What Do We Know About Staphylococcus aureus and Oxidative Stress? Resistance, Virulence, New Targets, and Therapeutic Alternatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Oxidative Stress and Its Involvement in the Virulence and Resistance of S. aureus
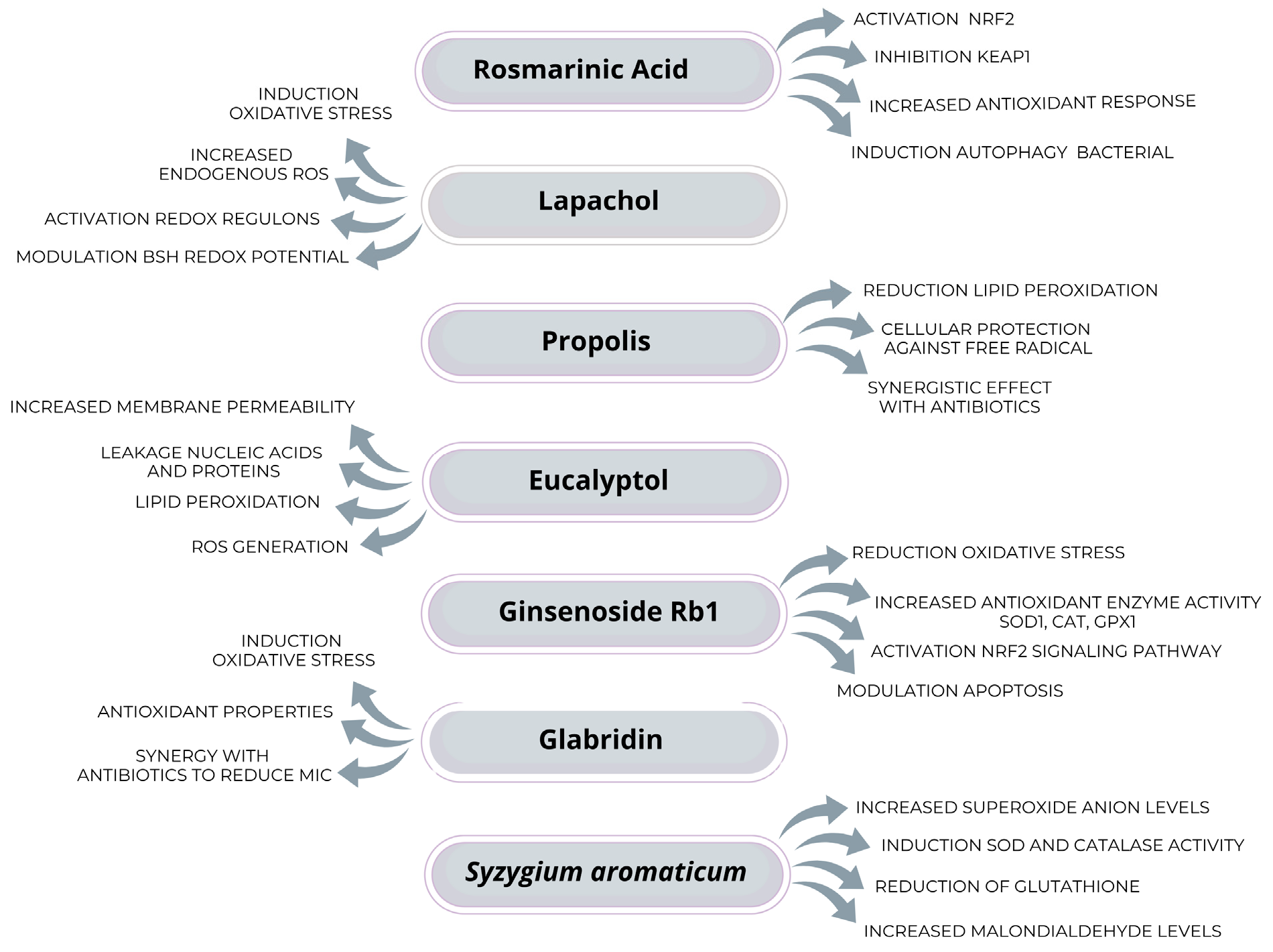
2.2. Possible Therapeutic Alternatives to Reduce the Impact of Oxidative Stress on S. aureus
| Process | Role in Pathogenesis | Protective Mechanisms | References |
|---|---|---|---|
| Production of ROS/RCS by the immune system | Neutrophils and macrophages release ROS/RCS to eliminate S. aureus, causing oxidative damage | S. aureus has developed resistance to oxidative stress through antioxidant systems and immune evasion | [34,35,36,37] |
| TLR2 activation and inflammatory pathways | TLR2 activation triggers the production of pro-inflammatory cytokines, increasing oxidative stress | Modulation of TLR2 and inflammatory pathways can minimize host damage and improve bacterial persistence | [18,19,20,21] |
| Oxidative damage to host cells | Excess ROS can damage immune cells, favoring infection persistence | Bacterial survival may be enhanced by reducing immune system efficacy | [28,29,30] |
| Biofilm production as a resistance mechanism | Biofilm formation protects S. aureus against ROS and antibiotics, increasing resistance | Biofilm prevents ROS and antibiotic penetration, enhancing bacterial resilience | [69,70] |
| Bacterial antioxidant response (SOD, CAT, Gpx) | S. aureus uses antioxidant enzymes to detoxify ROS and survive oxidative stress | Antioxidant enzymes neutralize ROS before they cause cellular damage | [31,32,33] |
| Role of BSH in ROS defense | Bacillithiol protects bacterial proteins from oxidative damage and contributes to virulence | BSH functions as a protective system against reactive species, enhancing bacterial resistance | [39,40] |
| Resistance mediated by operons (staphyloxathin msaABCR) | Resistance genes such as staphyloxanthin neutralize ROS, while msaABCR maintains membrane integrity | These operons facilitate adaptation and resistance to oxidative stress in hostile environments | [64,65,66,67,68] |
| Redox metabolism regulation (CymR, Trx, KatA) | Regulators such as CymR and Trx aid in adaptation to oxidative environments, increasing bacterial survival | Redox metabolism is regulated to prevent cellular damage and maintain bacterial homeostasis | [46,47,48,49,50,51,52,53,54,55] |
| Post-transcriptional modification of rRNA (KsgA) | KsgA improves the efficiency of translating proteins protective against oxidative stress | Translation regulation protects essential proteins for stress response | [71,72,73] |
| Enzymes involved in methionine maintenance (MsrA1, MsrB) | MsrA1 and MsrB regulate oxidative stress response and influence bacterial virulence | Self-regulation of MsrA1 and MsrB ensures balance in oxidative stress response | [74] |
| Influence of antimicrobial resistance on redox metabolism | Antibiotic-resistant strains, such as those resistant to ciprofloxacin, exhibit lower sensitivity to oxidative stress | Antimicrobial resistance can increase tolerance to oxidative stress, reducing antibiotic effectiveness | [75,76] |
| Regulatory Mechanism | Description | Role in Oxidative Stress/Pathogenesis | References |
|---|---|---|---|
| TLR2 Activation | Toll-like receptor 2 (TLR2) activation triggers pro-inflammatory cytokine production and oxidative stress. | Induces oxidative stress and inflammation, contributing to host cell damage. | [18,19,20,21] |
| NRF2 Pathway | Nuclear factor erythroid 2-related factor 2 (NRF2) activates antioxidant genes in response to oxidative stress. | Protects host cells from oxidative damage by upregulating antioxidant enzymes like HO-1 and NQO1. | [23,24,25,26,27] |
| Antioxidant Enzymes (SOD, CAT, GPx) | Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) neutralize reactive oxygen species (ROS). | Protects S. aureus from oxidative damage, enhancing survival and pathogenicity. | [31,32,33] |
| Bacillithiol (BSH) | A low-molecular-weight thiol that protects bacterial proteins from oxidative damage. | Enhances bacterial resistance to oxidative stress and contributes to virulence. | [39,40] |
| Redox-Sensing Regulators (SarZ, MgrA, HypR, QsrR) | Regulators that detect ROS, reactive chlorine species (RCS), and reactive electrophilic species (RES) through thiol modifications. | Activates specific operons to protect S. aureus from oxidative stress and maintain redox balance. | [47,48,49] |
| Catalase KatA | An enzyme that degrades hydrogen peroxide (H2O2), providing resistance to oxidative stress. | Protects S. aureus from oxidative damage, especially under microaerophilic conditions. | [50] |
| Thioredoxin (Trx) System | Thioredoxin and thioredoxin reductase (TrxR) maintain proteins in their reduced state, protecting against oxidative stress. | Essential for bacterial survival in adverse environments and a potential target for new antimicrobials. | [61,62] |
| Cysteine Metabolism Regulator (CymR) | Regulates cysteine metabolism and biofilm formation, influencing bacterial virulence and adaptation to oxidative stress. | Controls sulfur source utilization and biofilm formation, enhancing bacterial survival. | [53,54,55] |
| Post-Transcriptional Modification (KsgA) | Methyltransferase that modifies rRNA, influencing protein synthesis and oxidative stress response. | Enhances the translation of proteins involved in oxidative stress resistance. | [71,72,73] |
| Methionine Sulfoxide Reductases (MsrA1, MsrB) | Enzymes involved in repairing oxidative damage to methionine residues. | Regulates oxidative stress response and virulence factors in S. aureus. | [74] |
| Staphyloxanthin Biosynthesis Operon | Operon involved in the production of staphyloxanthin, a carotenoid pigment that neutralizes ROS. | Protects S. aureus from oxidative stress, enhancing virulence and resistance to immune defenses. | [64,65] |
| msaABCR Operon | Operon that maintains membrane integrity during oxidative stress and regulates biofilm formation. | Enhances bacterial resistance to oxidative stress and promotes biofilm formation, aiding in persistent infections. | [66,67,68] |
| Glucose-Inhibited Division Protein A (GbaA) | A regulator that inhibits biofilm-related gene transcription in S. aureus. | Modulates biofilm formation, influencing bacterial resistance to oxidative stress and antibiotics. | [70] |
| SigB and GraRS Regulons | Regulons involved in the response to cell wall stress and general stress. | Protects S. aureus from oxidative stress and other environmental stresses. | [87] |
| PerR, HypR, QsrR, MhqR, CtsR, HrcA Regulons | Regulons involved in the oxidative stress response and protein damage repair. | Protects S. aureus from oxidative damage and maintains redox balance. | [87] |
| Gene | Function | Role in Biofilm Formation |
|---|---|---|
| GbaA | TetR family regulator; acts as a negative regulator of biofilm-related gene transcription in S. aureus | Inhibits signaling pathways controlling the transcription of biofilm-related genes, modulating bacterial resistance |
| PIA | Main component of the biofilm matrix; contributes to S. aureus adhesion and biofilm resistance | Facilitates biofilm cohesion and structural stability, allowing increased bacterial persistence |
3. Conclusions
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AldA | aldehyde dehydrogenase |
| ALI | acute lung injury |
| AOEs | antioxidant enzymes |
| BSH | bacillithiol |
| CAT | catalase |
| CymR | Cysteine Metabolism Regulator |
| Cys | cysteine |
| DNA | Desoxyribonucleic Acid |
| GbaA | glucose-inhibited division protein A |
| GapDH | glycolytic glyceraldehyde-3-phosphate dehydrogenase |
| GPx | glutathione peroxidase |
| GPX1 | glutathione peroxidase 1 |
| H2O2 | hydrogen peroxide |
| HO | heme oxygenase or hydroxide |
| HO-1 | heme oxygenase 1 |
| HOCl | hypochlorous acid |
| HypR | hydrogen peroxide regulator |
| KEAP1 | kelch-like enoyl-coenzyme A hydratase-associated protein 1 |
| KatA | catalase A |
| Ksg | Kasugamycin |
| KsgA | Kasugamycin resistance A |
| MAKP | Mitogen-Activated Protein Kinase |
| MBC | minimum bactericidal concentration |
| MDA | malondialdehyde |
| MDR | developed multidrug resistance |
| MIC | minimum inhibitory concentration |
| MPO | myeloperoxidase |
| MgrA | multiple gene regulator A |
| mRNA | messenger ribonucleic acid |
| MRSA | methicillin-resistant Staphylococcus aureus |
| Msr | Methionine Sulfoxide Reductase |
| MrsA1 | Methionine Sulfoxide Reductase A1 |
| MrsA2 | Methionine Sulfoxide Reductase A2 |
| MrsA3 | Methionine Sulfoxide Reductase A3 |
| MrsB | Methionine Sulfoxide Reductase B |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NF-κB | Nuclear Factor Kappa B |
| NQO1 | quinone oxidoreductase 1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| O2− | superoxide anion |
| PIA | poly-N-acetylglucosamine intercellular adhesin |
| QsrR | quinone-sensing regulator |
| RA | rosmarinic acid |
| RES | electrophilic reactive species |
| RCS | reactive chlorine species |
| rRNA | ribosomal ribonucleic acid |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| SOD1 | superoxide dismutase 1 |
| SarZ | staphylococcal accessory regulator Z |
| TetR | tetracycline repressor family |
| regu2 | Toll-like receptor 2 |
| Trx | thioredoxin |
| TrxR | thioredoxin reductase |
| VRSA | vancomycin-resistant Staphylococcus aureus |
References
- Foster, T.J. The Staphylococcus aureus “superbug”. J. Clin. Investig. 2004, 114, 1693–1696. [Google Scholar] [CrossRef]
- Loi, V.V.; Busche, T.; Preuß, T.; Kalinowski, J.; Bernhardt, J.; Antelmann, H. The AGXX® Antimicrobial Coating Causes a Thiol-Specific Oxidative Stress Response and Protein S-bacillithiolation in Staphylococcus aureus. Front. Microbiol. 2018, 9, 3037. [Google Scholar] [CrossRef]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, J.; Flaxman, A.; Rollier, C.; O’Shea, M.K.; Fallowfield, J.; Lindsay, M.; Yamaguchi, Y. Population variation in anti-S. aureus IgG isotypes influences surface protein A mediated immune subversion. Vaccine 2016, 34, 1792–1799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Z.; Hu, Z.; Li, S.; Fang, R.; Ono, H.K.; Hu, D.L. Molecular Characteristics and Pathogenicity of Staphylococcus aureus Exotoxins. Int. J. Mol. Sci. 2023, 25, 395. [Google Scholar] [CrossRef]
- Tam, K.; Torres, V.J. Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol. Spectr. 2019, 7, 1128. [Google Scholar] [CrossRef]
- Cuevas, O.; Cercenado, E.; Vindel, A.; Guinea, J.; Sanchez-Conde, M.; Sanchez-Somolinos, M.; Bouza, E. Evolution of the antimicrobial resistance of Staphylococcus spp. in Spain: Five nationwide prevalence studies, 1986 to 2002. Antimicrob. Agents Chemother. 2004, 48, 4240–4245. [Google Scholar] [CrossRef]
- Suresh, L.; Sagar Vijay Kumar, P.; Poornachandra, Y.; Ganesh Kumar, C.; Babu, N.J.; Chandramouli, G.V. An expeditious four-component domino protocol for the synthesis of novel thiazolo[3,2-a]thiochromeno[4,3-d]pyrimidine derivatives as antibacterial and antibiofilm agents. Bioorg. Med. Chem. 2016, 24, 3808–3817. [Google Scholar] [CrossRef]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46, 350–359. [Google Scholar] [CrossRef]
- von Bubnoff, A. Seeking new antibiotics in nature’s backyard. Cell 2006, 127, 867–869. [Google Scholar] [CrossRef]
- Hiramatsu, K. Vancomycin-resistant Staphylococcus aureus: A new model of antibiotic resistance. Lancet Infect. Dis. 2001, 1, 147–155. [Google Scholar] [PubMed]
- MacLean, R.C.; San Millan, A. The evolution of antibiotic resistance. Science 2019, 365, 1082–1083. [Google Scholar] [CrossRef]
- Chopra, I.; Johnson, S.C.; Bennett, P.M. Inhibition of Providencia stuartii cell envelope enzymes by chlorhexidine. J. Antimicrob. Chemother. 1987, 19, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Weisblum, B.; Davies, J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol. Rev. 1968, 32, 493–528. [Google Scholar] [CrossRef]
- Drlica, K.; Zhao, X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997, 61, 377–392. [Google Scholar] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar]
- Ajiboye, T.O.; Naibi, A.M.; Abdulazeez, I.O.; Alege, I.O.; Mohammed, A.O.; Bello, S.A.; Yusuf, I.I.; Ibitoye, O.B.; Muritala, H.F. Microbial Pathogenesis Involvement of oxidative stress in bactericidal activity of 2-(2-nitrovinyl)furan against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Microb. Pathog. 2016, 91, 107–114. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, C.; Zheng, P.; Miao, L.; Yan, X.; Li, H.; Wang, Z.; Gao, B.; Li, Y. Ginsenoside Rb1 alleviates aluminum chloride-induced rat osteoblasts dysfunction. Toxicology 2016, 368–369, 183–188. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, T.; Yin, N.; Ma, X.; Zhang, Z.; Zhu, Z.; Shaukat, A.; Deng, G. Luteoloside protects the uterus from Staphylococcus aureus-induced inflammation, apoptosis and injury. Inflammation 2018, 41, 1702–1716. [Google Scholar] [CrossRef]
- Shaukat, A.; Guo, Y.-F.; Jiang, K.; Zhao, G.; Wu, H.; Zhang, T.; Yang, Y.; Guo, S.; Yang, C.; Zahoor, A.; et al. Ginsenoside Rb1 ameliorates Staphylococcus aureus-induced acute lung injury through attenuating NF-κB and MAPK activation. Microb. Pathog. 2019, 132, 302–312. [Google Scholar]
- Ma, X.; Guo, S.; Jiang, K.; Wang, X.; Yin, N.; Yang, Y.; Zahoor, A.; Deng, G. MiR-128 mediates negative regulation in Staphylococcus aureus induced inflammation by targeting MyD88. Int. Immunopharmacol. 2019, 70, 135–146. [Google Scholar] [CrossRef]
- Xing, J.; Moldobaeva, N.; Birukova, A.A. Atrial natriuretic peptide protects against Staphylococcus aureus-induced lung injury and endothelial barrier dysfunction. J. Appl. Physiol. 2011, 110, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Yang, C.; Yang, Y.; Guo, Y.-F.; Jiang, K.; Guo, S.; Liu, J.; Zhang, T.; Zhao, G.; Ma, X.; et al. Ginsenoside Rb1: A novel therapeutic agent in Staphylococcus aureus induced acute lung injury with special reference to oxidative stress and apoptosis. Microb. Pathog. 2020, 143, 104109. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Mao, J.; Luo, B.; Qin, Z. Role of transcriptional factor Nrf2 in the acute lung injury of mice. Int. J. Clin. Exp. Pathol. 2015, 8, 10929–10934. [Google Scholar] [PubMed]
- Athale, J.; Ulrich, A.; MacGarvey, N.C.; Bartz, R.R.; Welty-Wolf, K.E.; Suliman, H.B.; Piantadosi, C.A. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic. Biol. Med. 2012, 53, 1584–1594. [Google Scholar] [CrossRef]
- MacGarvey, N.C.; Suliman, H.B.; Bartz, R.R.; Fu, P.; Withers, C.M.; Welty-Wolf, K.E.; Piantadosi, C.A. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NFE2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am. J. Respir. Crit. Care Med. 2012, 185, 851–861. [Google Scholar] [CrossRef]
- Yi, E.H.; Xu, F.; Li, P. (3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one alleviates lipoteichoic acid-induced photoreceptor cell damage. Cutan. Ocul. Toxicol. 2018, 37, 367–373. [Google Scholar] [CrossRef]
- Leuner, K.; Pantel, J.; Frey, C.; Schindowski, K.; Schulz, K.; Wegat, T.; Maurer, K.; Eckert, A.; Müller, W.E. Enhanced apoptosis, oxidative stress and mitochondrial dysfunction in lymphocytes as potential biomarkers for Alzheimer’s disease. J. Neural. Transm. Suppl. 2007, 72, 207–215. [Google Scholar]
- Pereira, C.; Coelho, R.; Grácio, D.; Dias, C.; Silva, M.; Peixoto, A.; Lopes, P.; Costa, C.; Teixeira, J.P.; Macedo, G.; et al. DNA damage and oxidative DNA damage in inflammatory bowel disease. J. Crohns Colitis 2016, 10, 1316–1323. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, D.; Xia, Y.; Zhang, H.; Wang, Y.; Tang, T.; Xu, S. Synergistic effect of vancomycin and l-homocarnosine alleviates Staphylococcus aureus-induced osteomyelitis in rats. Biomed. Pharmacother. 2019, 111, 31–35. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, J.; Wang, Y.; Zhang, H.; Xia, Y.; Zhang, Y.; Xu, S. The synergistic therapeutic efficacy of vancomycin and omega-3 fatty acids alleviates Staphylococcus aureus-induced osteomyelitis in rats. Biomed. Pharmacother. 2019, 111, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Yang, J.J.; Yang, M.L.; Li, Y.C.; Kuan, Y.H. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free Radic. Biol. Med. 2014, 69, 249–257. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 2013, 18, 642–660. [Google Scholar] [CrossRef]
- Hillion, M.; Antelmann, H. Thiol-based redox switches in prokaryotes. Biol. Chem. 2015, 396, 415–444. [Google Scholar] [CrossRef] [PubMed]
- Beavers, W.N.; Skaar, E.P. Neutrophil-generated oxidative stress and protein damage in Staphylococcus aureus. Pathog. Dis. 2016, 74, ftw060. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Sultana, S.; Foti, A.; Dahl, J.U. Bacterial defense systems against the neutrophilic oxidant hypochlorous acid. Infect. Immun. 2020, 88, e00964-19. [Google Scholar] [CrossRef]
- Posada, A.C.; Kolar, S.L.; Dusi, R.G.; Francois, P.; Roberts, A.A.; Hamilton, C.J.; Liu, G.Y.; Cheung, A. Importance of bacillithiol in the oxidative stress response of Staphylococcus aureus. Infect. Immun. 2014, 82, 316–332. [Google Scholar] [CrossRef]
- Perera, V.R.; Newton, G.L.; Pogliano, K. Bacillithiol: A key protective thiol in Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 1089–1107. [Google Scholar] [CrossRef]
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Investig. 2003, 111, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.T.; Marnett, L.J. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc. Chem. Res. 2010, 43, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Delmastro-Greenwood, M.; Freeman, B.A.; Wendell, S.G. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu. Rev. Physiol. 2014, 76, 79–105. [Google Scholar] [CrossRef]
- Davey, M.E.; O’Toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A.; Falkow, S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc. Natl. Acad. Sci. USA 1990, 87, 4304–4308. [Google Scholar] [CrossRef]
- Uziel, O.; Borovok, I.; Schreiber, R.; Cohen, G.; Aharonowitz, Y. Transcriptional regulation of the Staphylococcus aureus thioredoxin and thioredoxin reductase genes in response to oxygen and disulfide stress. J. Bacteriol. 2004, 186, 326–334. [Google Scholar] [CrossRef]
- Antelmann, H.; Helmann, J.D. Thiol-based redox switches and gene regulation. Antioxid. Redox Signal. 2011, 14, 1049–1063. [Google Scholar] [CrossRef]
- Leichert, L.I.; Dick, T.P. Incidence and physiological relevance of protein thiol switches. Biol. Chem. 2015, 396, 389–399. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, L.; Jones, M.B.; Sun, F.; Deng, X.; Liang, H.; Cho, H.; Brugarolas, P.; Gao, Y.N.; Peterson, S.N.; et al. Molecular mechanism of quinone signaling mediated through S-quinonization of a YodB family repressor QsrR. Proc. Natl. Acad. Sci. USA 2013, 110, 5010–5015. [Google Scholar] [CrossRef]
- Linzner, N.; Loi, V.V.; Antelmann, H. The Catalase KatA Contributes to Microaerophilic H2O2 Priming to Acquire an Improved Oxidative Stress Resistance in Staphylococcus aureus. Antioxidants 2022, 11, 1793. [Google Scholar] [CrossRef]
- Zeller, T.; Klug, G. Thioredoxins in bacteria: Functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften 2006, 93, 259–266. [Google Scholar] [CrossRef] [PubMed]
- delCardayre, S.B.; Stock, K.P.; Newton, G.L.; Fahey, R.C.; Davies, J.E. Coenzyme A disulfide reductase, the primary low molecular weight disulfide reductase from Staphylococcus aureus. Purification and characterization of the native enzyme. J. Biol. Chem. 1998, 273, 5744–5751. [Google Scholar] [CrossRef] [PubMed]
- Soutourina, O.; Dubrac, S.; Poupel, O.; Msadek, T.; Martin-Verstraete, I. The Pleiotropic CymR Regulator of Staphylococcus aureus Plays an Important Role in Virulence and Stress Response. PLoS Pathog. 2010, 6, e1000894. [Google Scholar] [CrossRef] [PubMed]
- Soutourina, O.; Poupel, O.; Coppée, J.Y.; Danchin, A.; Msadek, T.; Martin-Verstraete, I. CymR, the master regulator of cysteine metabolism in Staphylococcus aureus, controls host sulfur source utilization and plays a role in biofilm formation. Mol. Microbiol. 2009, 73, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Tanous, C.; Soutourina, O.; Raynal, B.; Hullo, M.-F.; Mervelet, P.; Gilles, A.-M.; Noirot, P.; Danchin, A.; England, P.; Martin-Verstraete, I. The CymR Regulator in Complex with the Enzyme CysK Controls Cysteine Metabolism in Bacillus subtilis. J. Biol. Chem. 2008, 283, 35551–35560. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Loi, V.V.; Antelmann, H.; Helmann, J.D. The role of bacillithiol in Gram-positive Firmicutes. Antioxid. Redox Signal. 2018, 28, 445–462. [Google Scholar] [CrossRef]
- Chi, B.K.; Gronau, K.; Mäder, U.; Hessling, B.; Becher, D.; Antelmann, H. S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Mol. Cell. Proteom. 2011, 10, M111.009506. [Google Scholar] [CrossRef]
- Imber, M.; Huyen, N.T.T.; Pietrzyk-Brzezinska, A.J.; Van Loi, V.; Hillion, M.; Bernhardt, J.; Thärichen, L.; Kolšek, K.; Saleh, M.; Hamilton, C.J.; et al. Protein S-bacillithiolation functions in thiol protection and redox regulation of the glyceraldehyde-3-phosphate dehydrogenase Gap in Staphylococcus aureus under hypochlorite stress. Antioxid. Redox Signal. 2018, 28, 410–430. [Google Scholar] [CrossRef]
- Imber, M.; Van Loi, V.; Reznikov, S.; Fritsch, V.N.; Pietrzyk-Brzezinska, A.J.; Prehn, J.; Hamilton, C.; Wahl, M.C.; Bronowska, A.K.; Antelmann, H. The aldehyde dehydrogenase AldA contributes to the hypochlorite defense and is redox-controlled by protein S-bacillithiolation in Staphylococcus aureus. Redox Biol. 2018, 15, 557–568. [Google Scholar] [CrossRef]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in Gram-positive bacteria. Redox Biol. 2018, 20, 130–145. [Google Scholar] [CrossRef]
- Arner, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Austin, C.M.; Garabaglu, S.; Krute, C.N.; Ridder, M.J.; Seawell, N.A.; Markiewicz, M.A.; Boyd, J.M. Contribution of YjbIH to Virulence Factor Expression and Host Colonization in Staphylococcus aureus. Infect. Immun. 2019, 87, e00155-19. [Google Scholar] [CrossRef]
- Paudel, A.; Panthee, S.; Hamamoto, H.; Grunert, T.; Sekimizu, K. YjbH regulates virulence genes expression and oxidative stress resistance in Staphylococcus aureus. Virulence 2021, 12, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Hamamoto, H.; Panthee, S.; Matsumoto, Y.; Sekimizu, K. Large-scale screening and identification of novel pathogenic Staphylococcus aureus genes using a silkworm infection model. J. Infect. Dis. 2020, 221, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef]
- Sahukhal, G.S.; Elasri, M.O. Identification and characterization of an operon, msaABCR, that controls virulence and biofilm development in Staphylococcus aureus. BMC Microbiol. 2014, 14, 154. [Google Scholar] [CrossRef]
- Sahukhal, G.S.; Pandey, S.; Elasri, M.O. msaABCR operon is involved in persister cell formation in Staphylococcus aureus. BMC Microbiol. 2017, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Sahukhal, G.S.; Elasri, M.O. The msaABCR operon regulates the response to oxidative stress in Staphylococcus aureus. J. Bacteriol. 2019, 201, e00417-19. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar]
- You, Y.; Xue, T.; Cao, L.; Zhao, L.; Sun, H.; Sun, B. Staphylococcus aureus glucose-induced biofilm accessory proteins, GbaAB, influence biofilm formation in a PIA-dependent manner. Int. J. Med. Microbiol. 2014, 304, 603–612. [Google Scholar] [CrossRef]
- Sergeeva, O.V.; Bogdanov, A.A.; Sergiev, P.V. What do we know about ribosomal RNA methylation in Escherichia coli? Biochimie 2015, 117, 110–118. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, H.C.; Rife, J.P. Staphylococcus aureus and Escherichia coli have disparate dependences on KsgA for growth and ribosome biogenesis. BMC Microbiol. 2012, 12, 244. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- AlSaleh, A.; Shahid, M.; Farid, E.; Kamal, N.; Bindayna, K. Synergistic antimicrobial effect of ascorbic acid and nicotinamide with rifampicin and vancomycin against SCC mec type IV methicillin-resistant Staphylococcus aureus (MRSA). Access Microbiol. 2023, 5, 000475-v4. [Google Scholar] [CrossRef]
- Kyuma, T.; Kizaki, H.; Ryuno, H.; Sekimizu, K.; Kaito, C. 16S rRNA methyltransferase KsgA contributes to oxidative stress resistance and virulence in Staphylococcus aureus. Biochimie 2015, 119, 166–174. [Google Scholar] [CrossRef]
- Singh, V.K.; Vaish, M.; Johansson, T.R.; Baum, K.R.; Ring, R.P.; Singh, S.; Shukla, S.K.; Moskovitz, J. Significance of Four Methionine Sulfoxide Reductases in Staphylococcus aureus. PLoS ONE 2015, 10, e0117594. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Das, S.; Chattopadhyay, S.; Tripathy, S.; Dash, S.K.; Pramanik, P.; Roy, S. Staphylococcus aureus infection induced redox signaling and DNA fragmentation in T lymphocytes: Possible ameliorative role of nanoconjugated vancomycin. Toxicol. Mech. Methods 2012, 22, 193–204. [Google Scholar] [CrossRef]
- Becerra, M.C.; Albesa, I. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochem. Biophys. Res. Commun. 2002, 297, 1003–1007. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Shi, T.; Du, G.; Liu, W.; Yin, X.-F.; Sun, X.; Pan, Y.; He, Q.-Y. iTRAQ-Based Proteomics Revealed the Bactericidal Mechanism of Sodium New Houttuyfonate against Streptococcus pneumoniae. J. Agric. Food Chem. 2016, 64, 6375–6382. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Wu, M.; Kenry; Huang, Z.; Lee, C.S.; Liu, B. Membrane-Anchoring Photosensitizer with Aggregation-Induced Emission Characteristics for Combating Multidrug-Resistant Bacteria. Angew. Chem. Int. Ed. 2020, 59, 632–636. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Kohanski, M.A.; Hayete, B.; Collins, J.J. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 2007, 3, 91. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-W.; Hung, Y.-J.; Yang, C.-H.; Chen, Y.-C. The antimicrobial activity of gramicidin A is associated with hydroxyl radical formation. PLoS ONE 2015, 10, e0117065. [Google Scholar] [CrossRef] [PubMed]
- Lobritz, M.A.; Belenky, P.; Porter, C.B.; Gutierrez, A.; Yang, J.H.; Schwarz, E.G.; Dwyer, D.J.; Khalil, A.S.; Collins, J.J. Antibiotic efficacy is linked to bacterial cellular respiration. Proc. Natl. Acad. Sci. USA 2015, 112, 8173–8180. [Google Scholar] [CrossRef]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobiş, O.; Lassoued, M.A. 1,8-Cineol (Eucalyptol) disrupts membrane integrity and induces oxidative stress in methicillin-resistant Staphylococcus aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef]
- Shaukat, A.; Shaukat, I.; Rajput, S.A.; Shukat, R.; Hanif, S.; Jiang, K.; Zhang, T.; Akhtar, M.; Shaukat, I.; Ma, X.; et al. Ginsenoside Rb1 protects from Staphylococcus aureus-induced oxidative damage and apoptosis through endoplasmic reticulum-stress and death receptor-mediated pathways. Ecotoxicol. Environ. Saf. 2021, 219, 112353. [Google Scholar] [CrossRef]
- Singh, V.; Pal, A.; Darokar, M.P. A polyphenolic flavonoid glabridin: Oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic. Biol. Med. 2015, 87, 48–57. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Mohammed, A.O.; Bello, S.A.; Yusuf, I.I.; Ibitoye, O.B.; Muritala, H.F.; Onajobi, I.B. Antibacterial activity of Syzygium aromaticum seed: Studies on oxidative stress biomarkers and membrane permeability. Microb. Pathog. 2016, 95, 208–215. [Google Scholar] [CrossRef]
- Kong, A.S.-Y.; Maran, S.; Yap, P.S.-X.; Lim, S.-H.E.; Yang, S.-K.; Cheng, W.-H.; Tan, Y.-H.; Lai, K.-S. Anti- and Pro-Oxidant Properties of Essential Oils against Antimicrobial Resistance. Antioxidants 2022, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Linzner, N.; Fritsch, V.N.; Busche, T.; Tung, Q.N.; Van Loi, V.; Bernhardt, J.; Kalinowski, J.; Antelmann, H. The plant-derived naphthoquinone lapachol causes an oxidative stress response in Staphylococcus aureus. Free Radic. Biol. Med. 2020, 158, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Kumar, N.R.; Kaur, J. Therapeutic effect of propolis on Staphylococcus aureus induced oxidative stress in spleen of Balb/c mice: A biochemical and histopathological study. Indian J. Nat. Prod. Resour. 2022, 13, 346–355. [Google Scholar]
- Zhang, W.; Cheng, C.; Sha, Z.; Chen, C.; Yu, C.; Lv, N.; Ji, P.; Wu, X.; Ma, T.; Cheng, H.; et al. Rosmarinic acid prevents refractory bacterial pneumonia through regulating Keap1/Nrf2-mediated autophagic pathway and mitochondrial oxidative stress. Free Radic. Biol. Med. 2021, 168, 247–257. [Google Scholar] [CrossRef]
- Wang, X.; Koffi, P.F.; English, O.F.; Lee, J.C. Staphylococcus aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020. Toxins 2021, 13, 75. [Google Scholar] [CrossRef]
- Singh, V.; Pal, A.; Darokar, M.P. Glabridin synergy with norfloxacin induces ROS in multidrug resistant Staphylococcus aureus. J. Gen. Appl. Microbiol. 2021, 67, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Faujdar, S.S.; Bisht, D.; Sharma, A. Antibacterial activity of Syzygium aromaticum (clove) against uropathogens producing ESBL, MBL, and AmpC beta-lactamase: Are we close to getting a new antibacterial agent? J. Fam. Med. Prim. Care 2020, 9, 180–186. [Google Scholar] [CrossRef]
- Lima, R.G.; Flores, R.S.; Miessi, G.; Pulcherio, J.H.V.; Aguilera, L.F.; Araujo, L.O.; Oliveira, S.L.; Caires, A.R.L. Determination of Photosensitizing Potential of Lapachol for Photodynamic Inactivation of Bacteria. Molecules 2024, 29, 5184. [Google Scholar] [CrossRef]
- Shim, K.-S.; Kim, H.J.; Ji, K.-Y.; Jung, D.H.; Park, S.H.; Song, H.-K.; Kim, T.; Kim, K.M. Rosmarinic Acid Ameliorates Dermatophagoides farinae Extract-Induced Atopic Dermatitis-like Skin Inflammation by Activating the Nrf2/HO-1 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 12737. [Google Scholar] [CrossRef]
- Kohanski, M.; Dwyer, D.; Collins, J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Nizer, W.S.; Adams, M.E.; Allison, K.N.; Montgomery, M.C.; Mosher, H.; Cassol, E.; Overhage, J. Oxidative stress responses in biofilms. Biofilm 2024, 7, 100203. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, M.L.C.; Ferreira, G.G.; Kosmiscky, I.d.O.; Guedes, I.M.L.; Muniz, J.A.P.C.; Carneiro, L.A.; Peralta, Í.L.d.C.; Bahia, M.N.M.; Souza, C.d.O.; Dolabela, M.F. What Do We Know About Staphylococcus aureus and Oxidative Stress? Resistance, Virulence, New Targets, and Therapeutic Alternatives. Toxics 2025, 13, 390. https://doi.org/10.3390/toxics13050390
Bastos MLC, Ferreira GG, Kosmiscky IdO, Guedes IML, Muniz JAPC, Carneiro LA, Peralta ÍLdC, Bahia MNM, Souza CdO, Dolabela MF. What Do We Know About Staphylococcus aureus and Oxidative Stress? Resistance, Virulence, New Targets, and Therapeutic Alternatives. Toxics. 2025; 13(5):390. https://doi.org/10.3390/toxics13050390
Chicago/Turabian StyleBastos, Mírian Letícia Carmo, Gleison Gonçalves Ferreira, Isis de Oliveira Kosmiscky, Ieda Maria Louzada Guedes, José Augusto Pereira Carneiro Muniz, Liliane Almeida Carneiro, Ísis Lins de Carvalho Peralta, Marcia Nazaré Miranda Bahia, Cintya de Oliveira Souza, and Maria Fâni Dolabela. 2025. "What Do We Know About Staphylococcus aureus and Oxidative Stress? Resistance, Virulence, New Targets, and Therapeutic Alternatives" Toxics 13, no. 5: 390. https://doi.org/10.3390/toxics13050390
APA StyleBastos, M. L. C., Ferreira, G. G., Kosmiscky, I. d. O., Guedes, I. M. L., Muniz, J. A. P. C., Carneiro, L. A., Peralta, Í. L. d. C., Bahia, M. N. M., Souza, C. d. O., & Dolabela, M. F. (2025). What Do We Know About Staphylococcus aureus and Oxidative Stress? Resistance, Virulence, New Targets, and Therapeutic Alternatives. Toxics, 13(5), 390. https://doi.org/10.3390/toxics13050390






