Human Health Risk and Bioaccessibility of Arsenic in Wadis and Marine Sediments in a Coastal Lagoon (Mar Menor, Spain)
Abstract
1. Introduction
2. Materials and Methods
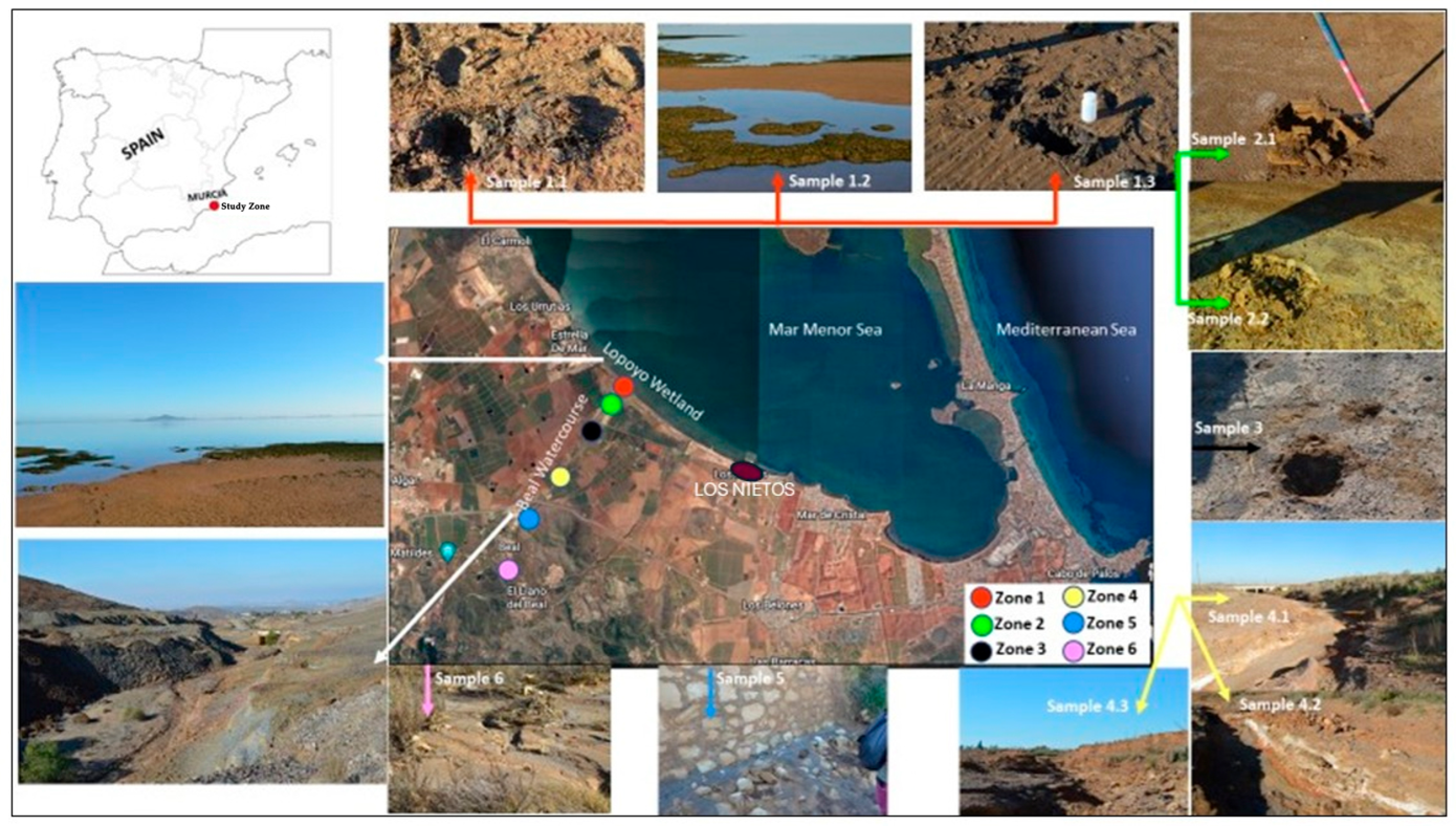
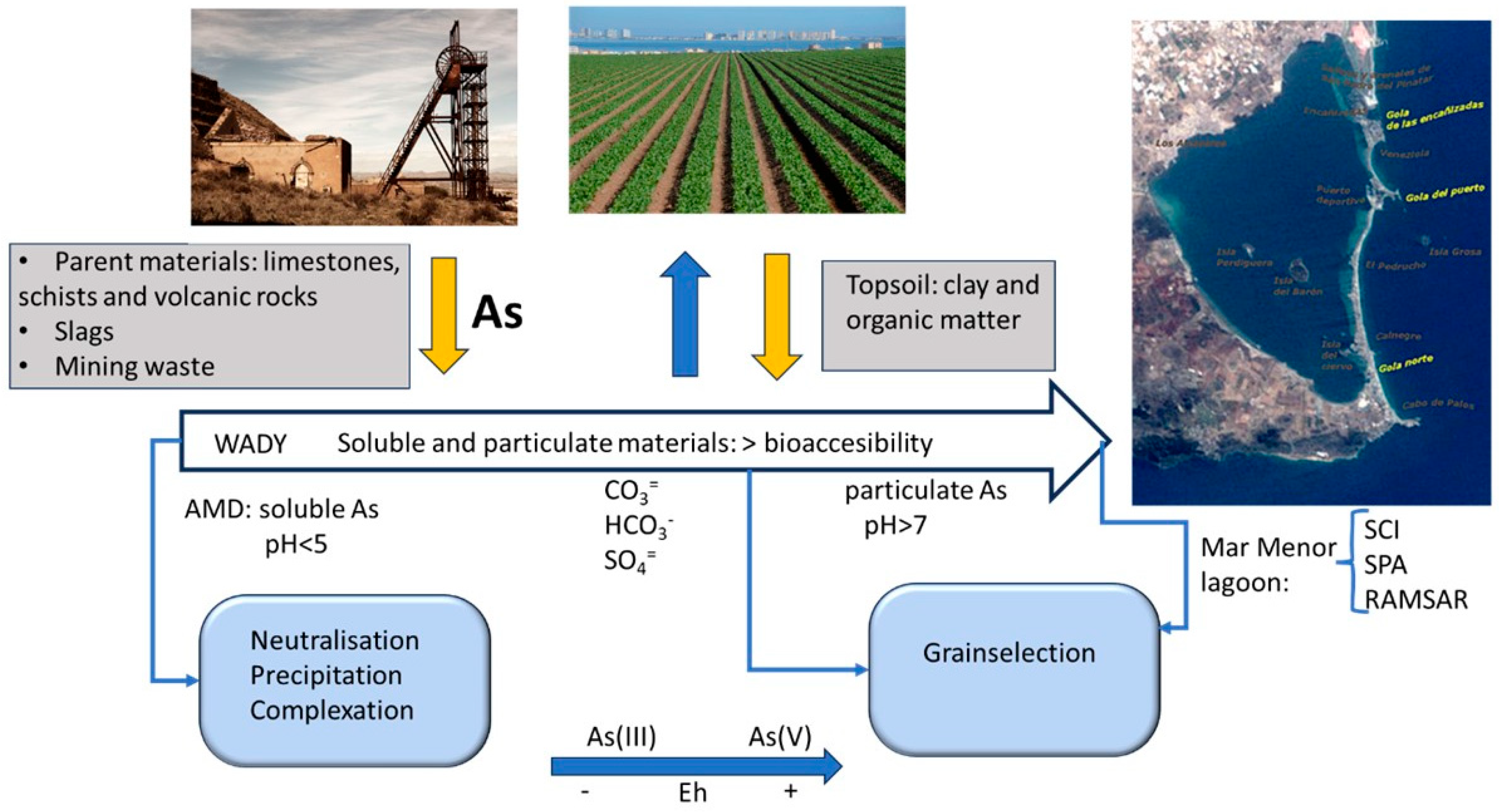
2.1. Study Area
2.2. Materials
2.3. Methodology
2.3.1. Characteristics of the Soil, Marine Sediments, and Waters
2.3.2. Determination of As
- Determination of the total As content
- Determination of As bioaccessibility and Extraction in the gastrointestinal tract.
2.3.3. Mineralogical Composition
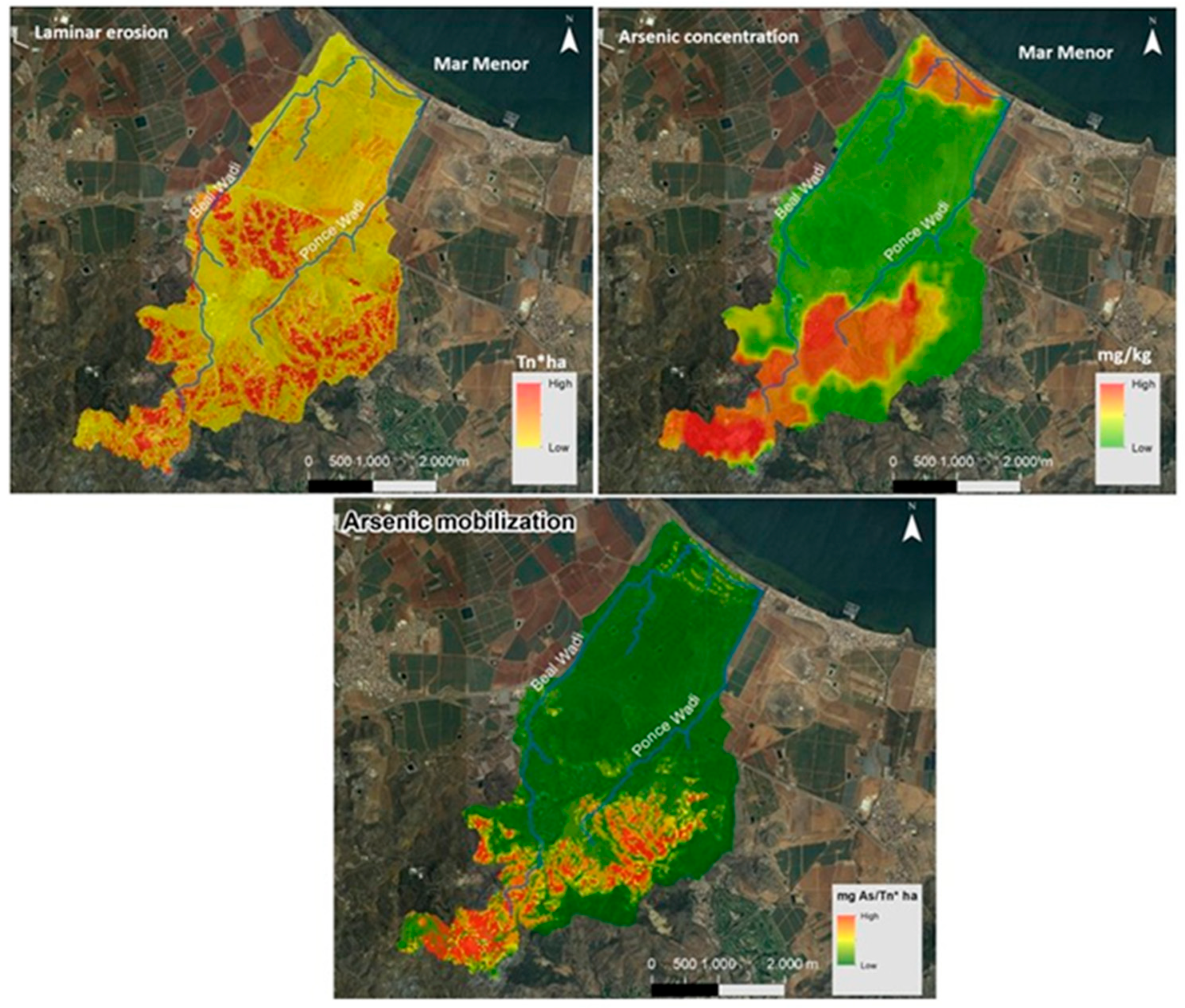
2.3.4. Analysis for the Development of Cartography: Laminar Erosion and As Concentration
2.3.5. Risk Analysis
- Calculation of the Oral Chemical Daily Intake (CDI)
- Risk characterisation for carcinogenic pollutants.
- Hazard characterisation for non-carcinogenic contaminants.
2.3.6. Statistics
3. Results and Discussion
3.1. General Analytical Features
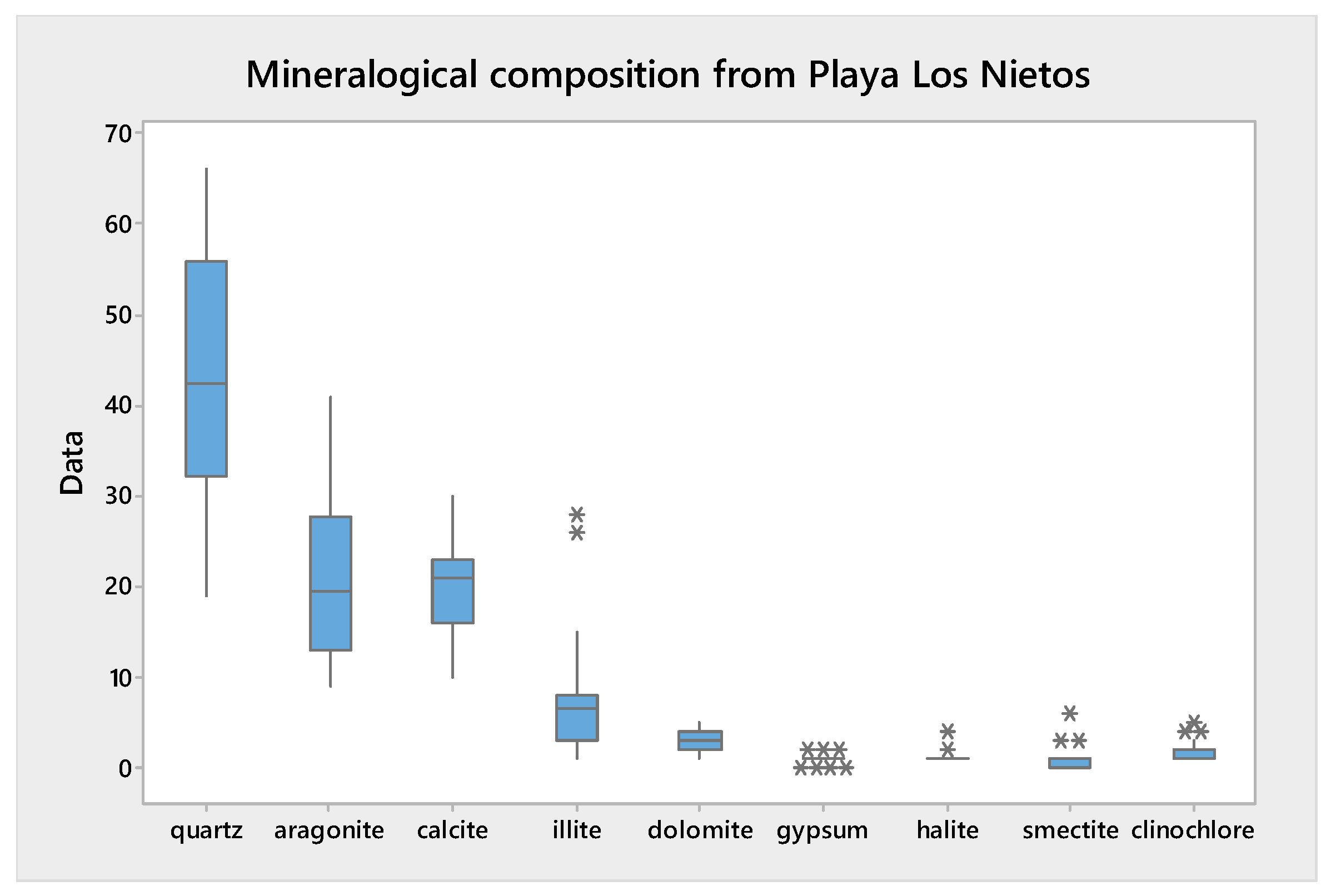
3.2. Mineralogical Composition
3.3. Arsenic Content
3.3.1. Arsenic in Particulate Material
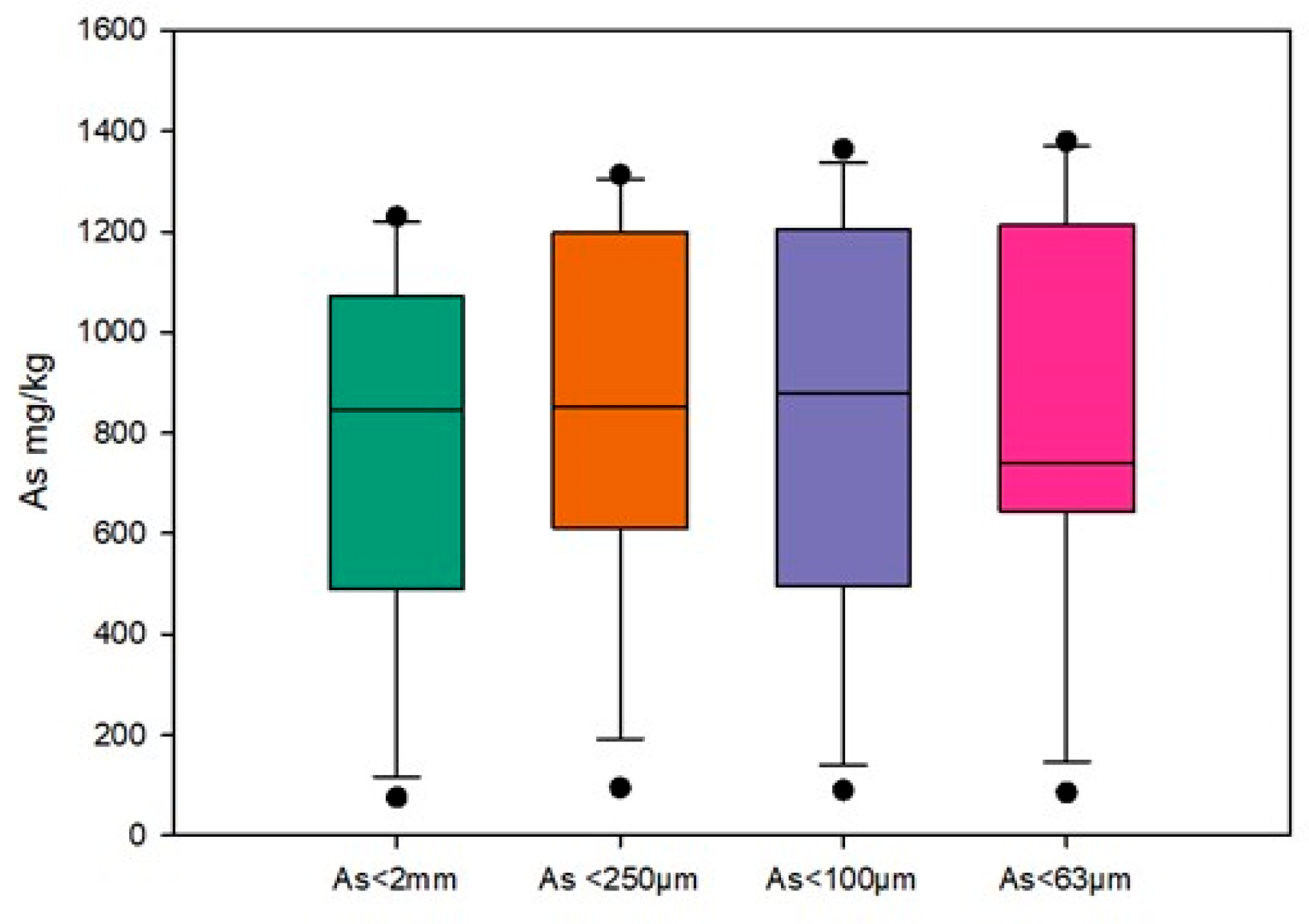
3.3.2. Total Arsenic
3.3.3. Soluble Arsenic
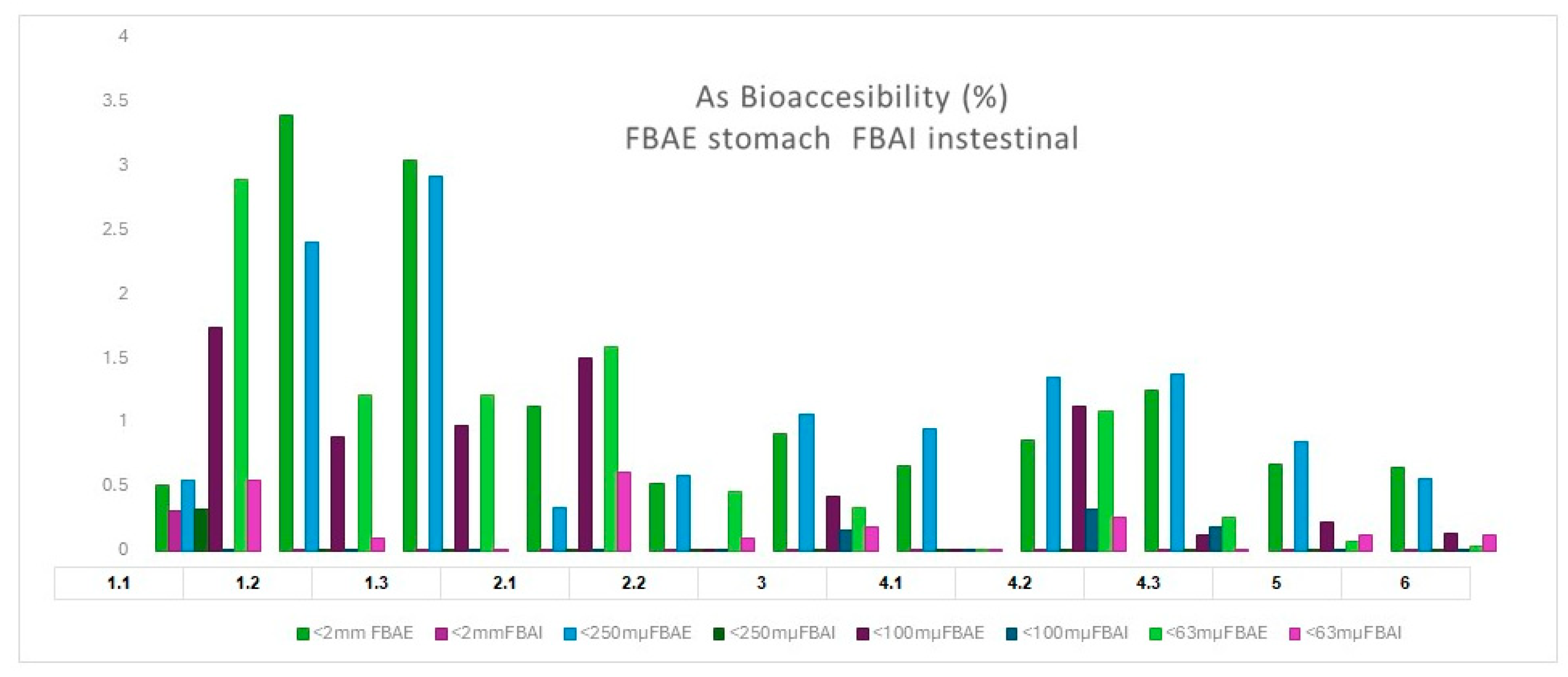
3.3.4. Bioaccessible Arsenic
3.4. Chemical Daily Intake and Risk Characterisation for Public Health
3.5. Relationship Between the Variables Determined
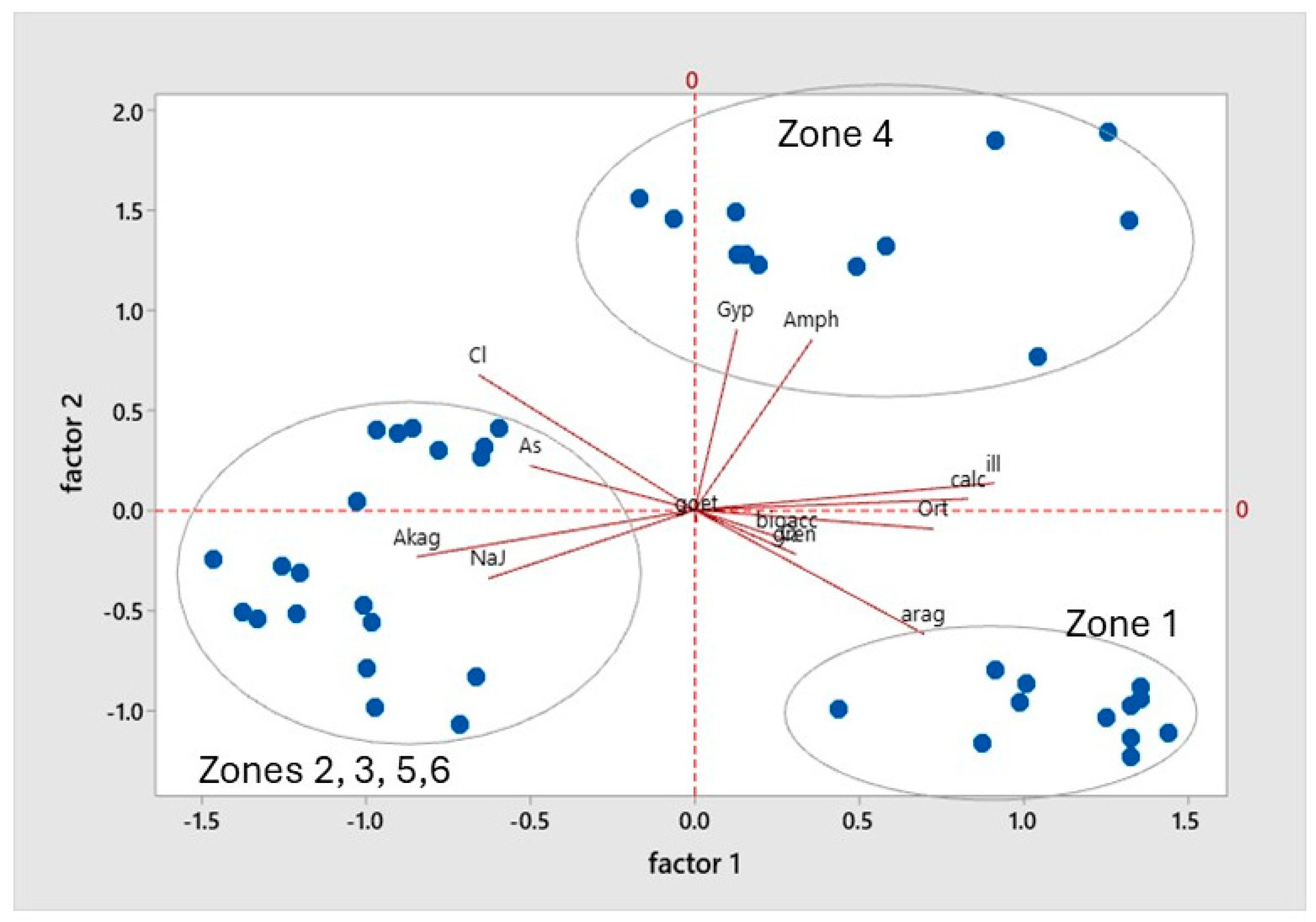
3.5.1. Relationship of the As Content with the Mineralogical Composition of the Soil
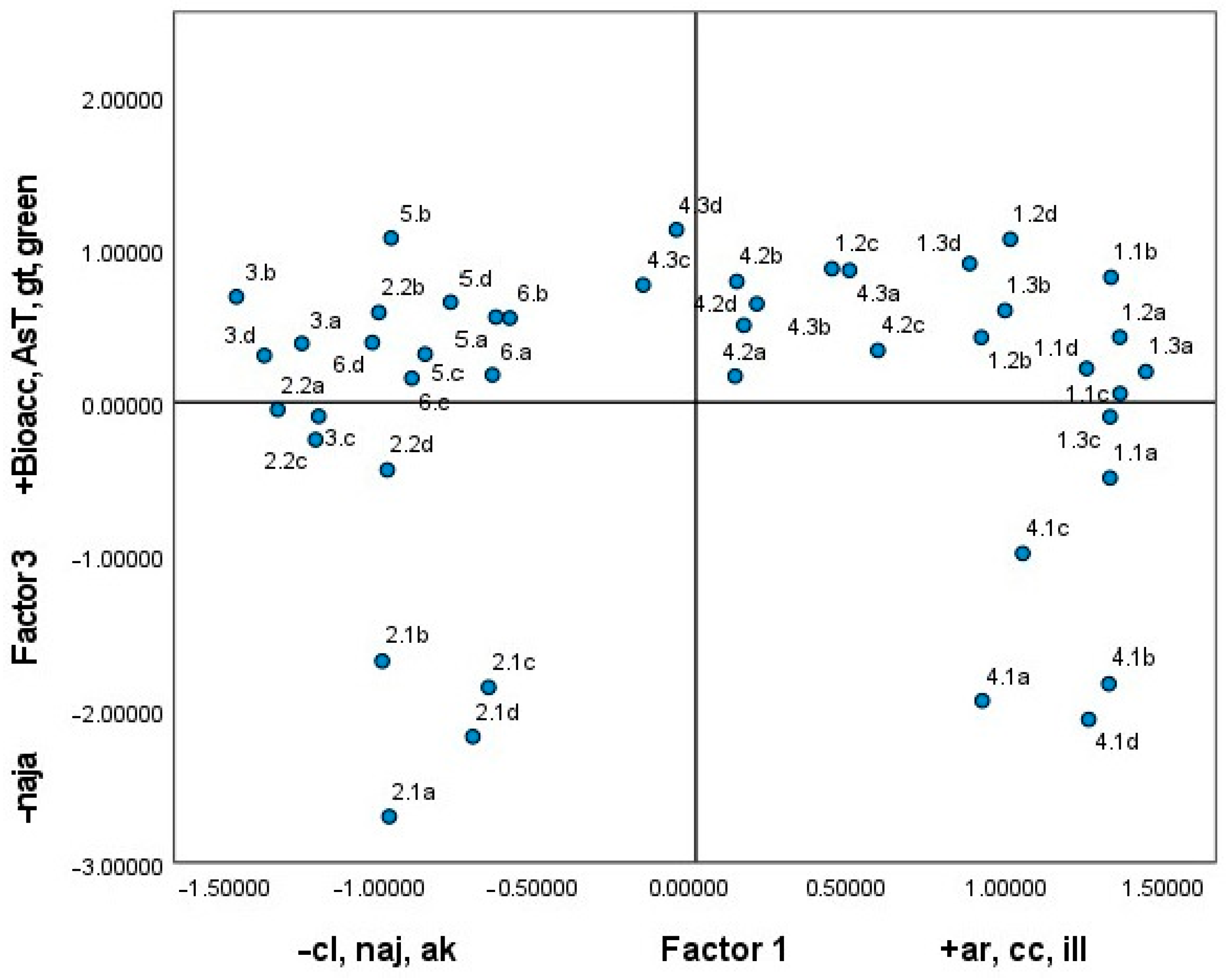
3.5.2. Relationship Between the As Content of the Soil and the Risk Analysis Determined
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhowmick, S.; Pramanik, S.; Singh, P.; Mondal, P.; Chatterjee, D.; Nriagu, J. Arsenic in groundwater of West Bengal, India: A review of human health risks and assessment of possible intervention options. Sci. Total Environ. 2018, 612, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Q.; Yao, J.; Lv, C.; Gao, Y.; Sun, D.; Yang, Y. MiR-96-5p Suppresses Progression of Arsenite-Induced Human Keratinocyte Proliferation and Malignant Transformation by Targeting Denticleless E3 Ubiquitin Protein Ligase Homolog. Toxics 2023, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Somsunun, K.; Prapamontol, T.; Kuanpan, T.; Santijitpakdee, T.; Kohsuwan, K.; Jeytawan, N.; Thongjan, N. Health Risk Assessment of Heavy Metals in Indoor Household Dust in Urban and Rural Areas of Chiang Mai and Lamphun Provinces, Thailand. Toxics 2023, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Escot-Espinoza, V.M.; Rodríguez-Márquez, S.; Briseño-Bugarín, J.; López-Luna, M.A.; Flores de la Torre, J.A. Presence of Potentially Toxic Elements in Historical Mining Areas in the North-Center of Mexico and Possible Bioremediation Strategies. Toxics 2024, 12, 813. [Google Scholar] [CrossRef]
- WHO (World Health Organization). As. 2018. Available online: https://www.who.int/publications/i/item/9789241565585 (accessed on 2 April 2024).
- Majumder, S.; Powell, M.A.; Biswas, P.K.; Banik, P. The impact of Arsenic induced stress on soil enzyme activity in different rice agroecosystems. Environ. Technol. Innov. 2022, 26, 102282. [Google Scholar] [CrossRef]
- Yao, Y.; Han, X.; Chen, Y.; Li, D. The variations of labile arsenic diffusion driven by algal bloom decomposition in eutrophic lake ecosystems. Sci. Total Environ. 2022, 842, 156703. [Google Scholar] [CrossRef]
- Raessler, M. The arsenic contamination of drinking and groundwaters in Bangladesh: Featuring biogeochemical aspects and implications on public health. Arch. Environ. Contam. Toxicol. 2018, 75, 1–7. [Google Scholar] [CrossRef]
- Buekers, J.; Baken, K.; Govarts, E.; Martin, L.R.; Vogel, N.; Kolossa-Gehring, M.; Šlejkovec, Z.; Falnoga, I.; Horvat, M.; Lignell, S.; et al. Human urinary arsenic species, associated exposure determinants and potential health risks assessed in the HBM4EU Aligned Studies. Int. J. Hyg. Environ. Health 2023, 248, 114115. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Wang, H.; Yang, J.; Zhang, X.; Li, Z.; Martín, J.D. A hydrochemical and isotopic approach for source identification and health risk assessment of groundwater arsenic pollution in the central Yinchuan basin. Environ. Res. 2023, 231 Pt 2, 116153. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Speciation and surface structure of inorganic arsenic in solid phases: A review. Environ. Int. 2008, 34, 867–879. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Chatterjee, D.; Singh, K.K.; Giri, A.K. Systems biology approaches to evaluate arsenic toxicity and carcinogenicity: An overview. Int. J. Hyg. Environ. Health 2013, 216, 574–586. [Google Scholar] [CrossRef]
- Han, L.; Zhai, Y.; Chen, R.; Fan, Y.; Liu, Z.; Zhao, Y.; Li, R.; Xia, L. Characteristics of Soil Arsenic Contamination and the Potential of Pioneer Plants for Arsenic Remediation in Gold Mine Tailings. Toxics 2023, 11, 1025. [Google Scholar] [CrossRef]
- EPA (Environmental Protection Agency). Guidance for Evaluating the Oral Bioavailability of Metals in Soils for Use in Human Health Risk Assessment. 2007. Available online: https://clu-in.org/conf/tio/bioavailability3/prez/beringer_maddaloni_combinedbw.pdf (accessed on 9 January 2024).
- IARC (International Arctic Research Center). Some Drinking-Water Disinfectants and Contaminants, Including Arsenic; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2004; Volume 84, pp. 1–477. [Google Scholar]
- Fazle Bari, A.S.M.; Lamb, D.; Choppala, G.; Seshadri, B.; Islam, M.R.; Sanderson, P.; Rahman, M.M. Arsenic bioaccessibility and fractionation in abandoned mine soils from selected sites in New South Wales, Australia and human health risk assessment. Ecotoxicol. Environ. Saf. 2021, 223, 112611. [Google Scholar] [CrossRef]
- Helser, J.; Vassilieva, E.; Cappuyns, V. Environmental and human health risk assessment of sulfidic mine waste: Bioaccessibility, leaching and mineralogy. J. Hazard. Mater. 2022, 424, 127313. [Google Scholar] [CrossRef]
- Hussain, S.; Khanam, T.; Ullah, S.; Aziz, F.; Sattar, A.; Hussain, I.; Saddique, M.A.B.; Maqsood, A.; Ding, C.; Wang, X.; et al. Assessment and Exposure Analysis of Trace Metals in Different Age Groups of the Male Population in Southern Punjab, Pakistan. Toxics 2023, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- NRC. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications; The National Academies Press: Washington, DC, USA, 2003; p. 432. [Google Scholar]
- Ng, J.; Juhasz, A.; Smith, E.; Naidu, R. Contaminant Bioavailability and Bioaccessibility. Part 1: A Scientific and Technical Review; CRC CARE for Contamination Assessment and Remediation of the Environment: Adelaide, Australia, 2010. [Google Scholar]
- O’Connor, K.P.; Montgomery, M.; Rosales, R.A.; Whiteman, K.K.; Kim, C.S. Wetting/drying cycles increase arsenic bioaccessibility in mine-impacted sediments. Sci. Total Environ. 2021, 774, 145420. [Google Scholar] [CrossRef]
- Xie, K.; Ou, J.; He, M.; Peng, W.; Yuan, Y. Predicting the Bioaccessibility of Soil Cd, Pb, and AS with Advanced Machine Learning for Continental-Scale Soil Environmental Criteria Determinatin I China. Environ. Health 2024, 2, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Zeng, J.-Y.; Ding, S.; Li, J.; Liu, X.; Guan, D.-X.; Ma, L.Q. Arsenic contents, speciation and bioaccessibility in rice grains from China: Regional and variety differences. J. Hazard. Mater. 2022, 437, 129431. [Google Scholar] [CrossRef] [PubMed]
- Griggs, J.L.; Chi, L.; Hanley, N.M.; Kohan, M.; Herbin-Davis, K.; Thomas, D.J.; Lu, K.; Fry, R.C.; Bradham, K.D. Bioaccessibility of arsenic from contaminated soils and alteration of the gut microbiome in an in vitro gastrointestinal model. Environ. Pollut. 2022, 309, 119753. [Google Scholar] [CrossRef]
- Barrio-Parra, F.; Izquierdo-Díaz, M.; Dominguez-Castillo, A.; Medina, R.; De Miguel, E. Human-health probabilistic risk assessment: The role of exposure factors in an urban garden scenario. Landsc. Urban Plan. 2019, 185, 191–199. [Google Scholar] [CrossRef]
- Martínez-Sánchez, M.J.; García Lorenzo, M.L.; Martínez-López, S.; Martínez-Martínez, L.B.; Hernández Pérez, C.; Pérez-Sirvent, C. El análisis de riesgos para la salud humana, en el paradigma de la gestión de suelos contmiandos: El caso de la Bahía de Portman. Rev. Salud Ambient. 2015, 15, 103–112. [Google Scholar]
- Liu, Y.; Ma, J.; Yan, H.; Ren, Y.; Wang, B.; Lin, C.; Liu, X. Bioaccessibility and health risk assessment of arsenic in soil and indoor dust in rural and urban areas of Hubei province, China. Ecotoxicol. Environ. Saf. 2016, 126, 14–22. [Google Scholar] [CrossRef]
- Pérez-Sirvent, C.; Martínez-Martínez, L.B.; Martínez-Lopez, S.; Hernández-Perez, C.; García-Lorenzo, M.L.; Bech, J.; Martínez-Sánchez, M.J. Assessment of risk from lead intake in mining areas: Proposal of indicators. Environ. Geochem. Health 2022, 44, 447–463. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, Y.; Mu, L.; Liu, W.; Wang, Q.; Su, T.; Yang, Q.; Milinga, A.; Zhang, Y. Health Risk Assessment of Heavy Metals in Agricultural Soils Based on Multi-Receptor Modeling Combined with Monte Carlo Simulation. Toxics 2024, 12, 643. [Google Scholar] [CrossRef]
- Hamel, S.C.; Buckley, B.; Lioy, P.J. Bioaccessibility of Metals in Soils for Different Liquid to Solid Ratios in Synthetic Gastric Fluid. Environ. Sci. Technol. 1998, 32, 358–362. [Google Scholar] [CrossRef]
- Ruby, M.V.; Schoof, R.; Brattin, W.; Goldade, M.; Post, G.; Harnois, M.; Mosby, D.E.; Casteel, S.W.; Berti, W.; Carpenter, M.; et al. Advances in Evaluating the Oral Bioavailability of Inorganics in Soil for Use in Human Health Risk Assessment. Environ. Sci. Technol. 1999, 33, 3697–3705. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; p. 867. [Google Scholar]
- Litter, M.I.; Armienta, M.A.; Farías, S.S. IBEROARSEN Metodologías Analíticas Para la Determinación y Especiación de Arsénico en Aguas y Suelos; CYTED: Buenos Aires, Argentina, 2009; ISBN 978-84-96023-71-0. [Google Scholar]
- Drahota, P.; Venhauerova, P.; Strnad, L. Speciation and mobility of arsenic and antimony in soils and mining wastes from an abandoned Sb–Au mining area. Appl. Geochem. 2023, 152, 105665. [Google Scholar] [CrossRef]
- Drahota, P.; Raus, K.; Rychlíková, E.; Rohovec, J. Bioaccessibility of As, Cu, Pb, and Zn in mine waste, urban soil, and road dust in the historical mining village of Kaňk, Czech Republic. Environ. Geochem. Health 2018, 40, 1495–1512. [Google Scholar] [CrossRef]
- Ma, M.; Wang, L.; Huang, W.; Zhong, X.; Li, L.; Wang, H.; Peng, B.; Mao, M. Meta-analysis of the correlation between serum uric acid level and carotid intima-media thickness. PLoS ONE 2021, 16, e0246416. [Google Scholar] [CrossRef]
- Xie, K.; Xie, N.; Liao, Z.; Luo, X.; Peng, W.; Yuan, Y. Bioaccessibility of arsenic, lead, and cadmium in contaminated m ing/smelting soils: Assessment, modeling, and application for soil environment criteria derivation. J. Hazard. Mater. 2023, 443, 130321. [Google Scholar] [CrossRef]
- Martínez-López, S.; Martínez-Sánchez, M.J.; Gómez-Martínez, M.d.C.; Pérez-Sirvent, C. Arsenic zoning in a coastal area of the Mediterranean Sea as a base for management and recovery of areas contaminated by old mining activities. Appl. Clay Sci. 2020, 199, 105881. [Google Scholar] [CrossRef]
- Martínez-Sánchez, M.J.; Martínez-López, S.; García-Lorenzo, M.L.; Martínez-Martínez, L.B.; Pérez-Sirvent, C. Evaluation of arsenic in soils and plant uptake using various chemical extraction methods in soils affected by old mining activities. Geoderma 2011, 160, 535–541. [Google Scholar] [CrossRef]
- Martínez-López, S.; Martínez-Sánchez, M.J.; Pérez-Sirvent, C. Do Old Mining Areas Represent an Environmental Problem and Health Risk? A Critical Discussion through a Particular Case. Minerals 2021, 11, 594. [Google Scholar] [CrossRef]
- Ruby, M.V.; Davis, A.; Link, T.E.; Schoof, R.; Chaney, R.L.; Freeman, G.B.; Bergstrom, P. 1993. Development of an in vitro screening-test to evaluate the in-vivo bioaccessibility of ingested mine-waste lead. Environ. Sci. Technol. 1993, 27, 2870–2877. [Google Scholar] [CrossRef]
- Meunier, L.; Walker, S.R.; Wragg, J.; Parsons, M.B.; Koch, I.; Jamieson, H.E.; Reimer, K.J. Effects of soil composition and mineralogy on the bioaccessibility of arsenic from tailings and soil in gold mine districts of Nova Scotia. Environ. Sci. Technol. 2010, 44, 2667–2674. [Google Scholar] [CrossRef]
- USEPA. Risk Based Concentration Table; United States Environmental Protection Agency: Philadelphia, PA, USA; Washington, DC, USA, 2000. [Google Scholar]
- Yamamoto, N.; Takahashi, Y.; Yoshinaga, J.; Tanaka, A.; Shibata, Y. Size distributions of soil particles adhered to children’s hands. Arch. Environ. Contam. Toxicol. 2006, 51, 157–163. [Google Scholar] [CrossRef]
- US EPA. Air Pollution: Current and Future Challenges. 2017. Available online: https://www.epa.gov/clean-air-act-overview/air-pollution-current-and-future-challenges (accessed on 3 April 2024).
- US EPA. Acid Rain. 2017. Available online: https://www.epa.gov/acidrain (accessed on 7 July 2024).
- Billmann, M.; Hulot, C.; Pauget, B.; Badreddine, R.; Papin, A.; Pelfrêne, A. Oral bioaccessibility of PTEs in soils: A review of data, influencing factors and application in human health risk assessment. Sci. Total Environ. 2023, 896, 165263. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lei, M.; Weng, L.; Li, Y.; Chen, Y.; Islam, M.S.; Zhao, J.; Chen, T. Fractions and colloidal distribution of arsenic associated with iron oxide minerals in lead-zinc mine-contaminated soils: Comparison of tailings and smelter pollution. Chemosphere 2019, 227, 614–623. [Google Scholar] [CrossRef]
- Martínez Sánchez, M.J.; Martínez López, S.; Martínez Martínez, L.B.; Pérez Sirvent, C. Importance of the oral arsenic bioaccessibility factor for characterising the risk associated with soil ingestion in a mining-influenced zone. J. Environ. Manag. 2013, 116, 10–17. [Google Scholar] [CrossRef]
- Directrices Para la Caracterización del Material Dragado y su Reubicación en Aguas del Dominio Público Marítimo-Terrestre. Comisión Interministerial de Estrategias Marinas. 2021. Available online: https://www.miteco.gob.es/content/dam/miteco/es/costas/temas/proteccion-medio-marino/220121_directrices_2021_final_tcm30-157006.pdf (accessed on 3 April 2025).
- ISO 14235:1998; Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. ISO: Geneva, Switzerland, 1998. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/iso?c=023140 (accessed on 4 June 2024).
- FAO. Soil Description Guide. In Guide to Soil Description, 4th ed.; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2009. [Google Scholar]
- El-Hadri, F.; Morales-Rubio, A.; de la Guardia, M. Atomic fluorescence spectrometric determination of trace amounts of arsenic and antimony in drinking water by continuous hydride generation. Talanta 2000, 52, 653–662. [Google Scholar] [CrossRef]
- Cava-Montesinos, P.; Cervera, M.L.; Pastor, A.; de la Guardia, M. Hydride generation atomic fluorescence spectrometric determination of ultratraces of selenium and tellurium in cow milk. Anal. Chim. Acta 2003, 481, 291–300. [Google Scholar] [CrossRef]
- Michon, G.; de Foresta, H.; Levang, P.; Verdeaux, F. Domestic Forests. A New Paradigm for Integrating Local Communities’ Forestry into Tropical Forest Science. Ecol. Soc. 2007, 12, 1. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Weber, J.; Smith, E.; Naidu, R.; Rees, M.; Rofe, A.; Kuchel, T.; Sansom, L. Assessment of Four Commonly Employed in Vitro Arsenic Bioaccessibility Assays for Predicting in Vivo Relative Arsenic Bioavailability in Contaminated Soils. Environ. Sci. Technol. 2009, 43, 9487–9494. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.R.; Basta, N.T.; Casteel, S.W.; Pace, L.W. An in-vitro gastro-intestinal method to assess bioavailable arsenic in contaminated soils and solid media. Environ. Sci. Technol. 1999, 33, 642–649. [Google Scholar] [CrossRef]
- Yang, J.K.; Barnett, M.O.; Jardine, P.M.; Basta, N.T.; Casteel, S.W. Adsorption, Sequestration, and Bioaccessibility of As (V) in Soils. Environ. Sci. Technol. 2002, 36, 4562–4569. [Google Scholar] [CrossRef]
- Martin, D. Qualitative, Quantitative and Microtextural Powder X-Ray Diffraction Analysis. 2019. Available online: http://www.xpowder.com/ (accessed on 21 July 2024).
- Gómez Martinez, M.A.; Martínez López, S. Repercusión de los Sistemas de Información Geográfica Como Herramienta Para la Investigación Ambiental. Innovación en la Gestión e Investigación Ambiental; Editorial Diego Marin-Librero: Murcia, Spain, 2015. [Google Scholar]
- Martínez López, S. El Arsénico en Suelos con Influencia Minera en Ambientes Semiáridos. Ph.D. Thesis, University of Murcia, Murcia, Spain, 2010. [Google Scholar]
- Inventario Nacional de Erosión de Suelos. 2022. Available online: https://www.miteco.gob.es/es/biodiversidad/servicios/banco-datos-naturaleza/informacion-disponible/inventario_nacional_erosion.html (accessed on 2 January 2024).
- EPA. Environmental Protection Agency. Superfund Risk Assessment. 2024. Available online: https://www.epa.gov/risk/superfund-risk-assessment (accessed on 8 September 2024).
- Basta, N.; Rodriguez, R.; Casteel, S. Bioavailability and risk of arsenic exposure by the soil ingestion pathway. In Environmental Chemistry of Arsenic; William, T.F., Jr., Ed.; CRC Press: New York, NY, USA, 2001; Volume 1, pp. 117–139. [Google Scholar]
- Veiga del Baño, J.M.; Martínez-López, S.; Pérez Sirvent, C.; Martínez Sánchez, M.J.; Andreo Martínez, P. Optimisation of the chemical immobilisation by limestone filler of heavy metals and metalloids in contaminated soils via response surface methodology (RSM). Min. Eng. 2023, 201, 108211. [Google Scholar] [CrossRef]
- Martínez Sánchez, M.J.; Pérez Sirvent, C. Niveles de Fondo y Niveles Genéricos de Referencia de Metales Pesados en Suelos de la Región de Murcia; Comunidad Autónoma de la Región de Murcia, Consejería de Desarrollo Sostenible y Ordenación del Territorio: Murcia, Spain, 2007; ISBN 978-84-6909-104. [Google Scholar]
- García Oliva, M.; Pérez Ruzafa, Á.; Umgiesser, G.; McKiver, W.; Ghezzo, M.; De Pascalis, F.; Marcos, C. Assessing the Hydrodynamic Response of the Mar Menor Lagoon to Dredging Inlets Interventions through Numerical Modelling. Water 2018, 10, 959. [Google Scholar] [CrossRef]

| Adults (Use) | Children (Use) | |||||
|---|---|---|---|---|---|---|
| Variable | E1 Residential | E2 Tourism | E3 Agriculture | E1 Residential | E2 Tourism | |
| Cs: arsenic concentration soil. (mg/kg). Total/bioaccessible | - | - | - | - | - | |
| IR: Soil ingestion rate (mg/day) | 100 | 100 | 200 | 200 | 200 | |
| EF: Exposure frequency (days) | 350 | 60 | 150 | 350 | 60 | |
| ED: exposure duration (years) | 30 | 30 | 30 | 6 | 6 | |
| CF: Conversion Factor (dimensionless) | 1.10−6 | 1.10−6 | 1.10−6 | 1.10−6 | 1.10−6 | |
| FI: ingestion factor (dimensionless) | 1 | 1 | 1 | 1 | 1 | |
| BW: body weight (Kg) | 70 | 70 | 70 | 15 | 15 | |
| AT: averaging time (days) | Carcinogenic | 25550 | 25550 | 25550 | 25550 | 25550 |
| Hazard index | 2190.ED | 10950.ED | 10950.ED | 2190.ED | 2190.ED | |
| Arsenic | Non-Carcinogen Toxicity | Carcinogen Toxicity | ||
| RfD Oral (mg/kg·day) | SF Oral (mg/kg·day) | |||
| 0.0003 | EPA | 1.5 | EPA | |
| Samples | pH | E.C. (mS/cm) | O.M. (%) | Texture (100%) | ||
|---|---|---|---|---|---|---|
| Clay | Silt | Sand | ||||
| 1.1 | 7.6 | 10.0 | 2.5 | 1.8 | 45.3 | 52.9 |
| 1.2 | 7.3 | 25.8 | 2.9 | 2.5 | 41.1 | 56.4 |
| 1.3 | 7.7 | 10.0 | 1.6 | 4.8 | 28.2 | 67 |
| 2.1 | 2.8 | 16.6 | N.D. | 22.3 | 57.5 | 20.2 |
| 2.2 | 3.3 | 21.8 | N.D. | 20.9 | 52.6 | 26.5 |
| 3 | 4.4 | 2.2 | N.D. | 5.5 | 18.1 | 76.4 |
| 4.1 | 5.4 | 3.1 | N.D. | 6.2 | 29.7 | 64.1 |
| 4.2 | 6.1 | 2.2 | N.D. | 4.9 | 25.2 | 69.9 |
| 4.3 | 5.2 | 2.5 | N.D. | 65.2 | 28.6 | 6.2 |
| 5 | 5.4 | 1.9 | N.D. | 3.1 | 8.4 | 88.5 |
| 6 | 4.6 | 2.14 | N.D. | 1.5 | 12.8 | 85.7 |
| Mean ± SD | 5.4 ± 1.6 | 8.9 ± 8.8 | - | 12.4 ± 18.9 | 26.9 ± 15.4 | 6.06 ± 28.8 |
| [As] mg/kg | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Total < 2 mm | Stomach Phase < 2 mm | Intestinal Phase < 2 mm | Total < 250 µm | Stomach Phase < 250 µm | Intestinal Phase < 250 µm | Total < 100 µm | Stomach Phase < 100 µm | Intestinal Phase < 100 µm | Total < 63 µm | Stomach Phase < 63 µm | Intestinal Phase < 63 µm |
| 1.1 | 489 | 2.5 | 1.5 | 580 | 3.2 | 1.9 | 495 | 8.6 | <ld | 644 | 18.6 | 3.5 |
| 1.2 | 539 | 18.3 | <ld | 809 | 19.5 | <ld | 845 | 7.5 | <ld | 728 | 8.8 | 0.7 |
| 1.3 | 528 | 16.1 | <ld | 611 | 17.8 | <ld | 690 | 6.7 | <ld | 712 | 8.6 | <ld |
| 2.1 | 283 | 3.2 | <ld | 1269 | 4.3 | <ld | 339 | 5.1 | <ld | 395 | 6.3 | 2.4 |
| 2.2 | 1184 | 6.2 | <ld | 1070 | 6.3 | <ld | 880 | <ld | <ld | 1199 | 5.5 | 1.2 |
| 3 | 959 | 8.7 | <ld | 853 | 9.1 | <ld | 1055 | 4.5 | 1.64 | 1040 | 3.5 | 1.9 |
| 4.1 | 75 | 0.5 | <ld | 95 | 0.9 | <ld | 90 | <ld | <ld | 85 | <ld | <ld |
| 4.2 | 1072 | 9.2 | <ld | 699 | 9.4 | <ld | 1135 | 12.8 | 3.60 | 1337 | 14.6 | 3.5 |
| 4.3 | 1230 | 15.3 | <ld | 1314 | 18.1 | <ld | 1364 | 1.66 | 2.49 | 740 | 1.9 | <ld |
| 5 | 945 | 6.4 | <ld | 1089 | 9.2 | <ld | 1204 | 2.65 | <ld | 1214 | 0.9 | 1.4 |
| 6 | 845 | 5.5 | <ld | 1199 | 6.7 | <ld | 1230 | 1.62 | <ld | 1380 | 0.5 | 1.6 |
| Range | 75–1184 | 0.5–18.3 | <0.5–1.5 | 95–1314 | 0.9–19.5 | <0.5–1.9 | 90–1364 | <0.5–12.8 | <0.5–3.6 | 85–1380 | 0.5–18.6 | 0.7–3.5 |
| Media ± SD | 740.8 ± 380.5 | 8.3 ± 6.1 | - | 871.6 ± 365.7 | 9.5 ± 6.3 | - | 847.9 ± 408.1 | 4.7 ± 3.9 | 2.6 ± 0.9 | 861.3 ± 408.8 | 6.3 ± 6.0 | 1.6 ± 1.2 |
| Sample | pH | Eh | E.C. | [As] μg L−1 | ||
|---|---|---|---|---|---|---|
| mV | (mS/cm) | [As] Total | %[As] III | %[As] V | ||
| w-1 | 7.03 ± 0.56 | 410 ± 9 | 14.35 ± 0.09 | 15 ± 6 | 2 | 98 |
| w-2 | 4.39 ± 1.51 | 310 ± 6 | 12.56 ± 0.18 | 950 ± 320 | 4 | 96 |
| w-3 | 8.98 ± 0.75 | 430 ± 8 | 3.54 ± 0.17 | 5 ± 2 | 1 | 99 |
| Risk Characterisation for As for Children | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario E1: Residential Use | ||||||||||||||||
| <2 mm | <250 µm | <100 µm | <63 µm | |||||||||||||
| SAMPLES | Without bioaccessibility | With bioaccessibility | Without bioaccessibility | With bioaccessibility | Without bioaccessibility | With bioaccessibility | Without bioaccessibility | With bioaccessibility | ||||||||
| Carcinogenic risk | Hazard index | Carcinogenic Risk | Hazard index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | |
| 1.1 | 8.0 × 10−4 | 20.84 | 4.1 × 10−6 | 0.11 | 9.5 × 10−4 | 24.72 | 5.3 × 10−6 | 0.14 | 8.1 × 10−4 | 21.10 | 1.4 × 10−5 | 0.37 | 1.1 × 10−3 | 27.45 | 3.1 × 10−5 | 0.79 |
| 1.2 | 8.9 × 10−4 | 22.97 | 3.0 × 10−5 | 0.78 | 1.3 × 10−3 | 34.48 | 3.2 × 10−5 | 0.83 | 1.4 × 10−3 | 36.01 | 1.2 × 10−5 | 0.32 | 1.2 × 10−3 | 31.03 | 1.4 × 10−5 | 0.37 |
| 1.3 | 8.7 × 10−4 | 22.50 | 2.6 × 10−5 | 0.69 | 1.0 × 10−3 | 26.04 | 2.9 × 10−5 | 0.76 | 1.1 × 10−3 | 29.41 | 1.1 × 10−5 | 0.29 | 1.2 × 10−3 | 30.34 | 1.4 × 10−5 | 0.37 |
| 2.1 | 4.7 × 10−4 | 12.06 | 5.3 × 10−6 | 0.14 | 2.1 × 10−3 | 54.08 | 7.1 × 10−6 | 0.18 | 5.6 × 10−4 | 14.45 | 8.4 × 10−6 | 0.22 | 6.5 × 10−4 | 16.83 | 1.0 × 10−5 | 0.27 |
| 2.2 | 1.9 × 10−3 | 50.46 | 1.0 × 10−5 | 0.26 | 1.8 × 10−3 | 45.60 | 1.0 × 10−5 | 0.27 | 1.4 × 10−3 | 37.50 | 6.6 × 10−7 | 0.02 | 2.0 × 10−3 | 51.10 | 9.1 × 10−6 | 0.23 |
| 3 | 1.6 × 10−3 | 40.87 | 1.4 × 10−5 | 0.37 | 1.4 × 10−3 | 36.35 | 1.5 × 10−5 | 0.39 | 1.7 × 10−3 | 44.96 | 7.4 × 10−6 | 0.19 | 1.7 × 10−3 | 44.32 | 5.8 × 10−6 | 0.15 |
| 4.1 | 1.2 × 10−4 | 3.20 | 8.2 × 10−7 | 0.02 | 1.6 × 10−4 | 4.05 | 1.5 × 10−6 | 0.04 | 1.5 × 10−4 | 3.84 | 6.6 × 10−8 | 0.00 | 1.4 × 10−4 | 3.62 | 3.3 × 10−8 | 0.00 |
| 4.2 | 1.8 × 10−3 | 45.69 | 1.5 × 10−5 | 0.39 | 1.1 × 10−3 | 29.79 | 1.5 × 10−5 | 0.40 | 1.9 × 10−3 | 48.37 | 2.1 × 10−5 | 0.55 | 2.2 × 10−3 | 56.98 | 2.4 × 10−5 | 0.62 |
| 4.3 | 2.0 × 10−3 | 52.42 | 2.5 × 10−5 | 0.65 | 2.2 × 10−3 | 56.00 | 3.0 × 10−5 | 0.77 | 2.3 × 10−3 | 58.81 | 4.1 × 10−6 | 0.11 | 1.2 × 10−3 | 31.54 | 3.1 × 10−6 | 0.08 |
| 5 | 1.6 × 10−3 | 40.27 | 1.1 × 10−5 | 0.27 | 1.8 × 10−3 | 46.41 | 1.5 × 10−5 | 0.39 | 2.0 × 10−3 | 51.31 | 4.4 × 10−6 | 0.11 | 2.0 × 10−3 | 51.74 | 2.3 × 10−6 | 0.06 |
| 6 | 1.4 × 10−3 | 36.01 | 9.0 × 10−6 | 0.23 | 2.0 × 10−3 | 51.10 | 1.1 × 10−5 | 0.29 | 2.0 × 10−3 | 52.42 | 2.7 × 10−6 | 0.07 | 2.2 × 10−3 | 58.13 | 2.7 × 10−6 | 0.07 |
| Scenario E2: Tourism Use | ||||||||||||||||
| 1.1 | 1.4 × 10−4 | 3.57 | 7.0 × 10−7 | 0.02 | 1.6 × 10−4 | 4.24 | 9.0 × 10−7 | 0.02 | 1.4 × 10−4 | 3.62 | 2.4 × 10−6 | 0.06 | 1.8 × 10−4 | 4.71 | 5.2 × 10−6 | 0.14 |
| 1.2 | 1.5 × 10−4 | 3.94 | 5.2 × 10−6 | 0.13 | 2.3 × 10−4 | 5.91 | 5.5 × 10−6 | 0.14 | 2.4 × 10−4 | 6.17 | 2.1 × 10−6 | 0.05 | 2.1 × 10−4 | 5.32 | 2.5 × 10−6 | 0.06 |
| 1.3 | 1.5 × 10−4 | 3.86 | 4.5 × 10−6 | 0.12 | 1.7 × 10−4 | 4.46 | 5.0 × 10−6 | 0.13 | 1.9 × 10−4 | 5.04 | 1.9 × 10−6 | 0.05 | 2.0 × 10−4 | 5.20 | 2.4 × 10−6 | 0.06 |
| 2.1 | 8.0 × 10−5 | 2.07 | 9.0 × 10−7 | 0.02 | 3.6 × 10−4 | 9.27 | 1.2 × 10−6 | 0.03 | 9.6 × 10−5 | 2.48 | 1.4 × 10−6 | 0.04 | 1.1 × 10−4 | 2.89 | 1.8 × 10−6 | 0.05 |
| 2.2 | 3.3 × 10−4 | 8.65 | 1.7 × 10−6 | 0.05 | 3.0 × 10−4 | 7.82 | 1.8 × 10−6 | 0.05 | 2.5 × 10−4 | 6.43 | 1.1 × 10−7 | 0.00 | 3.4 × 10−4 | 8.76 | 1.6 × 10−6 | 0.04 |
| 3 | 2.7 × 10−4 | 7.01 | 2.5 × 10−6 | 0.06 | 2.4 × 10−4 | 6.23 | 2.6 × 10−6 | 0.07 | 3.0 × 10−4 | 7.71 | 1.3 × 10−6 | 0.03 | 2.9 × 10−4 | 7.60 | 9.9 × 10−7 | 0.03 |
| 4.1 | 2.1 × 10−5 | 0.55 | 1.4 × 10−7 | 0.00 | 2.7 × 10−5 | 0.69 | 2.5 × 10−7 | 0.01 | 2.5 × 10−5 | 0.66 | 1.1 × 10−8 | 0.00 | 2.4 × 10−5 | 0.62 | 5.6 × 10−9 | 0.00 |
| 4.2 | 3.0 × 10−4 | 7.83 | 2.6 × 10−6 | 0.07 | 2.0 × 10−4 | 5.11 | 2.6 × 10−6 | 0.07 | 3.2 × 10−4 | 8.29 | 3.6 × 10−6 | 0.09 | 3.8 × 10−4 | 9.77 | 4.1 × 10−6 | 0.11 |
| 4.3 | 3.5 × 10−4 | 8.99 | 4.3 × 10−6 | 0.11 | 3.7 × 10−4 | 9.60 | 5.1 × 10−6 | 0.13 | 3.9 × 10−4 | 10.08 | 7.0 × 10−7 | 0.02 | 2.1 × 10−4 | 5.41 | 5.3 × 10−7 | 0.01 |
| 5 | 2.7 × 10−4 | 6.90 | 1.8 × 10−6 | 0.05 | 3.1 × 10−4 | 7.96 | 2.6 × 10−6 | 0.07 | 3.4 × 10−4 | 8.80 | 7.5 × 10−7 | 0.02 | 3.4 × 10−4 | 8.87 | 4.0 × 10−7 | 0.01 |
| 6 | 2.4 × 10−4 | 6.17 | 1.5 × 10−6 | 0.04 | 3.4 × 10−4 | 8.76 | 1.9 × 10−6 | 0.05 | 3.5 × 10−4 | 8.99 | 4.6 × 10−7 | 0.01 | 3.8 × 10−4 | 9.97 | 4.6 × 10−7 | 0.01 |
| Risk Characterisation for As for Adults | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario E1: Residential Use | ||||||||||||||||
| <2 mm | <250 µm | <100 µm | <63 µm | |||||||||||||
| SAMPLES | Without bioaccessibility | With bioaccessibility | Without bioaccessibility | With bioaccessibility | Without bioaccessibility | With bioaccessibility | Without bioaccessibility | With bioaccessibility | ||||||||
| Carcinogenic risk | Hazard index | Carcinogenic Risk | Hazard index | Carcinogenic risk | Hazard Index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | Carcinogenic risk | Hazard index | |
| 1.1 | 4.3 × 10−4 | 2.23 | 2.2 × 10−6 | 0.01 | 5.1 × 10−4 | 2.65 | 2.8 × 10−6 | 0.01 | 4.4 × 10−4 | 2.26 | 7.6 × 10−6 | 0.04 | 5.7 × 10−4 | 2.94 | 1.6 × 10−5 | 0.08 |
| 1.2 | 4.7 × 10−4 | 2.46 | 1.6 × 10−5 | 0.08 | 7.1 × 10−4 | 3.69 | 1.7 × 10−5 | 0.09 | 7.4 × 10−4 | 3.86 | 6.6 × 10−6 | 0.03 | 6.4 × 10−4 | 3.32 | 7.7 × 10−6 | 0.04 |
| 1.3 | 4.6 × 10−4 | 2.41 | 1.4 × 10−5 | 0.07 | 5.4 × 10−4 | 2.79 | 1.6 × 10−5 | 0.08 | 6.1 × 10−4 | 3.15 | 5.9 × 10−6 | 0.03 | 6.3 × 10−4 | 3.25 | 7.6 × 10−6 | 0.04 |
| 2.1 | 2.5 × 10−4 | 1.29 | 2.8 × 10−6 | 0.01 | 1.1 × 10−3 | 5.79 | 3.8 × 10−6 | 0.02 | 3.0 × 10−4 | 1.55 | 4.5 × 10−6 | 0.02 | 3.5 × 10−4 | 1.80 | 5.6 × 10−6 | 0.03 |
| 2.2 | 1.0 × 10−3 | 5.41 | 5.5 × 10−6 | 0.03 | 9.4 × 10−4 | 4.89 | 5.5 × 10−6 | 0.03 | 7.7 × 10−4 | 4.02 | 3.5 × 10−7 | 0.00 | 1.1 × 10−3 | 5.47 | 4.9 × 10−6 | 0.03 |
| 3 | 8.4 × 10−4 | 4.38 | 7.7 × 10−6 | 0.04 | 7.5 × 10−4 | 3.89 | 8.0 × 10−6 | 0.04 | 9.3 × 10−4 | 4.82 | 4.0 × 10−6 | 0.02 | 9.2 × 10−4 | 4.75 | 3.1 × 10−6 | 0.02 |
| 4.1 | 6.6 × 10−5 | 0.34 | 4.4 × 10−7 | 0.00 | 8.4 × 10−5 | 0.43 | 7.9 × 10−7 | 0.00 | 7.9 × 10−5 | 0.41 | 3.5 × 10−8 | 0.00 | 7.5 × 10−5 | 0.39 | 1.8 × 10−8 | 0.00 |
| 4.2 | 9.4 × 10−4 | 4.89 | 8.1 × 10−6 | 0.04 | 6.2 × 10−4 | 3.19 | 8.3 × 10−6 | 0.05 | 1.0 × 10−3 | 5.18 | 1.1 × 10−5 | 0.06 | 1.2 × 10−3 | 6.11 | 1.3 × 10−5 | 0.07 |
| 4.3 | 1.1 × 10−3 | 5.62 | 1.3 × 10−5 | 0.07 | 1.2 × 10−3 | 6.00 | 1.6 × 10−5 | 0.08 | 1.2 × 10−3 | 6.30 | 2.2 × 10−6 | 0.01 | 6.5 × 10−4 | 3.38 | 1.7 × 10−6 | 0.01 |
| 5 | 8.3 × 10−4 | 4.32 | 5.6 × 10−6 | 0.03 | 9.6 × 10−4 | 4.97 | 8.1 × 10−6 | 0.04 | 1.1 × 10−3 | 5.50 | 2.3 × 10−6 | 0.01 | 1.1 × 10−3 | 5.54 | 1.2 × 10−6 | 0.01 |
| 6 | 7.4 × 10−4 | 3.86 | 4.8 × 10−6 | 0.03 | 1.1 × 10−3 | 5.47 | 5.9 × 10−6 | 0.03 | 1.1 × 10−3 | 5.62 | 1.4 × 10−6 | 0.01 | 1.2 × 10−3 | 6.23 | 1.4 × 10−6 | 0.01 |
| Scenario E2: tourism use | ||||||||||||||||
| 1.1 | 7.4 × 10−5 | 0.38 | 3.8 × 10−7 | 0.00 | 8.8 × 10−5 | 0.45 | 4.8 × 10−7 | 0.00 | 7.5 × 10−5 | 0.39 | 1.3 × 10−6 | 0.01 | 9.7 × 10−5 | 0.50 | 2.8 × 10−6 | 0.01 |
| 1.2 | 8.1 × 10−5 | 0.42 | 2.8 × 10−6 | 0.01 | 1.2 × 10−4 | 0.63 | 2.9 × 10−6 | 0.02 | 1.3 × 10−4 | 0.66 | 1.1 × 10−6 | 0.01 | 1.1 × 10−4 | 0.57 | 1.3 × 10−6 | 0.01 |
| 1.3 | 8.0 × 10−5 | 0.41 | 2.4 × 10−6 | 0.01 | 9.2 × 10−5 | 0.48 | 2.7 × 10−6 | 0.01 | 1.0 × 10−4 | 0.54 | 1.0 × 10−6 | 0.01 | 1.1 × 10−4 | 0.56 | 1.3 × 10−6 | 0.01 |
| 2.1 | 4.3 × 10−5 | 0.22 | 4.8 × 10−7 | 0.00 | 1.9 × 10−4 | 0.99 | 6.5 × 10−7 | 0.00 | 5.1 × 10−5 | 0.27 | 7.7 × 10−7 | 0.00 | 6.0 × 10−5 | 0.31 | 9.5 × 10−7 | 0.00 |
| 2.2 | 1.8 × 10−4 | 0.93 | 9.4 × 10−7 | 0.00 | 1.6 × 10−4 | 0.84 | 9.5 × 10−7 | 0.00 | 1.3 × 10−4 | 0.69 | 6.0 × 10−8 | 0.00 | 1.8 × 10−4 | 0.94 | 8.3 × 10−7 | 0.00 |
| 3 | 1.4 × 10−4 | 0.75 | 1.3 × 10−6 | 0.01 | 1.3 × 10−4 | 0.67 | 1.4 × 10−6 | 0.01 | 1.6 × 10−4 | 0.83 | 6.8 × 10−7 | 0.00 | 1.6 × 10−4 | 0.81 | 5.3 × 10−7 | 0.00 |
| 4.1 | 1.1 × 10−5 | 0.06 | 7.5 × 10−8 | 0.00 | 1.4 × 10−4 | 0.07 | 1.4 × 10−7 | 0.00 | 1.4 × 10−5 | 0.07 | 6.0 × 10−9 | 0.00 | 1.3 × 10−5 | 0.07 | 3.0 × 10−9 | 0.00 |
| 4.2 | 1.6 × 10−4 | 0.84 | 1.4 × 10−6 | 0.01 | 1.1 × 10−4 | 0.55 | 1.4 × 10−6 | 0.01 | 1.7 × 10−4 | 0.89 | 1.9 × 10−6 | 0.01 | 2.0 × 10−4 | 1.05 | 2.2 × 10−6 | 0.01 |
| 4.3 | 1.9 × 10−4 | 0.96 | 2.3 × 10−6 | 0.01 | 2.0 × 10−4 | 1.03 | 2.7 × 10−6 | 0.01 | 2.1 × 10−4 | 1.08 | 3.8 × 10−7 | 0.00 | 1.1 × 10−4 | 0.58 | 2.8 × 10−7 | 0.00 |
| 5 | 1.4 × 10−4 | 0.74 | 9.7 × 10−7 | 0.01 | 1.6 × 10−4 | 0.85 | 1.4 × 10−6 | 0.01 | 1.8 × 10−4 | 0.94 | 4.0 × 10−7 | 0.00 | 1.8 × 10−4 | 0.95 | 2.1 × 10−7 | 0.00 |
| 6 | 1.3 × 10−4 | 0.66 | 8.3 × 10−7 | 0.00 | 1.8 × 10−4 | 0.94 | 1.0 × 10−6 | 0.01 | 1.9 × 10−4 | 0.96 | 2.4 × 10−7 | 0.00 | 2.1 × 10−4 | 1.07 | 2.5 × 10−7 | 0.00 |
| Scenario E3: agriculture use | ||||||||||||||||
| 1.1 | 3.7 × 10−4 | 1.91 | 1.9 × 10−6 | 0.01 | 4.4 × 10−4 | 2.27 | 2.4 × 10−6 | 0.01 | 3.7 × 10−4 | 1.94 | 6.5 × 10−6 | 0.03 | 4.9 × 10−4 | 2.52 | 1.4 × 10−5 | 0.07 |
| 1.2 | 4.1 × 10−4 | 2.11 | 1.4 × 10−5 | 0.07 | 6.1 × 10−4 | 3.17 | 1.5 × 10−5 | 0.07 | 6.4 × 10−4 | 3.31 | 5.7 × 10−6 | 0.03 | 5.5 × 10−4 | 2.85 | 6.6 × 10−6 | 0.03 |
| 1.3 | 4.0 × 10−4 | 2.07 | 1.2 × 10−5 | 0.06 | 4.6 × 10−4 | 2.39 | 1.3 × 10−5 | 0.07 | 5.2 × 10−4 | 2.70 | 5.1 × 10−6 | 0.03 | 5.4 × 10−4 | 2.79 | 6.5 × 10−6 | 0.03 |
| 2.1 | 2.1 × 10−4 | 1.11 | 2.4 × 10−6 | 0.01 | 9.6 × 10−4 | 4.97 | 3.2 × 10−6 | 0.02 | 2.6 × 10−4 | 1.33 | 3.8 × 10−6 | 0.02 | 3.0 × 10−4 | 1.55 | 4.8 × 10−6 | 0.02 |
| 2.2 | 8.9 × 10−4 | 4.63 | 4.7 × 10−6 | 0.02 | 8.1 × 10−4 | 4.19 | 4.8 × 10−6 | 0.02 | 6.6 × 10−4 | 3.44 | 3.0 × 10−7 | 0.00 | 9.1 × 10−4 | 4.69 | 4.2 × 10−6 | 0.02 |
| 3 | 7.2 × 10−4 | 3.75 | 6.6 × 10−6 | 0.03 | 6.4 × 10−4 | 3.34 | 6.9 × 10−6 | 0.03 | 8.0 × 10−4 | 4.13 | 3.4 × 10−6 | 0.02 | 7.9 × 10−4 | 4.07 | 2.6 × 10−6 | 0.01 |
| 4.1 | 5.7 × 10−5 | 0.29 | 3.8 × 10−7 | 0.00 | 7.2 × 10−5 | 0.37 | 6.8 × 10−7 | 0.00 | 6.8 × 10−5 | 0.35 | 3.0 × 10−8 | 0.00 | 6.4 × 10−5 | 0.33 | 1.5 × 10−8 | 0.00 |
| 4.2 | 8.1 × 10−4 | 4.20 | 6.9 × 10−6 | 0.04 | 5.3 × 10−4 | 2.74 | 7.1 × 10−6 | 0.04 | 8.6 × 10−4 | 4.44 | 9.7 × 10−6 | 0.05 | 1.0 × 10−3 | 5.23 | 1.1 × 10−5 | 0.06 |
| 4.3 | 9.3 × 10−4 | 4.81 | 1.2 × 10−5 | 0.06 | 9.9 × 10−4 | 5.14 | 1.4 × 10−5 | 0.07 | 1.0 × 10−3 | 5.40 | 1.9 × 10−6 | 0.01 | 5.6 × 10−4 | 2.90 | 1.4 × 10−6 | 0.01 |
| 5 | 7.1 × 10−4 | 3.70 | 4.8 × 10−6 | 0.03 | 8.2 × 10−4 | 4.26 | 6.9 × 10−6 | 0.04 | 9.1 × 10−4 | 4.71 | 2.0 × 10−6 | 0.01 | 9.2 × 10−4 | 4.75 | 1.1 × 10−6 | 0.01 |
| 6 | 6.4 × 10−4 | 3.31 | 4.2 × 10−6 | 0.02 | 9.1 × 10−4 | 4.69 | 5.1 × 10−6 | 0.03 | 9.3 × 10−4 | 4.81 | 1.2 × 10−6 | 0.01 | 1.0 × 10−3 | 5.34 | 1.2 × 10−6 | 0.01 |
| arag | calc | ill | gren | Cl | NaJ | akag | As | bioac | |
|---|---|---|---|---|---|---|---|---|---|
| Q | 0.492 ** | 0.276 | 0.182 | 0.330 * | −0.336 * | −0.544 ** | −0.183 | 0.095 | 0.276 |
| ort | 0.539 ** | 0.620 ** | 0.656 ** | 0.333 * | −0.479 ** | −0.527 ** | −0.476 ** | 0.204 | 0.290 |
| arag | 0.621 ** | 0.566 ** | 0.499 ** | −0.835 ** | −0.387 ** | −0.455 ** | 0.300 * | 0.402 ** | |
| calc | 0.740 ** | 0.467 ** | −0.421 ** | −0.688 ** | −0.766 ** | 0.155 | 0.302 * | ||
| ill | 0.346 * | −0.492 ** | −0.687 ** | −0.781 ** | 0.305 * | 0.392 ** | |||
| gren | −0.188 | −0.513 ** | −0.298 * | 0.321 * | 0.563 ** | ||||
| Cl | 0.115 | 0.373 * | 0.583 ** | −0.260 | |||||
| NaJ | 0.500 ** | 0.160 | −0.293 |
| Model Summary | R | R Squared | Adjusted R Squared | Std. Error of the Estimate | Sig. |
| 0.713 | 0.509 | 0.458 | 3.89805 | ||
| Unstandardised Coefficients | Standardised Coefficients | t | |||
| B | Std. Error | Beta | |||
| (Constant) | −2.812 | 2.624 | −1.072 | 0.290 | |
| illite | 0.184 | 0.079 | 0.314 | 2.315 | 0.026 |
| greenalite | 0.752 | 0.214 | 0.484 | 3.515 | 0.01 |
| As total content | 0.004 | 0.002 | 0.290 | 2.116 | 0.041 |
| goethite | −0.350 | 0.168 | −0.249 | −2.088 | 0.43 |
| ANOVA Model Summary Regression Residual Total | Sum of squares | gl | Mean quadratic | F | Sig. |
| 613.639 | 4 | 153.410 | 10.096 | 0.000 | |
| 592.596 | 39 | 15.195 | |||
| 1206.235 | 43 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez López, S.; Pérez Sirvent, C.; Martínez Sánchez, M.J.; Esteban Abad, M.Á. Human Health Risk and Bioaccessibility of Arsenic in Wadis and Marine Sediments in a Coastal Lagoon (Mar Menor, Spain). Toxics 2025, 13, 647. https://doi.org/10.3390/toxics13080647
Martínez López S, Pérez Sirvent C, Martínez Sánchez MJ, Esteban Abad MÁ. Human Health Risk and Bioaccessibility of Arsenic in Wadis and Marine Sediments in a Coastal Lagoon (Mar Menor, Spain). Toxics. 2025; 13(8):647. https://doi.org/10.3390/toxics13080647
Chicago/Turabian StyleMartínez López, Salvadora, Carmen Pérez Sirvent, María José Martínez Sánchez, and María Ángeles Esteban Abad. 2025. "Human Health Risk and Bioaccessibility of Arsenic in Wadis and Marine Sediments in a Coastal Lagoon (Mar Menor, Spain)" Toxics 13, no. 8: 647. https://doi.org/10.3390/toxics13080647
APA StyleMartínez López, S., Pérez Sirvent, C., Martínez Sánchez, M. J., & Esteban Abad, M. Á. (2025). Human Health Risk and Bioaccessibility of Arsenic in Wadis and Marine Sediments in a Coastal Lagoon (Mar Menor, Spain). Toxics, 13(8), 647. https://doi.org/10.3390/toxics13080647







