Effects of Lead Exposure on 1573 Male Workers’ Sex Hormones in China
Abstract
1. Introduction
2. Materials and Methods
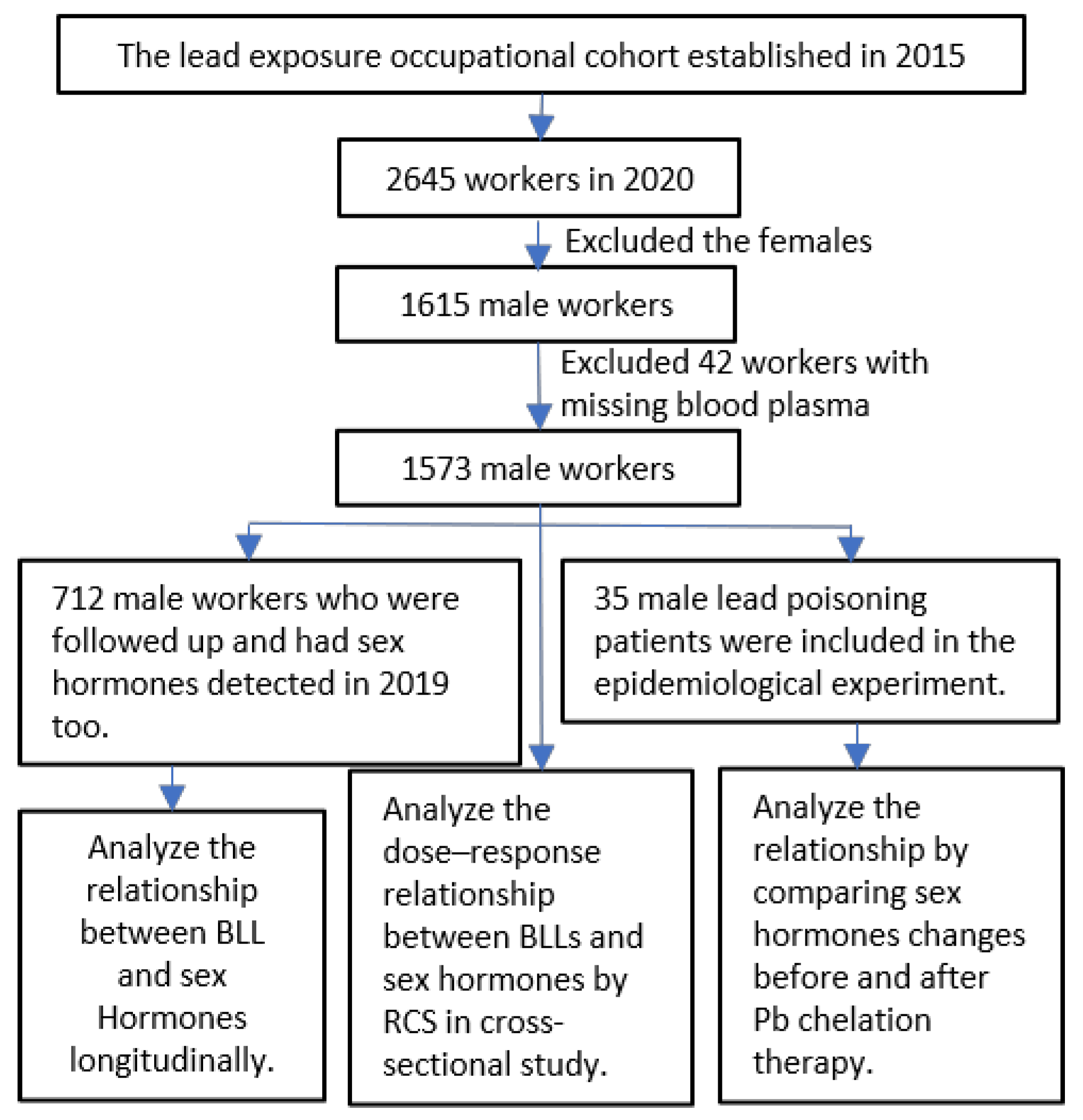
2.1. Study Population
2.2. Experimental Design
2.3. Hormone Measurement
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics, Blood Pb Levels, and Sex Hormones
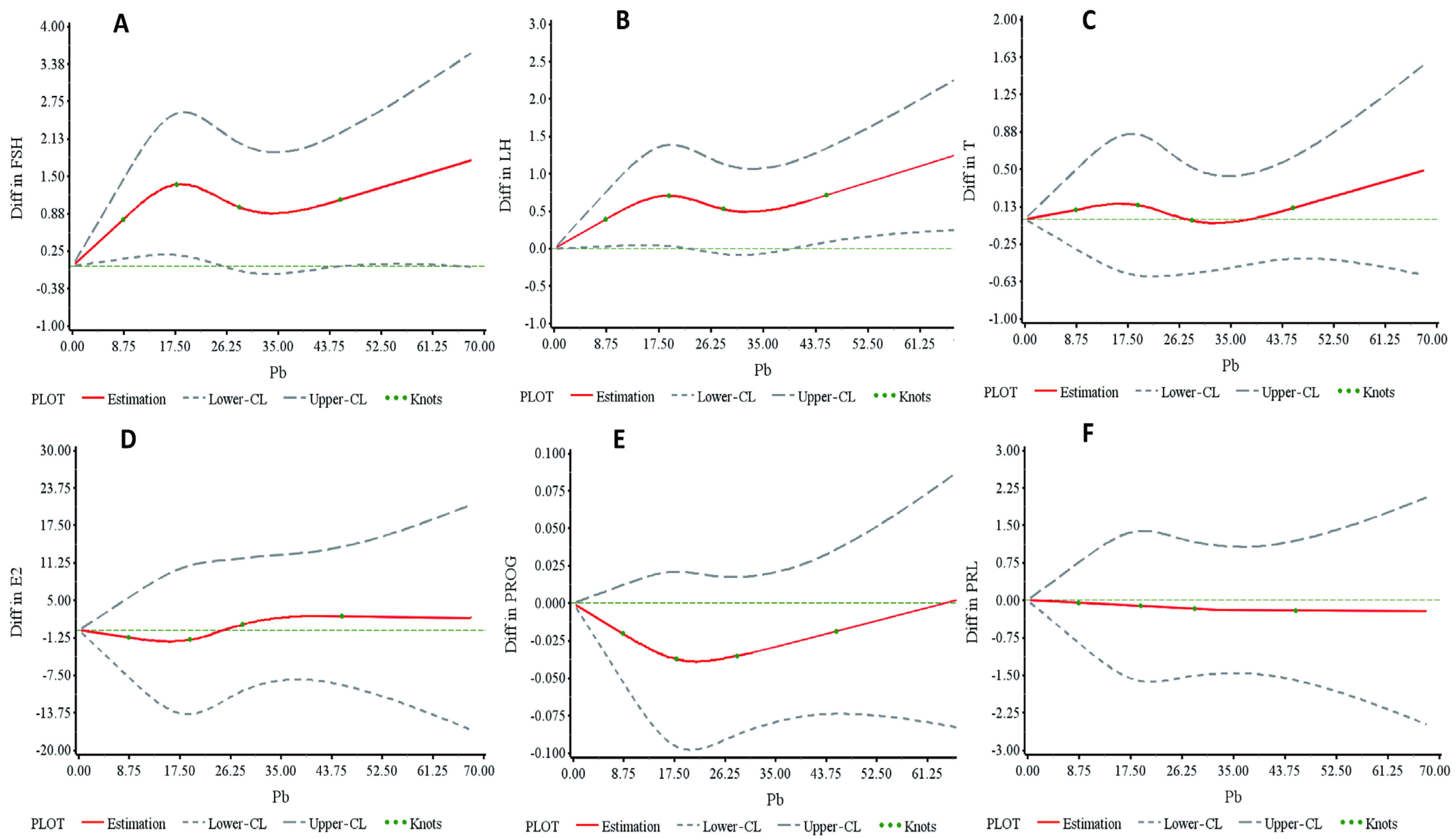
3.2. Dose–Response Relationship Between Blood Pb Levels and Hormones in the Cross-Sectional Study
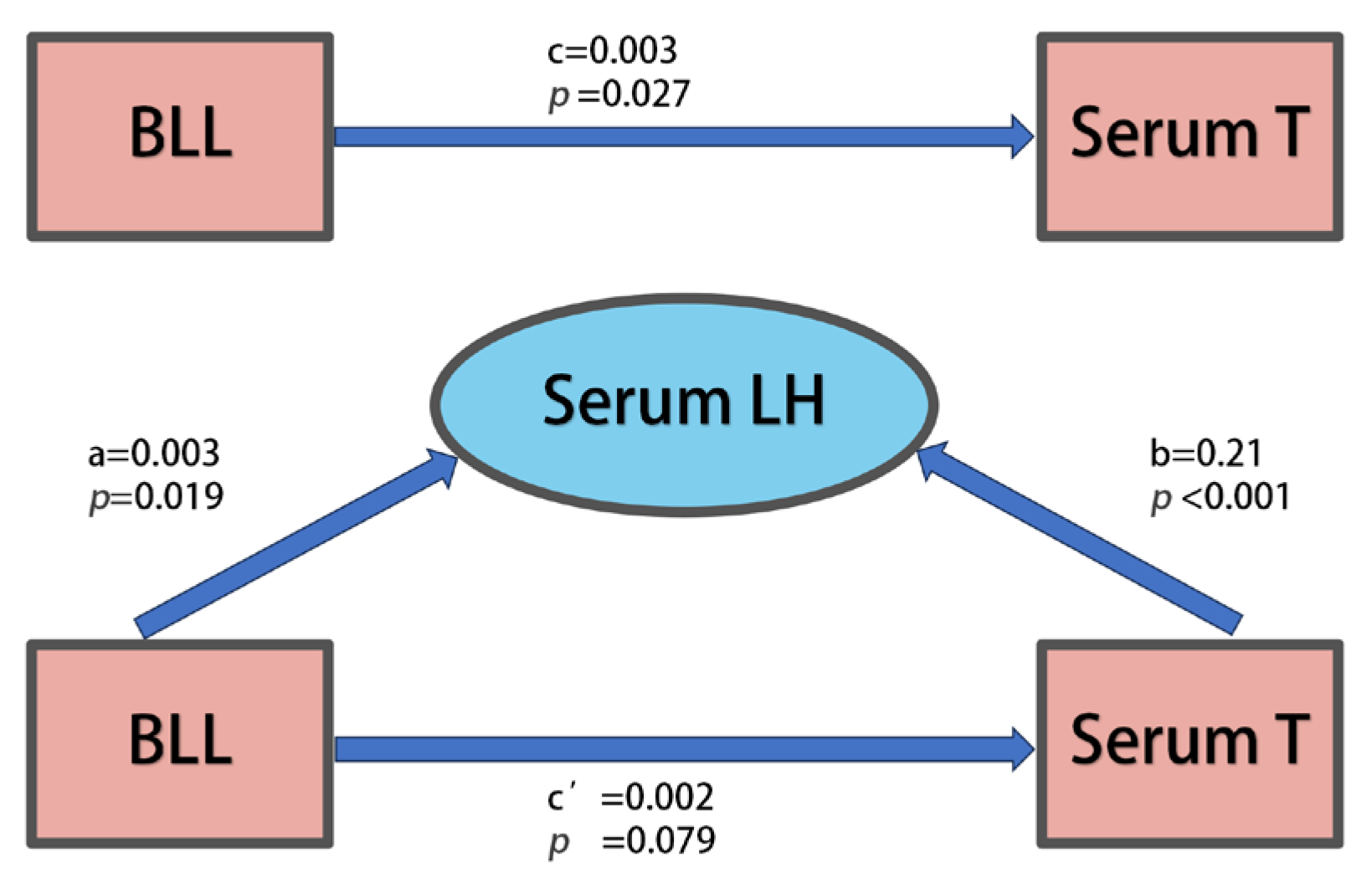
3.3. Relationships Between Blood Pb Levels and Hormones in Repeated Cross-Sectional Study
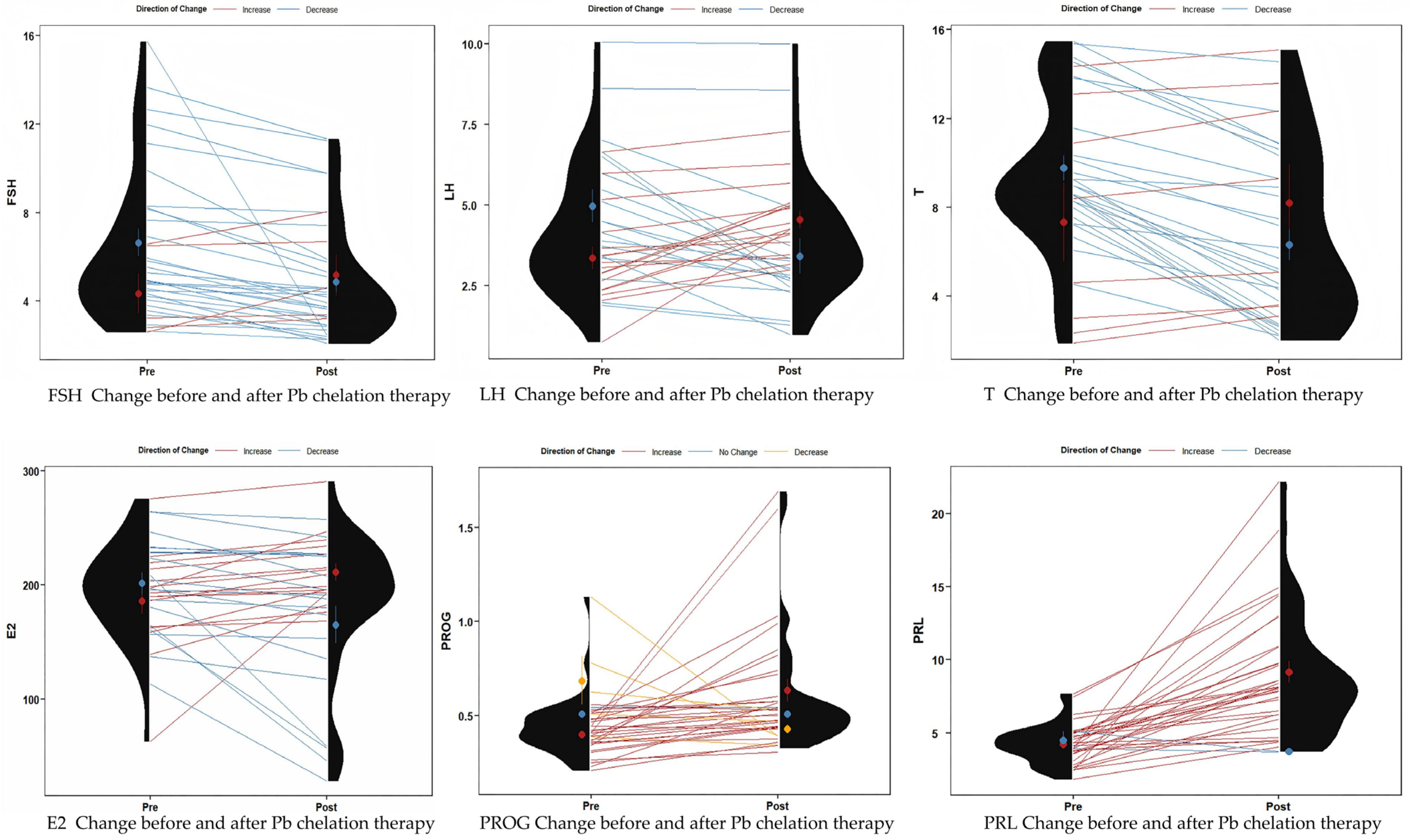
3.4. Sex Hormone Changes in Patients Before and After Pb Chelation Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Galiciolli, M.E.A.; Lima, L.S.; da Costa, N.S.; de Andrade, D.P.; Irioda, A.C.; Oliveira, C.S. IQ alteration induced by lead in developed and underdeveloped/developing countries: A systematic review and a meta-analysis. Environ. Pollut. 2022, 292, 118316. [Google Scholar] [CrossRef]
- Falck, A.J.; Mooney, S.; Kapoor, S.S.; White, K.M.; Bearer, C.; El Metwally, D. Developmental Exposure to Environmental Toxicants. Pediatr. Clin. N. Am. 2015, 62, 1173–1197. [Google Scholar] [CrossRef]
- Shen, Y.; Laue Hannah, E.; Shrubsole Martha, J.; Wu, H.; Bloomquist Tessa, R.; Larouche, A.; Zhao, K.; Gao, F.; Boivin, A.; Prada, D.; et al. Associations of Childhood and Perinatal Blood Metals with Children’s Gut Microbiomes in a Canadian Gestation Cohort. Environ. Health Perspect. 2022, 130, 017007. [Google Scholar] [CrossRef]
- Ericson, B.; Hu, H.; Nash, E.; Ferraro, G.; Sinitsky, J.; Taylor, M.P. Blood lead levels in low-income and middle-income countries: A systematic review. Lancet Planet Health 2021, 5, e145–e153. [Google Scholar] [CrossRef]
- Rolland, M.; Le Moal, J.; Wagner, V.; Royère, D.; De Mouzon, J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum. Reprod. 2013, 28, 462–470. [Google Scholar] [CrossRef]
- Li, C.; Zhao, K.; Zhang, H.; Liu, L.; Xiong, F.; Wang, K.; Chen, B. Lead exposure reduces sperm quality and DNA integrity in mice. Environ. Toxicol. 2018, 33, 594–602. [Google Scholar] [CrossRef]
- Ren, J.; Cui, J.; Chen, Q.; Zhou, N.; Zhou, Z.; Zhang, G.H.; Wu, W.; Yang, H.; Cao, J. Low-level lead exposure is associated with aberrant sperm quality and reproductive hormone levels in Chinese male individuals: Results from the MARHCS study low-level lead exposure is associated with aberrant sperm quality. Chemosphere 2020, 244, 125402. [Google Scholar] [CrossRef]
- Telisman, S.; Cvitković, P.; Jurasović, J.; Pizent, A.; Gavella, M.; Rocić, B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ. Health Perspect. 2000, 108, 45–53. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Shalaby, A.A.; Abd Elaziz, R.T.; Bahr, H.I. Chlorella vulgaris or Spirulina platensis mitigate lead acetate-induced testicular oxidative stress and apoptosis with regard to androgen receptor expression in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 39126–39138. [Google Scholar] [CrossRef] [PubMed]
- Ezejiofor, A.N.; Orisakwe, O.E. The protective effect of Costus afer Ker Gawl aqueous leaf extract on lead-induced reproductive changes in male albino Wistar rats. JBRA Assist. Reprod. 2019, 23, 215–224. [Google Scholar] [CrossRef] [PubMed]
- El-Fakharany, Y.M.; Mohamed, E.M.; Etewa, R.L.; Abdel Hamid, O.I. Selenium nanoparticles alleviate lead acetate-induced toxicological and morphological changes in rat testes through modulation of calmodulin-related genes expression. J. Biochem. Mol. Toxicol. 2022, 36, e23017. [Google Scholar] [CrossRef]
- Soleimanzadeh, A.; Kian, M.; Moradi, S.; Malekifard, F. Protective effects of hydro-alcoholic extract of Quercus brantii against lead-induced oxidative stress in the reproductive system of male mice. Avicenna J. Phytomed. 2018, 8, 448–456. [Google Scholar]
- Kolawole, T.A.; Asiwe, J.N.; Buduburisi, B.R.; Akintade, V.A.; Adebayo, O.G.; Ojetola, A.A.; Dapper, D.V. Cabbage (Brassica oleracea) mitigates lead (II) acetate-induced testicular dysfunction in Wistar rats via up-regulation of Bcl-2 protein expression, pituitary-testicular hormonal axis and down-regulation of oxido-inflammatory reactions. Andrologia 2022, 54, e14476. [Google Scholar] [CrossRef]
- Hassan, E.; El-Neweshy, M.; Hassan, M.; Noreldin, A. Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: Possible mechanisms are involved. Life Sci. 2019, 230, 132–140. [Google Scholar] [CrossRef]
- Abdel-Emam, R.A.; Ahmed, E.A. Ameliorative effect of L-carnitine on chronic lead-induced reproductive toxicity in male rats. Vet. Med. Sci. 2021, 7, 1426–1435. [Google Scholar] [CrossRef]
- Balachandar, R.; Bagepally, B.S.; Kalahasthi, R.; Haridoss, M. Blood lead levels and male reproductive hormones: A systematic review and meta-analysis. Toxicology 2020, 443, 152574. [Google Scholar] [CrossRef]
- Lee, T.W.; Kim, D.H.; Ryu, J.Y. The effects of exposure to lead, cadmium and mercury on follicle-stimulating hormone levels in men and postmenopausal women: Data from the Second Korean National Environmental Health Survey (2012–2014). Ann. Occup. Environ. Med. 2019, 31, e21. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Argos, M.; Turyk, M.E. Associations of lead and cadmium with sex hormones in adult males. Environ. Res. 2015, 142, 25–33. [Google Scholar] [CrossRef]
- Gandhi, J.; Hernandez, R.J.; Chen, A.; Smith, N.L.; Sheynkin, Y.R.; Joshi, G.; Khan, S.A. Impaired hypothalamic-pituitary-testicular axis activity, spermatogenesis, and sperm function promote infertility in males with lead poisoning. Zygote 2017, 25, 103–110. [Google Scholar] [CrossRef] [PubMed]
- WS/T 174-1999; Blood—Determination of Lead and Cadmium—Graphite Furnace Atomic Absorption Spectrophotometric Method. Ministry of Health of China: Beijing, China, 1999. (In Chinese)
- Shvachiy, L.; Amaro-Leal, A.; Outeiro, T.F.; Rocha, I.; Geraldes, V. Intermittent Lead Exposure Induces Behavioral and Cardiovascular Alterations Associated with Neuroinflammation. Cells 2023, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jing, J.; Shao, Y.; Zeng, R.; Wang, C.; Yao, B.; Hang, D. Circulating sex hormone levels in relation to male sperm quality. BMC Urol. 2020, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Tamegart, L.; Abbaoui, A.; El Khiat, A.; Bouyatas, M.M.; Gamrani, H. Lead (Pb) exposure induces physiological alterations in the serotoninergic and vasopressin systems causing anxiogenic-like behavior in Meriones shawi: Assessment of BDMC as a neuroprotective compound for Pb-neurotoxicity and kidney damages. J. Trace Elem. Med. Biol. 2021, 65, 126722. [Google Scholar] [CrossRef]
- Alva, S.; Parithathvi, A.; Harshitha, P.; Dsouza, H.S. Influence of lead on cAMP-response element binding protein (CREB) and its implications in neurodegenerative disorders. Toxicol. Lett. 2024, 400, 35–41. [Google Scholar] [CrossRef]
- Mei, Z.; Liu, G.; Zhao, B.; He, Z.; Gu, S. Emerging roles of epigenetics in lead-induced neurotoxicity. Environ. Int. 2023, 181, 108253. [Google Scholar] [CrossRef]
- Scorrano, G.; La Bella, S.; Matricardi, S.; Chiarelli, F.; Giannini, C. Neuroendocrine Effects on the Risk of Metabolic Syndrome in Children. Metabolites 2023, 13, 810. [Google Scholar] [CrossRef]
- Goel, M.; Mittal, A.; Jain, V.R.; Bharadwaj, A.; Modi, S.; Ahuja, G.; Jain, A.; Kumar, K. Integrative Functions of the Hypothalamus: Linking Cognition, Emotion and Physiology for Well-being and Adaptability. Ann. Neurosci. 2025, 32, 128–142. [Google Scholar] [CrossRef]
- Black, S.R.; Scalco, M.D.; Mackin, D.; Shirtcliff, E.A.; Klein, D.N. Longitudinal patterns of neuroendocrine coupling from middle childhood to early adolescence. Dev. Psychobiol. 2022, 64, e22340. [Google Scholar] [CrossRef]
- Perez-Gomez, J.M.; Montero-Hidalgo, A.J.; Luque, R.M. GHRH and reproductive systems: Mechanisms, functions, and clinical implications. Rev. Endocr. Metab. Disord. 2024, 25, 157–168. [Google Scholar] [CrossRef]
- Starrett, J.R.; Moenter, S.M. Hypothalamic kisspeptin neurons as potential mediators of estradiol negative and positive feedback. Peptides 2023, 163, 170963. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vazquez, E.; Aranda-Torrecillas, A.; Lopez-Sancho, M.; Castellano, J.M.; Tena-Sempere, M. Emerging roles of lipid and metabolic sensing in the neuroendocrine control of body weight and reproduction. Front. Endocrinol. 2024, 15, 1454874. [Google Scholar] [CrossRef] [PubMed]
- Chabchoub, I.; Nouioui, M.A.; Araoud, M.; Mabrouk, M.; Amira, D.; Ben Aribia, M.H.; Mahmoud, K.; Zhioua, F.; Merdassi, G.; Hedhili, A. Effects of lead, cadmium, copper and zinc levels on the male reproductive function. Andrologia 2021, 53, e14181. [Google Scholar] [CrossRef]
- Dos Santos, N.R.; Rodrigues, J.L.G.; Bandeira, M.J.; Anjos, A.; Araújo, C.; Adan, L.F.F.; Menezes-Filho, J.A. Manganese and Lead Exposure and Early Puberty Onset in Children Living near a Ferromanganese Alloy Plant. Int. J. Environ. Res. Public Health 2022, 19, 7158. [Google Scholar] [CrossRef]
- Li, S.; Yang, C.; Yi, X.; Wei, R.; Aschner, M.; Jiang, Y.; Ou, S.; Yao, C. Effects of Sub-chronic Lead Exposure on Essential Element Levels in Mice. Biol. Trace Elem. Res. 2023, 201, 282–293. [Google Scholar] [CrossRef]
- Sokol, R.Z.; Berman, N.; Okuda, H.; Raum, W. Effects of lead exposure on GnRH and LH secretion in male rats: Response to castration and alpha-methyl-p-tyrosine (AMPT) challenge. Reprod. Toxicol. 1998, 12, 347–355. [Google Scholar] [CrossRef]
- Sokol, R.Z.; Wang, S.; Wan, Y.J.; Stanczyk, F.Z.; Gentzschein, E.; Chapin, R.E. Long-term, low-dose lead exposure alters the gonadotropin-releasing hormone system in the male rat. Environ. Health Perspect. 2002, 110, 871–874. [Google Scholar] [CrossRef]

| Groups | Mean ± SD/No. (%) | ||
|---|---|---|---|
| Cross-Sectional Study (N = 1573) | Repeated Cross-Sectional Study from 2019 to 2020 (N = 712) | Pb Chelation Therapy Experiment (N = 35) | |
| Age (years) a | 36.8 ± 7.2 | 36.4 ± 7.1 | 36.9 ± 5.7 |
| BMI (kg/m2) a | 24.43 ± 3.56 | 24.44 ± 3.46 | 23.86 ± 2.80 |
| Smoking status b | |||
| Never smoker | 637 (41) | 298(42) | 15 (43) |
| Smoker | 928 (59) | 411 (58) | 20 (57) |
| Alcohol drinking b | |||
| Never drinker | 699 (44) | 301 (43) | 14 (40) |
| Alcohol user | 842 (54) | 397 (57) | 21 (60) |
| Blood Pb (μg/dL) a | 24.96 ± 11.47 | 24.62 ± 11.86 | 61.66 ± 6.32 |
| Pituitary hormones a | |||
| FSH (mlU/mL) | 6.15 ± 3.47 | 6.16 ± 3.21 | 6.32 ± 3.34 |
| LH (mlU/mL) | 4.16 ± 1.98 | 4.21 ± 2.31 | 4.19 ± 2.12 |
| Prolactin (ng/mL) | 5.78 ± 4.29 | 5.80 ± 3.56 | 4.25 ± 1.34 |
| Testis hormones a | |||
| Estradiol (pg/mL) | 72.91 ± 36.01 | 73.23 ± 35.66 | 194.29 ± 43.74 |
| Testosterone (ng/mL) | 4.58 ± 1.99 | 4.61 ± 2.02 | 9.21 ± 3.62 |
| Renicapsule hormones a | |||
| Progesterone (ng/mL) | 0.53 ± 0.17 | 0.54 ± 0.22 | 0.44 ± 0.16 |
| FSH | LH | E2 | T | PROG | |
|---|---|---|---|---|---|
| Low-BLL group (N) | 616 | 616 | 236 | 421 | 659 |
| Concentration (μg/dL) | <20.5 | <20.5 | ≤13.0 | <16.89 | ≤21.26 |
| Model I | |||||
| β (95%CI) | 0.069 (0.002, 0.137) | 0.030 (−0.006, 0.067) | −0.402 (−2.023, 1.219) | 0.034 (−0.019, 0.086) | −0.002 (−0.006, 0.001) |
| t value | 2.03 | 1.62 | −0.49 | 1.25 | −1.37 |
| p value | 0.043 | 0.105 | 0.626 | 0.213 | 0.171 |
| Model II | |||||
| β (95%CI) | 0.069 (0.002, 0.137) | 0.030 (−0.007, 0.067) | −0.179 (−1.8863, 1.504) | 0.005 (−0.048, 0.057) | −0.003 (−0.007, 0) |
| t value | 2.02 | 1.57 | −0.21 | 0.17 | −1.82 |
| p value | 0.044 | 0.118 | 0.834 | 0.862 | 0.069 |
| Middle-level group (N) | 468 | 468 | - | 590 | - |
| Dose (μg/dL) | 20.5–30.0 | 20.5–30.0 | - | 16.89–28.09 | - |
| Model I | |||||
| β (95%CI) | −0.041 (−0.159, 0.072) | 0.011 (−0.057, 0.079) | 0.007 (−0.044, 0.059) | ||
| t value | −0.75 | 0.31 | 0.28 | ||
| p value | 0.456 | 0.753 | 0.779 | ||
| Model II | |||||
| β (95%CI) | −0.017 (−0.130, 0.096) | 0.019 (−0.049, 0.086) | −0.008 (−0.059, 0.043) | ||
| t value | −0.30 | 0.54 | −0.31 | ||
| p value | 0.765 | 0.588 | 0.755 | ||
| High-BLL group (N) | 489 | 489 | 1337 | 562 | 914 |
| Dose (μg/dL) | >30.0 | >30.0 | >13.0 | >28.09 | >21.26 |
| Model I | |||||
| β (95%CI) | 0.020 (−0.019, 0.060) | 0.028 (0.005, 0.052) | 0.155 (−0.037, 0.347) | 0.023 (0.002, 0.045) | 0.001 (0, 0.002) |
| t value | 1.00 | 2.35 | 1.58 | 2.12 | 1.09 |
| p value | 0.318 | 0.019 | 0.113 | 0.035 | 0.278 |
| Model II | |||||
| β (95%CI) | 0.019 (−0.021, 0.058) | 0.027 (0.003, 0.051) | 0.152 (−0.043, 0.348) | 0.027 (0.005, 0.049) | 0.001 (0, 0.002) |
| t value | 0.92 | 2.20 | 1.53 | 2.41 | 1.18 |
| p value | 0.359 | 0.028 | 0.127 | 0.016 | 0.240 |
| Before Pb Chelation Therapy | After Pb Chelation Therapy | t a | p a | Z b | p b | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | P50 (P25, P75) | Mean ± SD | P50 (P25, P75) | |||||
| BLL (μg/dL) | 61.66 ± 6.32 | 61.13 (58.59, 64.75) | 36.30 ± 5.52 | 35.47 (32.39, 39.15) | 25.86 | <0.001 | −5.16 | <0.001 |
| FSH (mlU/mL) | 6.32 ± 3.34 | 4.95 (4.09, 8.04) | 4.92 ± 2.59 | 4.06 (3.01, 5.87) | 3.45 | 0.002 | −4.14 | <0.001 |
| LH (mlU/mL) | 4.19 ± 2.02 | 3.70 (2.76, 5.85) | 3.97 ± 1.91 | 3.61 (2.77, 4.92) | 0.75 | 0.460 | −0.52 | 0.6000 |
| T (ng/mL) | 9.21 ± 3.62 | 8.77 (7.32, 11.39) | 6.75 ± 3.97 | 5.17 (3.22, 10.08) | 6.19 | <0.001 | −4.32 | <0.001 |
| E2 (pg/mL) | 194.30 ± 43.74 | 196.86 (163.54, 224.63) | 186.14 ± 60.94 | 195.90 (174.09, 227.00) | 0.97 | 0.336 | −0.59 | 0.555 |
| PROG (ng/mL) | 0.44 ± 0.16 | 0.41 (0.34, 0.51) | 0.60 ± 0.31 | 0.51 (0.42, 0.59) | −2.68 | 0.011 | −3.58 | <0.001 |
| PRL (ng/mL) | 4.25 ± 1.34 | 4.42 (3.58, 5.04) | 8.85 ± 4.28 | 8.10 (6.01, 10.92) | −6.39 | <0.001 | −5.06 | <0.001 |
| Reference | Study Type | Sample Characteristics | Blood Pb Level (BLL) | Key Findings |
|---|---|---|---|---|
| This Study | Cross-sectional study | 1753 male Pb-exposed workers | Mean: 24.96 μg/dL; median: 23.63 μg/dL | Positive correlation between Pb exposure level and testosterone (T) levels; LH levels significantly linked to BLL and serum T |
| Repeated cross-sectional study | 712 male Pb-exposed workers | 2019 mean: 19.75 μg/dL; 2020 mean: 24.62 μg/dL | BLL, LH, and PRL were significantly higher in 2020 compared to 2019, while T and E2 were significantly lower in 2019 | |
| Clinical study | 35 male workers with occupational Pb poisoning | BLL decreased from 61.66 μg/dL to 36.30 μg/dL | Significant decline in serum T and FSH; no significant change in LH | |
| [24] | Cross-sectional study | 338 men from infertile couples | / | LH, FSH, and T levels inversely correlated with sperm motility |
| [10] | Cross-sectional study | 98 subjects with slight to moderate occupational exposure to Pb and 51 reference subjects | Control group mean: 109 μg/dL; Pb-exposed workers mean: 387 μg/dL | Increased serum T and E2 in Pb-exposed group |
| [20] | Cross-sectional study | 869 non-hospitalized civilians | Median BLL: 2.0 μg/dL | Positive correlation between BLL and T |
| [34] | Cross-sectional study | One hundred and two infertile men with occupational exposure and thirty fertile men. | Exposed group median: 43.80 μg/dL; control group median: 24.50 μg/dL | Higher FSH and lower T in heavy metal-exposed group compared to controls |
| [35] | Cross-sectional study | 225 school-aged children (113 boys) near a ferromanganese plant | Boys’ median BLL: 1.20 μg/dL | Testosterone and LH concentrations were statistically higher in boys with an increased BLL |
| [18] | Systematic review and meta-analysis | 26 studies on occupational exposure to heavy metals (e.g., Cd, Cr, Se, and Zn) | Significantly higher BLL in Pb-exposed groups vs. controls | Pb-exposed males showed comparable testosterone, FSH, and LH levels to unexposed controls |
| [36] | Animal study | 32 male mice | / | Hypothalamus identified as a Pb accumulation site |
| [37] | In vivo and in vitro experiments | 36 Pb-exposed male rats; 6 controls | The blood Pb levels of Pb-dosed animals were significantly higher than control animals in all experiments | Pb exposure caused abnormal GnRH levels and significant LH elevation |
| [38] | Animal study | Pb-exposed male rats | Control group: <3 µg/mL; Pb-exposed group: higher than controls | The signals within and between the hypothalamus and pituitary gland appear to be disrupted by long-term, low-dose Pb exposure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wu, Z.; Li, J.; Li, Y.; Wang, X.; Ma, M.; Wei, W.; Wang, Y.; Liu, Y.; Sun, Y.; et al. Effects of Lead Exposure on 1573 Male Workers’ Sex Hormones in China. Toxics 2025, 13, 415. https://doi.org/10.3390/toxics13050415
Wang P, Wu Z, Li J, Li Y, Wang X, Ma M, Wei W, Wang Y, Liu Y, Sun Y, et al. Effects of Lead Exposure on 1573 Male Workers’ Sex Hormones in China. Toxics. 2025; 13(5):415. https://doi.org/10.3390/toxics13050415
Chicago/Turabian StyleWang, Ping, Zhiling Wu, Ju Li, Yue Li, Xuefeng Wang, Mengya Ma, Wenkai Wei, Yijun Wang, Yi Liu, Yi Sun, and et al. 2025. "Effects of Lead Exposure on 1573 Male Workers’ Sex Hormones in China" Toxics 13, no. 5: 415. https://doi.org/10.3390/toxics13050415
APA StyleWang, P., Wu, Z., Li, J., Li, Y., Wang, X., Ma, M., Wei, W., Wang, Y., Liu, Y., Sun, Y., Tao, L., Yang, Y., Zhou, Z., Ren, J., Cao, J., & Zhang, G. (2025). Effects of Lead Exposure on 1573 Male Workers’ Sex Hormones in China. Toxics, 13(5), 415. https://doi.org/10.3390/toxics13050415






