia (2010 to 2022): A Review. Asian Pac. J. Trop. Med. 2023, 16, 434–445. [Google Scholar] [CrossRef]
- Chandor-Proust, A.; Bibby, J.; Régent-Kloeckner, M.; Roux, J.; Guittard-Crilat, E.; Poupardin, R.; Riaz, M.A.; Paine, M.; Dauphin-Villemant, C.; Reynaud, S.; et al. The Central Role of Mosquito Cytochrome P450 CYP6Zs in Insecticide Detoxification Revealed by Functional Expression and Structural Modelling. Biochem. J. 2013, 455, 75–85. [Google Scholar] [CrossRef]
- Rocha dos Santos, C.; Rodovalho, C.M.; Jablonka, W.; Martins, A.J.; Pereira Lima, J.B.; dos Santos Dias, L.; da Silva Neto, M.A.C.; Atella, G.C. Insecticide Resistance, Fitness and Susceptibility to Zika Infection of an Interbred Aedes aegypti Population from Rio de Janeiro, Brazil. Parasites Vectors 2020, 13, 496. [Google Scholar] [CrossRef]
- Vera-Maloof, F.Z.; Saavedra-Rodriguez, K.; Penilla-Navarro, R.P.; Rodriguez-Ramirez, A.D.; Dzul, F.; Manrique-Saide, P.; Black, W.C., IV. Loss of pyrethroid resistance in newly established laboratory colonies of Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0007753. [Google Scholar] [CrossRef]
- Smith, L.B.; Silva, J.J.; Chen, C.; Harrington, L.C.; Scott, J.G. Fitness Costs of Individual and Combined Pyrethroid Resistance Mechanisms, kdr and CYP-Mediated Detoxification, in Aedes aegypti. PLoS Negl. Trop. Dis. 2021, 15, e0009271. [Google Scholar] [CrossRef]
- Fisher, C.R.; Dressel, A.E.; Silva, J.J.; Scott, J.G. A Globally Distributed Insecticide Resistance Allele Confers a Fitness Cost in the Absence of Insecticide in Aedes aegypti (Diptera: Culicidae), the Yellow Fever Mosquito. J. Med. Entomol. 2023, 60, 494–499. [Google Scholar] [CrossRef]
- Diniz, D.F.A.; de Melo-Santos, M.A.V.; Santos, E.M.M.; Beserra, E.B.; Helvecio, E.; de Carvalho-Leandro, D.; dos Santos, B.S.; de Menezes Lima, V.L.; Ayres, C.F.J. Fitness Cost in Field and Laboratory Aedes aegypti Populations Associated with Resistance to the Insecticide Temephos. Parasites Vectors 2015, 8, 662. [Google Scholar] [CrossRef]
- Penilla-Navarro, P.; Solis-Santoyo, F.; Lopez-Solis, A.; Rodriguez, A.D.; Vera-Maloof, F.; Lozano, S.; Contreras-Mejía, E.; Vázquez-Samayoa, G.; Torreblanca-Lopez, R.; Perera, R.; et al. Pyrethroid Susceptibility Reversal in Aedes aegypti: A Longitudinal Study in Tapachula, Mexico. PLoS Negl. Trop. Dis. 2024, 18, e0011369. [Google Scholar] [CrossRef]
- Rigby, L.M.; Rašić, G.; Peatey, C.L.; Hugo, L.E.; Beebe, N.W.; Devine, G.J. Identifying the Fitness Costs of a Pyrethroid-Resistant Genotype in the Major Arboviral Vector Aedes aegypti. Parasites Vectors 2020, 13, 358. [Google Scholar] [CrossRef]
- Vinauger, C.; Chandrasegaran, K. Context-Specific Variation in Life History Traits and Behavior of Aedes aegypti Mosquitoes. Front. Insect Sci. 2024, 4, 1426715. [Google Scholar] [CrossRef] [PubMed]
- Silalahi, C.N.; Yasin, A.; Chen, M.E.; Ahmad, I.; Neoh, K.B. Behavioral Responses and Life History Traits of Taiwanese and Indonesian Populations of Aedes aegypti Surviving Deltamethrin-Clothianidin Treatment. Parasites Vectors 2024, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti Vector Competence Studies: A Review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Smartt, C.T.; Shin, D. Permethrin Resistance in Aedes aegypti Affects Aspects of Vectorial Capacity. Insects 2021, 12, 71. [Google Scholar] [CrossRef]
- Deng, J.; Guo, Y.; Su, X.; Liu, S.; Yang, W.; Wu, Y.; Wu, K.; Yan, G.; Chen, X.G. Impact of deltamethrin-resistance in Aedes albopictus on its fitness cost and vector competence. PLoS Negl. Trop. Dis. 2021, 15, e0009391. [Google Scholar] [CrossRef] [PubMed]
- Tantowijoyo, W.; Tanamas, S.K.; Nurhayati, I.; Setyawan, S.; Budiwati, N.; Fitriana, I.; Ernesia, I.; Wardana, D.S.; Supriyati, E.; Arguni, E.; et al. Aedes aegypti Abundance and Insecticide Resistance Profiles in the Applying Wolbachia to Eliminate Dengue Trial. PLoS Negl. Trop. Dis. 2022, 16, e0010284. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary Status of Insecticide Resistance in the Major Aedes Vectors of Arboviruses Infecting Humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Beins, K.; Costa, D.N.; Coelho, G.E.; Bezerra, H.S.S. Patterns of Insecticide Resistance in Aedes aegypti: Meta-Analyses of Surveys in Latin America and the Caribbean. Pest Manag. Sci. 2020, 76, 2144–2157. [Google Scholar] [CrossRef]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent Trends in Global Insecticide Use for Disease Vector Control and Potential Implications for Resistance Management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef]
- Zheng, C.; Li, S.; Wu, M.; Li, J.; Ma, K.; You, H. Insecticide Binding Mode Analysis and Biological Effects of Acetylcholinesterase Target-Site Resistance Mutations in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 205, 106164. [Google Scholar] [CrossRef]
- Saladini di Rovetino, M.; Lueke, B.; Masawang, K.; Piyasaengthong, N.; Kaewwongse, M.; Nobsathian, S.; Fricaux, T.; Nam, K.; d’Alençon, E.; Bullangpoti, V.; et al. Monitoring the Molecular Mechanisms of Insecticide Resistance in Spodoptera frugiperda Populations from Thailand. Pestic. Biochem. Physiol. 2025, 214, 106599. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Khalid, M.Z.; Jiang, W.; Zhong, G. An Overview of Insecticide Resistance Mechanisms, Challenges, and Management Strategies in Spodoptera frugiperda. Crop Prot. 2025, 197, 107322. [Google Scholar] [CrossRef]
- Chen, Y.; Nguyen, D.T.; Spafford, H.; Herron, G.A. A High-Throughput Multilocus-Amplicon Sequencing Panel to Monitor Insecticide Resistance in Fall Armyworm (Spodoptera frugiperda) (Lepidoptera: Noctuidae). Pest Manag. Sci. 2024, 80, 1510–1522. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; El-Said, N.A.; Alfuhaid, N.A.; Abo-Elinin, F.M.A.; Mohamed, R.M.B.; Aioub, A.A.A. Monitoring and Detection of Insecticide Resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae): Evidence for Field-Evolved Resistance in Egypt. Insects 2024, 15, 705. [Google Scholar] [CrossRef]
- Samanta, S.; Barman, M.; Thakur, H.; Chakraborty, S.; Upadhyaya, G.; Roy, D.; Banerjee, A.; Samanta, A.; Tarafdar, J. Evidence of Population Expansion and Insecticide Resistance Mechanism in Invasive Fall Armyworm (Spodoptera frugiperda). BMC Biotechnol. 2023, 23, 17. [Google Scholar] [CrossRef]
- Bolzan, A.; Padovez, F.E.O.; Nascimento, A.R.B.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.A.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and inheritance of resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef]
- Okuma, D.M.; Cuenca, A.; Nauen, R.; Omoto, C. Large-Scale Monitoring of the Frequency of Ryanodine Receptor Target-Site Mutations Conferring Diamide Resistance in Brazilian Field Populations of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, K.; Evangelou, V.; Grigoriadou, A.M.; Papachristos, D.P.; Vontas, J. Molecular Surveillance of Resistance Mutations in Invasive Populations of Spodoptera frugiperda in Europe, for Evidence-Based Pest Control. Pest Manag. Sci. 2025, 81, 4821–4830. [Google Scholar] [CrossRef]
- Barbosa, M.G.; André, T.P.P.; Pontes, A.D.S.; Souza, S.A.; Oliveira, N.R.X.; Pastori, P.L. Insecticide Rotation and Adaptive Fitness Cost Underlying Insecticide Resistance Management for Spodoptera frugiperda (Lepidoptera: Noctuidae). Neotrop. Entomol. 2020, 49, 882–892. [Google Scholar] [CrossRef]
- Kafle, L.; Joshi, R. Fall Armyworm Threatens Asian Rice Security: A Review of Sustainable Management Strategies. CABI Rev. 2025, 20, 0017. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Akutse, K.S.; Amalin, D.M.; Araj, S.-E.; Barrera, G.; Beltran, M.J.B.; Ben Fekih, I.; Calatayud, P.-A.; Cicero, L.; Cokola, M.C.; et al. Global Scientific Progress and Shortfalls in Biological Control of the Fall Armyworm Spodoptera frugiperda. Biol. Control 2024, 191, 105460. [Google Scholar] [CrossRef]
- Idrees, A.; Afzal, A.; Qadir, Z.A.; Li, J. Bioassays of Beauveria bassiana Isolates against the Fall Armyworm, Spodoptera frugiperda. J. Fungi 2022, 8, 717. [Google Scholar] [CrossRef]
- Ratto, F.; Bruce, T.; Chipabika, G.; Mwamakamba, S.; Mkandawire, R.; Khan, Z.; Mkindi, A.; Pittchar, J.; Sallu, S.M.; Whitfield, S.; et al. Biological control interventions reduce pest abundance and crop damage while maintaining natural enemies in sub-Saharan Africa: A meta-analysis. Proc. Biol. Sci. 2022, 289, 20221695. [Google Scholar] [CrossRef]
- Midega, C.A.O.; Pittchar, J.O.; Pickett, J.A.; Hailu, G.W.; Khan, Z.R. A climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (J E Smith), in maize in East Africa. Crop Protect. 2018, 105, 10–15. [Google Scholar] [CrossRef]
- Kinyanjui, G.; Mawcha, K.T.; Malinga, L.N.; Soobramoney, K.; Ṋethononda, P.; Assefa, Y.; Okonkwo, C.O.; Ndolo, D. Managing African Armyworm Outbreaks in Sub-Saharan Africa: Current Strategies and Future Directions. Insects 2025, 16, 645. [Google Scholar] [CrossRef]
- Yin, J.; Su, X.; Yan, S.; Shen, J. Multifunctional Nanoparticles and Nanopesticides in Agricultural Application. Nanomaterials 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Ataide, L.M.S.; Vargas, G.; Velazquez-Hernandez, Y.; Reyes-Arauz, I.; Villamarin, P.; Canon, M.A.; Yang, X.; Riley, S.S.; Revynthi, A.M. Efficacy of Conventional and Biorational Insecticides against the Invasive Pest Thrips parvispinus (Thysanoptera: Thripidae) under Containment Conditions. Insects 2024, 15, 48. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Achal, V. A Comprehensive Review on Environmental and Human Health Impacts of Chemical Pesticide Usage. Emerg. Contam. 2025, 11, 100410. [Google Scholar] [CrossRef]
- Li, F.; Xiong, W.; Zhang, C.; Wang, D.; Zhou, C.; Li, W.; Zeng, G.; Song, B.; Zeng, Z. Neonicotinoid insecticides in non-target organisms: Occurrence, exposure, toxicity, and human health risks. J. Environ. Manag. 2025, 383, 125432. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Paxton, R.J. The Novel Insecticides Flupyradifurone and Sulfoxaflor Do Not Act Synergistically with Viral Pathogens in Reducing Honey Bee (Apis mellifera) Survival but Sulfoxaflor Modulates Host Immunocompetence. Microb. Biotechnol. 2021, 14, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Singavarapu, B.; Paxton, R.J.; Wubet, T. Bees under Interactive Stressors: The Novel Insecticides Flupyradifurone and Sulfoxaflor along with the Fungicide Azoxystrobin Disrupt the Gut Microbiota of Honey Bees and Increase Opportunistic Bacterial Pathogens. Sci. Total Environ. 2022, 849, 157941. [Google Scholar] [CrossRef] [PubMed]
- El-Din, H.S.; Helmy, W.S.; Al Naggar, Y.; Ahmed, F.S. Chronic Exposure to a Field-Realistic Concentration of Closer® SC (24% Sulfoxaflor) Insecticide Impacted the Growth and Foraging Activity of Honey Bee Colonies. Apidologie 2022, 53, 22. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Wubet, T. Chronic Exposure to Pesticides Disrupts the Bacterial and Fungal Co-Existence and the Cross-Kingdom Network Characteristics of Honey Bee Gut Microbiome. Sci. Total Environ. 2024, 906, 167530. [Google Scholar] [CrossRef] [PubMed]
- Barathi, S.; Sabapathi, N.; Kandasamy, S.; Lee, J. Present Status of Insecticide Impacts and Eco-Friendly Approaches for Remediation—A Review. Environ. Res. 2024, 240, 117432. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Liu, N.; Gao, X.; Liu, X. Effect of RNAi targeting CYP6CY3 on the growth, development and insecticide susceptibility of Aphis gossypii by using nanocarrier-based transdermal dsRNA delivery system. Pestic. Biochem. Physiol. 2021, 177, 104878. [Google Scholar] [CrossRef]
- Arjunan, N.; Thiruvengadam, V.; Sushil, S. Nanoparticle-Mediated dsRNA Delivery for Precision Insect Pest Control: A Comprehensive Review. Mol. Biol. Rep. 2024, 51, 355. [Google Scholar] [CrossRef]
- Kaduskar, B.; Kushwah, R.B.S.; Auradkar, A.; Guichard, A.; Li, M.; Bennett, J.B.; Julio, A.H.F.; Marshall, J.M.; Montell, C.; Bier, E. Reversing Insecticide Resistance with Allelic-Drive in Drosophila melanogaster. Nat. Commun. 2022, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Hancock, P.A.; Hendriks, C.J.M.; Tangena, J.A.; Gibson, H.; Hemingway, J.; Coleman, M.; Gething, P.W.; Cameron, E.; Bhatt, S.; Moyes, C.L. Mapping Trends in Insecticide Resistance Phenotypes in African Malaria Vectors. PLoS Biol. 2020, 18, e3000633. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a Promising Alternative to Synthetic Pesticides: A Case for Microbial Pesticides, Phytopesticides, and Nanobiopesticides. Front. Microbiol. 2023, 14, 1040901, Erratum in Front. Microbiol. 2024, 14, 1258968. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Alagumalai, A.; Balaji, D.; Song, H. Bio-based Agricultural Products: A Sustainable Alternative to Agrochemicals for Promoting a Circular Economy. RSC Sustain. 2023, 1, 746–762. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Kansman, J.T.; Jaramillo, J.L.; Ali, J.G.; Hermann, S.L. Chemical ecology in conservation biocontrol: New perspectives for plant protection. Trends Plant Sci. 2023, 28, 1166–1177. [Google Scholar] [CrossRef]
- Villavicencio-Vásquez, M.; Espinoza-Lozano, F.; Espinoza-Lozano, L.; Coronel-León, J. Biological Control Agents: Mechanisms of Action, Selection, Formulation and Challenges in Agriculture. Front. Agron. 2025, 7, 1578915. [Google Scholar] [CrossRef]
- Liu, T.-X.; Chen, X.-X. Biological control of aphids in China: Successes and prospects. Annu. Rev. Entomol. 2025, 70, 401–419. [Google Scholar] [CrossRef]
- Haase, S.; Sciocco-Cap, A.; Romanowski, V. Baculovirus Insecticides in Latin America: Historical Overview, Current Status and Future Perspectives. Viruses 2015, 7, 2230–2267. [Google Scholar] [CrossRef]
- Karthi, S.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Han, Y.S.; Shivakumar, M.S.; Murali-Baskaran, R.K.; Kalaivani, K.; Radhakrishnan, N.; Park, K.B.; Malafaia, G. Entomopathogenic Fungi Promising Biocontrol Agents for Managing Lepidopteran Pests: Review of Current Knowledge. Biocatal. Agric. Biotechnol. 2024, 58, 103146. [Google Scholar] [CrossRef]
- Pijnakker, J.; Vangansbeke, D.; Duarte, M.; Moerkens, R.; Wäckers, F.L. Predators and parasitoids-in-first: From inundative releases to preventative biological control in greenhouse crops. Front. Sustain. Food Syst. 2020, 4, 595630. [Google Scholar] [CrossRef]
- Angon, P.B.; Mondal, S.; Jahan, I.; Datto, M.; Antu, U.B.; Ayshi, F.J.; Islam, M.S. Integrated pest management (IPM) in agriculture and its role in maintaining ecological balance and biodiversity. Int. J. Agron. 2023, 2023, 5546373. [Google Scholar] [CrossRef]
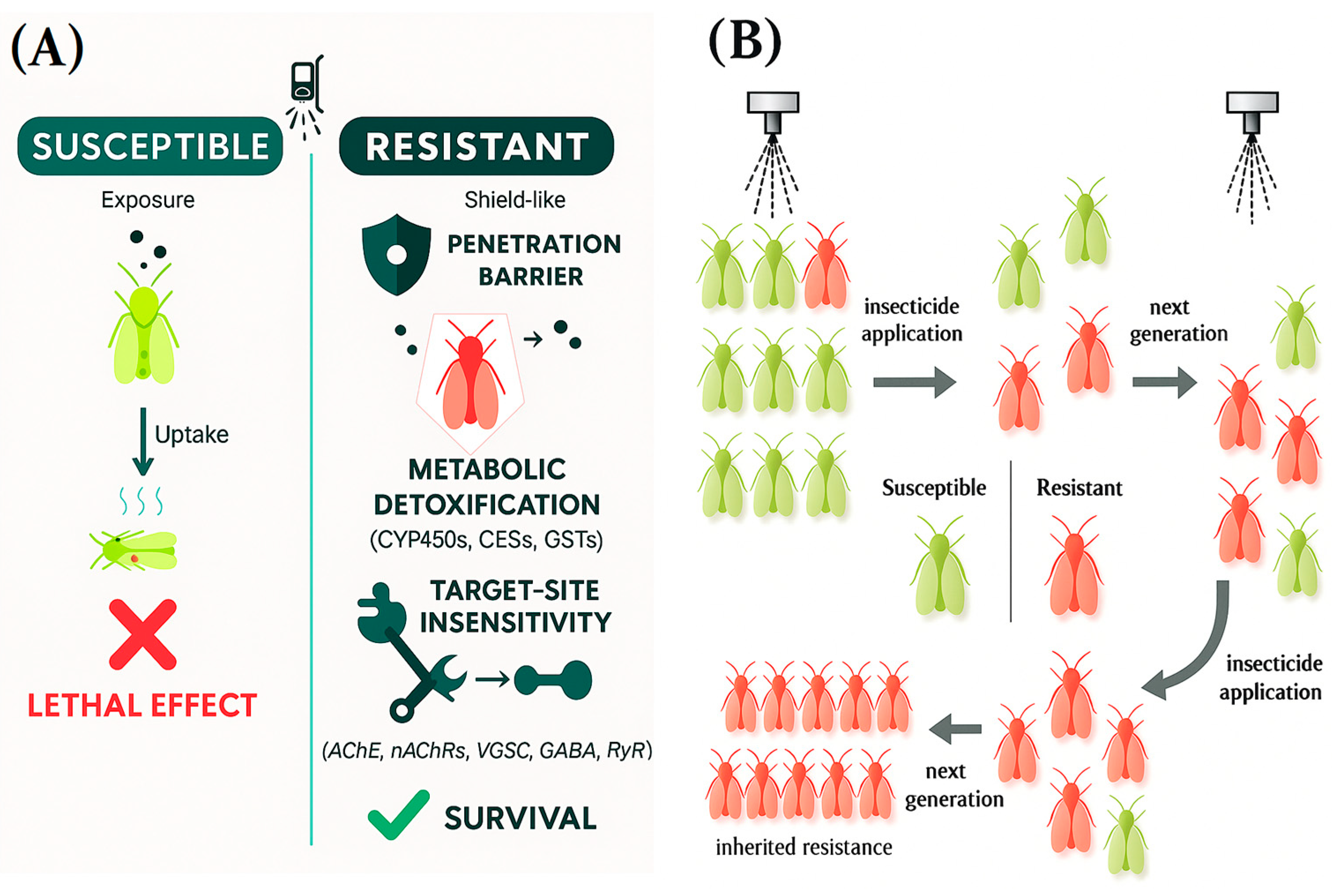
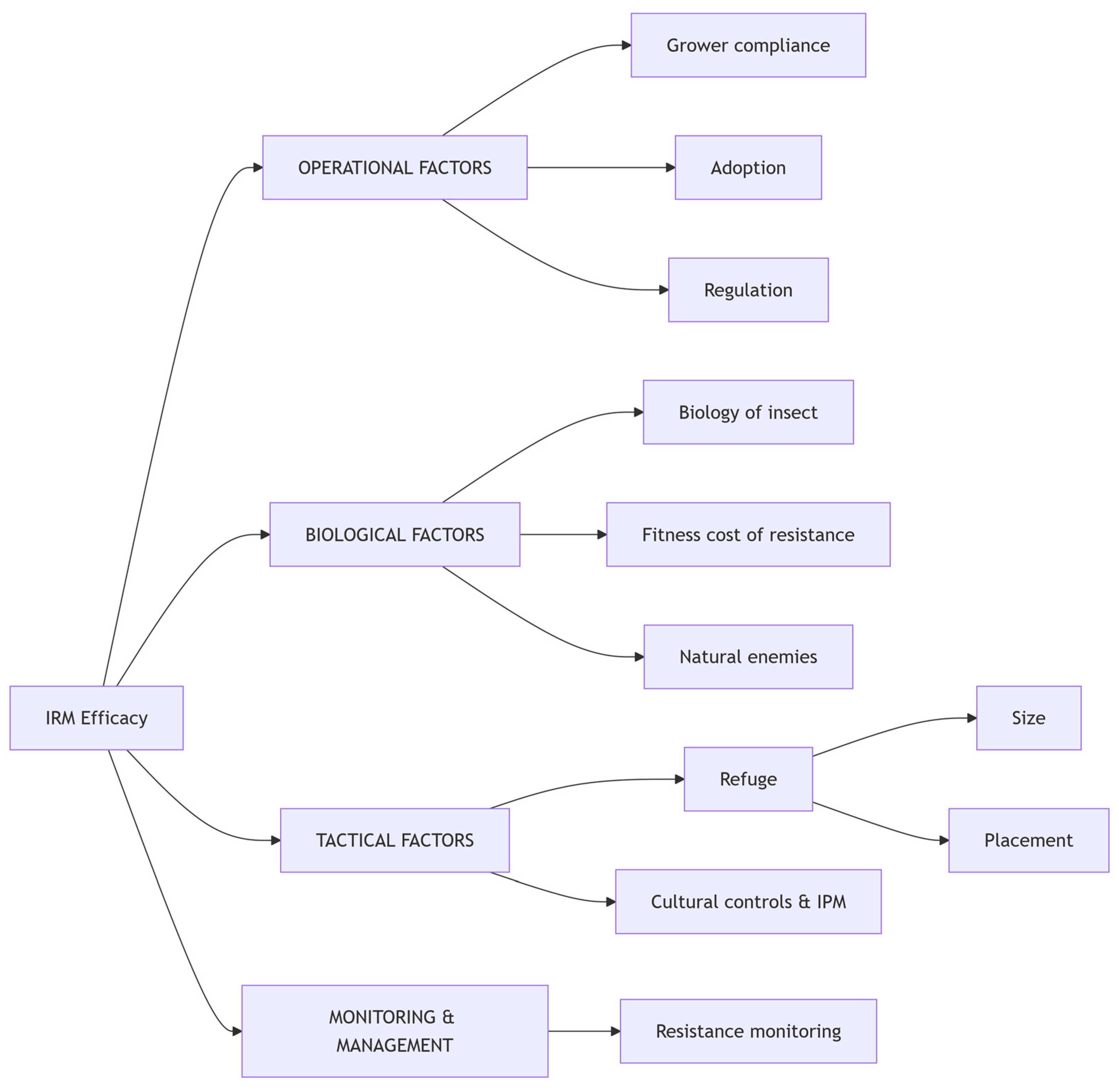
| Mechanism | Description | Molecular/Behavioral Basis & Key Examples | Level & Pattern of Resistance | References |
|---|---|---|---|---|
| Behavioral resistance | Modified behaviors that reduce contact with treated surfaces. |
| Low–moderate (2–10-fold); delays physiological resistance; compromises contact-dependent interventions | [19,20,21] |
| Penetration resistance | Thickened or altered cuticle reduces insecticide uptake. |
| Moderate (<5-fold); offers cross-class protection; synergizes with other mechanisms | [22,23] |
| Target-site insensitivity | Mutations at insecticide binding sites reduce compound efficacy. |
| High (100–1000-fold); class-specific; causes rapid control failure; detectable via molecular diagnostics | [24,25,26] |
| Metabolic resistance | Increased detoxification via overexpressed or amplified enzymes. |
| High (10–500-fold); often polygenic; causes broad cross-resistance; undermines new insecticide chemistries | [27,28,29,30] |
| Insect Species | nAChR Mutation(s) | Insecticide(s) | Resistance? | References |
|---|---|---|---|---|
| Aedes aegypti (yellow fever mosquito) | No known nAChR target-site mutation | Neonicotinoids | No nAChR target-site mutations found (metabolic resistance via CYPs is highlighted elsewhere (Section 5.1.2)) | [48] |
| Frameshift mutation in α6 subunit (32-bp deletion) | Spinosad | Yes (320-fold resistance) | [49] | |
| Anopheles gambiae (malaria mosquito) | Multiple subunits. Reduced expression (β1, α3, α7) | Neonicotinoids (clothianidin, acetamiprid) | Partial resistance; 15–23-fold downregulation in field populations | [50] |
| Drosophila melanogaster (fruit fly) | β1 subunit: R81T (engineered) | Neonicotinoids | Yes (Increased tolerance)—CRISPR/Cas9 genome editing; Fitness costs observed | [51] |
| α6 subunit: Knockout | Spinosad | Yes (High resistance)—CRISPR/Cas9 deletion; No fitness deficits | [51] | |
| Ceratitis capitata (medfly) | α6 subunit: 3aQ68 * and K352 * (* premature stops) | Spinosyns (spinosad) | Yes—truncated α6 isoforms (stop codons at Q68 and K352) confer resistance | [52] |
| Bemisia tabaci (silverleaf whitefly) | β1 subunit: A58T and R79E (target-site) [38] | Neonicotinoids (imidacloprid, thiamethoxam, etc.) | Yes—confers resistance [38]. (earlier studies suggested an absence of nAChR mutations [53]) | [38,53] |
| None identified | Spinosyns (spinosad) | No—no target-site mutations reported (resistance mainly metabolic) | [53] | |
| Tuta absoluta (tomato borer) | α6 subunit: G275E (target-site) | Spinosyns (spinosad, spinetoram) | Yes—high resistance via G275E | [54] |
| None identified | Neonicotinoids | No—no nAChR target-site mutations reported in recent studies | ||
| Spodoptera exigua (beet armyworm) | α6 subunit: G275E (target-site) | Spinosyns (spinosad, spinetoram) | Yes—CRISPR knock-in G275E confers resistance | [55] |
| None identified | Neonicotinoids | No—no target-site mutations reported | ||
| Spodoptera frugiperda (fall armyworm) | α6 subunit: G275E (low frequency) | Spinosyns | Potential transitional resistance—Amplicon sequencing (0.1–1% allele frequency) | [56] |
| Plutella xylostella (diamondback moth) | α6 subunit: 3-amino-acid deletion (TM4) | Spinosyns (spinosad, spinetoram) | Yes—3-aa deletion (IIA) in nAChR α6 underlies ~940-fold resistance | [57] |
| None identified | Neonicotinoids | No—no nAChR target-site mutation documented in recent literature | ||
| Frankliniella occidentalis (western flower thrips) | α6 subunit: G275E (target-site) | Spinosyns (spinosad) | Yes—G275E associated with spinosad resistance, but α6 knockout confers complete resistance to spinosad | [58] |
| Thrips palmi (melon thrips) | α6 subunit: G275E (target-site) | Spinosyns (spinosad) | Yes—G275E confers spinosad resistance | [59] |
| Myzus persicae (green peach aphid) | β1 subunit: R81T (major), V101I | Neonicotinoids (imidacloprid) | Yes—R81T and V101I linked to imidacloprid resistance | [33] |
| Aphis gossypii (cotton aphid) | β1 subunit: R81T | Neonicotinoids (imidacloprid) | Yes—R81T confers neonicotinoid resistance | [60] |
| Nilaparvata lugens (brown planthopper) | Nlα2 subunit | Neonicotinoids (imidacloprid, dinotefuran) | Yes—CRISPR/Cas9 knockout. Nlα2 knockout confers cross-resistance to neonicotinoids | [61] |
| Mechanism | Insect Host | Symbiont(s) | Pesticide(s) | Evidence Type | Notes | References |
|---|---|---|---|---|---|---|
| Direct metabolism | Bactrocera dorsalis | Citrobacter sp. (CF-BD) | Trichlorfon | Genomics + Metabolomics | Degrades trichlorfon into chloral hydrate and dimethyl phosphite; enhances host survival | [158] |
| Direct metabolism | Riptortus pedestris | Burkholderia | Fenitrothion | Bioassays + Functional Enzyme Tests | Soil-acquired strains detoxify fenitrothion; induces resistance and modulates host gene expression | [161] |
| Direct metabolism | Nilaparvata lugens | Serratia marcescens | Buprofezin | Gain/loss of symbiont | Acquisition alters resistance; symbiont breaks down pesticide | [171] |
| Direct metabolism | Drosophila melanogaster | Mixed gut microbiota | Imidacloprid | Metabolic comparisons | Symbiont-mediated nitro-reduction complements host oxidative CYP6G1 pathway | [172] |
| Gene regulation | Nilaparvata lugens | Wolbachia, Arsenophonus | Imidacloprid | Gene expression profiling | Wolbachia upregulates CYPs and GSTs; Arsenophonus suppresses detox genes; response is strain-specific | [167,168] |
| Gene regulation | Apis mellifera | Gut bacteria (e.g., Pantoea, Enterobacter) | Clothianidin | Transcriptomics + Probiotic Rescue | Disrupted microbiota leads to P450 gene suppression; reintroduction restores detox response | [170] |
| Gene regulation + Enzyme activity | Tetranychus urticae | Wolbachia | Abamectin, Pyridaben, Cyflumetofen | RNAi, qPCR, Enzyme Assays | Upregulates TuCYP392D2 and TuGSTd05; increases GST activity; abamectin increases Wolbachia abundance | [166] |
| Direct + Indirect (dual) | Aphis gossypii | Sphingomonas | Imidacloprid | Dual-mode functional profiling | Chemical degradation and host P450 upregulation demonstrated | [173] |
| Immune modulation | Lymantria dispar | Mixed gut microbiota | Pyrethroids | Immune gene profiling | Microbial shifts affect Toll/IMD signaling and Nrf2-mediated detox enzyme expression | [175] |
| Trait | Aedes aegypti | Spodoptera frugiperda | Evolutionary Insight |
|---|---|---|---|
| Primary mechanism | Target-site (kdr) + P450s | Metabolic (P450s) + RyR mutation | Vector-herbivore divergence in adaptation |
| Resistance spread | ~10–20 years regionally | <5 years globally | Trade-facilitated gene flow accelerates resistance |
| Fitness cost | Reduced fecundity, prolonged development | Minimal (behavioral compensation) | Metabolic flexibility buffers trade-offs |
| Key vulnerability | Sequestration (OBPs) | RNAi susceptibility | Taxon-specific weaknesses enable precision control |
| Approach | Core Principle | Implementation Examples and Scientific Rationale |
|---|---|---|
| MoA rotation | Alternate MoA classes to disrupt resistance selection. |
|
| Mixtures & synergists | Combine compounds with distinct targets or inhibit detoxification. |
|
| Refugia & IPM | Maintain susceptible alleles; reduce pest density non-chemically. |
|
| Diagnostic monitoring | Deploy resistance data for threshold-based interventions. |
|
| Biological/genetic tools | Exploit natural enemies or genetic mechanisms. |
|
| Policy & stewardship | Enforce regulations to delay resistance. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Naggar, Y.; Fahmy, N.M.; Alkhaibari, A.M.; Al-Akeel, R.K.; Alharbi, H.M.; Mohamed, A.; Eleftherianos, I.; El-Seedi, H.R.; Giesy, J.P.; Alharbi, H.A. Mechanisms and Genetic Drivers of Resistance of Insect Pests to Insecticides and Approaches to Its Control. Toxics 2025, 13, 681. https://doi.org/10.3390/toxics13080681
Al Naggar Y, Fahmy NM, Alkhaibari AM, Al-Akeel RK, Alharbi HM, Mohamed A, Eleftherianos I, El-Seedi HR, Giesy JP, Alharbi HA. Mechanisms and Genetic Drivers of Resistance of Insect Pests to Insecticides and Approaches to Its Control. Toxics. 2025; 13(8):681. https://doi.org/10.3390/toxics13080681
Chicago/Turabian StyleAl Naggar, Yahya, Nedal M. Fahmy, Abeer M. Alkhaibari, Rasha K. Al-Akeel, Hend M. Alharbi, Amr Mohamed, Ioannis Eleftherianos, Hesham R. El-Seedi, John P. Giesy, and Hattan A. Alharbi. 2025. "Mechanisms and Genetic Drivers of Resistance of Insect Pests to Insecticides and Approaches to Its Control" Toxics 13, no. 8: 681. https://doi.org/10.3390/toxics13080681
APA StyleAl Naggar, Y., Fahmy, N. M., Alkhaibari, A. M., Al-Akeel, R. K., Alharbi, H. M., Mohamed, A., Eleftherianos, I., El-Seedi, H. R., Giesy, J. P., & Alharbi, H. A. (2025). Mechanisms and Genetic Drivers of Resistance of Insect Pests to Insecticides and Approaches to Its Control. Toxics, 13(8), 681. https://doi.org/10.3390/toxics13080681









