Health Risks from Microplastics in Intravenous Infusions: Evidence from Italy, Spain, and Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. IV-MDs’ Pre-Treatment
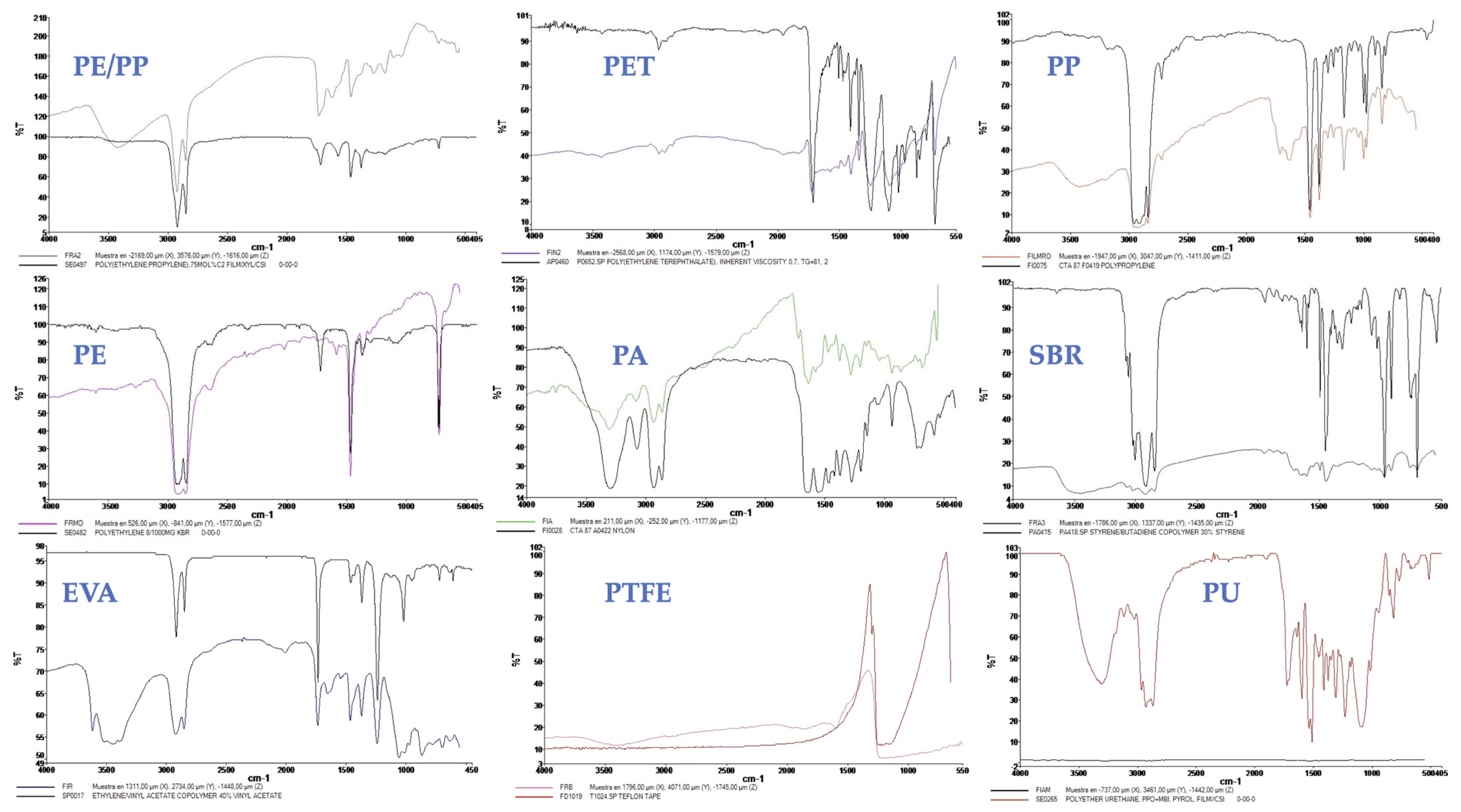
2.3. Microplastic Analysis
2.4. Quality Assurance and Quality Control (QA/QC)
- Wearing face masks, fibre-free lab coats, and nitrile gloves that have been previously cleaned to stop the operator or clothing from producing particles. Before each use, it is advised that all lab equipment, such as forceps, funnels, and filters, be thoroughly cleaned with Type I ultrapure water. Laminar flow should then be used to dry any remaining residue.
- Blank controls: To assess any potential unintentional contamination throughout the process, procedural blanks, or control filters devoid of samples, were processed in conjunction with the experimental samples.
- To reduce their exposure to the environment, the filters should always be kept in closed or covered systems. A high level of confidence is provided by these approaches that the MPs found in the samples are solely from the IV-MDs under investigation and are not the result of contamination from outside sources or experimental artefacts.
2.5. Statistical Analysis
3. Results and Discussion
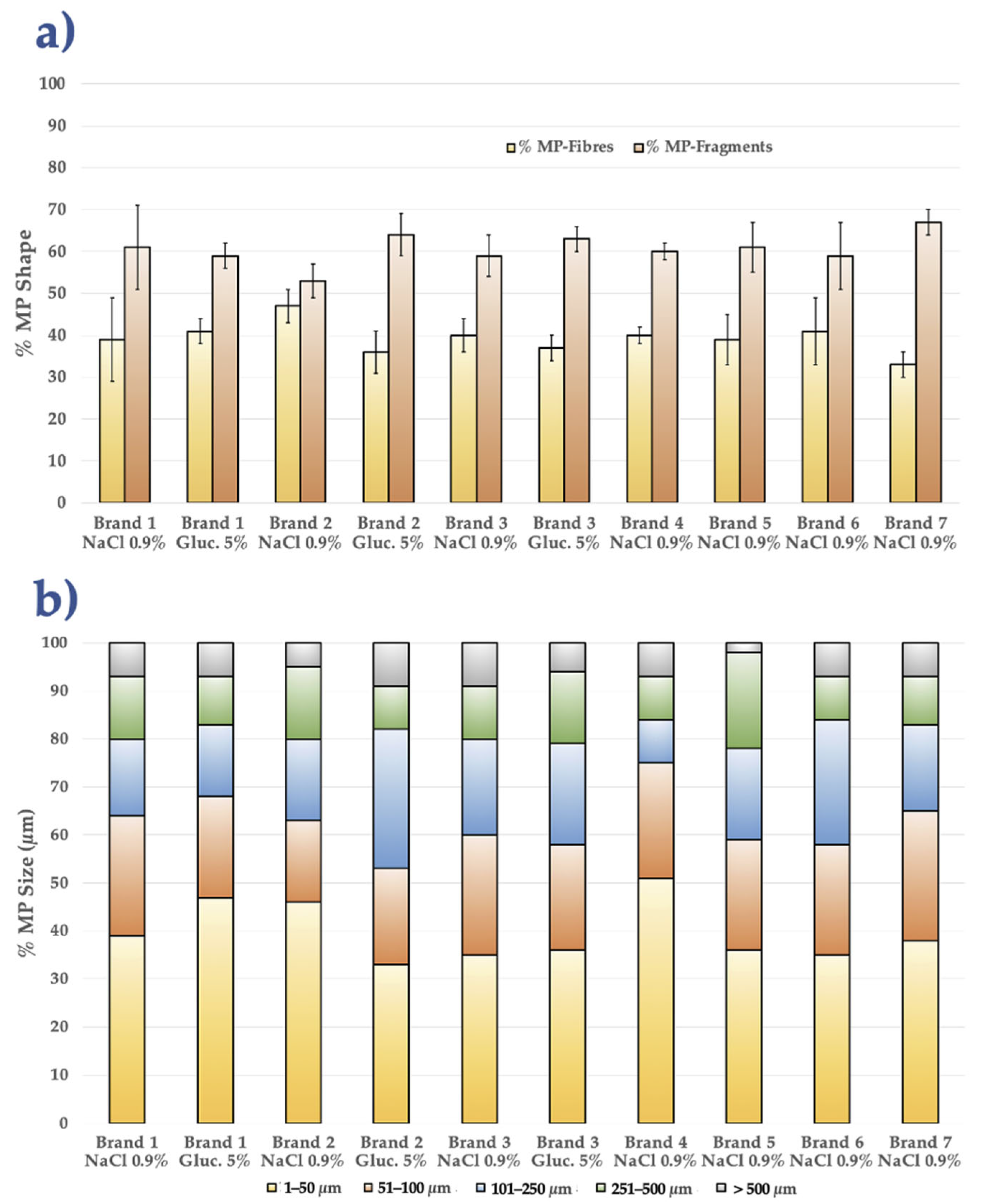
3.1. Comparative MP Analysis Among All IV-MDs Brands
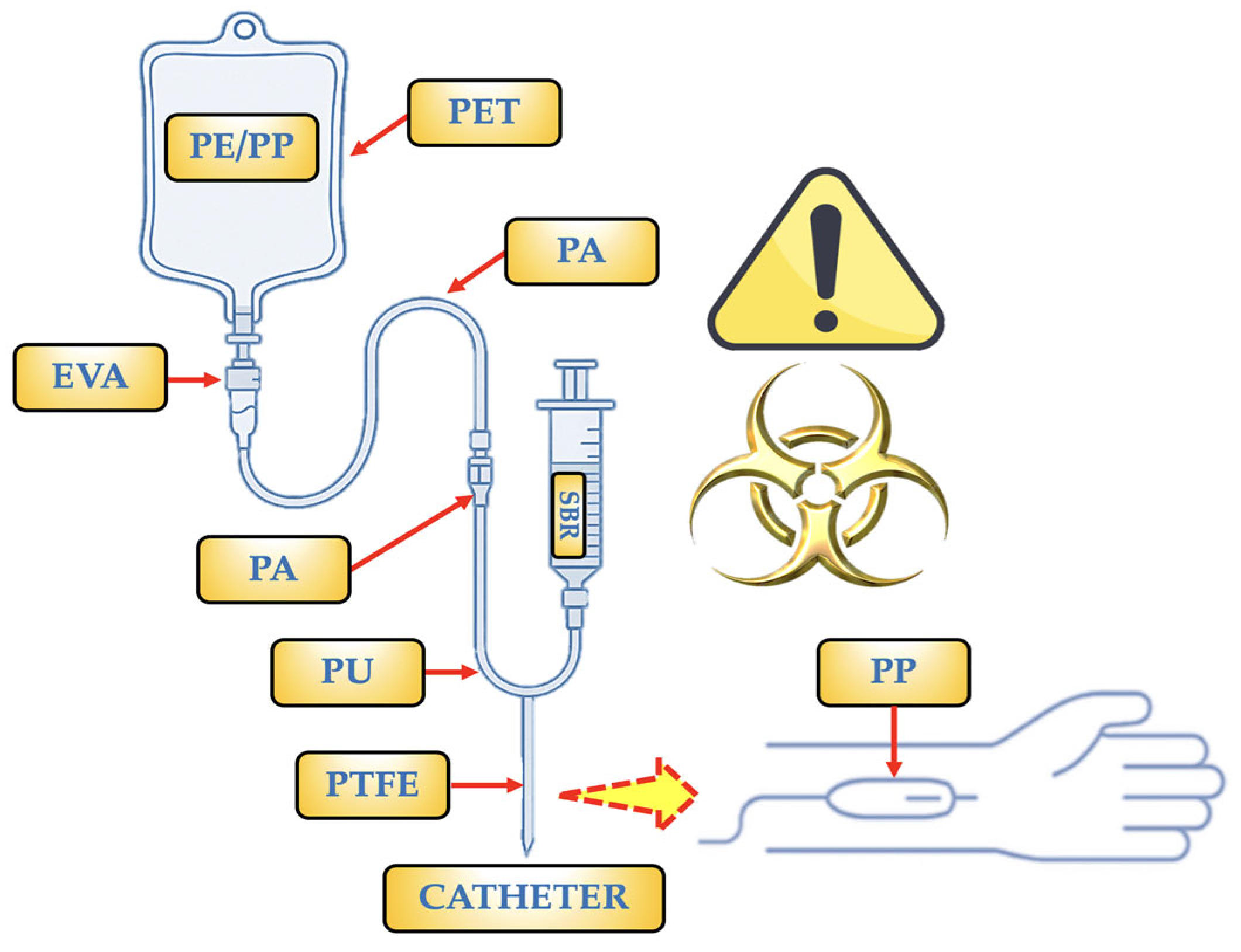
- PP: It is the polymer used to make bags and some barrier layers. It can also occasionally be found as a copolymer with PE (PE/PP). Only PP particles (1–62 µm) were found in a trial including two brands of saline bags, with an estimated 750 MPs per bag [20]. It is utilized for IV bags, particularly in items that require autoclaving (at pressure and temperature) for sterilization. NPs < 50 nm (≈2.1 × 10−4 NPs/mL) and particles of 2–10 µm (≈216 MPs/mL) were discovered as a copolymer [47]. It may be present due to fragments produced during production or assembly, as well as mechanical wear (component friction).
- PE/PP: It is utilized in IV bags because it makes it simple to close the bags during production, which is essential for preserving sterility. In clinical settings, PE/PP copolymer-based infusion bags are the preferred option because they guarantee IV treatment administration that is both safe and effective. They may be present as a result of fragments released during production or assembly, or mechanical wear, which is the result of friction between components.
- PE: Specifically, low-density polyethylene (LDPE). It is a component of syringes, caps, connections, and the inner layers of some multi-layer IV bags. This polymer is flexible, has strong chemical resistance, and—most importantly—works well with drugs. Both mechanical wear (component friction) and fragments discharged during production or assembly may be the cause of its presence.
- SBR: Infusion tubes devoid of Di(2-ethylhexyl)phthalate (DEHP) are made with it as a contemporary substitute for PVC in PVC-free products. In addition to being soft and flexible, this polymer is also very biocompatible and is devoid of plasticizers. Due to their elasticity and resilience to chemicals, rubber components are used in vial or bag stoppers or septa, as well as in connections or other portions of the infusion system, such as adapters, tubes, or connectors. It is employed as part of secondary packaging materials. Short fibres or irregular fragments are produced when SBR is used as a gasket or splice and is abrased by contact against hard materials (plastic or metal). Micrograms are more likely to be released during the sterilizing phase in SBR if radiation (such as gamma or electron radiation) is used to destroy the polymeric network.
- PU: Filter membranes and seals, as well as some high-performance flexible tubing and IV catheters, use it. It is highly elastic and biocompatible. PU develops surface cracks in tubing bends and places that are bent repeatedly; these cracks can separate as MPs that measure 20 to 100 µm (such as flex fatigue). Long-term exposure to disinfectants or solvent solutions (such as alcohols or H2O2) can also break down urethane bonds, causing MP fragments to be released.
- PTFE: Internal valve components, coatings for extremely reactive or sensitive drug systems, and occasionally specialized catheters are among its uses. It is employed due to its superior heat resistance and excellent chemical inertness. Additionally, its surface is non-stick. It is also used in technical or stiff components of infusion sets that need to be precise, transparent, stiff, or resistant to chemicals. The PTFE coating may deteriorate with repeated fluid flow and connecting device use, releasing micronized flakes or layers. Microcracks in the film can be caused by abrupt changes in temperature or pressure, and as these breaks spread, fragments are released.
- EVA: It is the perfect material for bags that need to endure handling and transportation because it is flexible and remains intact under stress. Medical fluids including saline solutions, glucose, and prescription drugs can all be used with it. It offers a moderate barrier to oxygen. Due to the absence of plasticizers (i.e., phthalates), which can migrate into the IV fluid and are frequently hazardous, it is utilized as a substitute for PVC. Very thin sheets or flakes may be released when MP-EVA cracks or peels in places where it is repeatedly folded, such as bends in soft tubing. Vinyl bonds in EVA can be broken by this phase if sterilized with radiation (such as gamma or electron radiation), creating particles that range in size from 10 to 50 µm.
- PA: It is applied as an outer or intermediate layer to increase the resistance of the bag to high temperatures and punctures (such as those that occur during autoclaving procedures). For oxidation-sensitive solutions, its high oxygen barrier is essential. During filling, storing, and using the bag, it helps keep its integrity and shape. Many IV bags are multilayered structures composed of combinations including PA/EVA or occasionally PP/PA/EVA. Each layer has a distinct purpose, such as PA (intermediate or outer layer, which provides strength and acts as a barrier) and EVA (inner layer, which is in touch with the solution). This combination guarantees the sterility and longevity of the bag. It prolongs the stability of drugs or liquids by not releasing known pollutants. They can be released after several cycles of insertion and flexing.
3.2. Polymer Hazard Index (PHI)
3.3. Possible Risks in Human Health
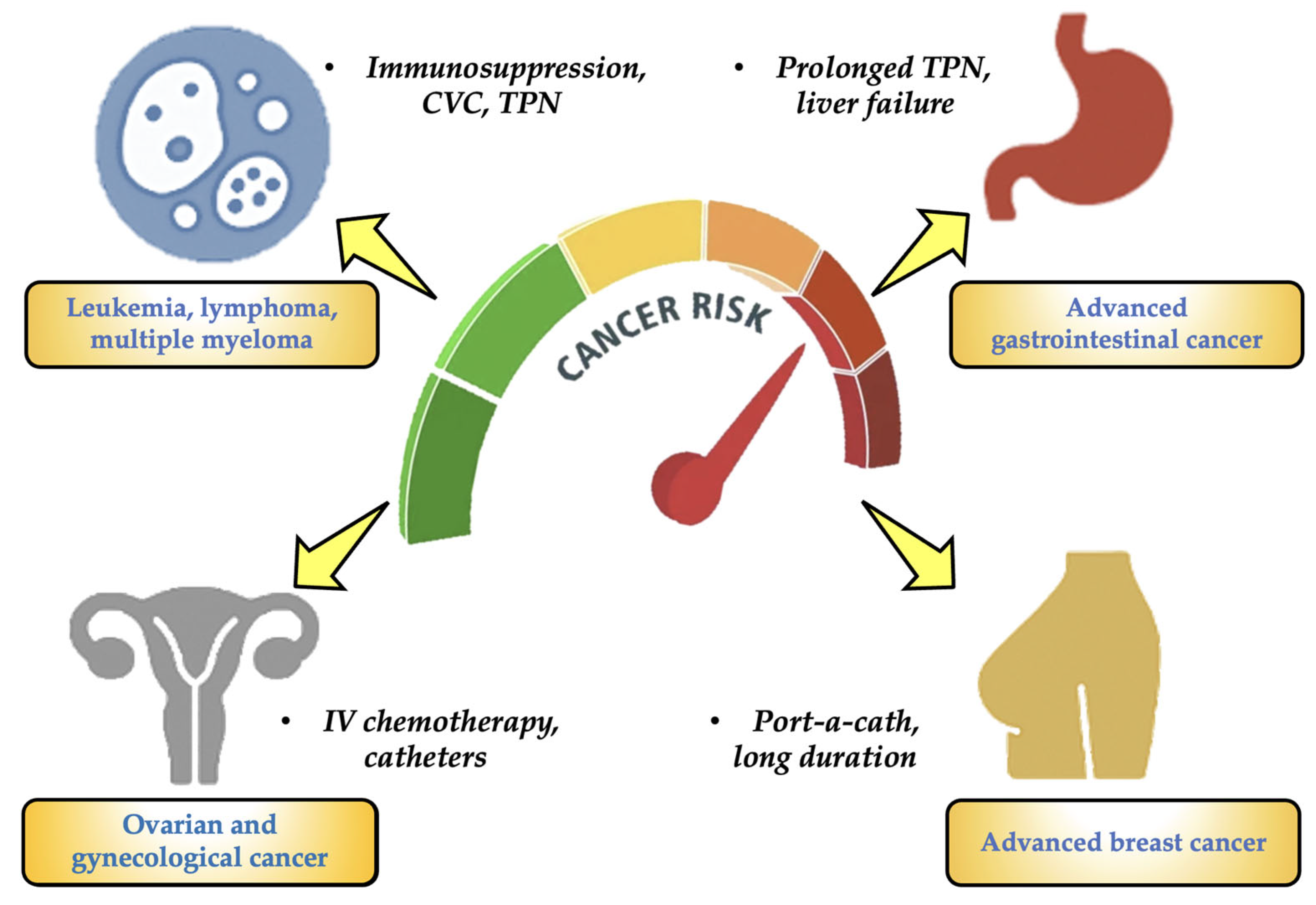
- Patients with blood cancers (such as leukemias, lymphomas, and multiple myeloma): They have received protracted (months to years) treatment with several cycles of IV chemotherapy and are at the highest risk for MPs in oncology. They also continue to employ port-a-catheters, peripherally inserted central catheters (PICCs), CVCs, and other devices. These patients also have severely weakened immune systems and often need IV antibiotics, transfusions, and parenteral nourishment [54,55].
- Advanced gastrointestinal cancer (liver, pancreas, colon, and stomach): It is another type of cancer that has a significant risk of MPs. Long-term TPN is necessary for many patients, and they are frequently exposed to plastic IV bags, which frequently contain lipid emulsions that can encourage the leaching of chemicals like DEHP. This particular type of patient also has compromised intestine or liver function, which lowers the removal of toxins [56,57].
- Gynecological and ovarian carcinomas: For this kind of cancer, the risk for MPs is considerable. This particular type of cancer requires intensive IV chemotherapy treatments. Catheters, continuous fluids, and peritoneal lavage are commonly employed. Patients may require TPN as a result of ascites and malabsorption events [58,59].
- Advanced breast cancer: It is the final stage of a high-risk cancer. Patients receive chemotherapy and IV targeted treatments for an extended period of time. Some people have been using port-a-catheters for years. Patients are exposed to medical plastics on a regular basis, but their functional status is typically better than that of patients with the previously described cancer categories [57,60].
3.4. Putative Limitations of This Study
- An accurate analytical method that clarifies molecular chemical bonds and offers comprehensive details on polymer types and their functional groups, μ-FTIR is essential for MP identification [31].
- Its ability to identify polymers containing polar functional groups, including hydroxyl (O–H) and carbonyl (C=O), gives it a distinct edge when analyzing particular MP categories.
- Since FTIR sample preparation is simple, the analysis is more thorough and efficient. Rapid examination of several samples is made possible by the measuring method’s exceptional speed, which is particularly useful in high-throughput situations like material characterization and environmental monitoring. Additionally, it reduces fluorescence and signals produced by pollutants, additives, pigments, and other materials [31].
4. Future Perspectives and Potential Solutions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile Butadiene Styrene |
| AFM-Raman | Atomic Force Microscopy-Raman |
| ARCSA | Agencia Nacional de Regulación, control y Vigilancia Sanitaria |
| CAGR | Compound Annual Growth Rate |
| CKD | Chronic Kidney Disease |
| CVC | Central Venous Catheter |
| CVD | Cardiovascular Diseases |
| DEHP | Di(2-ethylhexyl)phthalate |
| ECIS | European Cancer Information System |
| ECMO | Extracorporeal Membrane Oxygenation |
| EFSA | European Food Safety Authority |
| EPS | Expanded Polystyrene |
| EVA | Ethylene Vinyl Acetate |
| FDA | Food Drug of Administration |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GLP | Good Field and Laboratory Practices |
| ICH | International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use |
| IV | Intravenous Administration |
| IV-MDs | Intravenous Medical Devices |
| LDPE | Low Density Polyethylene |
| MDs | Medical Devices |
| MPs | Microplastics |
| NPs | Nanoplastics |
| OPEs | Organophosphate Esters |
| OS | Oxidative stress |
| PA | Polyamide |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCBs | Polychlorinated Biphenyls |
| PE | Polyethylene |
| PTFE | Polytetrafluoroethylene (Teflon) |
| PET | Polyethylene Terephthalate |
| PHI | Polymer Hazard Index |
| PICC | Peripherally Inserted Central Catheter |
| PM | Particulate Matter |
| PMMA | Polymethylmethacrylate |
| POPs | Persistent Organic Pollutants |
| PP | Polypropylene |
| PPN | Peripheral Parenteral Nutrition |
| PS | Polystyrene |
| PVA | Polyvinyl Alcohol |
| PVC | Polyvinyl Chloride |
| PU | Polyurethane |
| RH | Relative Humidity |
| ROS | Reactive Oxygen Species |
| SBR | Styrene-Butadiene |
| SEM | Scanning Electron Microscopy |
| TDCPP | Tris(1,3-dichloro-2-propyl)phosphate |
| TPN | Total Parenteral Nutrition |
| UV | Ultraviolet Light |
| WHO | World Health Organization |
| μFTIR | Micro Fourier Transform Infrared Spectroscopy |
References
- Casella, C.; Sol, D.; Laca, A.; Díaz, M. Microplastics in Sewage Sludge: A review. Environ. Sci. Pollut. Res. 2023, 30, 63382–63415. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.; Vadivel, D.; Dondi, D. The current situation of the legislative gap on microplastics (MPs) as new pollutants for the environment. Water Air Soil Pollut. 2024, 235, 778. [Google Scholar] [CrossRef]
- Casella, C.; Sol, D.; Laca, A.; Díaz, M. Microplastic Retention in Secondary Sewage Sludge: Characterization and Influence of Solid Concentration. Appl. Sci. 2025, 15, 3557. [Google Scholar] [CrossRef]
- Casella, C.; Cornelli, U.; Ballaz, S.; Zanoni, G.; Merlo, G.; Ramos-Guerrero, L. Plastic Smell: A Review of the Hidden Threat of Airborne Micro and Nanoplastics to Human Health and the Environment. Toxics 2025, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe 2024—The Fast Facts 2024 Plastics Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023 (accessed on 25 March 2025).
- Li, B.; Li, M.; Du, D.; Tang, B.; Yi, W.; He, M.; Zheng, J. Characteristics and influencing factors of microplastics entering human blood through intravenous injection. Environ. Int. 2025, 198, 109377. [Google Scholar] [CrossRef] [PubMed]
- Simionov, I.A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.L.; Faggio, C.; Petrea, Ş.M. Human health risk assessment of potentially toxic elements and microplastics accumulation in products from the Danube River Basin fish market. Environ. Toxicol. Pharmacol. 2023, 104, 104307. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, N.N.; Călmuc, M.; Milea, Ș.A.; Georgescu, P.L.; Iticescu, C. Micro and nano plastics in fruits and vegetables: A review. Heliyon 2024, 10, e28291. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Chakraborty, S. Hidden hazards: Microplastics in intravenous admixtures and their path into the body. Environ Monit. Assess. 2025, 197, 400. [Google Scholar] [CrossRef] [PubMed]
- Rotchell, J.M.; Jenner, L.C.; Chapman, E.; Bennett, R.T.; Bolanle, I.O.; Loubani, M.; Palmer, T.M. Detection of microplastics in human saphenous vein tissue using μFTIR: A pilot study. PLoS ONE 2023, 18, e0280594. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Yang, X. Detection of various microplastics in patients undergoing cardiac surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Feng, Y.; Wang, R.; Jiang, J.; Guan, Q.; Yang, X.; Luo, Y. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J. Adv. Res. 2023, 49, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D. Microplastic diagnostics in humans:“The 3Ps” Progress, problems, and prospects. Sci. Total Environ. 2023, 856, 159164. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.; Ballaz, S.J. Genotoxic and neurotoxic potential of intracellular nanoplastics: A review. J. Appl. Toxicol. 2024, 44, 1657–1678. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Matta, M.; Cristiano, L.; Matassa, R.; Battaglione, E.; Svelato, A.; Nottola, S.A. Deeply in plasticenta: Presence of microplastics in the intracellular compartment of human placentas. Int. J. Environ. Res. Public Health 2022, 19, 11593. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.M.; Parvathi, V.D.; Yoghalakshmi, N.; Kumar, S.M.; Athulya, P.A.; Mukherjee, A.; Chandrasekaran, N. Plastic particles in medicine: A systematic review of exposure and effects to human health. Chemosphere 2022, 303, 135227. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, Y.; Wang, L.; Ruan, X.; Ge, Q.; Ma, M.; Zhang, L. MPs Entering Human Circulation through Infusions: A Significant Pathway and Health Concern. Environ. Health 2025, 3, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Du, S.; Liu, Z.; Li, W.; Tao, F.; Qie, X. Systematic characterisation of microplastics released from disposable medical devices using laser direct infrared spectroscopy. Anal. Chim. Acta 2025, 1355, 343982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ma, M.; Sun, X.; Wu, Z.; Yu, Y.; Kang, Y.; An, L. Microplastics entry into the blood by infusion therapy: Few but a direct pathway. Environ. Sci. Technol. Lett. 2023, 11, 67–72. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, L.; Wen, X.; Jiang, C.; Long, Y.; Zhang, J.; Liu, R. Organophosphate esters in China: Fate, occurrence, and human exposure. Toxics 2021, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, D.; Casella, C.; Laca, A.; Díaz, M.; Dondi, D. A Ray of Hope: Gamma Radiation for Microplastic Remediation. Glob. Chall. 2025, 9, 2500117. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, M.R.; Abdallah, M.A.E.; Harrad, S. A critical review of human exposure to organophosphate esters with a focus on dietary intake. Sci. Total Environ. 2021, 771, 144752. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, P.; Ma, S.; Lu, S.; Yu, Y.; An, T. A critical review of human internal exposure and the health risks of organophosphate ester flame retardants and their metabolites. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1528–1560. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Liao, C.; Jiang, G. Human internal exposure to organophosphate esters: A short review of urinary monitoring on the basis of biological metabolism research. J. Hazard. Mater. 2021, 418, 126279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.; Li, X.; Liu, C.; Wang, M.; Zhang, S.; Liu, S. Reactive oxygen species drive aging-associated microplastic release in diverse infusion ingredients. J. Hazard. Mater. 2025, 490, 137728. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Xie, J.; Gowen, A.; O’Higgins, A.C.; Xu, J. Microplastic Transfer into the Bloodstream from Intravenous Fluid Infusion Systems. 2024. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4709116 (accessed on 25 March 2025).
- Zheng, X.; Feng, Q.; Guo, L. Quantitative analysis of microplastics and nanoplastics released from disposable PVC infusion tubes. J. Hazard. Mater. 2024, 465, 133246. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Liu, K.; Zhu, L.; Wang, X.; Dong, X.; Jabeen, K.; Li, D. Is intravenous infusion an unrecognized route for internal microplastic human exposure? A general assessment. J. Hazard. Mater. 2024, 480, 135769. [Google Scholar] [CrossRef] [PubMed]
- Mou, L.; Wu, C.; Li, R.; Zhu, Y.; Su, G.; Zhang, Y. Rapid detection of microplastics/nanoplastics directly exposed to blood during intravenous injections via mie scattering spectra. J. Hazard. Mater. 2024, 480, 136193. [Google Scholar] [CrossRef] [PubMed]
- Çağlayan, U.; Gündoğdu, S.; Ramos, T.M.; Syberg, K. Intravenous hypertonic fluids as a source of human microplastic exposure. Environ. Toxicol. Pharmacol. 2024, 107, 104411. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández, C.; Matikainen, E.; McGuigan, K.G.; Andrade, J.M.; Marugán, J. Evaluation of microplastics release from solar water disinfection poly (ethylene terephthalate) and polypropylene containers. J. Hazard. Mater. 2024, 465, 133179. [Google Scholar] [CrossRef] [PubMed]
- Mordor Intelligence. Forecast 2024. Available online: https://www.mordorintelligence.com/industry-reports/glucose-iv-solutions-market (accessed on 25 March 2025).
- EU Country Cancer Profile: Italy. 2025. Available online: https://www.oecd.org/en/publications/eu-country-cancer-profile-italy-2025_1e742c63-en.html (accessed on 25 March 2025).
- Moreira, M.G.M.; Baldeon, G.A.O.; del Jesús Azúa Menéndez, M. Enfermedades crónicas no transmisibles y la calidad de vida en el Ecuador. MQRInvestigar 2023, 7, 1592–1612. [Google Scholar] [CrossRef]
- WHO Mortality Database. 2023. Available online: https://platform.who.int/mortality/countries/country-details/MDB/ecuador (accessed on 25 March 2025).
- WHO International Agency for Research on Cancer. 2023. Available online: https://canscreen5.iarc.fr (accessed on 25 March 2025).
- Casella, C.; Ramos-Guerrero, L.; Cornelli, U. Microplastics in Spanish Eye Drops: Hidden contaminants in ophthalmic formulations. Res. Chem. 2025, 15, 102332. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G. Blanks and bias in microplastic research: Implications for future quality assurance. Trends Environ. Anal. Chem. 2023, 38, e00203. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D.; Elizalde-Martínez, I. Common laboratory reagents: Are they a double-edged sword in microplastics research? Sci. Total Environ. 2023, 875, 162610. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Xie, J.; Gowen, A.; O’Higgins, A.C.; Xu, J.L. Advanced optical photothermal infrared spectroscopy for comprehensive characterization of microplastics from intravenous fluid delivery systems. Sci. Total Environ. 2024, 929, 172648. [Google Scholar] [CrossRef] [PubMed]
- Dewika, M.; Kantha, N.; Markandan, K.; Nagaratnam, S.; Irfan, N.A.; Khalid, M. Microplastics release from coronary catheters: Insights from catheter analysis. Chemosphere 2024, 366, 143428. [Google Scholar] [CrossRef] [PubMed]
- ARCSA-DE-2024-047-DASP. 2024. Available online: https://www.controlsanitario.gob.ec/wp-content/uploads/downloads/2025/06/25.06.06_borrador-de-IE_Requisitos-para-la-certificacion-BPADT.pdf (accessed on 25 March 2025).
- Regulation (EU) 2017/745 of the European Parliament and of the Council. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745 (accessed on 25 March 2025).
- Wang, J.; Xie, L.G.; Wu, X.F.; Zhao, Z.G.; Yang, H.Y.; Sun, H.M. Identification and quantification of micro–nano-plastics in polypropylene-bottled injections. Heliyon 2024, 10, e35101. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and oxidative stress—Current problems and prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, Y.; Bai, L.; Cui, J. Microplastics: An often-overlooked issue in the transition from chronic inflammation to cancer. J. Transl. Med. 2024, 22, 959. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Zheng, C.M.; Wang, Y.J.; Wang, Y.L.; Chiu, H.W. Effects of microplastics and nanoplastics on the kidney and cardiovascular system. Nat. Rev. Nephrol. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Passos, R.S.; Davenport, A.; Busquets, R.; Selden, C.; Silva, L.B.; Baptista, J.S.; Campos, L.C. Microplastics and nanoplastics in haemodialysis waters: Emerging threats to be in our radar. Environ. Toxicol. Pharmacol. 2023, 102, 104253. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Winiarska, E.; Jutel, M.; Zemelka-Wiacek, M. The potential impact of nano-and microplastics on human health: Understanding human health risks. Environ. Res. 2024, 251, 118535. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dong, C.; Yu, Z.; Ozaki, Y.; Hu, Z.; Zhang, B.; Xie, Y. Detection and analysis of microplastics in tissues and blood of human cervical cancer patients. Environ. Res. 2024, 259, 119498. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Jung, J.; Park, S.A.; Lee, Y.; Kim, J.; Han, C.; Hong, Y.C. Microplastic particles in human blood and their association with coagulation markers. Sci. Rep. 2024, 14, 30419. [Google Scholar] [CrossRef] [PubMed]
- Rafazi, P.; Haghi-Aminjan, H.; Bagheri, Z.; Rahimifard, M. Microplastics and cancer progression: A comparative study of 2D and 3D gastric cancer models using ISOCompliant protocols. Res. Eng. 2024, 24, 103329. [Google Scholar] [CrossRef]
- Scuto, M.; Lombardo, C.M.G.; Lo Sasso, B.; Di Fatta, E.; Ferri, R.; Trovato Salinaro, A. Microplastics as Emerging Contaminants and Human Health: Exploring Functional Nutrition in Gastric–Colon–Brain Axis Cancer. Toxics 2025, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Raimondo, S.; Piscopo, M.; Ricciardi, M.; Guglielmino, A.; Chamayou, S.; Motta, O. First evidence of microplastics in human ovarian follicular fluid: An emerging threat to female fertility. Ecotoxicol. Environ. Saf. 2025, 291, 117868. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dong, C.; Xiang, T.; Shentu, X.; Yu, Z.; Xu, J.; Xie, Y. Microplastic changes during the developent of cervical cancer and its effects on the metabolomic profiles of cancer tissues. J. Hazard. Mater. 2025, 483, 136656. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.; Jha, N.; Precilla, D.; Sivachandran, R.; Ramprasath, A.; Rajkumar, M.; Anitha, T.S. Microplastics and Cancer: A Comprehensive Review of Their Impact on Tumor Progression and Mechanisms of Carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2025. [Google Scholar] [CrossRef]
- Li, P.; Li, Q.; Lai, Y.; Yang, S.; Yu, S.; Liu, R.; Liu, J. Direct entry of micro (nano) plastics into human blood circulatory system by intravenous infusion. Iscience 2023, 26, 108454. Available online: https://www.cell.com/action/showCitFormats?doi=10.1016%2Fj.isci.2023.108454&pii=S2589-0042%2823%2902531-2 (accessed on 25 March 2025). [CrossRef] [PubMed]




| By Type of Solution | By Final User | By Geographical Division |
|---|---|---|
| Total Parenteral Nutrition (TPN): Complete treatments that give patients who are unable to obtain enteral or oral feeding all the nutrients they need. In 2023, this market category dominated the European market, and through 2030, it is anticipated to continue growing at the quickest rate. Peripheral Parenteral Nutrition (PPN): Used for patients who need temporary or partial nutritional support. Crystalloids: Aqueous electrolyte solutions, including 5% glucose and saline (0.9% NaCl), are frequently used to maintain electrolyte balance and hydrate the body. Colloids: High-molecular-weight molecule-containing solutions that are utilized to increase plasma volume in hypovolemic shock and other conditions. Others: Contains customized solutions for particular situations and blood products. |
|
|
| MPs | Hi * | Distribution (%) |
|---|---|---|
| PE | 1 | 25 |
| PP | 1 | 20 |
| PET | 2 | 15 |
| PA | 2 | 10 |
| SBR | 3 | 10 |
| PU | 3 | 10 |
| PTFE | 2 | 5 |
| EVA | 1 | 5 |
| Brand | IV-MD | MPs/L (Mean ± SD) | PHI Value | PHI Level | Exposition Level | Clinical Risk |
|---|---|---|---|---|---|---|
| Brand 1 | Glucose 5% | 12 ± 4 | 1.7 | Medium | Low | Low |
| Brand 1 | NaCl 0.9% | 16 ± 3 | 1.7 | Medium | Low | Low |
| Brand 2 | Glucose 5% | 17 ± 4 | 1.7 | Medium | Low | Low |
| Brand 2 | NaCl 0.9% | 227 ± 15 | 1.7 | Medium | High | High |
| Brand 3 | Glucose 5% | 185 ± 17 | 1.7 | Medium | High | High |
| Brand 3 | NaCl 0.9% | 219 ± 22 | 1.7 | Medium | High | High |
| Brand 4 | NaCl 0.9% | 22 ± 6 | 1.7 | Medium | Moderate | Moderate |
| Brand 5 | NaCl 0.9% | 259 ± 57 | 1.7 | Medium | High | High |
| Brand 6 | NaCl 0.9% | 191 ± 42 | 1.7 | Medium | High | High |
| Brand 7 | NaCl 0.9% | 240 ± 37 | 1.7 | Medium | High | High |
| MP Type | Potential Risk | Reasoning |
|---|---|---|
| PVC | High | Contains harmful substances that can cause chlorine release, like phthalates (i.e., plasticizer), which are endocrine disruptors |
| PS | High | It is easily fragmented into NPs, can induce oxidative stress, cell damage, inflammation |
| SBR | Moderate-High | Can leach residual monomers (styrene, butadiene) and additives (antioxidants, accelerators); prone to oxidative degradation into NPs, triggering oxidative stress, inflammation, and potential cytotoxicity |
| PET | Moderate | It can release Sb (i.e., used in its production) associated with oxidative stress and intracellular accumulation |
| PU | Moderate | It can degrade, releasing toxic diisocyanates and cause inflammation |
| PA | Moderate | Less investigated, but it might have inflammatory and physical impacts |
| EVA | Moderate | Contains vinyl acetate units that may leach monomer (a probable carcinogen) and additives; prone to mechanical fragmentation into NPs, potentially inducing oxidative stress and inflammatory responses |
| PP | Low-moderate | Generally thought to be inert, they may trigger immunological reactions if they break apart into NPs |
| PE | Low-moderate | Chemically inert, whereas cumulative and physical effects are not completely ruled out, just like PP |
| PTFE | Unknow potential | Extremely resistant to chemicals, but capable of releasing some harmful fluorinated compounds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casella, C.; Cornelli, U.; Zanoni, G.; Moncayo, P.; Ramos-Guerrero, L. Health Risks from Microplastics in Intravenous Infusions: Evidence from Italy, Spain, and Ecuador. Toxics 2025, 13, 597. https://doi.org/10.3390/toxics13070597
Casella C, Cornelli U, Zanoni G, Moncayo P, Ramos-Guerrero L. Health Risks from Microplastics in Intravenous Infusions: Evidence from Italy, Spain, and Ecuador. Toxics. 2025; 13(7):597. https://doi.org/10.3390/toxics13070597
Chicago/Turabian StyleCasella, Claudio, Umberto Cornelli, Giuseppe Zanoni, Pablo Moncayo, and Luis Ramos-Guerrero. 2025. "Health Risks from Microplastics in Intravenous Infusions: Evidence from Italy, Spain, and Ecuador" Toxics 13, no. 7: 597. https://doi.org/10.3390/toxics13070597
APA StyleCasella, C., Cornelli, U., Zanoni, G., Moncayo, P., & Ramos-Guerrero, L. (2025). Health Risks from Microplastics in Intravenous Infusions: Evidence from Italy, Spain, and Ecuador. Toxics, 13(7), 597. https://doi.org/10.3390/toxics13070597







