A Comprehensive Ecotoxicological Evaluation of a Treated Olive Mill Wastewater and Obtained Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Precipitation Technique in Olive Oil Mill Wastewater (OMWW)
2.2. Ecotoxicological Test Model Species
2.2.1. Freshwater Species
2.2.2. Soil Species
2.3. Ecotoxicological Assays
2.3.1. Freshwater and Soil Test Samples Preparation
Raphidocelis subcapitata Bioassay
Daphnia magna Bioassay
Danio rerio Bioassay
2.3.2. Soil Bioassays
Folsomia candida Bioassay
Enchytraeus crypticus Bioassay
Brassica oleracea and Lolium perenne Plant Bioassays
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. Olive Mill Wastewater and Sludge Physicochemical Characterization
3.2. Freshwater Ecotoxicity of Olive Mill Wastewater
3.2.1. Raphidocelis subcapitata
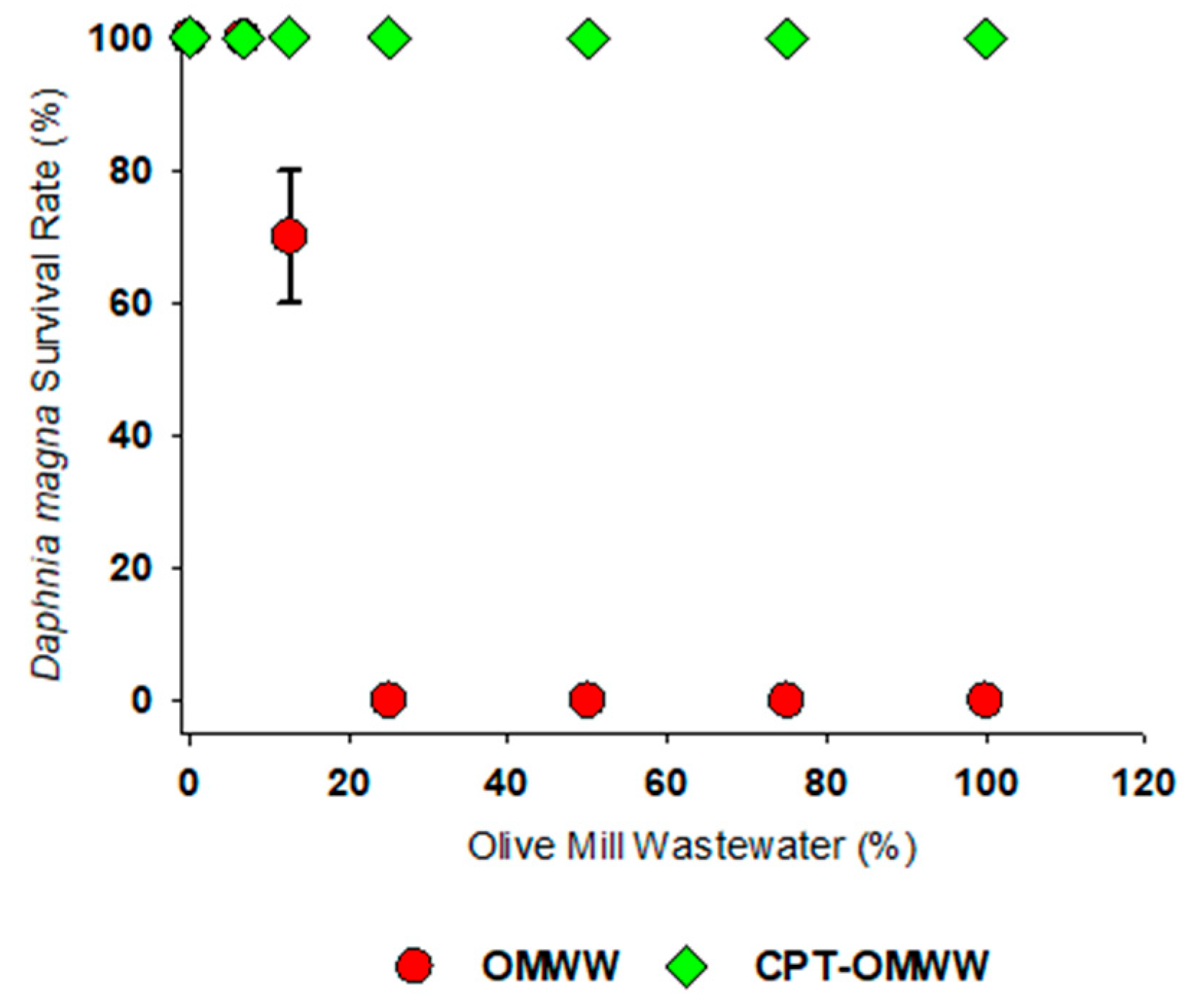
3.2.2. Daphnia magna
3.2.3. Danio rerio
3.3. Soil Ecotoxicity After Sludge Application
3.3.1. Folsomia candida and Enchytraeus crypticus
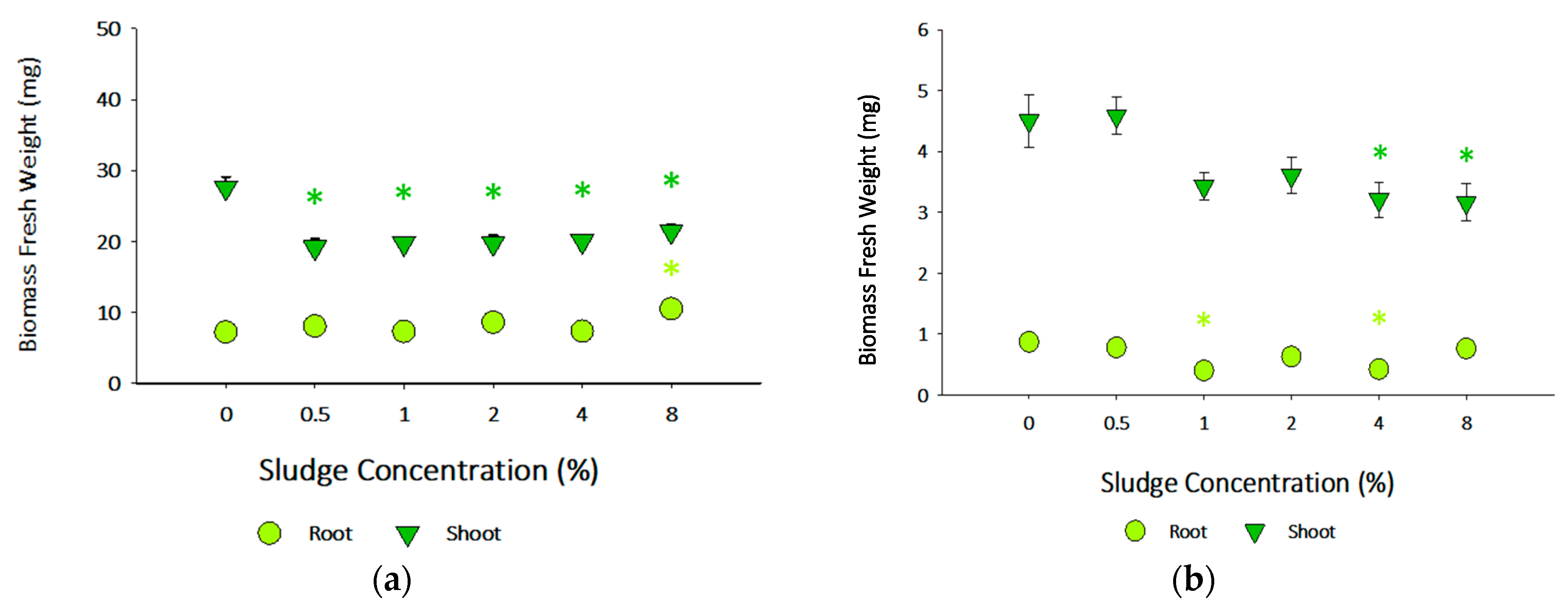
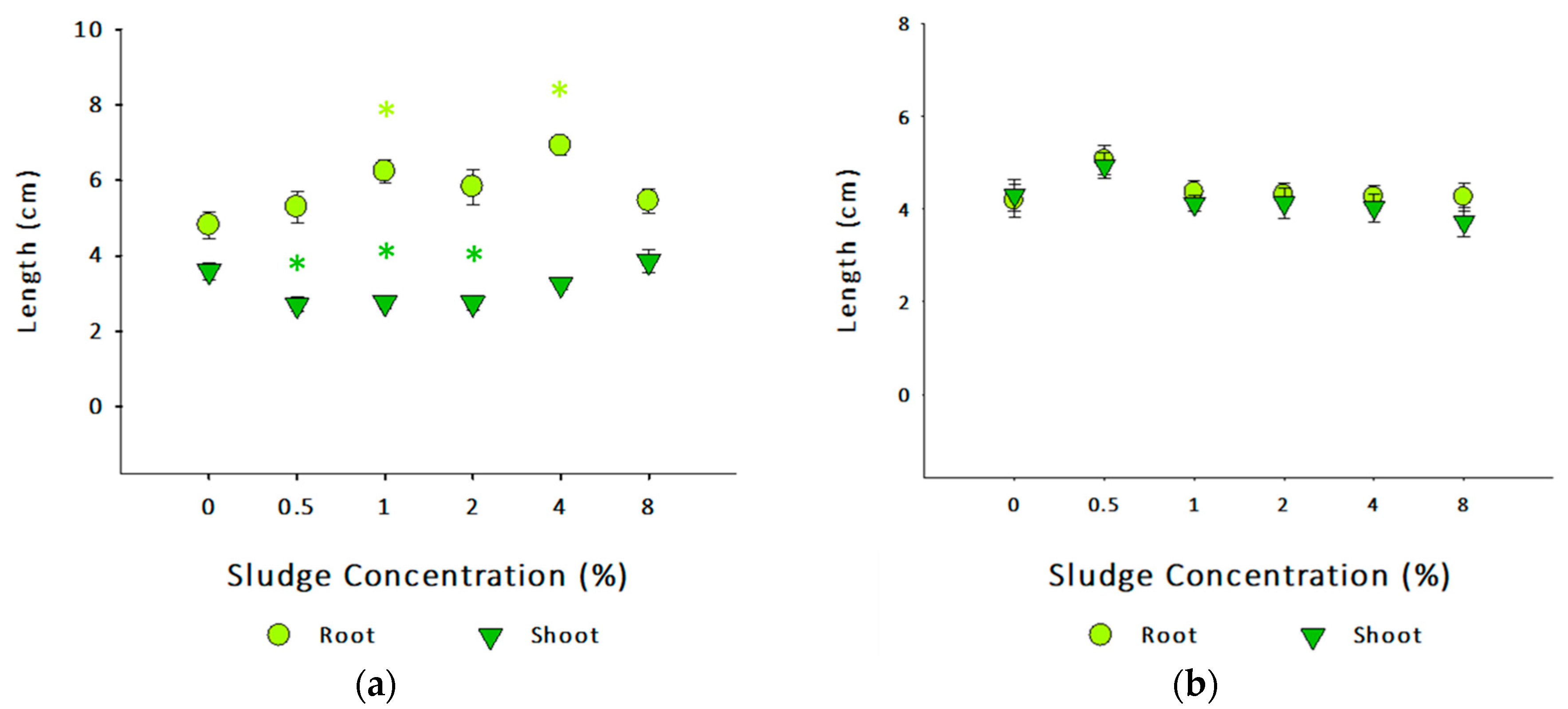
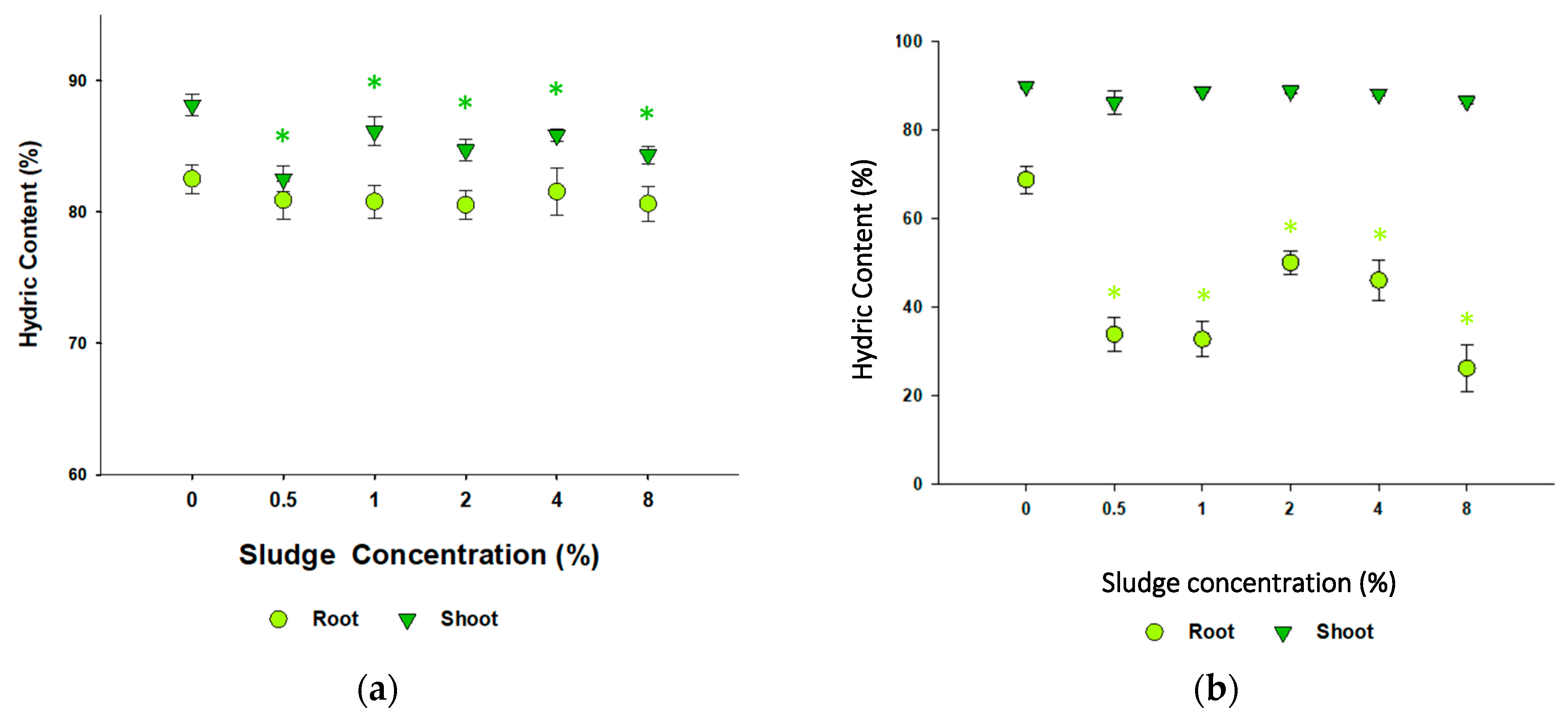
3.3.2. Brassica oleracea and Lolium perenne
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atamer Balkan, B.; Meral, S. Olive oil value-chain dynamics: The Turkish olive oil industry case. Acta Hortic. 2018, 1199, 195–202. [Google Scholar] [CrossRef]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental effects caused by olive mill wastewaters: Toxicity comparison of low-molecular-weight phenol components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Cerretani, L.; Toschi, T.G.; Bendini, A. Phenolic Fraction of Virgin Olive Oil: An Overview on Identified Compounds and Analytical Methods for Their Determination. Funct. Plant Sci. Biotechnol. 2009, 3, 69–80. [Google Scholar]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Hanafi, F.; Belaoufi, A.; Mountadar, M.; Assobhei, O. Augmentation of biodegradability of olive mill wastewater by electrochemical pre-treatment: Effect on phytotoxicity and operating cost. J. Hazard. Mater. 2011, 190, 94–99. [Google Scholar] [CrossRef]
- Paredes, M.J.; Monteoliva-Sanchez, M.; Moreno, E.; Perez, J.; Ramos-Cormenzana, A.; Martinez, J. Effect of waste waters from olive oil extraction plants on the bacterial population of soil. Chemosphere 1986, 15, 659–664. [Google Scholar] [CrossRef]
- Arienzo, M.; Capasso, R. Analysis of metal cations and inorganic anions in olive oil mill waste waters by atomic absorption spectroscopy and ion chromatography: Detection of metals bound mainly to the organic polymeric fraction. J. Agric. Food Chem. 2000, 48, 1405–1410. [Google Scholar] [CrossRef]
- Federici, F.; Bongi, G. Improved method for isolation of bacterial inhibitors from oleuropein hydrolysis. Appl. Environ. Microbiol. 1983, 46, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Vilhena, A.; Novais, J.M.; Martins-Dias, S. Modelling of olive mill wastewater characteristics. Prog. Water Resour. 2003, 9, 313–322. [Google Scholar]
- Gizgis, N.; Georgiou, M.; Diamadopoulos, E. Sequential anaerobic/aerobic biological treatment of olive mill wastewater and municipal wastewater. J. Chem. Technol. Biotechnol. 2006, 81, 1563–1569. [Google Scholar] [CrossRef]
- Di Serio, M.; Lanza, B.; Mucciarella, M.; Russi, F.; Iannucci, E.; Marfisi, P.; Madeo, A. Effects of olive mill wastewater spreading on the physico-chemical and microbiological characteristics of soil. Int. Biodeterior. Biodegrad. 2008, 62, 403–407. [Google Scholar] [CrossRef]
- Meftah, O.; Guergueb, Z.; Braham, M.; Sayadi, S.; Mekki, A. Long-term effects of olive mill wastewaters application on soil properties and phenolic compounds migration under arid climate. Agric. Water Manag. 2019, 212, 119–125. [Google Scholar] [CrossRef]
- Bertin, L.; Berselli, S.; Fava, F.; Petrangeli-Papini, M.; Marchetti, L. Anaerobic digestion of olive mill wastewaters in biofilm reactors packed with granular activated carbon and ‘Manville’ silica beads. Water Res. 2004, 38, 3167–3178. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Codigestion of olive oil mill wastewaters with manure, household waste or sewage sludge. Biodegradation 1997, 8, 221–226. [Google Scholar] [CrossRef]
- Bertin, L.; Majone, M.; Di Gioia, D.; Fava, F. An aerobic fixed-phase biofilm reactor system for the degradation of the low-molecular weight aromatic compounds occurring in the effluents of anaerobic digestors treating olive mill wastewaters. J. Biotechnol. 2001, 87, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Paraskeva, C.A.; Papadakis, V.G.; Tsarouchi, E.; Kanellopoulou, D.G.; Koutsoukos, P.G. Membrane processing for olive mill wastewater fractionation. Desalination 2007, 213, 218–229. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Luz, S.; Fernandes, F.; Jerónimo, E. Cheese wastewater treatment by acid and basic precipitation: Application of H2SO4, HNO3, HCl, Ca(OH)2 and NaOH. J. Environ. Chem. Eng. 2020, 8, 103556. [Google Scholar] [CrossRef]
- Correia, T.; Regato, M.; Almeida, A.; Santos, T.; Amaral, L.; Carvalho, F. Manual treatment of urban wastewater by chemical precipitation for production of hydroponic nutrient solutions. J. Ecol. Eng. 2020, 21, 143–152. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Afonso, A.; Guerreiro, R.; Jerónimo, E. Contamination reduction of real olive oil mill wastewater using innovative acid and basic chemical precipitation processes. Int. J. Environ. Sci. Technol. 2021, 18, 799–808. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Concha-Lozano, N.; Renard, F.; Quirico, E. Removal of oxyanions from synthetic wastewater via carbonation process of calcium hydroxide: Applied and fundamental aspects. J. Hazard. Mater. 2009, 166, 788–795. [Google Scholar] [CrossRef]
- Aktas, E.S.; Imre, S.; Ersoy, L. Characterization and lime treatment of olive mill wastewater. Water Res. 2001, 35, 2336–2340. [Google Scholar] [CrossRef]
- Afonso, A.; Guerreiro, R.; Prazeres, A.R.; Jerónimo, E. Reuse of pretreated agro-industrial wastewaters for hydroponic production of lettuce. Water 2023, 15, 1856. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; Gonçalves, A.; Baião, N.; Fernandes, R.; de Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A. Assessment of chemical, biochemical and ecotoxicological aspects in a mine soil amended with sludge of either urban or industrial origin. Chemosphere 2008, 72, 1774–1781. [Google Scholar] [CrossRef]
- Roig, N.; Sierra, J.; Martí, E.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Relationship between pollutant content and ecotoxicity of sewage sludges from Spanish wastewater treatment plants. Sci. Total Environ. 2012, 425, 99–109. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion performance and life table of black soldier fly (Hermetia illucens) on fermented maize straw. J. Clean. Prod. 2019, 230, 974–980. [Google Scholar] [CrossRef]
- Altieri, R.; Esposito, A. Olive orchard amended with two experimental olive mill wastes mixtures: Effects on soil organic carbon, plant growth and yield. Bioresour. Technol. 2008, 99, 8390–8393. [Google Scholar] [CrossRef] [PubMed]
- Belaqziz, M.; El-Abbassi, A.; Lakhal, E.K.; Agrafioti, E.; Galanakis, C.M. Agronomic application of olive mill wastewater: Effects on maize production and soil properties. J. Environ. Manag. 2016, 171, 158–165. [Google Scholar] [CrossRef]
- Benaddi, R.; Osmane, A.; Zidan, K.; El Harfi, K.; Ouazzani, N.; Aziz, F.; Bouriqi, A.; El Harfi, K.; Ouazzani, N. A Review on Processes for Olive Mill Waste Water Treatment. Ecol. Eng. Environ. Technol. 2023, 24, 196–207. [Google Scholar] [CrossRef]
- Afonso, A.; Ribeiro, C.; Carvalho, M.J.; Correia, T.; Correia, P.; Regato, M.; Costa, I.; Fernandes, A.; Almeida, A.; Lopes, A.; et al. Pretreated agro-industrial effluents as a source of nutrients for tomatoes grown in a dual function hydroponic system: Tomato quality assessment. Sustainability 2024, 16, 315. [Google Scholar] [CrossRef]
- Ramalho, M.; Jovanović, T.; Afonso, A.; Baía, A.; Lopes, A.; Fernandes, A.; Almeida, A.; Carvalho, F. Landfill leachate treatment by immediate one-step lime precipitation, carbonation, and phytoremediation fine-tuning. Environ. Sci. Pollut. Res. 2023, 30, 8647–8656. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Zyad, A.; Amechrouq, A.; Oukerrou, M.A.; Mouse, H.A.; Mbarki, M. Identification and characterisation of phenolic compounds extracted from Moroccan olive mill wastewater. Food Sci. Technol. 2014, 34, 249–257. [Google Scholar] [CrossRef]
- NP EN ISO 6869:2007; Animal Feeding Stuffs—Determination of the Contents of Calcium, Sodium, Magnesium and Potassium. Instituto Português da Qualidade: Caparica, Portugal, 2007.
- NP 874:1998; Fertilizers—Determination of Phosphorus Content. Instituto Português da Qualidade: Caparica, Portugal, 1998.
- NP ISO 5984:2014; Animal Feeding Stuffs—Determination of Crude Ash. Instituto Português da Qualidade: Caparica, Portugal, 2014.
- NP ISO 6496:2018; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. Instituto Português da Qualidade: Caparica, Portugal, 2018.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-chloro-3-indolyl Beta-D-glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6579:2002; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2002.
- Stein, J.R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- ASTM E729-96; Standard Guide for Conducting Acute Toxicity Tests on Test Materials with Fishes, Macroinvertebrates, and Amphibians. ASTM International: West Conshohocken, PA, USA, 2002.
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Guidelines for the Testing of Chemicals Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Test No. 232: Collembolan Reproduction Test in Soil; OECD Guidelines for the Testing of Chemicals Section 2; OECD Publishing: Paris, France, 2009. [Google Scholar] [CrossRef]
- Decreto-Lei n.º 276/2009, de 2 de outubro. Diário da República, 1.ª série, n.º 193, 2 outubro 2009. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/276-2009-490974 (accessed on 28 July 2025).
- OECD. Test No. 201: Alga, Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar] [CrossRef]
- Eisentraeger, A.; Dott, W.; Klein, J.; Hahn, S. Comparative studies on algal toxicity testing using fluorometric microplate and Erlenmeyer flask growth-inhibition assays. Ecotoxicol. Environ. Saf. 2003, 54, 346–354. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 220: Enchytraeid Reproduction Test; OECD Guidelines for the Testing of Chemicals Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; OECD Guidelines for the Testing of Chemicals Section 2; OECD Publishing: Paris, France, 2006. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; Bertoldi, M. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Achak, M.; Elayadi, F.; Boumya, W. Chemical coagulation/flocculation processes for removal of phenolic compounds from olive mill wastewater: A comprehensive review. Am. J. Appl. Sci. 2019, 16, 59–91. [Google Scholar] [CrossRef]
- Decreto-Lei n.º 119/2019, de 21 de agosto. Diário da República, 1.ª série, n.º 159, 21 agosto 2019. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/119-2019-124097549 (accessed on 28 July 2025).
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Madeira, L.; Ribau Teixeira, M.; Almeida, A.; Santos, T.; Carvalho, F. Reuse of lime sludge from immediate one-step lime precipitation process as a coagulant (aid) in slaughterhouse wastewater treatment. J. Environ. Manag. 2023, 342, 118278. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, A.R.; Rivas, J.; Paulo, Ú.; Ruas, F.; Carvalho, F. Sustainable treatment of different high-strength cheese whey wastewaters: An innovative approach for atmospheric CO2 mitigation and fertilizer production. Environ. Sci. Pollut. Res. 2016, 23, 13062–13075. [Google Scholar] [CrossRef]
- Brown, R. Relationships between suspended solids, turbidity, light attenuation, and algal productivity. Lake Reserv. Manag. 1984, 1, 198–205. [Google Scholar] [CrossRef]
- DellaGreca, M.; Monaco, P.; Pinto, G.; Pollio, A.; Previtera, L.; Temussi, F. Phytotoxicity of low-molecular-weight phenols from olive mill waste waters. Bull. Environ. Contam. Toxicol. 2001, 67, 352–359. [Google Scholar] [CrossRef]
- Mekki, A.; Dhouib, A.; Sayadi, S. Polyphenols dynamics and phytotoxicity in a soil amended by olive mill wastewaters. J. Environ. Manag. 2007, 84, 134–140. [Google Scholar] [CrossRef]
- Babić, S.; Malev, O.; Pflieger, M.; Lebedev, A.T.; Mazur, D.M.; Kužić, A.; Čož-Rakovac, R.; Trebše, P. Toxicity evaluation of olive oil mill wastewater and its polar fraction using multiple whole-organism bioassays. Sci. Total Environ. 2019, 686, 903–914. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R.; Sacco, O. Effect of combined physico-chemical processes on the phytotoxicity of olive mill wastewaters. Water Res. 2008, 42, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Lopes, M.; Ferreira, J.P.; Belo, I. Biological treatment of olive mill wastewater by non-conventional yeasts. Bioresour. Technol. 2009, 100, 3759–3763. [Google Scholar] [CrossRef] [PubMed]
- Rharrabti, Y.; El Yamani, M. Olive mill wastewater: Treatment and valorization technologies. In Handbook of Environmental Materials Management; Springer: Cham, Switzerland, 2019; pp. 1659–1686. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A. Chemical and toxic evaluation of a biological treatment for olive-oil mill wastewater using commercial microbial formulations. Appl. Microbiol. Biotechnol. 2004, 64, 735–739. [Google Scholar] [CrossRef]
- Oztekin, R.; Sponza, D.T. Treatment of wastewaters from the olive mill industry by sonication. J. Chem. Technol. Biotechnol. 2013, 88, 212–225. [Google Scholar] [CrossRef]
- Cho, H.; Ryu, C.S.; Lee, S.-A.; Adeli, Z.; Meupea, B.T.; Kim, Y.; Kim, Y.J. Endocrine-disrupting potential and toxicological effect of para-phenylphenol on Daphnia magna. Ecotoxicol. Environ. Saf. 2022, 243, 113965. [Google Scholar] [CrossRef]
- Tyagi, V.; Chopra, A.; Durgapal, N.; Kumar, A. Evaluation of Daphnia magna as an indicator of toxicity and treatment efficacy of municipal sewage treatment plant. J. Appl. Sci. Environ. Manag. 2009, 11, 61–67. [Google Scholar] [CrossRef]
- Weltens, R.; Goossens, R.; Van Puymbroeck, S. Ecotoxicity of contaminated suspended solids for filter feeders (Daphnia magna). Arch. Environ. Contam. Toxicol. 2000, 39, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Herbrandson, C.; Bradbury, S.P.; Swackhamer, D.L. Influence of suspended solids on acute toxicity of carbofuran to Daphnia magna: I. Interactive effects. Aquat. Toxicol. 2003, 63, 333–342. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Vis. Exp. 2012, 69, e4196. [Google Scholar] [CrossRef]
- Rouvalis, A.; Theodoropoulos, C.; Iliopoulou-Georgudaki, J. Assessment of toxicity of the untreated and Pleurotus ostreatus treated olive mill wastewater by using microbiotests. Int. J. Environ. Eng. 2013, 5, 373–386. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, Y.; Zhang, L.; Chen, L.; Zhang, H. Toxicity of urban highway runoff in Shanghai to zebrafish (Danio rerio) embryos and luminous bacteria (Vibrio qinghaiensis Q67). Environ. Sci. Pollut. Res. 2014, 21, 2663–2676. [Google Scholar] [CrossRef]
- Luz, S.; Rivas, J.; Afonso, A.; Carvalho, F. Immediate one-step lime precipitation process for the valorization of winery wastewater to agricultural purposes. Environ. Sci. Pollut. Res. 2021, 28, 18382–18391. [Google Scholar] [CrossRef]
- Paredes, C.; Bernal, M.P.; Cegarra, J.; Roig, A. Bio-degradation of olive mill wastewater sludge by its co-composting with agricultural wastes. Bioresour. Technol. 2002, 85, 1–8. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.; Jurado, M.; López-González, J.; Toribio, A.; Suárez-Estrella, F.; Sáez, J.; Moral, R.; Andreu-Rodríguez, F.; López, M. Biorecovery of olive mill wastewater sludge from evaporation ponds. J. Environ. Manag. 2022, 319, 115647. [Google Scholar] [CrossRef] [PubMed]
- Natal-Da-Luz, T.; Tidona, S.; Jesus, B.; Morais, P.V.; Sousa, J.P. The use of sewage sludge as soil amendment: The need for an ecotoxicological evaluation. J. Soils Sediments 2009, 9, 246–260. [Google Scholar] [CrossRef]
- Kovačević, M.; Stjepanović, N.; Trigui, S.; Hackenberger, D.K.; Lončarić, Ž.; Glavaš, O.J.; Kallel, A.; Hackenberger, B.K. Assessment of adverse effects of olive mill wastewater and olive mill waste contaminated soil on springtail Folsomia candida. Chemosphere 2022, 300, 134651. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mu, Q.; Kimura, H.; Murugadoss, V.; He, M.; Du, W.; Hou, C. Oxidative degradation of phenols and substituted phenols in the water and atmosphere: A review. Adv. Compos. Hybrid Mater. 2022, 5, 627–640. [Google Scholar] [CrossRef]
- Conesa, H.M.; María-Cervantes, A.; Álvarez-Rogel, J.; González-Alcaraz, M.N. Influence of soil properties on trace element availability and plant accumulation in a Mediterranean salt marsh polluted by mining wastes: Implications for phytomanagement. Sci. Total Environ. 2011, 409, 4470–4479. [Google Scholar] [CrossRef]
- Arif, M.R.; Thoihidul Islam, M.; Robin, A.H.K. Salinity stress alters root morphology and root hair traits in Brassica napus. Plants 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Pagès, L. Branching patterns of root systems: Comparison of monocotyledonous and dicotyledonous species. Ann. Bot. 2016, 118, 1337–1346. [Google Scholar] [CrossRef]
- Pérez-Murcia, M.D.; Moral, R.; Moreno-Caselles, J.; Pérez-Espinosa, A.; Paredes, C. Use of composted sewage sludge in growth media for broccoli. Bioresour. Technol. 2006, 97, 123–130. [Google Scholar] [CrossRef]
- Brito, L.M. Lettuce (Lactuca sativa L.) and cabbage (Brassica oleracea L. var. capitata L.) growth in soil mixed with municipal solid waste compost and paper mill sludge composted with bark. Acta Hortic. 2001, 563, 131–137. [Google Scholar] [CrossRef]
- Wurst, S.; Langel, R.; Rodger, S.; Scheu, S. Effects of belowground biota on primary and secondary metabolites in Brassica oleracea. Chemoecology 2006, 16, 69–73. [Google Scholar] [CrossRef]
- Ryser, P. The mysterious root length. Plant Soil 2006, 286, 1–6. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Plant morphology and root hydraulics are altered by nutrient deficiency in Pistacia lentiscus (L.). Trees 2006, 20, 334–339. Available online: https://www.academia.edu/3733430 (accessed on 28 July 2025). [CrossRef]
- Rusan, M.J.M.; Malkawi, H.I. Dilution of olive mill wastewater (OMW) eliminates its phytotoxicity and enhances plant growth and soil fertility. Desalination Water Treat. 2016, 57, 27945–27953. [Google Scholar] [CrossRef]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Di Serio, M.G.; Di Giovacchino, L. Long-term spreading of olive mill wastewater on olive orchard: Effects on olive production, oil quality, and soil properties. Commun. Soil Sci. Plant Anal. 2017, 48, 2420–2433. [Google Scholar] [CrossRef]
- Chatterjee, J.; Chatterjee, C. Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ. Pollut. 2000, 109, 69–74. [Google Scholar] [CrossRef]
- Li, Y.L.; Stanghellini, C. Analysis of the effect of EC and potential transpiration on vegetative growth of tomato. Sci. Hortic. 2001, 89, 9–21. [Google Scholar] [CrossRef]
- Koutsos, T.M.; Chatzistathis, T.; Balampekou, E.I. A new framework proposal, towards a common EU agricultural policy, with the best sustainable practices for the re-use of olive mill wastewater. Sci. Total Environ. 2018, 622–623, 942–953. [Google Scholar] [CrossRef]
- El-Hussiny, M.A.; Hassan, S.A.; El-Sebae, A.A.; Al-Araby, R.E. Integrated assessment of olive mill wastewater as a sustainable soil amendment: Effects on some soil properties in semi-arid agricultural systems. Catrina Int. J. Environ. Sci. 2024, 33, 83–95. [Google Scholar] [CrossRef]
- Iticescu, C.; Georgescu, L.P.; Murariu, G.; Circiumaru, A.; Timofti, M. The characteristics of sewage sludge used on agricultural lands. AIP Conf. Proc. 2018, 2022, 020001. [Google Scholar] [CrossRef]
- Khalil, J.; Jaafar, A.A.K.; Habib, H.; Bouguerra, S.; Nogueira, V.; Rodríguez-Seijo, A. The impact of olive mill wastewater on soil properties, nutrient and heavy metal availability—A study case from Syrian vertisols. J. Environ. Manag. 2024, 351, 119861. [Google Scholar] [CrossRef] [PubMed]


| Physico-Chemical | OMWW Sample | CPT-OMWW Sample |
|---|---|---|
| pH | 6.5 ± 0.10 | 7.8 ± 0.02 |
| EC (mS cm−1) | 0.7 ± 0.003 | 2.6 ± 0.004 |
| COD (mg L−1) | 1504 ± 350 | 238 ± 45 |
| BOD5 (mg L−1) | 35 ± 3 | 2.7 ± 0.6 |
| TSS (mg L−1) | 222 ± 0.6 | 32 ± 0.2 |
| Nt (mg L−1) | 439 ± 7 | 0 |
| Pt (mg L−1) | 84.3 ± 4.2 | 2.33 ± 010 |
| DO (%) | 5.6 ± 0.8 | 20.5 ± 0.5 |
| Alkalinity (CaCO3 mg L−1) | 0 | 17,676 ± 69 |
| Turbidity (NTU) | 417 ± 5 | 0.3 ± 0.03 |
| N-NH4 (mg L−1) | 234 ± 1 | 0 |
| N-NO3 (mg L−1) | 2.5 ± 0.1 | 0.8 ± 0.1 |
| N-NO2 (mg L−1) | 0.018 ± 0.00 | 0.003 ± 0.00 |
| Phenols (mg GAE L−1) | 56 ± 6 | 13 ±2 |
| Fecal Coliforms (Nº/100 mL) | 93 ± 2 | 0 |
| E. coli (Nº/100 mL) | 9.1 ± 0.2 | 0 |
| Parasite eggs (Nº/L) | 9.1 ± 0.1 | 0 |
| Chemical and Biological Characteristics | CPT-Sludge Sample |
|---|---|
| pH | 10 |
| Ca (g kg−1) | 154.1 |
| P (g kg−1) | 1.22 |
| Na (g kg−1) | 0.962 |
| K (g kg−1) | 1.44 |
| Mg (g kg−1) | 4.09 |
| Cu (g kg−1) | 0.117 |
| Fe (g kg−1) | 8.87 |
| Mn (g kg−1) | 0.099 |
| Zn (g kg−1) | 0.54 |
| N-Kjeldahl (g/kg−1) | 14.5 |
| Ashes (%) | 47 |
| Dry matter (%) | 94.1 |
| Escherichia coli (number/g) | <1 |
| Salmonella spp./50 g | Not present |
| OMWW | CPT-OMWW | |||
|---|---|---|---|---|
| Wastewater (%) | Average Daily Growth (Day−1) | Growth Reduction (%) | Average Daily Growth (Day−1) | Growth Reduction (%) |
| 0 | 1.462 ± 0.006 SE | - | 1.371 ± 0.010 SE | - |
| 6.75 | 1.322 ± 0.025 SE | 9.55 | 1.349 ± 0.025 SE | 1.57 |
| 12.5 | 1.174 ± 0.089 SE * | 19.67 | 1.337 ± 0.089 SE * | 2.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.N.; Pereira, A.; Silva, A.R.R.; Cardoso, D.N.; Mostafaie, A.; Campos, F.; Rehan, I.; Moreira, O.; Lopes, I.G.; Murta, D.; et al. A Comprehensive Ecotoxicological Evaluation of a Treated Olive Mill Wastewater and Obtained Sludge. Toxics 2025, 13, 648. https://doi.org/10.3390/toxics13080648
Pinto JN, Pereira A, Silva ARR, Cardoso DN, Mostafaie A, Campos F, Rehan I, Moreira O, Lopes IG, Murta D, et al. A Comprehensive Ecotoxicological Evaluation of a Treated Olive Mill Wastewater and Obtained Sludge. Toxics. 2025; 13(8):648. https://doi.org/10.3390/toxics13080648
Chicago/Turabian StylePinto, José N., Andreia Pereira, Ana Rita R. Silva, Diogo N. Cardoso, Amid Mostafaie, Fábio Campos, Iryna Rehan, Olga Moreira, Ivã Guidini Lopes, Daniel Murta, and et al. 2025. "A Comprehensive Ecotoxicological Evaluation of a Treated Olive Mill Wastewater and Obtained Sludge" Toxics 13, no. 8: 648. https://doi.org/10.3390/toxics13080648
APA StylePinto, J. N., Pereira, A., Silva, A. R. R., Cardoso, D. N., Mostafaie, A., Campos, F., Rehan, I., Moreira, O., Lopes, I. G., Murta, D., Afonso, A., Oliveira, M., Silvério, K. S., Santos, M. T., Carvalho, F., Almeida, A., & Loureiro, S. (2025). A Comprehensive Ecotoxicological Evaluation of a Treated Olive Mill Wastewater and Obtained Sludge. Toxics, 13(8), 648. https://doi.org/10.3390/toxics13080648














