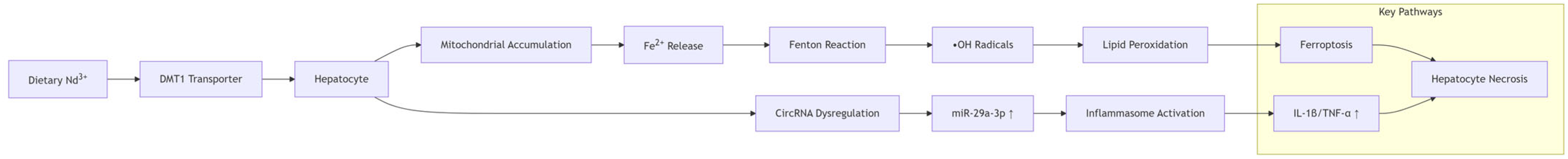
1. Introduction
The strategic importance of rare earth elements (REEs) is underscored by China’s dominance in global reserves (37%) and production (70% in 2024) [
1]. Recent market analyses reveal accelerated REE consumption growth at 8.2% CAGR (2023–2025), driven primarily by high-tech applications (
Figure 1) [
2]. Neodymium (Nd), constituting 18–23% of total REE output, has seen demand surge 150% since 2020 due to NdFeB magnets in electric vehicles and robotics [
3]. Critical concern arises from environmental exposure pathways: Dietary intake accounts for >60% of human Nd absorption, with bioaccumulation coefficients reaching 8.3 in liver tissue [
4]. This triggers hepatotoxic cascades through oxidative stress induction (ROS ↑300%), ferroptosis activation (GPX4 ↓70%), and non-coding RNA dysregulation (e.g., miR-29a-3p ↑5.8-fold) [
5]. Additionally, the accumulation of REEs has been detected in the liver, lungs, nails, and kidneys, with varying concentrations in different organs [
6]. Therefore, it is urgent to study the impact of REE accumulation on animals. Comprehensive mechanistic insights are visualized in
Figure 1.
1.1. Sources and Applications of Neodymium
Neodymium (Nd) is one of the most abundant REEs on Earth [
7]. It is primarily sourced from minerals such as monazite and bastnäsite, which are rich in REEs. Monazite, a rare earth–thorium phosphate mineral, typically contains 9% to 20% neodymium. Bastnäsite, a rare earth fluorocarbonate mineral, contains 2% to 15% neodymium. These minerals are widely distributed globally, with significant deposits in China and other regions. Neodymium is also extracted from ion-adsorption clay deposits, which are found in South China, Myanmar, South America, and Africa. In these deposits, REEs, including neodymium, are adsorbed onto clay minerals through ion exchange.
Accounting for >70% of global supply [
8] (USGS 2024), China dominates rare earth production, with neodymium output exceeding 25,000 tons annually, with substantial reserves of monazite and bastnäsite. The Bayan Obo mine in Inner Mongolia is one of the largest rare earth mines in the world. Neodymium is extracted primarily through electrolysis or chemical reduction methods. For example, neodymium can be obtained by reducing anhydrous neodymium chloride with metallic calcium or by electrolyzing molten neodymium chloride.
Neodymium has a wide range of applications, particularly in the manufacturing of high-performance permanent magnets. Neodymium–iron–boron (Nd2Fe14B) magnets, known as the “king of permanent magnets,” are widely used in the electronics and mechanical industries due to their high magnetic energy product (
Table 1).
1.2. Current Research Landscape
Numerous studies have been conducted to explore the biological effects of REEs, including neodymium. Research has shown that REEs can induce oxidative stress, inflammation, and cytotoxicity in different cell types and organisms. For instance, studies have demonstrated that neodymium exposure can lead to the generation of reactive oxygen species (ROS) and subsequent oxidative damage in cells. Additionally, the accumulation of REEs in tissues has been associated with various adverse health effects, such as hepatotoxicity and nephrotoxicity [
11]. The accumulation of neodymium in the environment and its potential health impacts highlight the need for further research into its toxicological effects.
1.3. Role of Non-Coding RNAs in Environmental Pollution Effects
Despite these findings, there are still significant gaps in our understanding of the molecular mechanisms underlying REE-induced toxicity. The role of non-coding RNAs (ncRNAs), such as miRNAs, circRNAs, and lncRNAs, in mediating the toxic effects of REEs remains largely unexplored [
12]. Furthermore, the potential long-term effects of REE exposure on human health, especially through environmental pathways, are not yet fully elucidated [
13]. To provide a clearer understanding of the different classes of non-coding RNAs (ncRNAs) mentioned in the study, we have added a brief introduction to distinguish between miRNA, circRNA, and lncRNA:
miRNA: These are small non-coding RNAs that primarily function by binding to complementary sequences on target mRNAs, leading to gene silencing through mRNA degradation or translational repression.
circRNA: Circular RNAs are covalently closed-loop structures that often act as molecular sponges for miRNAs, thereby regulating gene expression by sequestering miRNAs and preventing them from binding to their target mRNAs.
lncRNA: Long non-coding RNAs are typically longer than 200 nucleotides and have diverse functions, including the regulation of transcription, modification of chromatin structure, and modulation of protein activity.
This suggests that ncRNAs may also be involved in the hepatotoxic effects of neodymium nitrate (Nd(NO3)3), potentially contributing to the observed gene expression changes and liver injury. Understanding the involvement of ncRNAs in Nd(NO3)3-induced liver injury could provide valuable insights into the underlying mechanisms and potential biomarkers for early detection and intervention.
1.4. Epidemiological Evidence
Although there is a growing body of experimental research on REE toxicity, epidemiological evidence linking REE exposure to specific health outcomes is relatively limited. A few studies have reported associations between REE exposure and certain health issues in occupational settings or communities living near REE mining and processing sites. However, these studies often face challenges such as small sample sizes, lack of direct exposure measurements, and potential confounding factors, which limit the strength of the conclusions that can be drawn.
To investigate the effects of neodymium nitrate on hepatocytes, we selected the AML12 mouse hepatocyte cell line as our model. The AML12 cell line is a well-established and widely used model for studying hepatotoxicity and the mechanisms of liver injury. This cell line closely mimics the physiological and metabolic functions of primary hepatocytes, making it an ideal choice for investigating the effects of neodymium nitrate on liver cells.
1.5. Research Gaps and Objectives
Given the increasing use of REEs and the potential health risks associated with their exposure, it is imperative to conduct more comprehensive and in-depth research to fill the existing gaps in knowledge. Our study aims to investigate the effects of neodymium nitrate on gene expression and cellular pathways in AML12 hepatocytes, with a particular focus on the role of ncRNAs in mediating neodymium-induced liver injury. By identifying key genes and regulatory pathways involved in this process, we hope to provide valuable insights into the underlying mechanisms and potential biomarkers for early detection and intervention.
The aforementioned studies suggest that changes in genes and epigenetics may be related to the hepatotoxic effects of Nd(NO
3)
3. Given the widespread application of Nd(NO
3)
3 and its health risks to various systems, it is urgent to reveal the hepatotoxicity and expression changes of key genes in mouse liver tissue exposed to Nd(NO
3)
3. More researchers have used sequencing technology to analyze specific samples exposed to toxins to identify key genes and regulatory pathways that cause diseases. Highly upregulated or induced expression of non-coding RNAs, such as circRNA 003914, circRNA 009773, lncRNA H19, and miR-29a-3p [
14], have been discovered in mouse liver cells through RNA-Seq. Some lncRNAs, circRNAs, and mRNAs have potential value as candidate markers for Nd(NO
3)
3-induced hepatotoxicity in male mice [
15]. Based on the differences in expression levels of mRNA, miRNA, lncRNA, and circRNA, some studies have proposed the formation of an endogenous RNA competition mechanism (ceRNA) interaction network to explore the regulatory mechanisms of liver injury [
16]. ceRNA networks are essential tools for understanding the post-transcriptional regulatory mechanisms involving non-coding RNAs (ncRNAs) and messenger RNAs (mRNAs). In our study, the ceRNA network analysis helps to reveal how miRNAs, lncRNAs, and circRNAs interact with each other and with mRNAs to modulate gene expression. These differentially expressed genes obtained through RNA-Seq analysis will be particularly important candidate genes for exploring the mechanisms of liver injury toxicity. Therefore, we conducted an RNA-Seq analysis of miRNA/lncRNAs/circRNAs/mRNAs in AML12 cells exposed to Nd(NO
3)
3 to identify key genes and regulatory pathways for male liver injury and to explore early biomarkers and mechanisms of liver injury caused by Nd(NO
3)
3.
2. Materials and Methods
2.1. Cell Culture
AML12 cells were maintained in DMEM/F12 (Thermo Fisher Scientific, Waltham, MA, USA) with 10% FBS (Gibco, 10099141C, Melbourne, Australia), 100 mg/mL streptomycin, and 100 U/mL penicillin (Biyuntian, Shanghai, China). Cells are cultured at 37 °C with 5% CO2 and passaged at a 1:2 ratio every 3 days. After reaching 70–80% confluence, AML12 cells are washed with PBS and then cultured in DMEM/F12 supplemented with 10% FBS and exposed to 0 and 0.8 µmol/L Nd(NO3)3 for 24 h.
2.2. Rationale for Dose Selection
In our study, we selected a concentration of 0.8 μmol/L for neodymium nitrate exposure. This choice was based on previous cytotoxicity studies [
17]. In the referenced cytotoxicity study, it was demonstrated that a concentration of 0.8 μmol/L did not significantly impact cell survival rates or basic physiological functions within a 24 h exposure period. However, this concentration was sufficient to induce significant changes in gene expression. Given these findings, we concluded that 0.8 μmol/L is an appropriate concentration to investigate the biological effects of neodymium nitrate at environmentally relevant levels. This concentration allows us to study the gene expression profile changes directly attributable to neodymium nitrate exposure while maintaining cellular viability for mechanistic studies. The 24 h treatment duration was chosen based on the typical response time of cells to heavy metals and chemical substances, which is sufficient to reflect changes in gene expression. Additionally, 0 µmol/L was selected as the control group to assess the direct impact of neodymium nitrate exposure on gene expression, while the 0.8 µmol/L treatment group was used to investigate the biological effects of neodymium nitrate at environmentally relevant concentrations. This dose design can clearly demonstrate the impact of neodymium nitrate exposure on the cellular gene expression profile and provide a basis for further mechanistic studies.
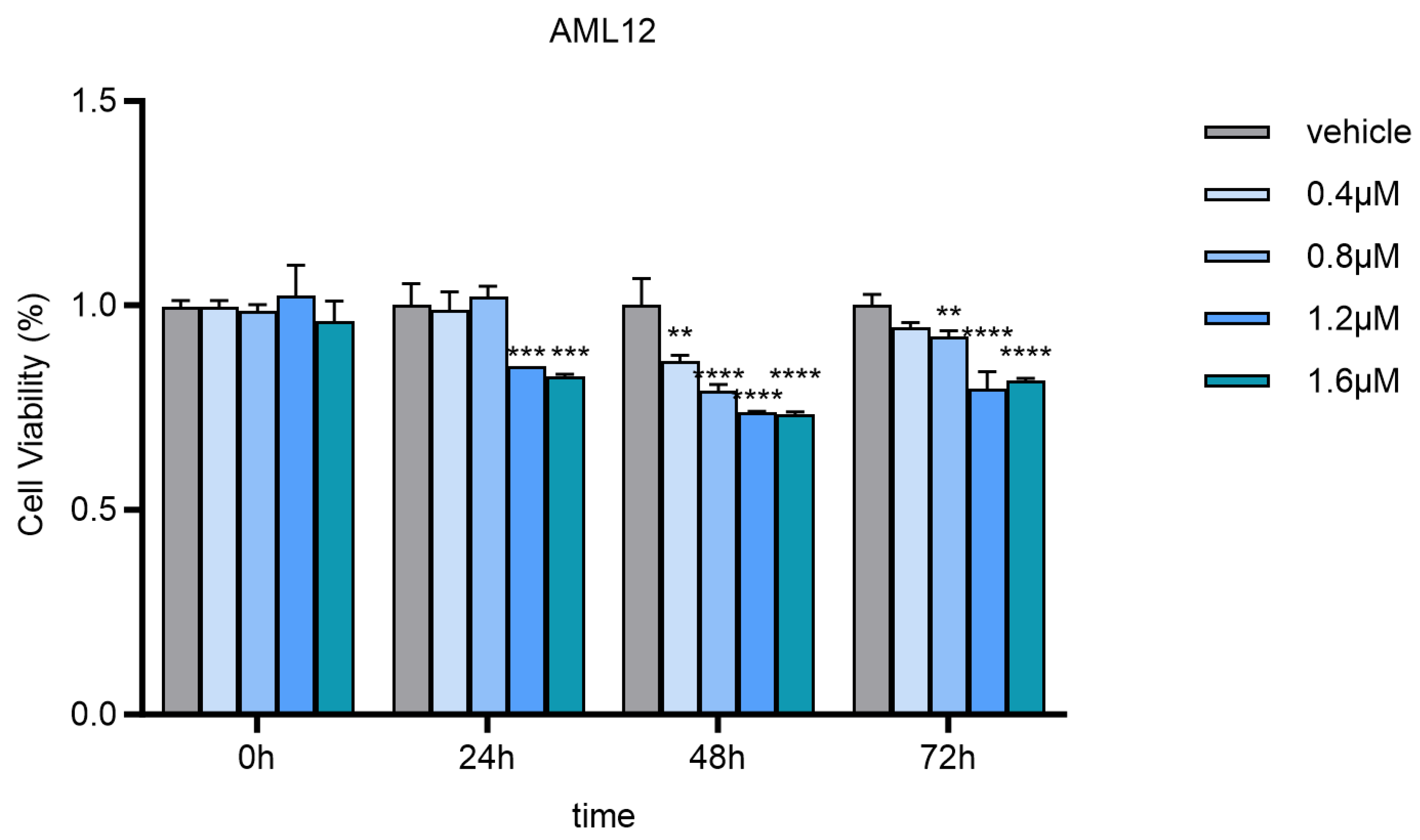
2.3. Cytotoxicity Assay
To assess the cytotoxic effects of neodymium nitrate on AML12 cells, we performed a cytotoxicity assay using the Cell Counting Kit-8 (CCK-8) (Biyuntian, C0038, Shanghai, China). The assay was conducted according to the manufacturer’s instructions with minor modifications.
2.3.1. Cell Seeding and Treatment:
AML12 cells were seeded at a density of 3 × 103 cells per well in a 96-well plate. The cells were allowed to adhere and grow for 24 h at 37 °C with 5% CO2. After the initial incubation, the cells were treated with various concentrations of neodymium nitrate (0, 0.4, 0.8, 1.2, and 1.6 µmol/L) for 24, 48, and 72 h.
2.3.2. CCK-8 Assay Procedure:
Following the treatment, 10 µL of CCK-8 solution was added to each well, and the plate was incubated at 37 °C with 5% CO2 for 2 h. The optical density (OD) was measured at 450 nm using a microplate reader (Tecan SPARK, Grödig, Austria). The cell survival rate was calculated based on the OD values, with the untreated control group set as 100%.
2.4. RNA Library Construction and Sequencing
Total RNA extraction was performed with TRIzol reagent (Tiangen, Beijing, China) following standardized procedures. RNA integrity (RIN > 8.0) and concentration were verified via Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). RNA-seq libraries were constructed as follows:
2.4.1. Small RNA
1 μg Total RNA → NEBNext Small RNA Library Prep Kit (Illumina, San Diego, CA, USA).
2.4.2. mRNA/lncRNA/circRNA
rRNA depletion (Ribo-Zero Kit, Illumina, San Diego, CA, USA).
RNA fragmentation and cDNA synthesis.
dUTP strand marking → UNG digestion.
Adapter ligation and size selection.
Libraries were sequenced on Illumina platforms (150 bp paired-end).
2.5. RNA Identification and Expression Analysis
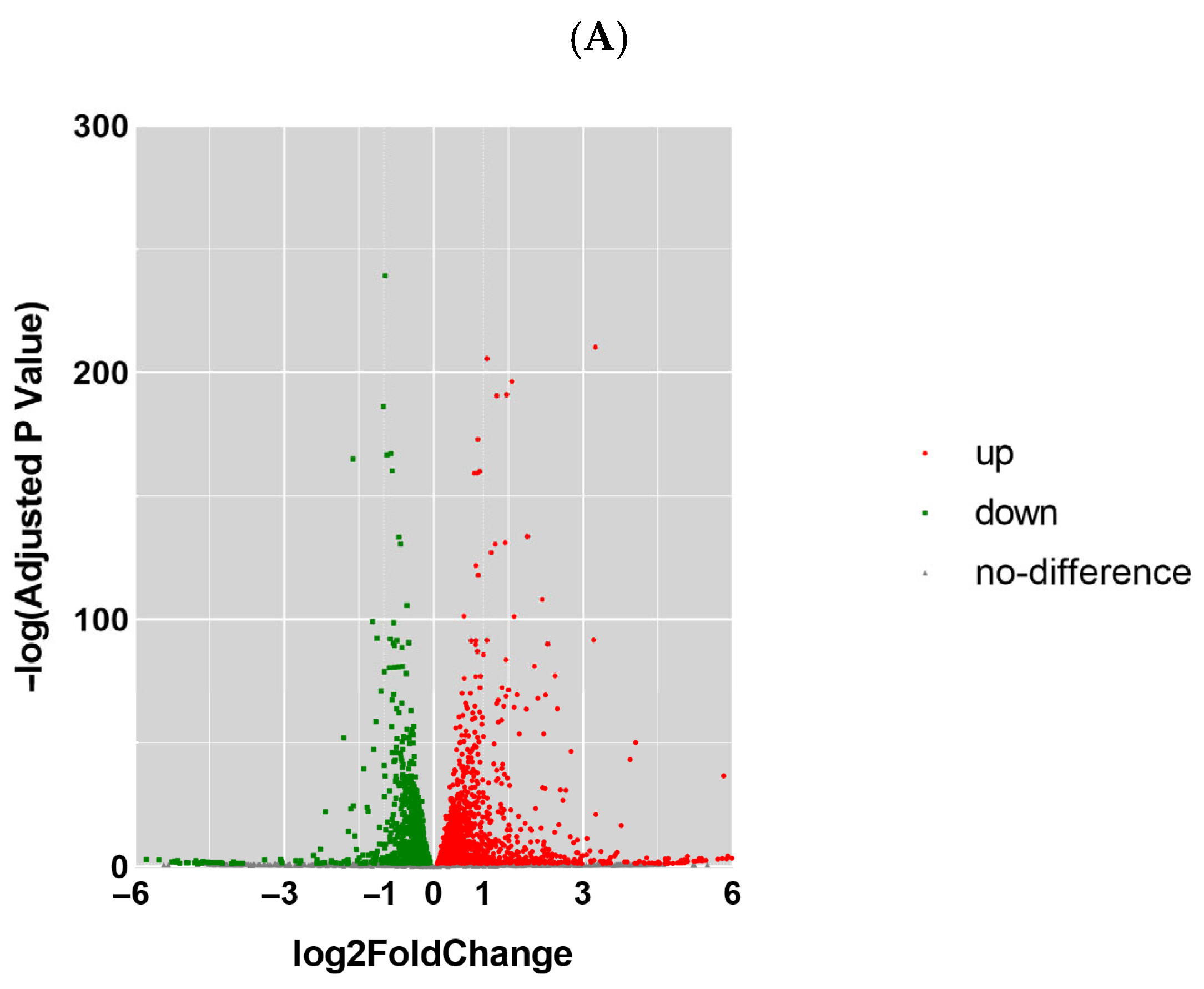
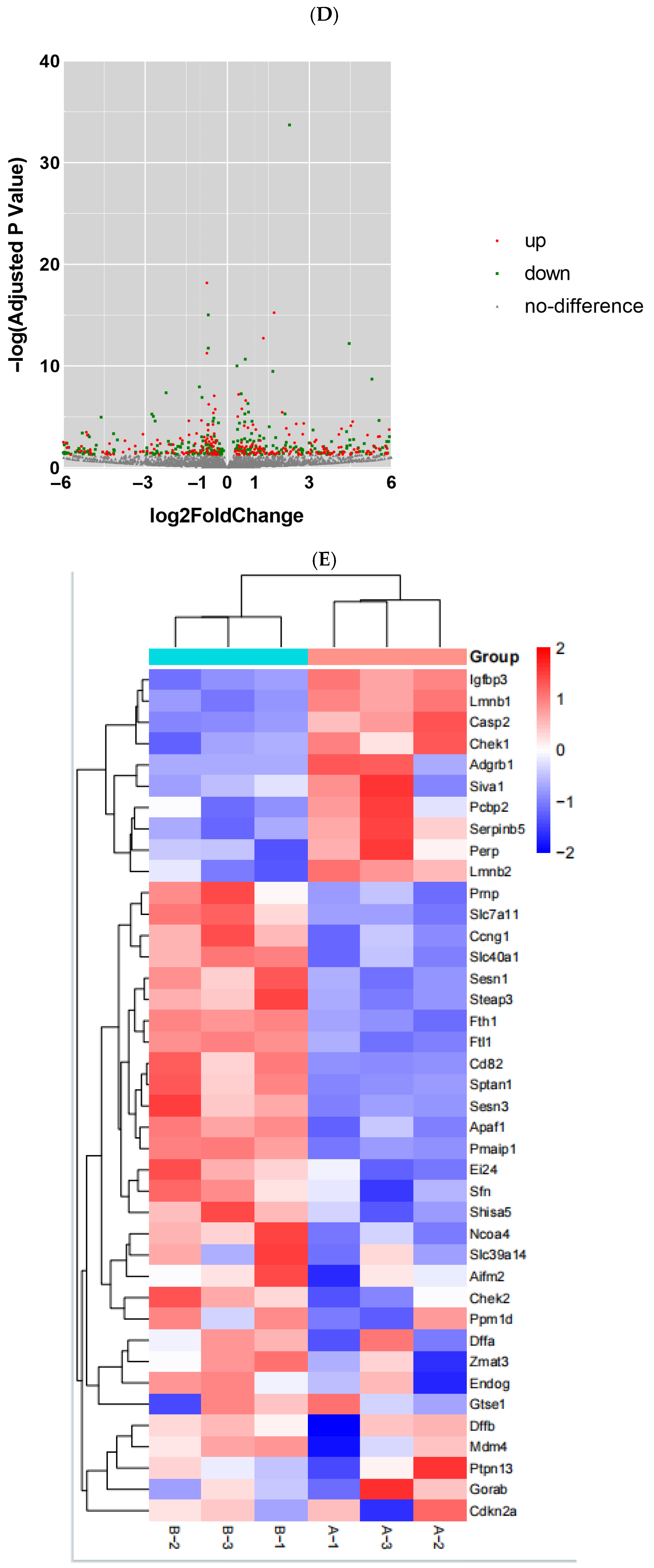
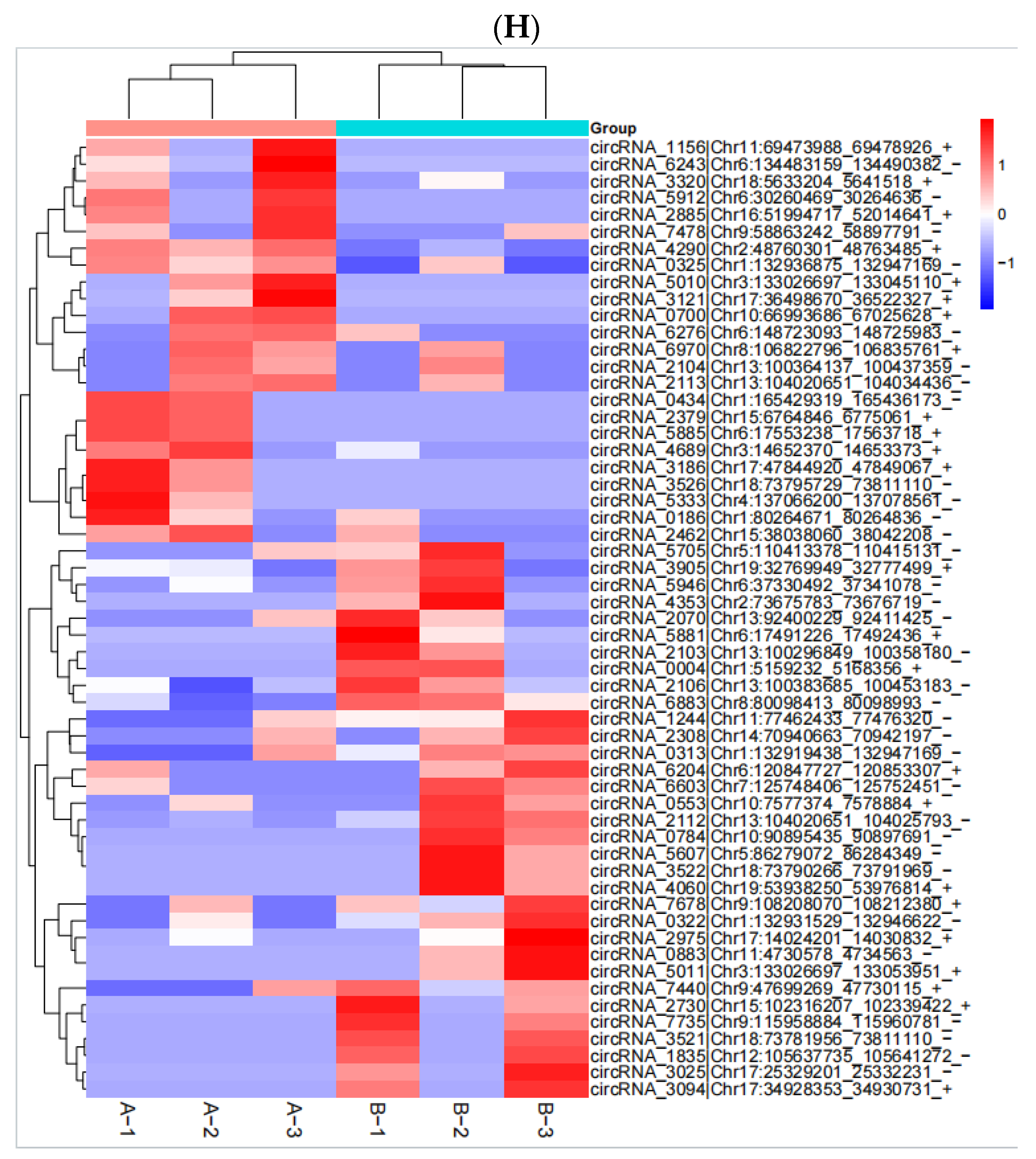
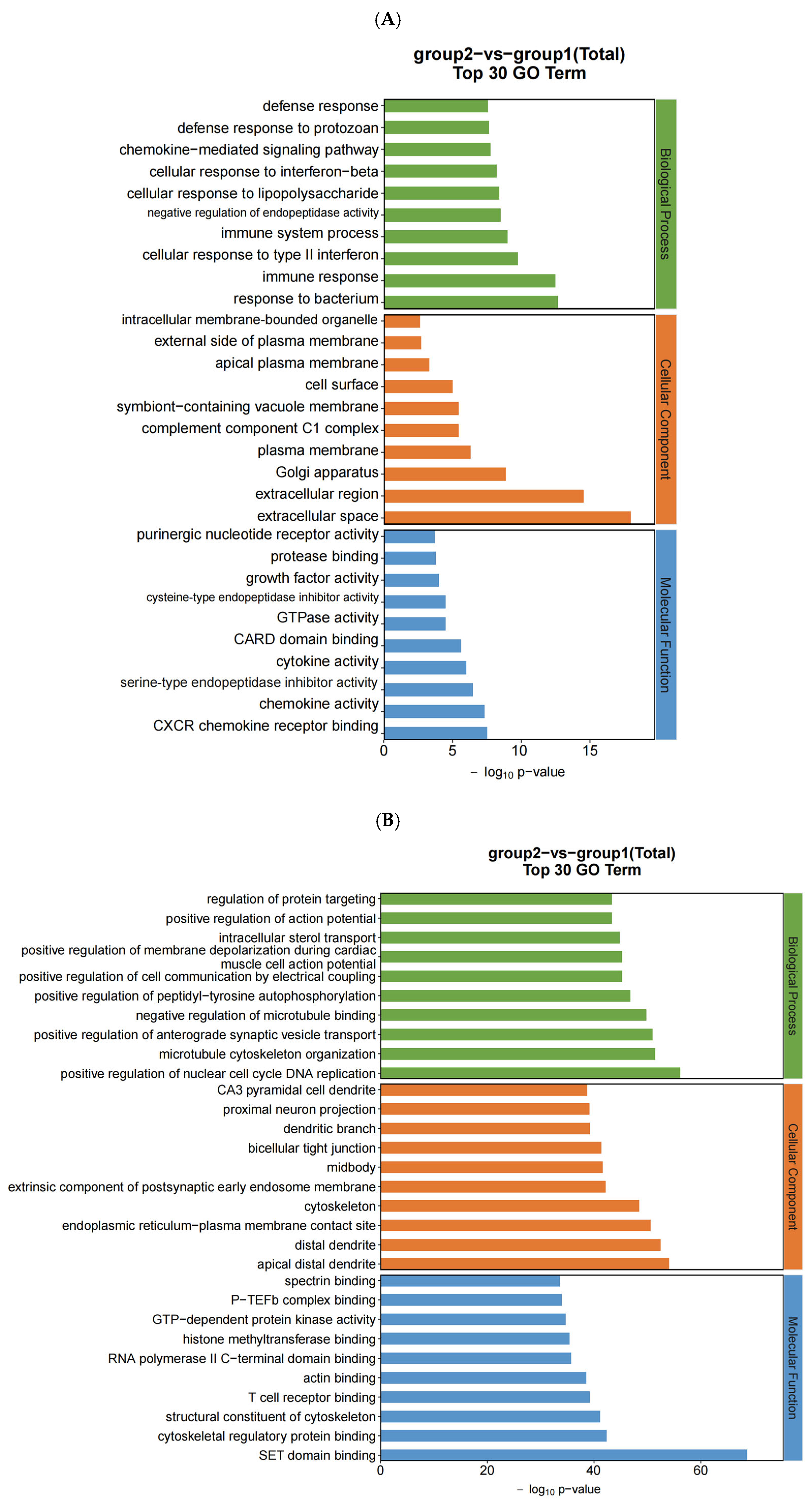
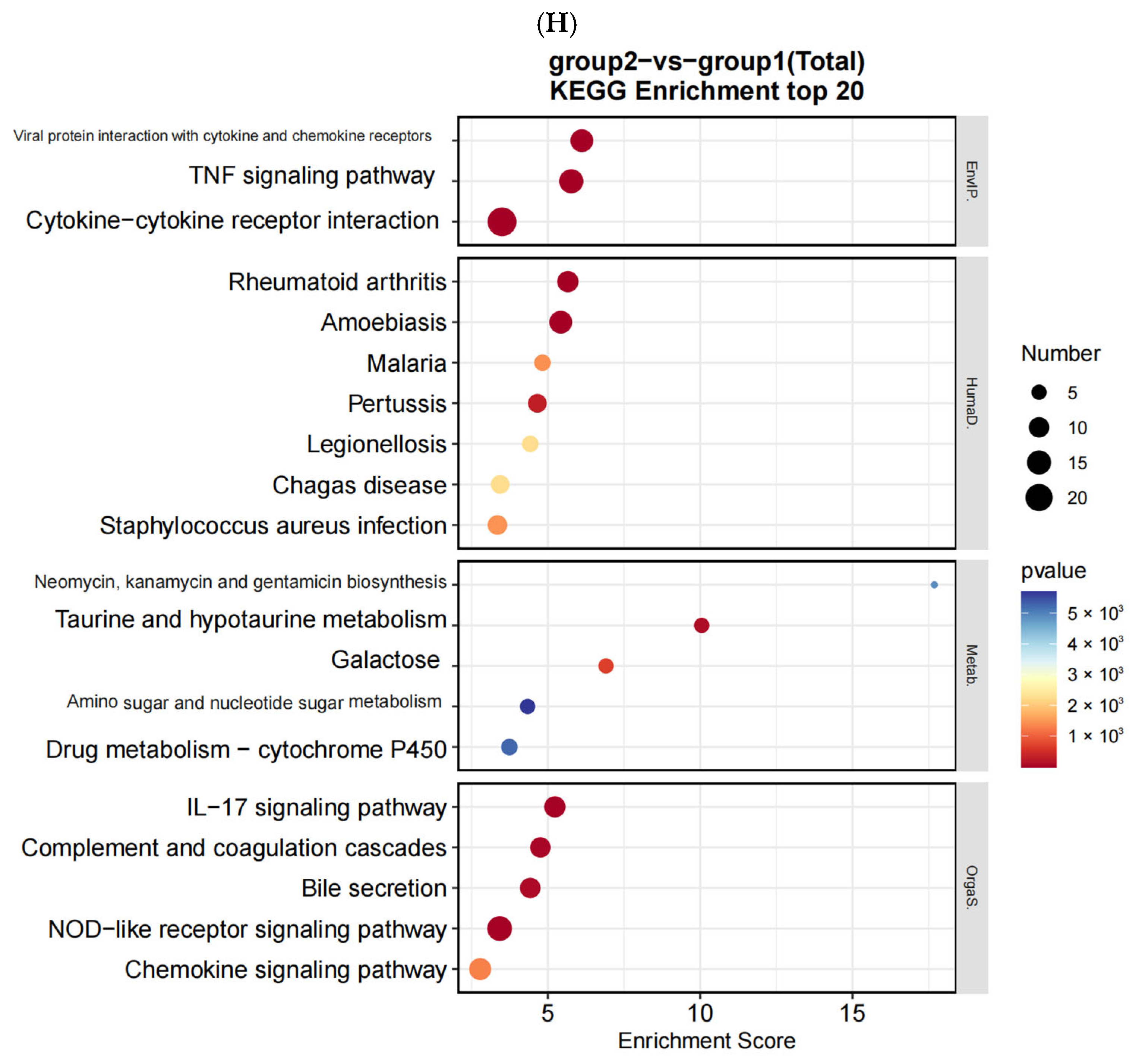
After the sequencing was completed, we used Hisat (v 2.0.4) and ACGT101-miR software (LC Science, Houston, TX, USA) for quality control, genome alignment, and identification of the selected raw data. We identified differentially expressed genes (DEGs) using a threshold of ≥ 1 and a p-value ≤ 0.05. The expression of differentially expressed genes (DEGs) was clustered using log10 (FPKM + 1) to display their expression patterns and differences between sample groups. We performed enrichment analysis of biological significance for DEGs, including Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. A p-value of ≤0.05 was considered significantly enriched, and the enrichment results were then represented using ggplot scatter plots.
2.6. Construction of Protein–Protein Interaction (PPI) Network
We conducted a protein–protein interaction network (PPI) analysis for differentially expressed mRNA using the STRING database (
https://string-db.org/ (accessed on 2 July 2025)). The PPI network was visualized using Cytoscape (version 3.9.1).
2.7. GSEA Enrichment Analysis of Gene Sets in the Reactome Database
We utilize the GSEA (Gene Set Enrichment Analysis) tool to assess the correlation between differentially expressed mRNA and selected gene sets. We calculate the enrichment scores and significance levels using the Normalized Enrichment Score (NES), p-value, and False Discovery Rate (FDR). Analyzing the enrichment results, a higher NES indicates a more significant enrichment of the gene set; a smaller p-value provides stronger evidence to reject the null hypothesis; and a lower FDR suggests a lower proportion of false positive results.
2.8. qPCR Confirmation
We used the FastKing One-Step RT-PCR Kit (Tiangen, KR123, Beijing, China) to reverse transcribe the obtained RNA samples into corresponding cDNA. Real-time PCR analysis was performed on the Mx_3000P fluorescence quantitative PCR instrument (Jiatai, Shanghai, China) using the SuperReal Color Fluorescence Quantitative Premix Reagent (SYBR Green) (Tiangen, FP215, Beijing, China). The relative expression of mRNAs was normalized to β-actin. The primer sequences are shown in
Table S2. These were designed and synthesized by Sangon Biotech, Shanghai, China.
2.9. Intracellular ROS and Mitochondrial ROS Assay
AML12 cells were cultured in 6-well plates and treated with toxins for 24 h as described above. 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, 10μM) (MedChemExpress, HY-D0940, Shanghai, China) was added to the cells and incubated for 20 min. The cells were then washed twice with PBS to remove unincorporated DCFH-DA and reduce background fluorescence. Fluorescence detection was performed using a fluorescence microscope (Leica DM3000, Wetzlar, Germany) with an excitation wavelength of 488 nm and an emission wavelength of 525 nm for DCFH-DA. Cells were harvested, washed once with PBS, and transferred to 1.5 mL centrifuge tubes. Relative Fluorescence Units (RFUs) were measured using a fully automated fluorescence plate reader (Tecan SPARK, Tecan Austria GmbH, Grodig, Austria), with three replicate wells for each sample. The remaining cell samples in the 1.5 mL centrifuge tubes were treated with an appropriate amount of hypotonic potassium chloride buffer, incubated on ice for about 1 h, and mixed frequently. Cells were manually homogenized using a cell sonicator, and cell viability was checked with trypan blue during the process. Homogenization was stopped when approximately 50% of the cells were lysed. The samples were centrifuged at 3000 rpm (approximately 1000× g) at 4 °C for 10 min, and the supernatant was transferred to a new tube. Further centrifugation at 12,000 rpm for 15 min resulted in the pellet being the mitochondrial fraction. The extracted mitochondria were assayed using the same method as for intracellular ROS measurement. The content of intracellular and mitochondrial ROS was quantitatively analyzed by comparing the fluorescence intensity of DCFH-DA.
2.10. Detection of Ferroptosis-Related Markers
The supernatant of the treated AML12 cells was used for the assay. A BCA protein assay kit (Biyuntian, P0012S, Shanghai, China) was used to prepare a protein standard curve, and the protein concentration in each sample was calculated using the standard curve, followed by normalization of protein concentrations. Superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) levels were measured using superoxide dismutase (Biyuntian, S0109, Shanghai, China), malondialdehyde (Biyuntian, S0131S, Shanghai, China), and reduced glutathione assay kits (Biyuntian, S0052, Shanghai, China), respectively.
2.11. Statistical Analysis
All data are presented as mean ± SD. All statistical analyses were performed using GraphPad Prism 10.1.2 (GraphPad Software, Boston, MA, USA) with t-tests. Fold changes and p-values were used to evaluate differences in gene expression. A p-value of less than 0.05 was considered statistically significant, while a p-value of less than 0.01 was considered highly statistically significant. In addition to p-values, we also report F-values to provide additional statistical context for the observed differences in cell survival rates. The F-value is a measure of the variance between groups relative to the variance within groups, which helps in assessing the significance of the observed differences. A higher F-value indicates a greater difference between the groups, suggesting a more pronounced effect of the treatment.
4. Discussion
REEs, which include 17 elements with similar physicochemical properties, have been widely used in industry and modern technology [
7]. However, they also pose a range of health and environmental issues. Neodymium and other REEs can enter the human body through respiration, deposit in the alveoli, and cause dysfunction of alveolar epithelial cells, inflammatory responses, fibrosis, and apoptosis [
18]. These reactions may be related to the physicochemical properties of REEs, which can affect the local environment in the lungs and trigger a series of pathological changes. After entering the body through the digestive tract and entering the bloodstream, neodymium accumulates mostly in the liver, causing liver damage characterized by hepatocyte necrosis, inflammatory responses, and metabolic disorders [
19]. The liver, as the primary detoxification organ, is particularly sensitive to the accumulation of REEs, and its damage may lead to a series of metabolic and immune function impairments [
20]. In addition to the liver, REEs may also have toxic effects on other organs such as the heart, kidneys, and brain. These effects may be related to the bioaccumulation of REEs and their ability to interfere with normal cell functions. Neodymium also has certain toxic effects on the reproductive system, which may be related to its impact on oxidative stress and inflammatory responses in the body [
21].
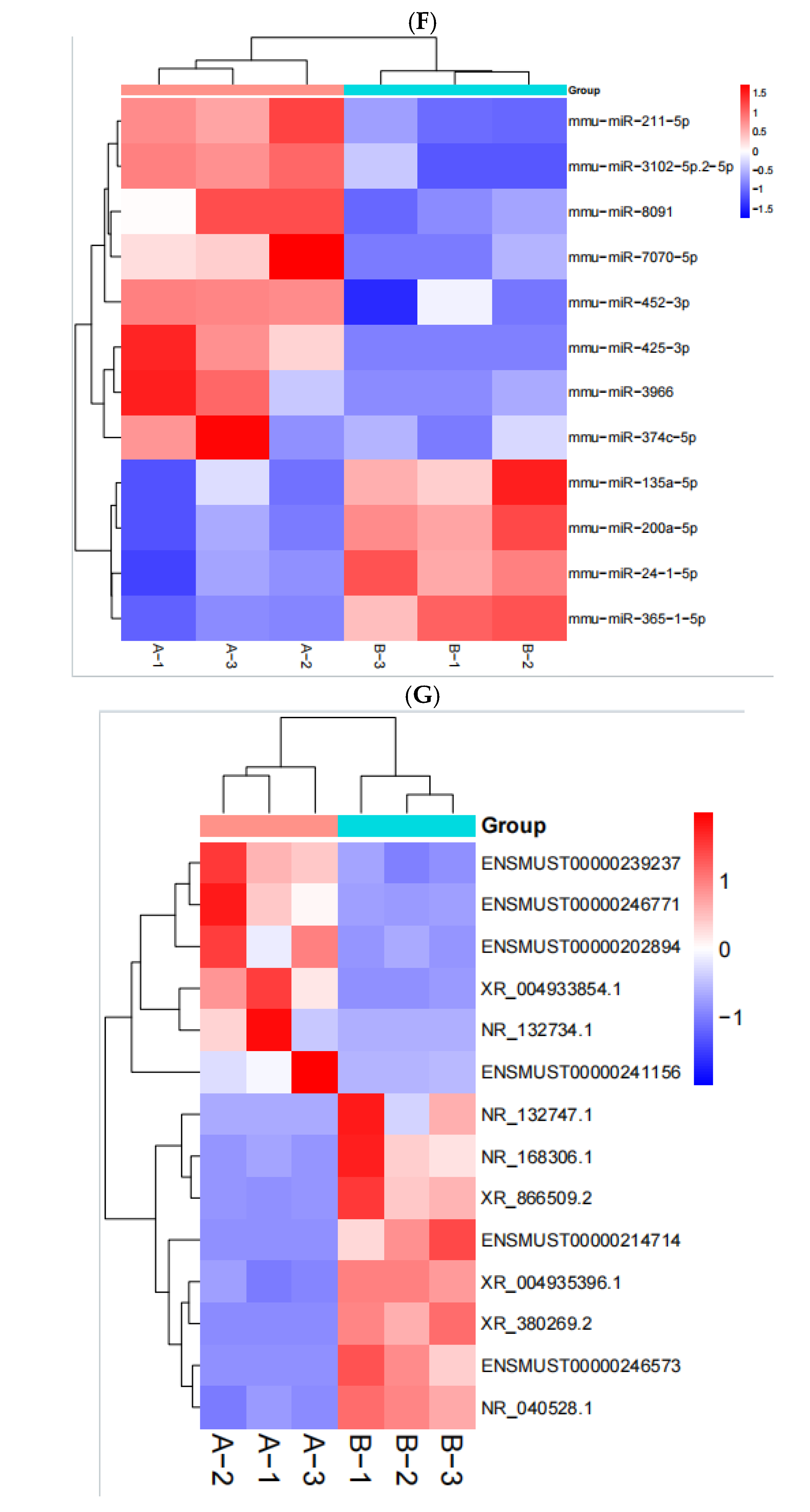
In this study, we utilized RNA sequencing to analyze the impact of neodymium nitrate on gene expression in AML12 cells. Our findings revealed significant changes in the expression of ferroptosis-related genes, as well as differentially expressed miRNAs, lncRNAs, and circRNAs. These results suggest that neodymium nitrate may induce hepatocyte injury by modulating iron metabolism and oxidative stress pathways.
In our study, we used a threshold of ≥ 1 and a p-value ≤ 0.05 to identify differentially expressed genes (DEGs). This approach allows us to capture genes with moderate expression changes that may still play a significant role in the biological processes underlying neodymium-nitrate-induced liver injury. While a stricter threshold like logFC > 2 could provide a more stringent filter for identifying DEGs, it may exclude genes with moderate expression changes that are biologically relevant. Future studies may consider using a combination of thresholds or additional validation methods to further refine the identification of DEGs.
In conclusion, the choice of threshold depends on the specific research question and the biological context. Our study aims to provide a comprehensive overview of the gene expression changes induced by neodymium nitrate, and we believe that using ≥ 1 strikes a balance between sensitivity and specificity.
In this study, we investigated the effects of neodymium nitrate on gene expression and cellular pathways in AML12 hepatocytes. Our findings reveal significant changes in the expression of genes related to ferroptosis and oxidative stress, indicating that neodymium nitrate induces liver injury through the modulation of iron metabolism and lipid peroxidation pathways.
In the context of liver injury, the accumulation of neodymium in hepatocytes can lead to significant oxidative stress, as evidenced by increased levels of ROS and oxidative damage markers such as 8-hydroxydeoxyguanosine (8-OHdG) and γ-H2AX. These changes can disrupt normal cellular functions and lead to hepatocyte necrosis and inflammation, ultimately contributing to liver damage [
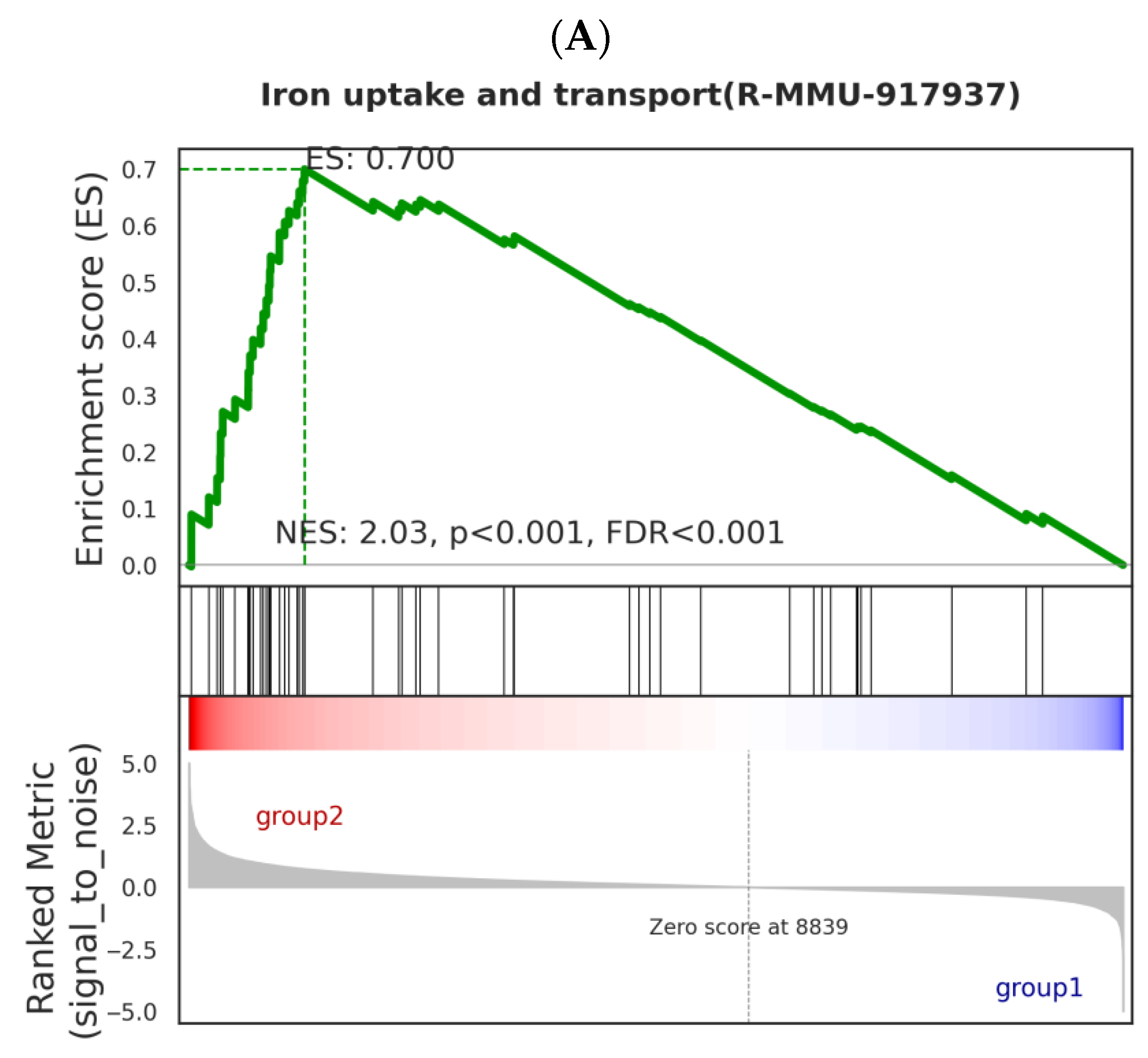
17]. Understanding the specific mechanisms by which neodymium induces oxidative stress and activates downstream signaling pathways can provide valuable insights into the development of therapeutic strategies to mitigate its toxic effects. As illustrated in
Figure 1, the ferroptosis axis (GPX4↓/MDA↑) constitutes a core mechanism in Nd hepatotoxicity, aligning with pathway enrichment analyses (
Section 3.3).
Our study identified significant changes in the expression of non-coding RNAs (ncRNAs) in AML12 cells exposed to neodymium nitrate (Nd(NO
3)
3). These findings are consistent with recent studies that have highlighted the role of small non-coding RNAs in mediating the effects of environmental pollutants. For instance, a study by [
12] demonstrated that small non-coding RNAs play a crucial role in the response to environmental pollution, influencing gene expression and cellular pathways involved in stress response and detoxification processes [
13]. This suggests that ncRNAs may also be involved in the hepatotoxic effects of Nd(NO
3)
3, potentially contributing to the observed gene expression changes and liver injury. Understanding the involvement of ncRNAs in Nd(NO
3)
3-induced liver injury could provide valuable insights into the underlying mechanisms and potential biomarkers for early detection and intervention. The strand-specific RNA-seq approach (
Figure S1) ensured accurate quantification of antisense transcripts, particularly critical for lncRNA analysis.
ceRNA and PPI network analyses provide a perspective on the complex interactions between genes and miRNAs in liver injury induced by Nd(NO
3)
3. These networks help us understand the molecular mechanisms regulating liver injury and reveal potential biomarkers [
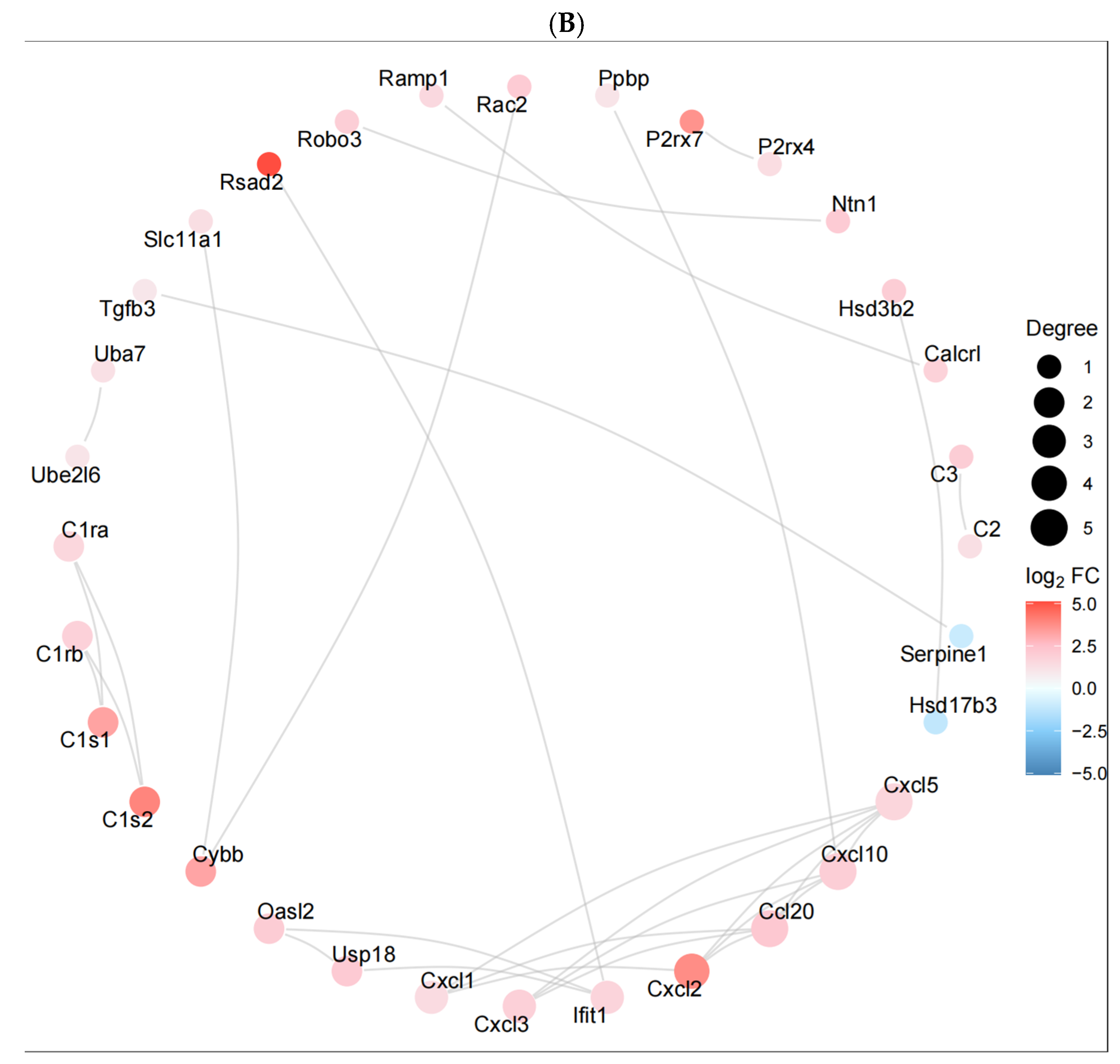
22]. By predicting the target binding relationships between miRNAs and mRNAs, lncRNAs, and circRNAs, ceRNA networks were constructed. This network reveals the complex regulatory relationships between non-coding RNAs and coding RNAs, with miRNAs in a central regulatory position, capable of regulating multiple target genes simultaneously. The ceRNA network shows the interactions between lncRNA–miRNA–mRNA and circRNA–miRNA–mRNA. These interactions may affect the availability of specific miRNAs, thereby influencing the expression of their target mRNAs and participating in the development of liver injury. PPI network analysis shows the physical interactions between proteins encoded by differentially expressed mRNAs, which may play a central role in liver injury. Key nodes in the network (such as the complement system C3, chemokine Cxcl10, Cxcl1, etc.) may represent key regulatory genes and signaling nodes in liver injury induced by Nd(NO
3)
3. These chemokines play a key role in inflammation and immune cell attraction and may play an important role in liver injury induced by Nd(NO
3)
3. Ackr3, as a non-signaling chemokine receptor, may be involved in regulating the availability of chemokines, affecting inflammatory responses. Bpi, bactericidal/permeability-increasing protein, may play a role in anti-infection and immune regulation. Ccl2, monocyte chemoattractant protein-1, may attract monocytes and other immune cells to the site of injury, exacerbating or regulating inflammatory responses. F5, coagulation factor V, may be involved in blood coagulation and wound healing processes, and its abnormalities may be related to liver injury. For miR-149-3p, miR-6975-5p, and miR-7235-5p, these miRNAs target the most genes in the ceRNA network, indicating that they may play a central role in regulating liver injury. They may regulate multiple biological processes by targeting multiple genes simultaneously [
18]. Differentially expressed genes and miRNAs, due to their key roles in liver injury induced by Nd(NO
3)
3, may serve as biomarkers for early diagnosis and prognosis assessment. The ceRNA network not only provides a comprehensive view of the regulatory interactions but also serves as a valuable resource for identifying potential biomarkers and therapeutic targets. By pinpointing key nodes in the network, we can identify critical regulatory elements that could be targeted for therapeutic intervention. These findings provide new directions for future research and may help develop new prevention and treatment strategies.
In the GSEA analysis, significant enrichment of pathways such as iron intake and transport was discovered, which has an important relationship with Nd(NO
3)
3-induced liver injury. The enrichment of iron intake and transport genes may indicate that Nd(NO
3)
3 exposure leads to an imbalance in iron metabolism within hepatocytes. Excessive accumulation of iron can trigger the production of reactive oxygen species (ROS) within cells, leading to oxidative stress. Oxidative stress is one of the important mechanisms of hepatocyte injury and death [
23]. Ferroptosis is a form of cell death that depends on iron, characterized by the accumulation of lipid peroxides within cells [
24]. The enrichment of iron intake and transport genes in the GSEA analysis suggests that Nd(NO
3)
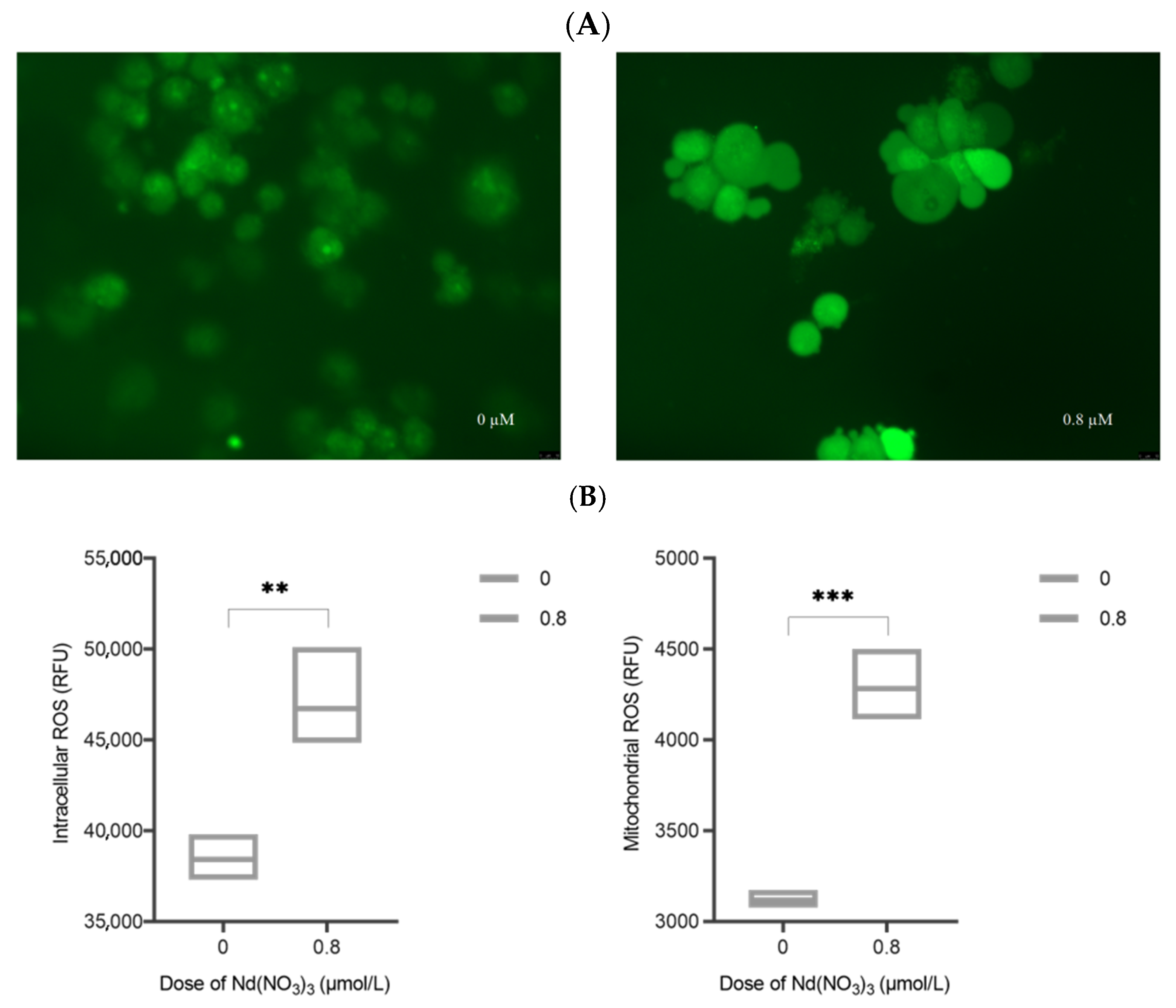
3 may induce ferroptosis by promoting iron intake or inhibiting iron excretion, leading to an excessive accumulation of iron. Our experimental results show that compared with the control group, the ROS level in Nd(NO
3)
3-treated AML12 cells increased significantly. This finding is consistent with the pathological characteristics of ferroptosis, which is a form of cell death associated with increased intracellular iron levels and lipid peroxidation. The accumulation of ROS is a key factor in the process of ferroptosis because it can react with polyunsaturated fatty acids in the cell membrane to form lipid peroxides, leading to cell membrane damage and cell death. Nd(NO
3)
3 exposure may lead to increased oxidative stress in AML12 cells, which may be due to the disruption of the intracellular redox balance by Nd(NO
3)
3. The increase in oxidative stress may be related to the disruption of iron metabolism within cells induced by Nd(NO
3)
3 as iron is a key metal ion that catalyzes the generation of reactive oxygen. In the context of ferroptosis, the accumulation of iron can promote the Fenton reaction, thereby producing more hydroxyl radicals, which are highly reactive ROS. One of the characteristics of ferroptosis is the increase in intracellular iron levels, which is consistent with our finding that the ROS level in Nd(NO
3)
3-treated AML12 cells increased. This increased ROS level may be due to the disruption of iron metabolism induced by Nd(NO
3)
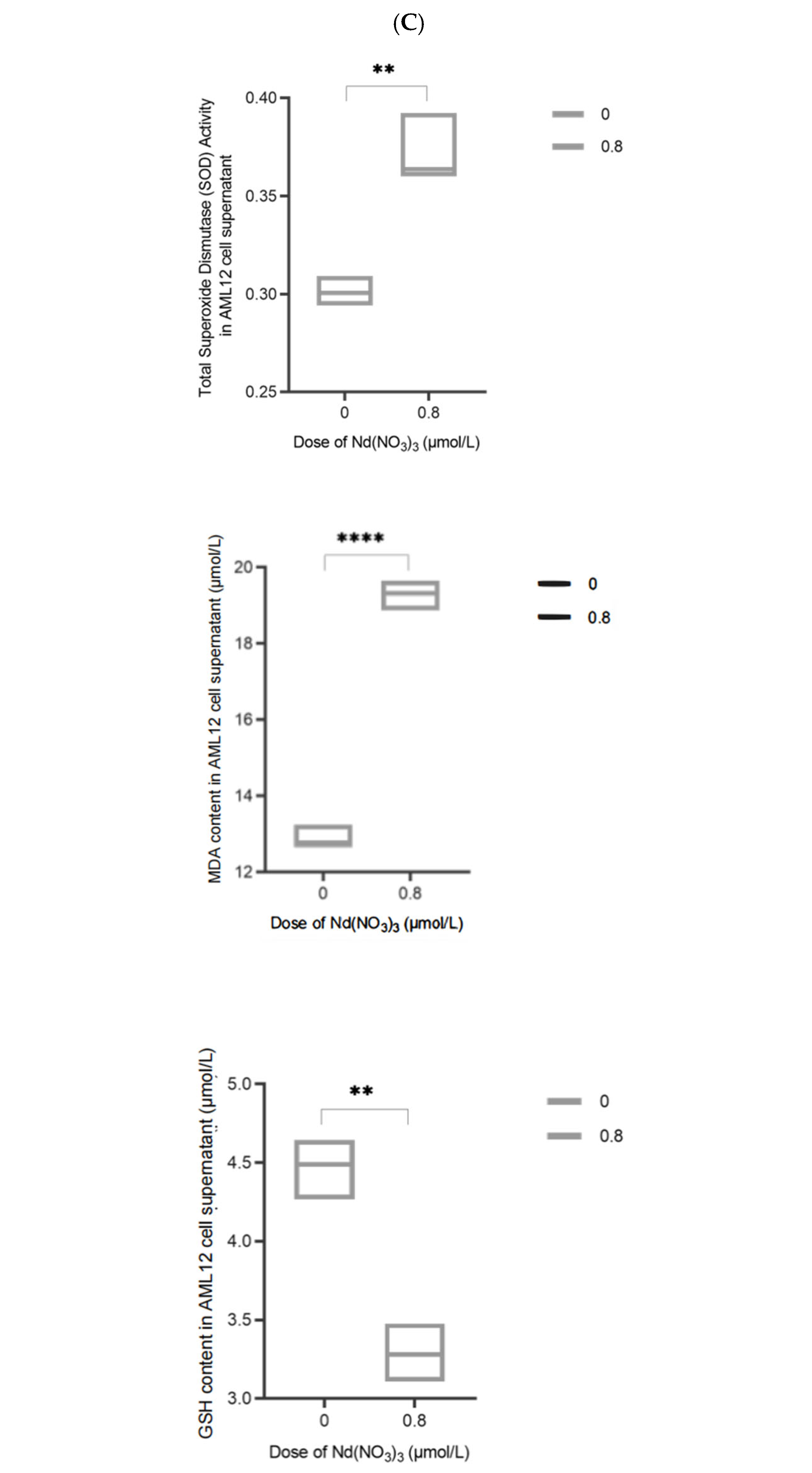
3, leading to the release of iron from storage proteins such as ferritin, increasing the free iron pool available for ROS production. The significant increase in ROS levels poses a challenge to the cell’s antioxidant defense system. We observed a significant increase in SOD activity in Nd(NO
3)
3-treated AML12 cells. SOD is the first line of defense for cells against oxidative stress, capable of catalyzing the dismutation of superoxide anions into oxygen and hydrogen peroxide, thereby reducing the level of ROS within cells. The increase in SOD activity may reflect the cell’s adaptive response to oxidative stress induced by Nd(NO
3)
3, attempting to mitigate oxidative damage by enhancing SOD activity. MDA is the end product of lipid peroxidation, and its increased level directly reflects the degree of lipid peroxidation of the cell membrane. In our study, the MDA level in Nd(NO
3)
3-treated AML12 cells increased significantly, indicating that the cell membrane suffered severe oxidative damage. This damage may be due to the accumulation of ROS induced by Nd(NO
3)
3, especially hydroxyl radicals and singlet oxygen, which can attack polyunsaturated fatty acids, triggering a chain reaction of lipid peroxidation. GSH is one of the most important antioxidants within cells, involved in a variety of antioxidant defense pathways, including the direct elimination of ROS and acting as a cofactor for glutathione peroxidase (GPx). We found that the level of GSH in Nd(NO
3)
3-treated AML12 cells changed, which may indicate a change in the cell’s antioxidant status. A decrease in GSH levels may weaken the cell’s antioxidant capacity, making the cell more susceptible to oxidative damage, while an increase in GSH levels may reflect the cell’s adaptive upregulation in response to oxidative stress. The changes in SOD, MDA, and GSH are closely related to the pathological characteristics of ferroptosis. Ferroptosis is characterized by iron-dependent lipid peroxidation, and the increased level of MDA directly reflects this process. In addition, for GSH, as a key regulator of ferroptosis, its level changes may affect the susceptibility to ferroptosis. The depletion of GSH may lead to increased susceptibility to ferroptosis, while an increase in GSH may provide additional antioxidant protection. The changes in these biomarkers are significant for understanding Nd(NO
3)
3-induced liver injury. The liver is the main metabolic organ and is particularly sensitive to oxidative stress. Oxidative stress induced by Nd(NO
3)
3 may lead to hepatocyte dysfunction, which can affect the liver’s detoxification, metabolism, and immune functions. In addition, sustained oxidative damage may lead to hepatocyte death, triggering inflammatory responses and fibrosis, and ultimately may lead to cirrhosis and liver cancer.
To place our findings within the broader context of current research, we have conducted an extensive review of the literature on neodymium-nitrate-induced liver injury. Our study aligns with previous findings that rare earth elements, including neodymium, can induce oxidative stress and ferroptosis in hepatocytes [
25,
26]. For instance, a study by [
27] demonstrated that neodymium exposure significantly increased the levels of reactive oxygen species (ROS) and lipid peroxidation markers in liver cells, leading to cell death [
28]. Similarly, [
29] reported that neodymium nitrate altered the expression of genes involved in iron metabolism, further supporting our observations [
21,
30].
Our study provides novel insights into the molecular mechanisms underlying neodymium-nitrate-induced liver injury. By identifying key genes and pathways involved in ferroptosis and oxidative stress, our research contributes to the growing body of literature on rare earth element toxicity. These findings have important implications for understanding the health risks associated with neodymium exposure and for developing strategies to mitigate its toxic effects.
In conclusion, our study demonstrates that neodymium nitrate induces liver injury through the modulation of iron metabolism and oxidative stress pathways. Future research should focus on further elucidating the molecular mechanisms involved and exploring potential therapeutic interventions.
Our RNA sequencing analysis identified numerous differentially expressed genes (DEGs) in response to neodymium nitrate exposure, primarily involved in ferroptosis, oxidative stress, and lipid metabolism pathways. Key genes such as Ptgs2, NOX1, and ACSL4 were upregulated, while Fth1 and Gpx4, involved in iron storage and antioxidant defense, were downregulated. These changes promote iron-dependent lipid peroxidation, leading to ferroptosis.
Our study provides valuable insights into the hepatotoxic effects of neodymium nitrate (Nd(NO3)3) and the role of non-coding RNAs in mediating these effects. However, limitations include the use of an in vitro model (AML12 cells) and a specific concentration (0.8 µmol/L) and exposure duration (24 h). Future studies should validate these findings in vivo, explore a wider range of doses and exposure times, and investigate epigenetic effects and the reproductive and neurological impacts of neodymium exposure.