Dual Effects of Maternal Diet and Perinatal Organophosphate Flame Retardant Treatment on Offspring Development, Behavior and Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Experimental Design
2.2. Flame Retardants
2.3. Tissue Preparation and Quantitative Real-Time PCR
2.4. Behavior Assays
2.5. Metabolic and Physiological Parameters
2.6. Data Analysis
3. Results
3.1. Maternal Diet-Induced Obesity Model
3.2. Anogenital Distance Measurements at PND 7
3.3. Blood–Brain Barrier Disruption in PND 0 Neonates
3.4. Hypothalamic Gene Expression in PND 0 and PND 14 Neonates
3.4.1. Neuropeptides
3.4.2. KNDy Neurons
3.4.3. Receptors
3.5. Hepatic Gene Expression in PND 0 and PND 14 Neonates
3.5.1. Receptors
3.5.2. Enzymes
3.5.3. Xenobiotic Targets
3.6. Adult Offspring Behavior Tests—Avoidance
3.6.1. Open Field Test (OFT)
3.6.2. Elevated Plus Maze (EPM)
3.6.3. Light/Dark Box Emergence Test (LDB)
3.7. Adult Offspring Behavior Test—Hippocampal-Dependent Memory
3.8. Adult Body Composition
3.9. Adult Metabolic Phenotyping
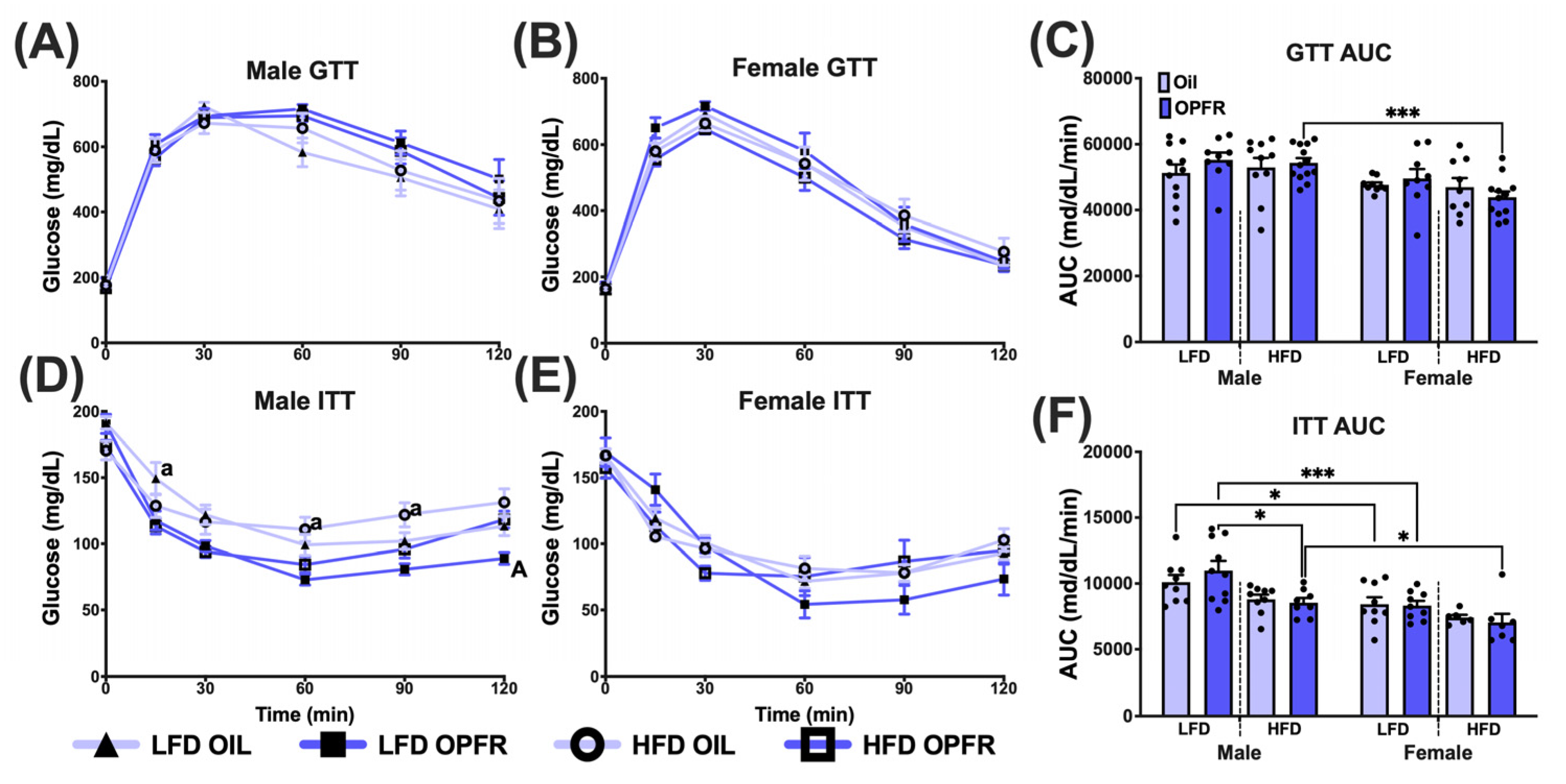
3.10. Adult Glucose Homeostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGD | Anogenital Distance |
| AUC | Area Under the Curve |
| BDNF | Brain-Derived Neurotrophic Factor |
| BDCPP | Bis(1,3-dichloro-2-propyl)phosphate |
| BMI | Body Mass Index |
| CANDLE | Conditions Affecting Neurocognitive Development and Learning in Early Childhood |
| CLAMS | Comprehensive Lab Animal Monitoring System |
| DBUP | Di-isobutyl Phosphate |
| DOHaD | Developmental Origins of Health and Disease |
| DIBP | Di-isobutyl Phthalate |
| DIO | Diet-Induced Obesity |
| EPM | Elevated Plus Maze |
| ERα | Estrogen Receptor Alpha |
| ERE | Estrogen Response Element |
| GD | Gestational Day |
| GTT | Glucose Tolerance Test |
| HFD | High-Fat Diet |
| ITT | Insulin Tolerance Test |
| LDB | Light-Dark Box |
| LFD | Low-Fat Diet |
| MBH | Mediobasal Hypothalamus |
| NHANES | National Health and Nutrition Examination Survey |
| OFT | Open Field Test |
| OPE | Organophosphate Ester |
| OPFR | Organophosphate Flame Retardants |
| PBDE | Polybrominated Diphenyl Ether |
| PND | Postnatal Day |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| RAR | Retinoic Acid Receptor |
| RER | Respiratory Exchange Ratio |
| TCP | Tricresyl Phosphate |
| TDCPP | Tris(1,3-dichloro-2-propyl) Phosphate |
| TPP | Triphenyl Phosphate |
References
- Cui, Y.; Zhou, R.; Yin, Y.; Liu, Y.; Zhao, N.; Li, H.; Zhang, A.; Li, X.; Fu, J. Occurrence of Organophosphate Esters in Food and Food Contact Materials and Related Human Exposure Risks. J. Agric. Food Chem. 2025, 73, 4455–4465. [Google Scholar] [CrossRef]
- Shi, S.; Feng, Q.; Zhang, J.; Wang, X.; Zhao, L.; Fan, Y.; Hu, P.; Wei, P.; Bu, Q.; Cao, Z. Global patterns of human exposure to flame retardants indoors. Sci. Total Environ. 2024, 912, 169393. [Google Scholar] [CrossRef]
- Tokumura, M.; Seo, M.; Wang, Q.; Miyake, Y.; Amagai, T.; Makino, M. Dermal exposure to plasticizers in nail polishes: An alternative major exposure pathway of phosphorus-based compounds. Chemosphere 2019, 226, 316–320. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Z.; Chen, X.; Xu, Z.; Ruan, J. Organophosphate flame retardants in air from formal e-waste recycling workshops in China: Size-distribution, gas-particle partitioning and exposure assessment. Environ. Pollut. 2024, 359, 124593. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, F.; Huang, C.; Hu, J. Exposure levels and maternal transfer of emerging organophosphate flame retardants (OPFRs) in pregnant women: Comparison with traditional OPFRs. Environ. Int. 2024, 191, 108996. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, P.; Zhao, L.; Zhu, L.; Wu, F. Transplacental Behaviors of Organophosphate Tri- and Diesters Based on Paired Human Maternal and Cord Whole Blood: Efficiencies and Impact Factors. Environ. Sci. Technol. 2021, 55, 3091–3100. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Wang, Y.; Li, W.; Kannan, K. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ. Int. 2019, 125, 342–349. [Google Scholar] [CrossRef]
- Hernández, A.F.; Romero-Molina, D.; Gonzalez-Alzaga, B.; López-Flores, I.; Lacasaña, M. Changes in molecular biomarkers of neurotoxicity in newborns following prenatal exposure to organophosphate compounds. Chemosphere 2025, 374, 144204. [Google Scholar] [CrossRef]
- Percy, Z.; Vuong, A.M.; Xu, Y.; Xie, C.; Ospina, M.; Calafat, A.M.; Hoofnagle, A.; Lanphear, B.P.; Braun, J.M.; Cecil, K.M.; et al. Maternal Urinary Organophosphate Esters and Alterations in Maternal and Neonatal Thyroid Hormones. Am. J. Epidemiol. 2021, 190, 1793–1802. [Google Scholar] [CrossRef]
- Lapehn, S.; Parenti, M.; Firsick, E.J.; Khodasevich, D.; Baker, B.H.; Day, D.B.; MacDonald, J.W.; Bammler, T.K.; Kannan, K.; Choi, H.Y.; et al. An assessment of organophosphate ester mixtures and the placental transcriptome. Environ. Int. 2025, 198, 109402. [Google Scholar] [CrossRef]
- Hoffman, K.; Stapleton, H.M.; Lorenzo, A.; Butt, C.M.; Adair, L.; Herring, A.H.; Daniels, J.L. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environ. Int. 2018, 116, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Liu, W.; Tao, Y.; Wang, L.; Yu, M.; Hu, L.; Zhou, A.; Covaci, A.; Xia, W.; Li, Y.; et al. Prenatal Exposure to Organophosphate Flame Retardants and the Risk of Low Birth Weight: A Nested Case-Control Study in China. Environ. Sci. Technol. 2020, 54, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Wiersielis, K.; Yasrebi, A.; Conde, K.; Armstrong, L.; Guo, G.L.; Roepke, T.A. Sex- and age-dependent effects of maternal organophosphate flame-retardant exposure on neonatal hypothalamic and hepatic gene expression. Reprod. Toxicol. 2020, 94, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Krumm, E.A.; Patel, V.J.; Tillery, T.S.; Yasrebi, A.; Shen, J.; Guo, G.L.; Marco, S.M.; Buckley, B.T.; Roepke, T.A. Organophosphate flame-retardants alter adult mouse homeostasis and gene expression in a sex-dependent manner potentially through interactions with ERα. Toxicol. Sci. 2018, 162, 212–224. [Google Scholar] [CrossRef]
- Chen, S.; Che, S.; Li, S.; Ruan, Z. The combined impact of decabromodiphenyl ether and high fat exposure on non-alcoholic fatty liver disease in vivo and in vitro. Toxicology 2021, 464, 153015. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Hoffman, K.; Stapleton, H.M. Characterization of Adipogenic Activity of House Dust Extracts and Semi-Volatile Indoor Contaminants in 3T3-L1 Cells. Environ. Sci. Technol. 2017, 51, 8735–8745. [Google Scholar] [CrossRef]
- Liu, Q.S.; Sun, Z.; Ren, X.; Ren, Z.; Liu, A.; Zhang, J.; Zhou, Q.; Jiang, G. Chemical Structure-Related Adipogenic Effects of Tetrabromobisphenol A and Its Analogues on 3T3-L1 Preadipocytes. Environ. Sci. Technol. 2020, 54, 6262–6271. [Google Scholar] [CrossRef]
- Okeke, E.S.; Feng, W.; Luo, M.; Mao, G.; Chen, Y.; Zhao, T.; Wu, X.; Yang, L. RNA-Seq analysis offers insight into the TBBPA-DHEE-induced endocrine-disrupting effect and neurotoxicity in juvenile zebrafish (Danio rerio). Gen. Comp. Endocrinol. 2024, 350, 114469. [Google Scholar] [CrossRef]
- Xu, M.; Wang, W.; Feng, J.; Ruan, Z.; Le, Y.; Liu, Y.; Zhang, Q.; Wang, C. The mechanism underlying pentabromoethylbenzene-induced adipogenesis and the obesogenic outcome in both cell and mouse model. Environ. Int. 2023, 178, 108088. [Google Scholar] [CrossRef]
- Tung, E.W.Y.; Ahmed, S.; Peshdary, V.; Atlas, E. Firemaster® 550 and its components isopropylated triphenyl phosphate and triphenyl phosphate enhance adipogenesis and transcriptional activity of peroxisome proliferator activated receptor (Pparγ) on the adipocyte protein 2 (aP2) promoter. PLoS ONE 2017, 12, e0175855. [Google Scholar] [CrossRef]
- Pillai, H.K.; Fang, M.; Beglov, D.; Kozakov, D.; Vajda, S.; Stapleton, H.M.; Webster, T.F.; Schlezinger, J.J. Ligand binding and activation of PPARγ by Firemaster® 550: Effects on adipogenesis and osteogenesis in vitro. Environ. Health Perspect. 2014, 122, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Driscoll AK, G.E. Increases in Prepregnancy Obesity: United States, 2016–2019; NCHS Data Brief, 2020 Nov:392:(1–8); 2020. Available online: https://pubmed.ncbi.nlm.nih.gov/33270551/ (accessed on 24 July 2025).
- Fisher, S.C.; Kim, S.Y.; Sharma, A.J.; Rochat, R.; Morrow, B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev. Med. 2013, 56, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Deputy, N.P.; Dub, B.; Sharma, A.J. Prevalence and Trends in Prepregnancy Normal Weight—48 States, New York City, and District of Columbia, 2011–2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 66, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Orós, M.; Lorenzo, M.; Serna, M.C.; Siscart, J.; Perejón, D.; Salinas-Roca, B. Obesity in Pregnancy as a Risk Factor in Maternal and Child Health-A Retrospective Cohort Study. Metabolites 2024, 14, 56. [Google Scholar] [CrossRef]
- Smith, C.J.; Ryckman, K.K. Epigenetic and developmental influences on the risk of obesity, diabetes, and metabolic syndrome. Diabetes Metab. Syndr. Obes. 2015, 8, 295–302. [Google Scholar] [CrossRef]
- Mustieles, V.; Fernández, M.F.; Messerlian, C. In Utero Metabolic Disruption—A Preventable Antecedent of Adult Disease? JAMA Netw. Open 2024, 7, e2412022. [Google Scholar] [CrossRef]
- Ross, M.G.; Desai, M. Developmental programming of offspring obesity, adipogenesis, and appetite. Clin. Obs. Gynecol. 2013, 56, 529–536. [Google Scholar] [CrossRef]
- Ribaroff, G.A.; Wastnedge, E.; Drake, A.J.; Sharpe, R.M.; Chambers, T.J.G. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: A meta-regression analysis. Obes. Rev. 2017, 18, 673–686. [Google Scholar] [CrossRef]
- Kim, D.W.; Glendining, K.A.; Grattan, D.R.; Jasoni, C.L. Maternal Obesity in the Mouse Compromises the Blood-Brain Barrier in the Arcuate Nucleus of Offspring. Endocrinology 2016, 157, 2229–2242. [Google Scholar] [CrossRef]
- Boyle, M.; Buckley, J.P.; Quirós-Alcalá, L. Associations between urinary organophosphate ester metabolites and measures of adiposity among U.S. children and adults: NHANES 2013–2014. Environ. Int. 2019, 127, 754–763. [Google Scholar] [CrossRef]
- Guo, X.; Ke, Y.; Wu, B.; Song, Q.; Sun, C.; Li, Y.; Wang, H.; Su, W.; Liang, Q.; Lowe, S.; et al. Exploratory analysis of the association between organophosphate ester mixtures with high blood pressure of children and adolescents aged 8–17 years: Cross-sectional findings from the National Health and Nutrition Examination Survey. Environ. Sci. Pollut. Res. 2023, 30, 22900–22912. [Google Scholar] [CrossRef]
- Peterson, A.K.; Alexeeff, S.E.; Ames, J.L.; Feng, J.; Yoshida, C.; Avalos, L.A.; Barrett, E.S.; Bastain, T.M.; Bennett, D.H.; Buckley, J.P.; et al. Gestational exposure to organophosphate ester flame retardants and risk of childhood obesity in the environmental influences on child health outcomes consortium. Environ. Int. 2024, 193, 109071. [Google Scholar] [CrossRef]
- Erkin-Cakmak, A.; Harley, K.G.; Chevrier, J.; Bradman, A.; Kogut, K.; Huen, K.; Eskenazi, B. In Utero and Childhood Polybrominated Diphenyl Ether Exposures and Body Mass at Age 7 Years: The CHAMACOS Study. Environ. Health Perspect. 2015, 123, 636–642. [Google Scholar] [CrossRef]
- Walley, S.N.; Krumm, E.A.; Yasrebi, A.; Kwiecinski, J.; Wright, V.; Baker, C.; Roepke, T.A. Maternal organophosphate flame-retardant exposure alters offspring energy and glucose homeostasis in a sexually dimorphic manner in mice. J. Appl. Toxicol. 2021, 41, 572–586. [Google Scholar] [CrossRef]
- Walley, S.N.; Krumm, E.A.; Yasrebi, A.; Wiersielis, K.R.; O’Leary, S.; Tillery, T.; Roepke, T.A. Maternal organophosphate flame-retardant exposure alters offspring feeding, locomotor and exploratory behaviors in a sexually-dimorphic manner in mice. J. Appl. Toxicol. 2021, 41, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. A Maternal High-Fat Diet Induces DNA Methylation Changes That Contribute to Glucose Intolerance in Offspring. Front. Endocrinol. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Mennitti, L.V.; Oyama, L.M.; Santamarina, A.B.; Nascimento, O.d.; Pisani, L.P. Influence of maternal consumption of different types of fatty acids during pregnancy and lactation on lipid and glucose metabolism of the 21-day-old male offspring in rats. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Gali Ramamoorthy, T.; Allen, T.-J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int. J. Obes. 2018, 42, 1431–1444. [Google Scholar] [CrossRef]
- Yonatan, E.; Shukha, O.N.; Golani, I.; Abu-ata, S.; Awad-Igbaria, Y.; Khatib, N.; Ginsberg, Y.; Palzur, E.; Beloosesky, R.; Shamir, A. Maternal N-acetylcysteine supplementation in lactation ameliorates metabolic and cognitive deficits in adult offspring exposed to maternal obesity. Neuropharmacology 2025, 271, 110390. [Google Scholar] [CrossRef]
- Stegmann, S.K.; Vohlen, C.; Im, N.G.; Niehues, J.; Selle, J.; Janoschek, R.; Kuiper-Makris, C.; Lang, S.; Demir, M.; Steffen, H.M.; et al. Perinatal obesity primes the hepatic metabolic stress response in the offspring across life span. Sci. Rep. 2025, 15, 6416. [Google Scholar] [CrossRef]
- Roepke, T.A.; Yasrebi, A.; Villalobos, A.; Krumm, E.A.; Yang, J.A.; Mamounis, K.J. Loss of ERα partially reverses the effects of maternal high-fat diet on energy homeostasis in female mice. Sci. Rep. 2017, 7, 6381. [Google Scholar] [CrossRef]
- Mamounis, K.J.; Shvedov, N.R.; Margolies, N.; Yasrebi, A.; Roepke, T.A. The effects of dietary fatty acids in the physiological outcomes of maternal high-fat diet on offspring energy homeostasis in mice. J. Dev. Orig. Health Dis. 2020, 11, 273–284. [Google Scholar] [CrossRef]
- Roepke, T.A.; Yasrebi, A.; Villalobos, A.; Krumm, E.A.; Yang, J.A.; Mamounis, K.J. The loss of ERE-dependent ERα signaling potentiates the effects of maternal high-fat diet on energy homeostasis in female offspring fed an obesogenic diet. J. Dev. Orig. Health Dis. 2020, 11, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Papalou, O.; Kandaraki, E.A.; Papadakis, G.; Diamanti-Kandarakis, E. Endocrine Disrupting Chemicals: An Occult Mediator of Metabolic Disease. Front. Endocrinol. 2019, 10, 112. [Google Scholar] [CrossRef]
- Chen, S.; Pan, L.; He, A.; Wang, Y.; Xiao, N.; Luo, H.; Yao, Y.; Sun, H. Detection of novel organophosphorus flame retardants and plasticizers in children urines: Associations with oxidative stress damage and thyroid nodule risk. Environ. Int. 2025, 199, 109445. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, M.; Jin, R.; Zhao, H.; Xia, C.; Liu, M.; Liu, X.; Yang, Y.; Wu, Y. Multimedia screening of conventional and emerging organophosphate esters alongside urbanized coasts. Mar. Pollut. Bull. 2025, 216, 117994. [Google Scholar] [CrossRef]
- Muglia, L.J.; Benhalima, K.; Tong, S.; Ozanne, S. Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med. 2022, 20, 418. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, X.; Huo, X. Anogenital distance and its application in environmental health research. Environ. Sci. Pollut. Res. 2014, 21, 5457–5464. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.L.; Basu, S.; Minium, J.; Hauguel-de-Mouzon, S.; Catalano, P. 48: Obesity in pregnancy is associated with decreased placental estradiol biosynthesis. Am. J. Obstet. Gynecol. 2014, 210, S32. [Google Scholar] [CrossRef]
- Kloboves, M.E.; Pacyga, D.C.; Gardiner, J.C.; Flaws, J.A.; Schantz, S.L.; Strakovsky, R.S. Associations of maternal anthropometrics with newborn anogenital distance and the 2:4 digit ratio. Hum. Reprod. 2022, 37, 2154–2166. [Google Scholar] [CrossRef]
- Rhea, E.M.; Salameh, T.S.; Logsdon, A.F.; Hanson, A.J.; Erickson, M.A.; Banks, W.A. Blood-Brain Barriers in Obesity. Aaps J. 2017, 19, 921–930. [Google Scholar] [CrossRef]
- Denzer, L.; Muranyi, W.; Schroten, H.; Schwerk, C. The role of PLVAP in endothelial cells. Cell Tissue Res. 2023, 392, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhang, B.; Fu, S.; Cai, Y.; Shi, Y. Penetration of Organophosphate Triesters and Diesters across the Blood-Cerebrospinal Fluid Barrier: Efficiencies, Impact Factors, and Mechanisms. Env. Sci. Technol. 2022, 56, 8221–8230. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Feng, C.; Jin, X.; Wang, F.; Liu, C.; Li, N.; Qiao, Y.; Bai, Y.; Wu, F.; Giesy, J.P. Organophosphate esters cause thyroid dysfunction via multiple signaling pathways in zebrafish brain. Environ. Sci. Ecotechnol 2022, 12, 100198. [Google Scholar] [CrossRef]
- Tozuka, Y.; Kumon, M.; Wada, E.; Onodera, M.; Mochizuki, H.; Wada, K. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 2010, 57, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Asadi, Y.; Moundounga, R.K.; Chakroborty, A.; Pokokiri, A.; Wang, H. FOXOs and their roles in acute and chronic neurological disorders. Front. Mol. Biosci. 2025, 12, 1538472. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Wang, Y.; Hong, J.; Shi, M.; Pfaff, D.; Guo, L.; Tang, H. Triphenyl phosphate permeates the blood brain barrier and induces neurotoxicity in mouse brain. Chemosphere 2020, 252, 126470. [Google Scholar] [CrossRef]
- Skowronski, A.A.; Shaulson, E.D.; Leibel, R.L.; LeDuc, C.A. The postnatal leptin surge in mice is variable in both time and intensity and reflects nutritional status. Int. J. Obes. 2022, 46, 39–49. [Google Scholar] [CrossRef]
- Toste, F.P.; de Moura, E.G.; Lisboa, P.C.; Fagundes, A.T.; de Oliveira, E.; Passos, M.C.F. Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rat. Br. J. Nutr. 2006, 95, 830–837. [Google Scholar] [CrossRef]
- Kirk, S.L.; Samuelsson, A.-M.; Argenton, M.; Dhonye, H.; Kalamatianos, T.; Poston, L.; Taylor, P.D.; Coen, C.W. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE 2009, 4, e5870. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, C.; Borgquist, A.; Nestor, C.C.; Smith, A.W.; Bosch, M.A.; Ku, S.; Wagner, E.J.; Rønnekleiv, O.K.; Kelly, M.J. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014, 19, 682–693. [Google Scholar] [CrossRef]
- Zhu, Y.; Jing, L.; Li, X.; Zheng, D.; Zhou, G.; Zhang, Y.; Sang, Y.; Shi, Z.; Sun, Z.; Zhou, X. Decabromodiphenyl ether disturbs hepatic glycolipid metabolism by regulating the PI3K/AKT/GLUT4 and mTOR/PPARγ/RXRα pathway in mice and L02 cells. Sci. Total Environ. 2021, 763, 142936. [Google Scholar] [CrossRef]
- MacTavish, B.S.; Zhu, D.; Shang, J.; Shao, Q.; He, Y.; Yang, Z.J.; Kamenecka, T.M.; Kojetin, D.J. Ligand efficacy shifts a nuclear receptor conformational ensemble between transcriptionally active and repressive states. Nat. Commun. 2025, 16, 2065. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Piccolo, B.D.; Mercer, K.E.; Andres, A.; Thakali, K.M.; Shankar, K. Maternal High-Fat Diet Programs Offspring Liver Steatosis in a Sexually Dimorphic Manner in Association with Changes in Gut Microbial Ecology in Mice. Sci. Rep. 2018, 8, 16502. [Google Scholar] [CrossRef]
- Tastet, V.; Le Vée, M.; Carteret, J.; Malnoë, D.; Bruyère, A.; Fardel, O. Repression of bile salt efflux pump expression by tri-ortho-cresyl phosphate in cultured human hepatic cells. Toxicol. Vitro 2025, 105, 106021. [Google Scholar] [CrossRef]
- Wang, D.; Yan, S.; Yan, J.; Teng, M.; Meng, Z.; Li, R.; Zhou, Z.; Zhu, W. Effects of triphenyl phosphate exposure during fetal development on obesity and metabolic dysfunctions in adult mice: Impaired lipid metabolism and intestinal dysbiosis. Environ. Pollut. 2019, 246, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, J.; Chen, Y.; Jiang, M.; Liu, Q.; Zhang, J.; Lu, X.; Hong, J.; Sun, W.; Sun, Y.; et al. Gestation and lactation triphenyl phosphate exposure disturbs offspring gut microbiota in a sex-dependent pathway. Food Chem. Toxicol. 2023, 172, 113579. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Taylor, R.E.; Guo, G.L. In vivo mouse models to study bile acid synthesis and signaling. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.R.; Phillips, A.L.; Horman, B.; Arambula, S.E.; Rebuli, M.E.; Stapleton, H.M.; Patisaul, H.B. Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Sci. Rep. 2017, 7, 7118. [Google Scholar] [CrossRef]
- Wiersielis, K.R.; Adams, S.; Yasrebi, A.; Conde, K.; Roepke, T.A. Maternal exposure to organophosphate flame retardants alters locomotor and anxiety-like behavior in male and female adult offspring. Horm. Behav. 2020, 122, 104759. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Lupu, D.S. High fat diet-induced maternal obesity alters fetal hippocampal development. Int. J. Dev. Neurosci. 2009, 27, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.M.; Wiersielis, K.R.; Roepke, T.A. Mechanisms of Flame Retardant Toxicity and Their Impacts on Anxiety and Cognition in the Brain. Endocrinology 2025, 166, bqaf074. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriades, A.; Koulouraki, S.; Belegrinos, A.; Eleftheriades, M.; Pervanidou, P. Maternal Obesity and Neurodevelopment of the Offspring. Nutrients 2025, 17, 891. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.; Zhu, M.; Impey, S.; Lambert, T.J.; Bland, T.; Karatsoreos, I.N.; Nakazawa, T.; Appleyard, S.M.; Wayman, G.A. Leptin Induces Hippocampal Synaptogenesis via CREB-Regulated MicroRNA-132 Suppression of p250GAP. Mol. Endocrinol. 2014, 28, 1073–1087. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef]
- Sun, B.; Purcell, R.H.; Terrillion, C.E.; Yan, J.; Moran, T.H.; Tamashiro, K.L.K. Maternal High-Fat Diet During Gestation or Suckling Differentially Affects Offspring Leptin Sensitivity and Obesity. Diabetes 2012, 61, 2833–2841. [Google Scholar] [CrossRef]
- Cordner, Z.A.; Khambadkone, S.G.; Boersma, G.J.; Song, L.; Summers, T.N.; Moran, T.H.; Tamashiro, K.L.K. Maternal high-fat diet results in cognitive impairment and hippocampal gene expression changes in rat offspring. Exp. Neurol. 2019, 318, 92–100. [Google Scholar] [CrossRef]
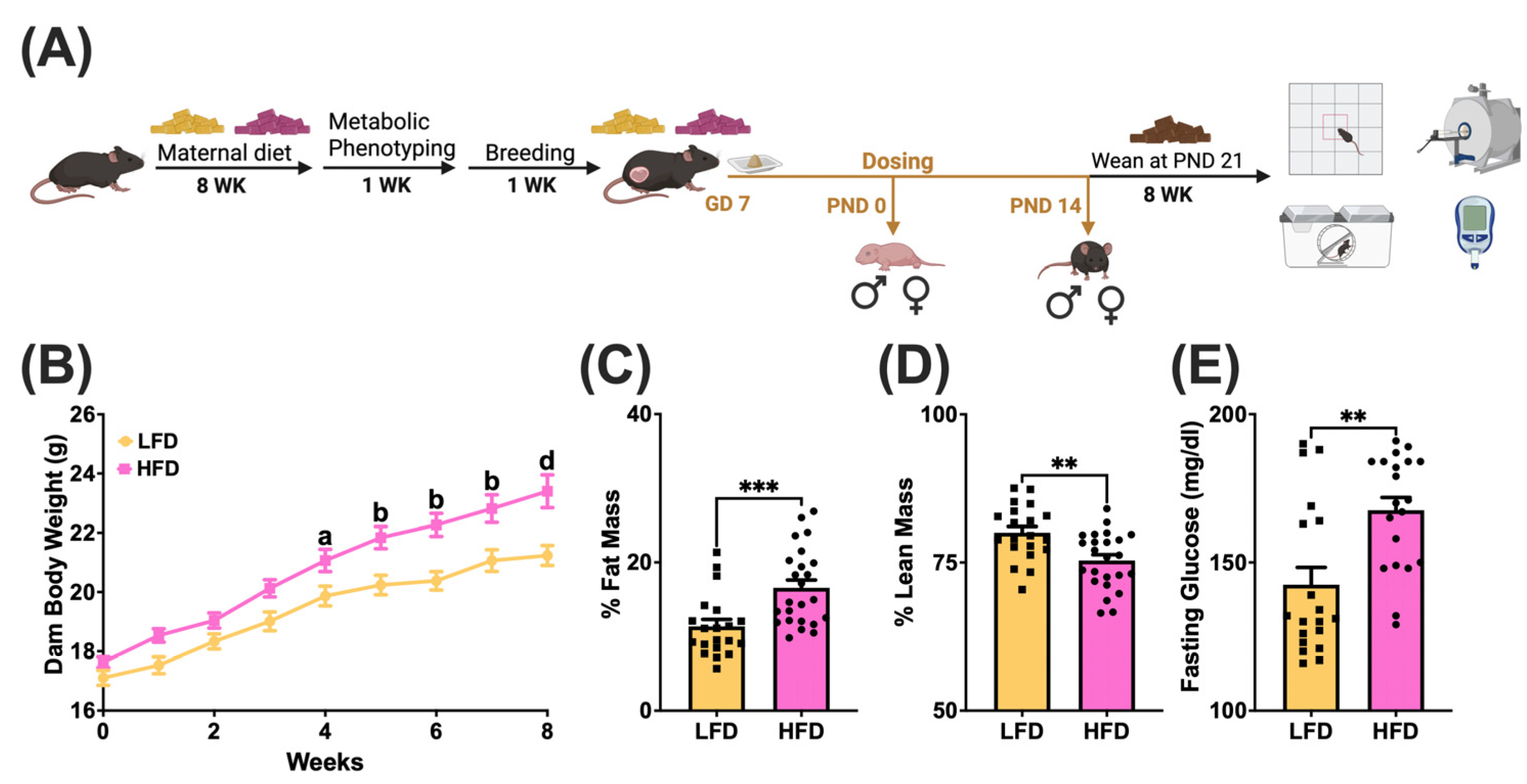
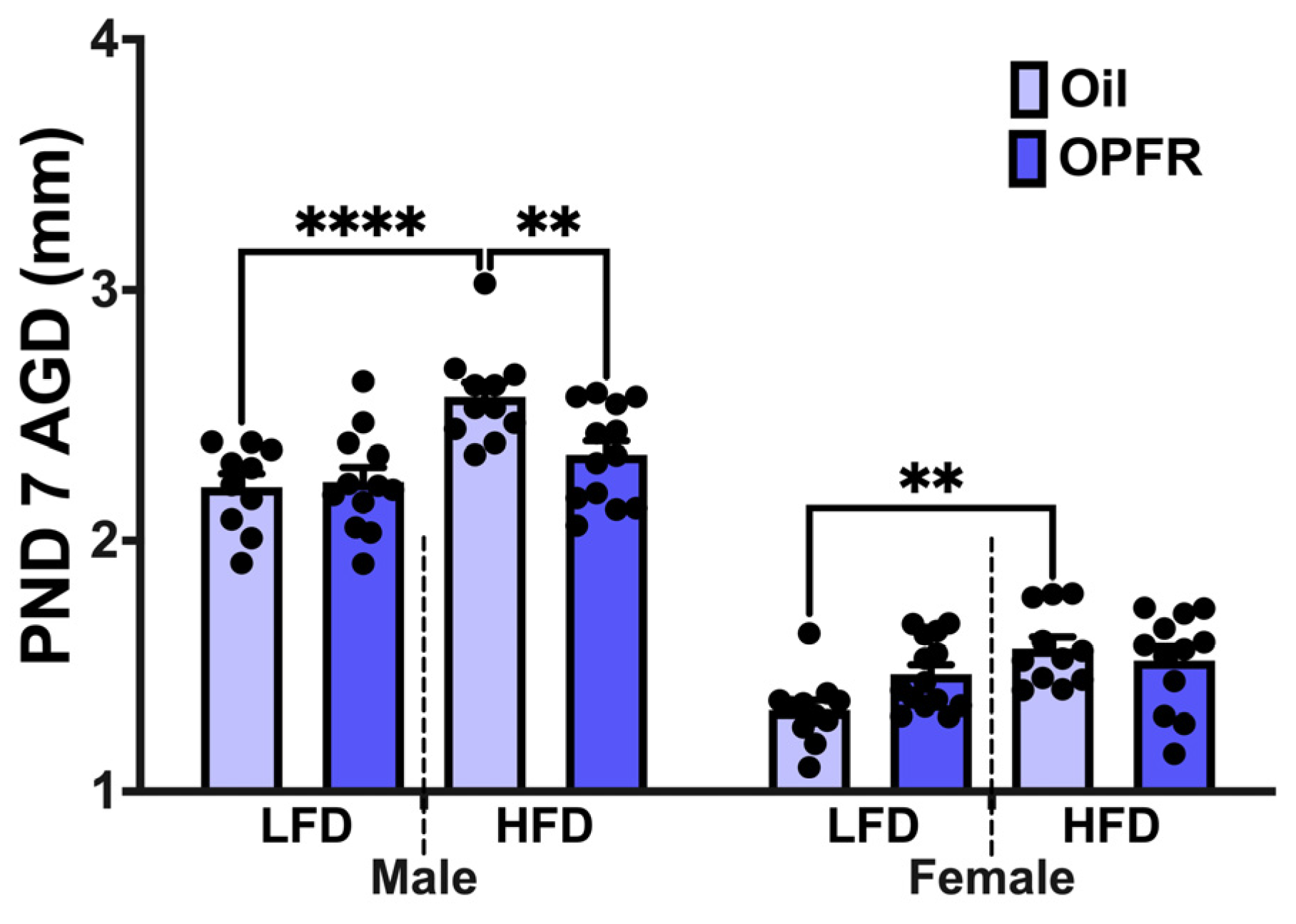


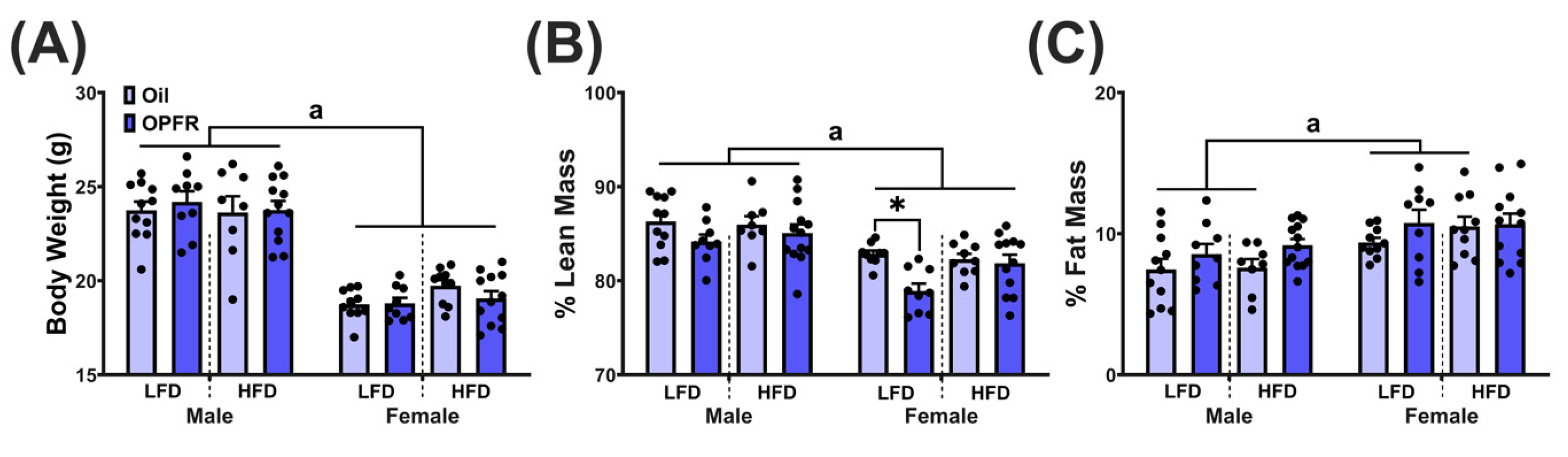
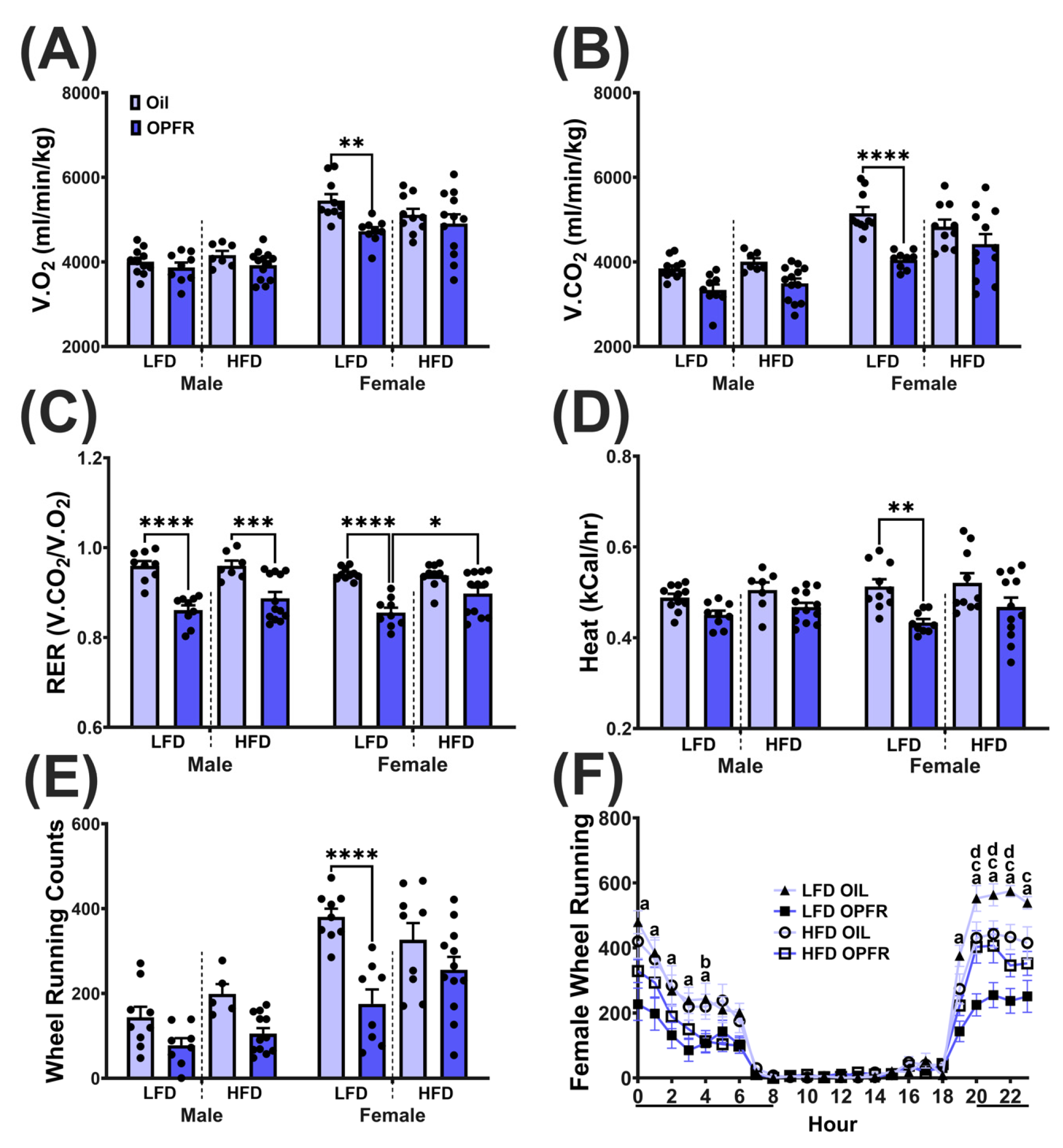
| LFD | HFD | |||
|---|---|---|---|---|
| Oil | OPFR | Oil | OPFR | |
| Dams | 10 * | 10 | 10 | 14 |
| Litters | 11 | 10 | 10 | 14 |
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Gene | Role | Maternal Diet | Oil | OPFR | Oil | OPFR |
| Claudin-3 | Tight junction | LFD | 0.4 ± 0.1 b | 1.9 ± 0.5 | 0.7 ± 0.1 b | 2.1 ± 0.3 |
| HFD | 0.9 ± 0.2 c | 2.3 ± 0.4 | 0.8 ± 0.1 c | 1.8 ± 0.4 | ||
| Claudin-5 | Tight junction | LFD | 21.2 ± 1.0 b,e | 12.5 ± 0.6 | 16.8 ± 1.8 b | 11.7 ± 1.8 |
| HFD | 20.9 ± 0.6 c | 11.4 ± 1.7 | 19.7 ± 1.3 c | 10.3 ± 1.1 | ||
| Dysferlin | Endothelial marker | LFD | 0.9 ± 0.2 | 0.9 ± 0.1 e | 0.8 ± 0.1 | 1.3 ± 0.2 |
| HFD | 0.5 ± 0.2 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | ||
| Lrp1 | Leptin transporter | LFD | 0.9 ± 0.0 e | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| HFD | 1.1 ± 0.2 c | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.6 ± 0.1 | ||
| Lrp2 | Leptin transporter | LFD | 0.4 ± 0.1 b | 1.7 ± 0.4 | 0.4 ± 0.0 b | 2.0 ± 0.5 |
| HFD | 0.5 ± 0.1 c | 2.6 ± 0.4 e | 0.4 ± 0.1 c | 1.6 ± 0.4 | ||
| Occludin | Tight junction | LFD | 0.5 ± 0.1 b | 2.9 ± 0.5 | 0.5 ± 0.0 b | 2.0 ± 0.3 d |
| HFD | 0.8 ± 0.1 c | 3.2 ± 0.4 | 0.5 ± 0.1 c | 3.3 ± 0.5 | ||
| Plvap | Endothelial marker | LFD | 0.7 ± 0.1 b,e | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 d |
| HFD | 0.6 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 c | 0.1 ± 0.0 | ||
| Vimentin | Tanycytic processes | LFD | 1.6 ± 0.1 b,e | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.1 |
| HFD | 1.3 ± 0.1 c | 0.9 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 | ||
| Zo1 | Tight junction | LFD | 1.2 ± 0.1 e | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 |
| HFD | 1.2 ± 0.1 | 1.1 ± 0.2 e | 0.9 ± 0.1 | 0.7 ± 0.1 | ||
| PND 0 | PND 14 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | |||||||
| Gene | Role | Maternal Diet | Oil | OPFR | Oil | OPFR | Oil | OPFR | Oil | OPFR |
| Agrp | Orexigenic neuropeptide | LFD | 1.2 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.2 | 0.5 ± 0.1 | 1.1 ± 0.2 | 0.7 ± 0.1 | 0.9 ± 0.2 a | 0.8 ± 0.1 |
| HFD | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.2 | 0.6 ± 0.1 | 1.6 ± 0.3 c | 0.7 ± 0.1 | ||
| Bdnf | Synaptic plasticity | LFD | 1.1 ± 0.1 | 1.0 ± 0.0 e | 1.0 ± 0.0 | 0.8 ± 0.1 | 0.8 ± 0.1 e | 0.7 ± 0.0 | 1.1 ± 0.1 b | 0.7 ± 0.0 |
| HFD | 1.3 ± 0.1 e | 1.1 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 c | 0.5 ± 0.0 | 1.1 ± 0.1 c | 0.6 ± 0.0 | ||
| Cart | Anorexigenic neuropeptide | LFD | 1.0 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 e | 0.6 ± 0.1 | 1.1 ± 0.1 b | 0.6 ± 0.1 |
| HFD | 0.9 ± 0.1 | 1.1 ± 0.1 | 0.7 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.2 c | 0.5 ± 0.0 | 1.1 ± 0.1 c | 0.7 ± 0.1 | ||
| Esr1 | Energy homeostasis | LFD | 1.9 ± 0.2 e | 1.5 ± 0.0 | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.3 ± 0.1 a | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 |
| HFD | 1.4 ± 0.3 | 1.5 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.1 | 2.2 ± 0.4 c,e | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | ||
| Foxo1 | Energy homeostasis | LFD | 1.0 ± 0.1 b | 1.5 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.2 b | 0.6 ± 0.0 | 1.1 ± 0.2 b | 0.6 ± 0.0 |
| HFD | 1.3 ± 0.2 | 1.4 ± 0.1 | 1.1 ± 0.1 c | 1.4 ± 0.1 | 1.4 ± 0.1 c | 0.5 ± 0.0 | 1.2 ± 0.1 c | 0.7 ± 0.1 | ||
| Ghsr | Ghrelin receptor | LFD | 0.8 ± 0.1 e | 0.7 ± 0.1 d | 0.5 ± 0.1 | 0.5 ± 0.1 d | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| HFD | 0.8 ± 0.2 | 1.1 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 c | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | ||
| Insr | Insulin receptor | LFD | 1.1 ± 0.1 b | 1.8 ± 0.2 | 1.0 ± 0.1 b | 1.7 ± 0.1 | 0.9 ± 0.1 e | 0.9 ± 0.0 | 0.6 ± 0.0 b | 0.8 ± 0.1 |
| HFD | 1.1 ± 0.0 c | 1.9 ± 0.1 | 0.8 ± 0.1 c | 1.6 ± 0.1 | 1.0 ± 0.1 c,e | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.0 | ||
| Kiss1 | Reproduction | LFD | 10 ± 0.7 a,b,e | 3.1 ± 0.5 e | 1.3 ± 0.2 | 0.6 ± 0.1 | 1.6 ± 0.2 | 1.5 ± 0.1 e | 1.5 ± 0.3 | 0.9 ± 0.1 |
| HFD | 4.7 ± 0.8 c,e | 2.5 ± 0.6 e | 1.1 ± 0.2 | 0.4 ± 0.1 | 1.6 ± 0.3 | 0.9 ± 0.2 | 1.4 ± 0.2 c | 0.6 ± 0.1 | ||
| Lepr | Leptin receptor | LFD | 1.4 ± 0.2 | 1.4 ± 0.3 | 1.1 ± 0.2 | 1.2 ± 0.2 | 0.8 ± 0.1 | 0.7 ± 0.1 d | 1.0 ± 0.1 | 0.8 ± 0.1 d |
| HFD | 1.1 ± 0.2 | 1.6 ± 0.1 e | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 c | 0.5 ± 0.1 | 0.9 ± 0.1 c | 0.6 ± 0.0 | ||
| Npy | Orexigenic neuropeptide | LFD | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.1 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 d |
| HFD | 1.6 ± 0.5 | 2.2 ± 0.4 | 1.1 ± 0.1 | 2.0 ± 0.3 | 1.2 ± 0.1 c,e | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.0 | ||
| Pdyn | Energy homeostasis | LFD | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.0 |
| HFD | 0.9 ± 0.1 c | 1.1 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 c | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | ||
| Pomc | Anorexigenic neuropeptide | LFD | 1.0 ± 0.1 a,e | 0.9 ± 0.1 e | 0.6 ± 0.1 | 0.6 ± 0.1 | 1.7 ± 0.2 b | 3.2 ± 0.2 | 1.9 ± 0.4 b | 3.3 ± 0.2 |
| HFD | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.1 | 2.0 ± 0.4 | 2.5 ± 0.3 | 2.2 ± 0.4 c | 3.4 ± 0.4 | ||
| Pparg | Energy homeostasis | LFD | 0.4 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.9 ± 0.4 | 0.5 ± 0.0 | 2.1 ± 0.7 | 0.6 ± 0.1 |
| HFD | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.4 ± 0.1 | 0.8 ± 0.3 e | 0.4 ± 0.1 | 3.4 ± 1.4 c | 0.7 ± 0.1 | ||
| Tac2 | Sexual maturation | LFD | 5.5 ± 0.4 b,e | 2.5 ± 0.2 e | 1.5 ± 0.2 | 0.9 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.2 b | 0.8 ± 0.0 |
| HFD | 4.5 ± 0.9 c,e | 2.7 ± 0.4 e | 1.2 ± 0.2 | 0.9 ± 0.1 | 1.6 ± 0.3 c | 0.9 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | ||
| PND 0 | PND 14 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | |||||||
| Gene | Role | Maternal Diet | Oil | OPFR | Oil | OPFR | Oil | OPFR | Oil | OPFR |
| Dgat2 | Triglyceride synthesis | LFD | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.0 | 1.1 ± 0.2 e | 1.3 ± 0.1 | 1.6 ± 0.2 a | 1.5 ± 0.1 |
| HFD | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.7 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | ||
| Esr1 | Lipid and | LFD | 1.2 ± 0.1 a | 1.4 ± 0.2 d | 1.3 ± 0.1 | 1.2 ± 0.2 | 1.0 ± 0.1 e | 1.2 ± 0.1 | 1.5 ± 0.3 a,b | 1.1 ± 0.0 |
| glucose metabolism | HFD | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | |
| Fasn | Lipogenesis | LFD | 1.1 ± 0.1 | 1.2 ± 0.2 d | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 | 1.3 ± 0.3 | 0.9 ± 0.2 |
| HFD | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 1.0 ± 0.3 | 1.1 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.1 | 1.0 ± 0.1 | ||
| Foxo1 | Lipogenesis and | LFD | 1.4 ± 0.4 | 1.3 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 e | 1.6 ± 0.4 | 2.0 ± 0.5 | 1.2 ± 0.1 |
| glucose metabolism | HFD | 1.3 ± 0.7 | 1.5 ± 0.2 | 0.8 ± 0.3 | 1.0 ± 0.2 | 1.4 ± 0.1 | 1.5 ± 0.3 | 1.4 ± 0.1 | 1.6 ± 0.4 | |
| G6pc | Glucose metabolism | LFD | 1.2 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.0 | 1.1 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.2 | 1.1 ± 0.2 |
| HFD | 0.9 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | ||
| Insr | Insulin receptor | LFD | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.2 | 1.0 ± 0.1 |
| HFD | 1.0 ± 0.2 | 1.1 ± 0.1 | 1.4 ± 0.2 c | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.0 | 1.0 ± 0.1 | ||
| Lepr | Leptin receptor | LFD | 0.8 ± 0.1 | 0.8 ± 0.1 d | 1.0 ± 0.1 | 0.7 ± 0.0 | 1.2 ± 0.3 b | 2.8 ± 0.4 d | 2.1 ± 0.5 | 2.5 ± 0.4 |
| HFD | 0.5 ± 0.1 c,e | 1.8 ± 0.2 e | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.6 ± 0.6 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.4 ± 0.3 | ||
| Pepck | Gluconeogenesis | LFD | 1.1 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.2 | 0.7 ± 0.0 | 1.1 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.9 ± 0.2 |
| HFD | 0.8 ± 0.1 | 1.2 ± 0.2 e | 0.9 ± 0.1 | 0.8 ± 0.2 | 1.2 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | ||
| Ppara | Fatty acid oxidation | LFD | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.4 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.3 | 1.0 ± 0.0 |
| HFD | 1.5 ± 0.4 | 1.2 ± 0.1 | 1.5 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.0 | ||
| Pparg | Lipid metabolism | LFD | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 |
| HFD | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.2 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 | ||
| PND 0 | PND 14 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | |||||||
| Gene | Role | Maternal Diet | Oil | OPFR | Oil | OPFR | Oil | OPFR | Oil | OPFR |
| Bsep | Bile salt export pump | LFD | 1.1 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.0 | 1.0 ± 0.1 | 0.9 ± 0.1 d | 0.9 ± 0.1 | 0.9 ± 0.1 |
| HFD | 1.0 ± 0.1 | 0.6 ± 0.1 | 1.2 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.0 | ||
| Cd36 | Lipid metabolism | LFD | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.2 | 0.9 ± 0.1 | 1.1 ± 0.2 e | 1.3 ± 0.3 | 1.6 ± 0.2 | 1.4 ± 0.2 |
| HFD | 0.7 ± 0.1 e | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.2 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.3 | ||
| Cyp2b10 | Target of Constitutive | LFD | 0.9 ± 0.3 | 1.2 ± 0.2 d | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.3 ± 0.2 | 0.8 ± 0.2 | 1.6 ± 0.5 |
| Androstane Receptor | HFD | 0.7 ± 0.2 c | 4.9 ± 1.4 e | 1.9 ± 0.5 | 2.5 ± 1.3 | 1.3 ± 0.2 | 1.0 ± 0.2 | 1.7 ± 0.5 | 1.6 ± 0.3 | |
| Cyp3a11 | Target of | LFD | 1.1 ± 0.2 | 1.2 ± 0.2 d | 0.6 ± 0.0 a | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.2 d | 1.4 ± 0.3 a | 1.2 ± 0.1 |
| Pregnane X Receptor | HFD | 0.8 ± 0.1 c,e | 2.3 ± 0.5 | 1.7 ± 0.5 | 1.1 ± 0.4 | 1.0 ± 0.0 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| Cyp4a10 | Target of PPARα | LFD | 1.3 ± 0.4 | 1.2 ± 0.3 | 0.7 ± 0.2 | 2.3 ± 0.6 | 1.1 ± 0.1 e | 1.8 ± 0.3 d | 2.1 ± 0.7 a | 1.2 ± 0.2 |
| HFD | 0.6 ± 0.1 c | 3.0 ± 0.8 | 0.6 ± 0.1 | 1.9 ± 0.7 | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 | 1.1 ± 0.3 | ||
| Cyp7a1 | Target of | LFD | 1.4 ± 0.4 | 1.5 ± 0.3 | 1.6 ± 0.3 | 1.2 ± 0.2 | 1.0 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.0 | 1.2 ± 0.4 |
| Farnesoid X Receptor | HFD | 1.6 ± 0.3 | 0.8 ± 0.1 | 1.6 ± 0.4 | 0.9 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | |
| Ostb | Bile acid transporter | LFD | 1.2 ± 0.2 | 1.4 ± 0.2 | 0.8 ± 0.2 | 1.6 ± 0.3 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 |
| HFD | 1.3 ± 0.2 | 1.0 ± 0.3 | 0.7 ± 0.1 | 1.4 ± 0.4 | 1.1 ± 0.1 c,e | 0.6 ± 0.0 | 0.8 ± 0.1 | 0.6 ± 0.0 | ||
| Shp | Bile acid synthesis | LFD | 1.3 ± 0.4 | 0.5 ± 0.1 d | 1.0 ± 0.2 | 0.4 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| HFD | 1.0 ± 0.3 c | 4.8 ± 2.2 e | 1.5 ± 0.4 | 1.4 ± 0.2 | 1.3 ± 0.2 c,e | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasrebi, A.; Rojas, C.M.; Anthony, S.; Feltri, S.; Evelyn, J.; Wiersielis, K.; Adams, S.; Basaly, V.; Guo, G.L.; Aleksunes, L.M.; et al. Dual Effects of Maternal Diet and Perinatal Organophosphate Flame Retardant Treatment on Offspring Development, Behavior and Metabolism. Toxics 2025, 13, 639. https://doi.org/10.3390/toxics13080639
Yasrebi A, Rojas CM, Anthony S, Feltri S, Evelyn J, Wiersielis K, Adams S, Basaly V, Guo GL, Aleksunes LM, et al. Dual Effects of Maternal Diet and Perinatal Organophosphate Flame Retardant Treatment on Offspring Development, Behavior and Metabolism. Toxics. 2025; 13(8):639. https://doi.org/10.3390/toxics13080639
Chicago/Turabian StyleYasrebi, Ali, Catherine M. Rojas, Shabree Anthony, Samantha Feltri, Jamilah Evelyn, Kimberly Wiersielis, Samantha Adams, Veronia Basaly, Grace L. Guo, Lauren M. Aleksunes, and et al. 2025. "Dual Effects of Maternal Diet and Perinatal Organophosphate Flame Retardant Treatment on Offspring Development, Behavior and Metabolism" Toxics 13, no. 8: 639. https://doi.org/10.3390/toxics13080639
APA StyleYasrebi, A., Rojas, C. M., Anthony, S., Feltri, S., Evelyn, J., Wiersielis, K., Adams, S., Basaly, V., Guo, G. L., Aleksunes, L. M., & Roepke, T. A. (2025). Dual Effects of Maternal Diet and Perinatal Organophosphate Flame Retardant Treatment on Offspring Development, Behavior and Metabolism. Toxics, 13(8), 639. https://doi.org/10.3390/toxics13080639









