Thermoset Polyester Resin Microplastics: Effects on Enzymatic Biomarkers and Toxicological Endpoint Responses of Eisenia fetida Earthworms
Abstract
1. Introduction
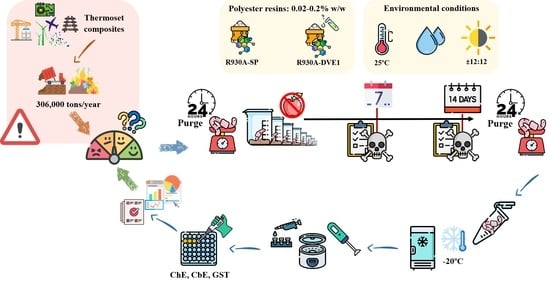
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Polyester Resins
2.3. Substrate Matrix
2.4. Test Organism
2.5. In Vivo Acute Toxicity Test
2.6. Sample Preparation
2.7. Enzymatic Biomarker Measurements
2.7.1. Protein Content Determination
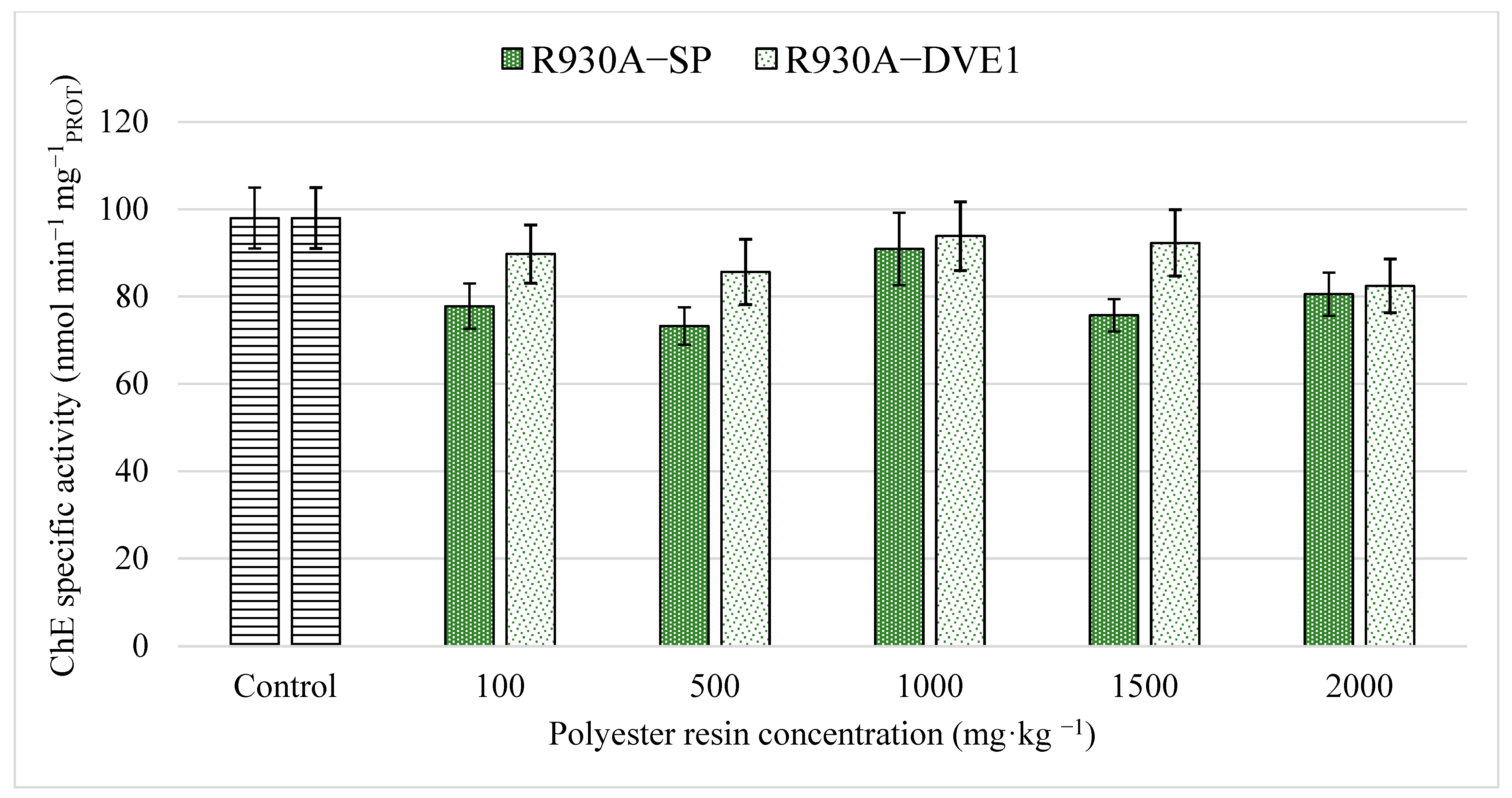
2.7.2. Cholinesterase (ChE) Activity
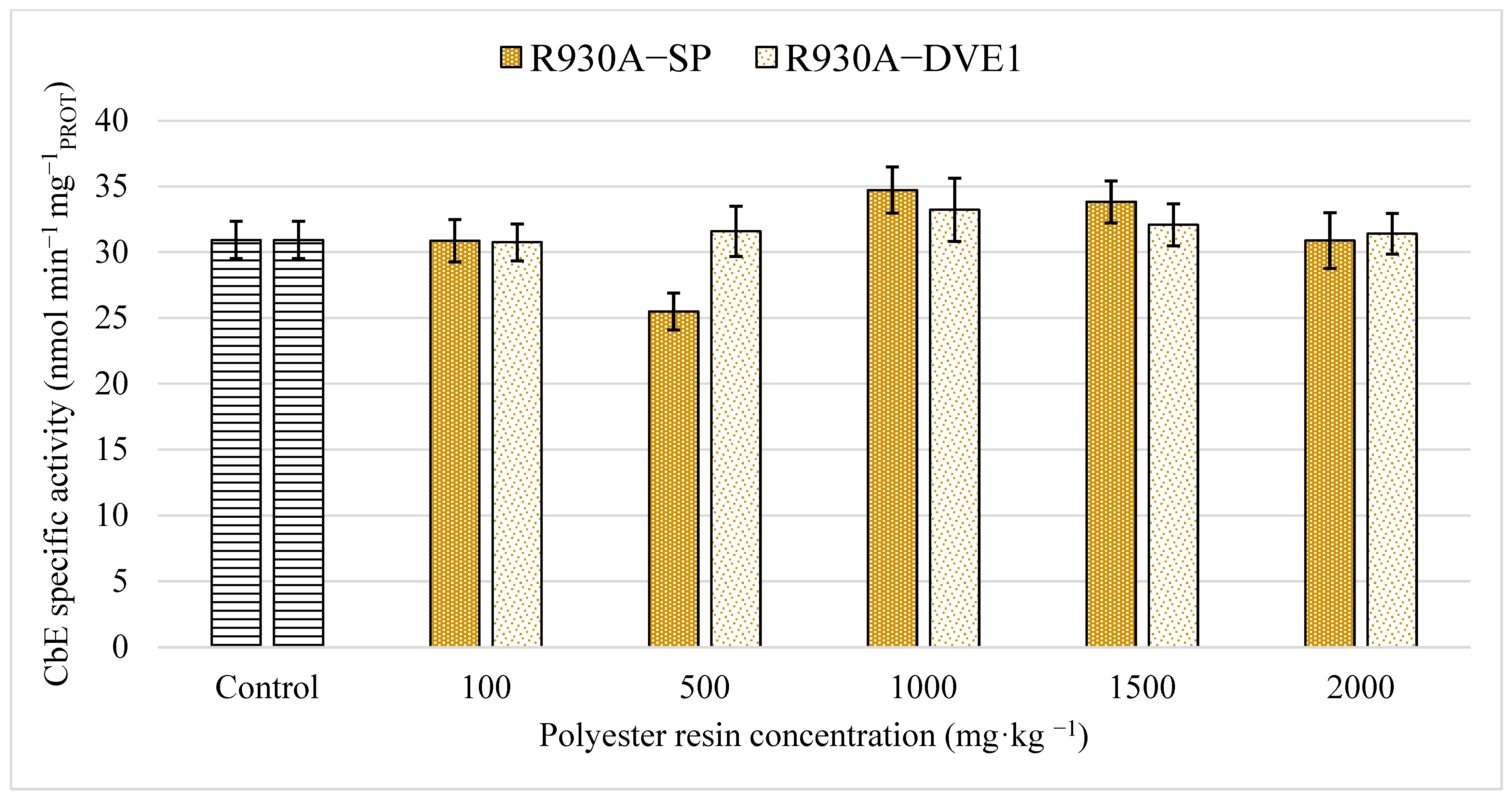
2.7.3. Carboxylesterase (CbE) Activity
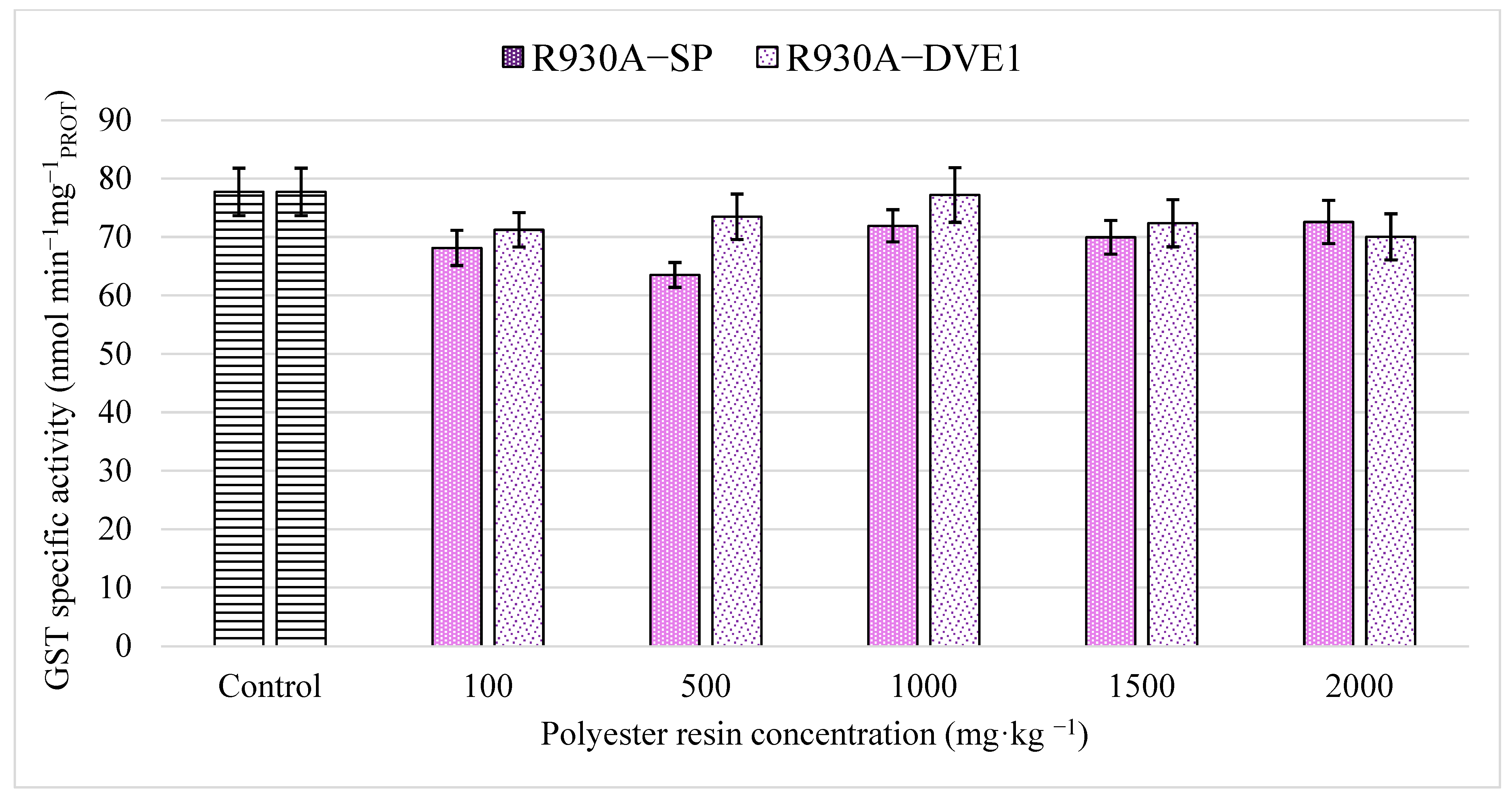
2.7.4. Glutathione S-Transferase (GST) Activity
2.8. Data Analysis
3. Results and Discussion
3.1. Individual Endpoints: Survival Rate, Morphological Changes and Behavioral Changes
3.2. Enzymatic Biomarkers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MEKP | Methyl ethyl ketone peroxide |
| VOC | Volatile organic compound |
| HAP | Hazardous air pollutant |
| IARC | International Agency for Research on Cancer |
| ChE | Cholinesterase |
| CbE | Carboxylesterase |
| GST | Glutathione S-transferase |
| GSH | L-Glutathione |
| DTNB | 5,5′-Dithiobis (2-nitrobenzoic acid) |
| CDNB | 1-Chlro-2,4-dinitrobenzene |
| PS-HBCD | Polystyrene with flame retardant |
| PP | Polypropylene |
| ROS | Reactive oxygen species |
References
- Zhu, Y.; Che, R.; Zong, X.; Wang, J.; Li, J.; Zhang, C.; Wang, F. A comprehensive review on the source, ingestion route, attachment and toxicity of microplastics/nanoplastics in human systems. J. Environ. Manag. 2024, 352, 120039. [Google Scholar] [CrossRef]
- Martín-García, A.P.; Egea-Corbacho, A.; Franco, A.A.; Rodríguez-Barroso, R.; Coello, M.D.; Quiroga, J.M. Grab and composite samples: Variations in the analysis of microplastics in a real wastewater treatment plant in the South of Spain. J. Environ. Chem. Eng. 2023, 11, 109486. [Google Scholar] [CrossRef]
- Cesarini, G.; Coppola, F.; Campos, D.; Venditti, I.; Battocchio, C.; Di Giulio, A.; Muzzi, M.; Pestana, J.L.T.; Scalici, M. Nanoplastic exposure inhibits feeding and delays regeneration in a freshwater planarian. Environ. Pollut. 2023, 332, 121959. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Hartmann, N.B.; Drobne, D.; Potthoff, A.; Kühnel, D. Quality of nanoplastics and microplastics ecotoxicity studies: Refining quality criteria for nanomaterial studies. J. Hazard. Mater. 2021, 415, 125751. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P.; Avellan, A.; Mouneyrac, C.; Duarte, A.; Rocha-Santos, T. Plastic additives and microplastics as emerging contaminants: Mechanisms and analytical assessment. Trends Anal. Chem. 2023, 158, 116898. [Google Scholar] [CrossRef]
- Vijay, N.; Rajkumara, V.; Bhattacharjee, P. Assessment of Composite Waste Disposal in Aerospace Industries. Procedia Environ. Sci. 2016, 35, 563–570. [Google Scholar] [CrossRef]
- Martín-García, A.P.; Egea-Corbacho, A.; Franco, A.A.; Albendín, G.; Arellano, J.M.; Rodríguez-Barroso, R.; Coello, M.D.; Quiroga, J.M. Application of intermittent sand and coke filters for the removal of microplastics in wastewater. J. Clean. Prod. 2022, 380, 134844. [Google Scholar] [CrossRef]
- Huang, D.; Tao, J.; Cheng, M.; Deng, R.; Chen, S.; Yin, L.; Li, R. Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mater. 2021, 407, 124399. [Google Scholar] [CrossRef]
- Crosta, A.; De Felice, B.; Minolfi, V.; Azzoni, R.S.; Pittino, F.; Franzetti, A.; Ortenzi, M.A.; Gazzotti, S.; Gianotti, V.; Roncoli, M.; et al. Latitudinal patterns of microplastic contamination in remote areas. Environ. Res. 2025, 277, 121553. [Google Scholar] [CrossRef]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic Products Leach Chemicals That Induce in Vitro Toxicity Under Realistic Use Conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef]
- Beiras, R.; Verdejo, E.; Campoy-López, P.; Vidal-Liñán, L. Aquatic toxicity of chemically defined microplastics can be explained by functional additives. J. Hazard. Mater. 2021, 406, 124338. [Google Scholar] [CrossRef] [PubMed]
- Egea-Corbacho, A.; Martín-García, A.P.; Franco, A.A.; Albendín, G.; Arellano, J.M.; Rodríguez, R.; Quiroga, J.M.; Coello, M.D. A method to remove cellulose from rich organic samples to analyse microplastics. J. Clean. Prod. 2022, 334, 130248. [Google Scholar] [CrossRef]
- Dogra, K.; Kumar, M.; Singh, S.; Bahukhandi, K.D. Decoding the interaction between microplastics, polyfluoroalkyl substances, and endocrine disruptors: Sorption kinetics and toxicity. Curr. Opin. Chem. Eng. 2025, 48, 101126. [Google Scholar] [CrossRef]
- Naqvi, S.Z.; Ramkumar, J.; Kar, K.K. Fly ash/glass fiber/carbon fiber-reinforced thermoset composites. In Handbook of Fly Ash; Elsevier: Amsterdam, The Netherlands, 2021; pp. 373–400. [Google Scholar] [CrossRef]
- Loos, M. Composites. In Carbon Nanotube Reinforced Composites: CNR Polymer Science and Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–72. [Google Scholar] [CrossRef]
- Jan, K.; Haque, M.A.; Cui, S.; Reimonn, G.; Dotan, A.; Lu, T.; Chen, W.T. Thermosets from renewable sources. In Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 679–718. [Google Scholar] [CrossRef]
- Morales-Ibarra, R. Recycling of thermosets and their composites. In Thermosets: Structure, Properties, and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 639–666. [Google Scholar] [CrossRef]
- Guggari, S.; Magliozzi, F.; Malburet, S.; Graillot, A.; Destarac, M.; Guerre, M. Vanillin-Based Epoxy Vitrimers: Looking at the Cystamine Hardener from a Different Perspective. ACS Sustain. Chem. Eng. 2023, 11, 6021–6031. [Google Scholar] [CrossRef]
- Wu, Y.; Fei, M.; Qiu, R.; Liu, W.; Qiu, J. A review on styrene substitutes in thermosets and their composites. Polymers 2019, 11, 1815. [Google Scholar] [CrossRef]
- Cousinet, S.; Ghadban, A.; Fleury, E.; Lortie, F.; Pascault, J.P.; Portinha, D. Toward replacement of styrene by bio-based methacrylates in unsaturated polyester resins. Eur. Polym. J. 2015, 67, 539–550. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Styrene-free unsaturated polyesters for hemp fibre composites. Compos. Sci. Technol. 2015, 120, 66–72. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Li, T.; Zhang, Q.; Cai, Y.; Bai, H.; Lv, Q. Application of validated migration models for the risk assessment of styrene and acrylonitrile in ABS plastic toys. Ecotoxicol. Environ. Saf. 2023, 252, 114570. [Google Scholar] [CrossRef]
- US EPA, (n.d.). Initial List of Hazardous Air Pollutants with Modifications. Available online: https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications (accessed on 9 May 2025).
- Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lambré, C.; Lampi, E.; et al. Assessment of the impact of the IARC Monograph Vol. 121 on the safety of the substance styrene (FCM No 193) for its use in plastic food contact materials. EFSA J. 2020, 18, 6247. [Google Scholar] [CrossRef]
- Viña-Gonzalez, J.; Dolz, M.; Sanchez-Moreno, I.; Mateljak, I.; Alcalde, M. Bizente: Enzymatic technologies in the value chain of thermoset materials. In III JORNADAS ESPAÑOLAS DE BIOCATÁLISIS, Sociedad Española de Biotecnología; Sebiot (Sociedad Española de Biotecnología/Spanish Society for Biotechnology): Madrid, Spain, 2021; p. 45. Available online: https://digital.csic.es/bitstream/10261/262076/1/Book%20JEB%20III.pdf (accessed on 27 May 2025).
- Dolz, M.; Mateljak, I.; Méndez-Sánchez, D.; Sánchez-Moreno, I.; Gomez de Santos, P.; Viña-Gonzalez, J.; Alcalde, M. Colorimetric High-Throughput Screening Assay to Engineer Fungal Peroxygenases for the Degradation of Thermoset Composite Epoxy Resins. Front. Catal. 2022, 2, 883263. [Google Scholar] [CrossRef]
- Noè, C.; Hakkarainen, M.; Malburet, S.; Graillot, A.; Adekunle, K.; Skrifvars, M.; Sangermano, M. Frontal-Photopolymerization of Fully Biobased Epoxy Composites. Macromol. Mater. Eng. 2022, 307, 2100864. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.D.; Bassett, A.W.; Sadler, J.M.; La Scala, J.J.; Stanzione, J.F. Synthesis and Characterization of Bio-based Epoxy Resins Derived from Vanillyl Alcohol. ACS Sustain. Chem. Eng. 2016, 4, 4328–4339. [Google Scholar] [CrossRef]
- Zhang, P.; Long, J.; Xue, K.; Liu, H.; Song, Z.D.; Liu, M.; Yao, T.; Liu, L. Preparation of degradable bio-based silicone/epoxy hybrid resins towards low dielectric composites. Eur. Polym. J. 2022, 181, 111691. [Google Scholar] [CrossRef]
- European Commission, (n.d.). Biopolymers with Advanced Functionalities for Building and Automotive Parts Processed Through Additive Manufacturing|Barbara|Project|Fact Sheet|H2020|Cordis. Available online: https://cordis.europa.eu/project/id/745578 (accessed on 27 May 2025).
- LIFE 3.0-LIFE Project Public Page, (n.d.). Available online: https://webgate.ec.europa.eu/life/publicWebsite/index.cfm?fuseaction=search.dspPage&n_proj_id=5738 (accessed on 27 May 2025).
- European Commission, (n.d.). Improving Recyclability of Thermoset Composite Materials Through a Greener Recycling Technology Based on Reversible Biobased Bonding Materials|Vibes|Project|Fact Sheet|H2020|Cordis. Available online: https://cordis.europa.eu/project/id/101023190 (accessed on 27 May 2025).
- European Commission, (n.d.). Apply Ligninases to Resolve End-Of-Life Issues of Thermoset Composite Plastics|Bizente|Project|Fact Sheet|H2020|Cordis|. Available online: https://cordis.europa.eu/project/id/886567 (accessed on 27 May 2025).
- Liu, Y.; Xu, G.; Yu, Y. Effects of polystyrene microplastics on accumulation of pyrene by earthworms. Chemosphere 2022, 296, 134059. [Google Scholar] [CrossRef]
- Chen, K.; Tang, R.; Luo, Y.; Chen, Y.; EI-Naggar, A.; Du, J.; Bu, A.; Yan, Y.; Lu, X.; Cai, Y.; et al. Transcriptomic and metabolic responses of earthworms to contaminated soil with polypropylene and polyethylene microplastics at environmentally relevant concentrations. J. Hazard. Mater. 2022, 427, 128176. [Google Scholar] [CrossRef]
- Gallego, S.; Nos, D.; Montemurro, N.; Sanchez-Hernandez, J.C.; Pérez, S.; Solé, M.; Martin-Laurent, F. Ecotoxicological impact of the antihypertensive valsartan on earthworms, extracellular enzymes and soil bacterial communities. Environ. Pollut. 2021, 275, 116647. [Google Scholar] [CrossRef]
- Rodríguez-Castellanos, L.; Sanchez-Hernandez, J.C. Chemical reactivation and aging kinetics of organophosphorus-inhibited cholinesterases from two earthworm species. Environ. Toxicol. Chem. 2007, 26, 1992–2000. [Google Scholar] [CrossRef]
- Lackmann, C.; Velki, M.; Šimić, A.; Müller, A.; Braun, U.; Ečimović, S.; Hollert, H. Two types of microplastics (polystyrene-HBCD and car tire abrasion) affect oxidative stress-related biomarkers in earthworm Eisenia andrei in a time-dependent manner. Environ. Int. 2022, 163, 107190. [Google Scholar] [CrossRef]
- OECD. Test No. 207: Earthworm, Acute Toxicity Tests; OECD Publishing: Paris, France, 1984. [Google Scholar] [CrossRef]
- Li, L.; Yang, D.; Song, Y.; Shi, Y.; Huang, B.; Bitsch, A.; Yan, J. The potential acute and chronic toxicity of cyfluthrin on the soil model organism, Eisenia fetida. Ecotoxicol. Environ. Saf. 2017, 144, 456–463. [Google Scholar] [CrossRef]
- Yang, X.; Song, Y.; Kai, J.; Cao, X. Enzymatic biomarkers of earthworms Eisenia fetida in response to individual and combined cadmium and pyrene. Ecotoxicol. Environ. Saf. 2012, 86, 162–167. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, S.; Zhu, L.; Liu, C.; Zhang, Y.; Zhang, Y. Effect of microplastics in sludge impacts on the vermicomposting. Bioresour. Technol. 2021, 326, 124777. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhou, D.; Wang, J.; Li, Y.; Liu, Y.; Ning, Y. Evaluation of the toxicity effects of microplastics and cadmium on earthworms. Sci. Total Environ. 2022, 836, 155747. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Panneerselvan, L.; Fang, C.; Naidu, R.; Megharaj, M. Chronic and transgenerational effects of polyethylene microplastics at environmentally relevant concentrations in earthworms. Environ. Technol. Innov. 2022, 25, 102226. [Google Scholar] [CrossRef]
- Lahive, E.; Cross, R.; Saarloos, A.I.; Horton, A.A.; Svendsen, C.; Hufenus, R.; Mitrano, D.M. Earthworms ingest microplastic fibres and nanoplastics with effects on egestion rate and long-term retention. Sci. Total Environ. 2022, 807, 151022. [Google Scholar] [CrossRef]
- Klimasz, M.; Grobelak, A. Effects of microplastics on selected earthworm species. Toxics 2025, 13, 201. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Cavanagh, J.; Yiming, A.; Wang, X.; Gao, W.; Matthew, C.; Qiu, J.; Li, Y. Toxicity of arsenite to earthworms and subsequent effects on soil properties. Soil Biol. Biochem. 2018, 117, 36–47. [Google Scholar] [CrossRef]
- Lackmann, C.; Velki, M.; Bjedov, D.; Ečimović, S.; Seiler, T.B.; Hollert, H. Commercial preparations of pesticides exert higher toxicity and cause changes at subcellular level in earthworm Eisenia andrei. Environ. Sci. Eur. 2021, 33, 12. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Narváez, C.; Cares, X.A.; Sabat, P.; Naidu, R. Predicting the bioremediation potential of earthworms of different ecotypes through a multi-biomarker approach. Sci. Total Environ. 2023, 862, 160547. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Albendín, M.G.; Mánuel-Vez, M.P.; Arellano, J.M. In vivo cholinesterase sensitivity of gilthead seabream (Sparus aurata) exposed to organophosphate compounds: Influence of biological factors. Ecol. Indic. 2021, 121, 107176. [Google Scholar] [CrossRef]
- Carr, R.L.; Chambers, J.E. Acute effects of the organophosphate paraoxon on schedule-controlled behavior and esterase activity in rats: Dose-response relationships. Pharmacol. Biochem. Behav. 1991, 40, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Soto-Mancera, F.; Arellano, J.M.; Albendín, M.G. Carboxylesterase in Sparus aurata: Characterisation and sensitivity to organophosphorus pesticides and pharmaceutical products. Ecol. Indic. 2020, 109, 105603. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Frasco, M.F.; Guilhermino, L. Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulate. Fish Physiol. Biochem. 2002, 26, 149–156. [Google Scholar] [CrossRef]
- Datta, S.; Singh, J.; Singh, J.; Singh, S.; Singh, S. Avoidance behavior of Eisenia fetida and Metaphire posthuma towards two different pesticides, acephate and atrazine. Chemosphere 2021, 278, 130476. [Google Scholar] [CrossRef]
- He, F.; Yu, H.; Shi, H.; Li, X.; Chu, S.; Huo, C.; Liu, R. Behavioral, histopathological, genetic, and organism-wide responses to phenanthrene-induced oxidative stress in Eisenia fetida earthworms in natural soil microcosms. Environ. Sci. Pollut. Res. 2022, 29, 40012–40028. [Google Scholar] [CrossRef]
- Shi, L.; Shen, C.; Zhang, P.; Xu, J.; Wu, X.; Pan, X.; He, L.; Dong, F.; Zheng, Y. Assessment on the stereoselective behavior of cyflumetofen to earthworms (Eisenia foetida): Degradation, bioaccumulation, toxicity mechanism, and metabolites. Sci. Total Environ. 2023, 892, 164541. [Google Scholar] [CrossRef]
- He, F.; Liu, R.; Tian, G.; Qi, Y.; Wang, T. Ecotoxicological evaluation of oxidative stress-mediated neurotoxic effects, genetic toxicity, behavioral disorders, and the corresponding mechanisms induced by fluorene-contaminated soil targeted to earthworm (Eisenia fetida) brain. Sci. Total Environ. 2023, 871, 162014. [Google Scholar] [CrossRef]
- Ellis, S.R.; Hodson, M.E.; Wege, P. The soil-dwelling earthworm Allolobophora chlorotica modifies its burrowing behaviour in response to carbendazim applications. Ecotoxicol. Environ. Saf. 2010, 73, 1424–1428. [Google Scholar] [CrossRef]
- Yadav, J.; Singh, D.; Shefalp, S. Effect of chlorpyrifos and carbofuran on morphology, behavior and acetylcholinesterase activity of earthworm (Eisenia fetida). Indian J. Agric. Sci. 2020, 90, 1871–1876. [Google Scholar] [CrossRef]
- Nadana, G.R.V.; Selvaraj, K.; Sivakumar, P.; Karuppaiah, P. Coelomic fluid of earthworms extruded by cold stress method has commercially significant compounds and trigger seed germination in Vigna radiata L. Environ. Technol. Innov. 2020, 19, 100814. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Singh, S.; Pandey, R.S. Assessment of acute toxicity and biochemical responses to chlorpyrifos, cypermethrin and their combination exposed earthworm, Eudrilus eugeniae. Toxicol. Rep. 2019, 6, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Henson-Ramsey, H.; Schneider, A.; Stoskopf, M.K. A comparison of multiple esterases as biomarkers of organophosphate exposure and effect in two earthworm species. Bull. Environ. Contam. Toxicol. 2011, 86, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Sales, S.; Ferreira Mannarino, C.; Maia Bila, D.; Taveira Parente, C.E.; Veríssimo Correia, F.; Mendes Saggioro, E. Lethal and long-term effects of landfill leachate on Eisenia andrei earthworms: Behavior, reproduction and risk assessment. J. Environ. Manag. 2021, 285, 112029. [Google Scholar] [CrossRef]
- Yao, X.; Qiao, Z.; Zhang, F.; Liu, X.; Du, Q.; Zhang, J.; Li, X.; Jiang, X. Effects of a novel fungicide benzovindiflupyr in Eisenia fetida: Evaluation through different levels of biological organization. Environ. Pollut. 2021, 271, 116336. [Google Scholar] [CrossRef]
- Zhao, S.; He, L.; Lu, Y.; Duo, L. The impact of modified nano-carbon black on the earthworm Eisenia fetida under turfgrass growing conditions: Assessment of survival, biomass, and antioxidant enzymatic activities. J. Hazard. Mater. 2017, 338, 218–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia fetida. J. Hazard. Mater. 2020, 392, 122273. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L.; Zhang, Y.; Zhang, P.; Jiang, H. Inhibition and recovery of biomarkers of earthworm Eisenia fetida after exposure to thiacloprid. Environ. Sci. Pollut. Res. Int. 2015, 22, 9475–9482. [Google Scholar] [CrossRef]
- Rizwan-Ul-Haq, M.; Zhenling, Z.; Yongxue, S.; Wenguang, X. Evaluation of glutathione s-transferase as toxicity indicator for roxarsone and arsanilic acid in Eisenia fetida. J. Appl. Toxicol. 2012, 32, 731–738. [Google Scholar] [CrossRef]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut. 2020, 259, 113896. [Google Scholar] [CrossRef]
- Cui, W.; Gao, P.; Zhang, M.; Wang, L.; Sun, H.; Liu, C. Adverse effects of microplastics on earthworms: A critical review. Sci. Total Environ. 2022, 850, 158041. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.; Jho, E.H.; Hwang, S.K.; Hyeon, Y.; Park, C. Responses of earthworms exposed to low-density polyethylene microplastic fragments. Chemosphere 2023, 333, 138945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Wang, W.; Cui, W.; Wang, L.; Sun, H.; Liu, C. Combined effects of microplastics and other contaminants on earthworms: A critical review. Appl. Soil Ecol. 2022, 180, 104626. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, L.; Mao, L.; Zhang, L.; Zhang, Y.; Chang, Y.; Liu, X.; Jiang, H. Combined ecotoxicological effects of different-sized polyethylene microplastics and imidacloprid on the earthworms (Eisenia fetida). Sci. Total Environ. 2023, 870, 161795. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, H.; Deng, H.; Xing, W.; Feng, D.; Li, J.; Ge, C.; Yu, H.; Zhang, Y.; Chen, H. Response of earthworms to microplastics in soil under biogas slurry irrigation: Toxicity comparison of conventional and biodegradable microplastics. Sci. Total Environ. 2023, 858, 160092. [Google Scholar] [CrossRef]
- Baihetiyaer, B.; Jiang, N.; Li, X.; He, B.; Wang, J.; Fan, X.; Sun, H.; Yin, X. Oxidative stress and gene expression induced by biodegradable microplastics and imidacloprid in earthworms (Eisenia fetida) at environmentally relevant concentrations. Environ. Pollution. 2023, 323, 121285. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaya-Vías, D.; Albendín, G.; Aranda-Quirós, V.; Rodríguez-Barroso, R.; Coello, D.; Arellano, J.M. Thermoset Polyester Resin Microplastics: Effects on Enzymatic Biomarkers and Toxicological Endpoint Responses of Eisenia fetida Earthworms. Toxics 2025, 13, 602. https://doi.org/10.3390/toxics13070602
Amaya-Vías D, Albendín G, Aranda-Quirós V, Rodríguez-Barroso R, Coello D, Arellano JM. Thermoset Polyester Resin Microplastics: Effects on Enzymatic Biomarkers and Toxicological Endpoint Responses of Eisenia fetida Earthworms. Toxics. 2025; 13(7):602. https://doi.org/10.3390/toxics13070602
Chicago/Turabian StyleAmaya-Vías, David, Gemma Albendín, Vanessa Aranda-Quirós, Rocío Rodríguez-Barroso, Dolores Coello, and Juana María Arellano. 2025. "Thermoset Polyester Resin Microplastics: Effects on Enzymatic Biomarkers and Toxicological Endpoint Responses of Eisenia fetida Earthworms" Toxics 13, no. 7: 602. https://doi.org/10.3390/toxics13070602
APA StyleAmaya-Vías, D., Albendín, G., Aranda-Quirós, V., Rodríguez-Barroso, R., Coello, D., & Arellano, J. M. (2025). Thermoset Polyester Resin Microplastics: Effects on Enzymatic Biomarkers and Toxicological Endpoint Responses of Eisenia fetida Earthworms. Toxics, 13(7), 602. https://doi.org/10.3390/toxics13070602