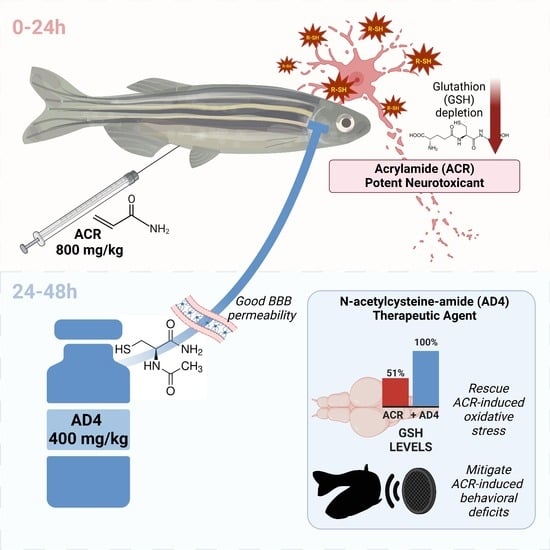
N-Acetylcysteine-Amide Protects Against Acute Acrylamide Neurotoxicity in Adult Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fish Husbandry
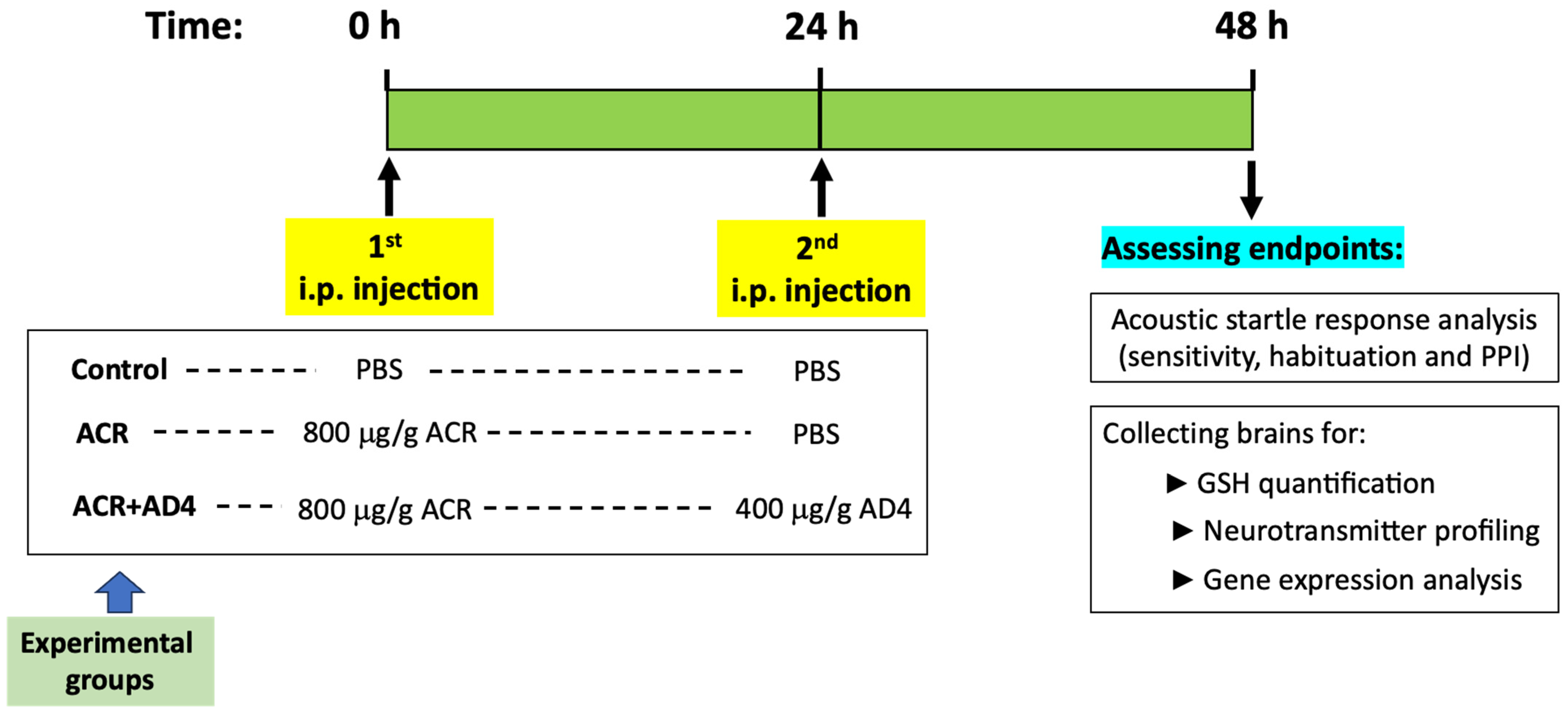
2.3. Experimental Design
2.4. GSH Determination
2.5. Neurotransmitters Assessment
2.6. Gene Expression Analysis
2.7. Kinematic Analysis of the Acoustic Startle Response
2.8. Data Analysis
3. Results
3.1. Identification of a Single Dose of Acrylamide Inducing Brain GSH Depletion
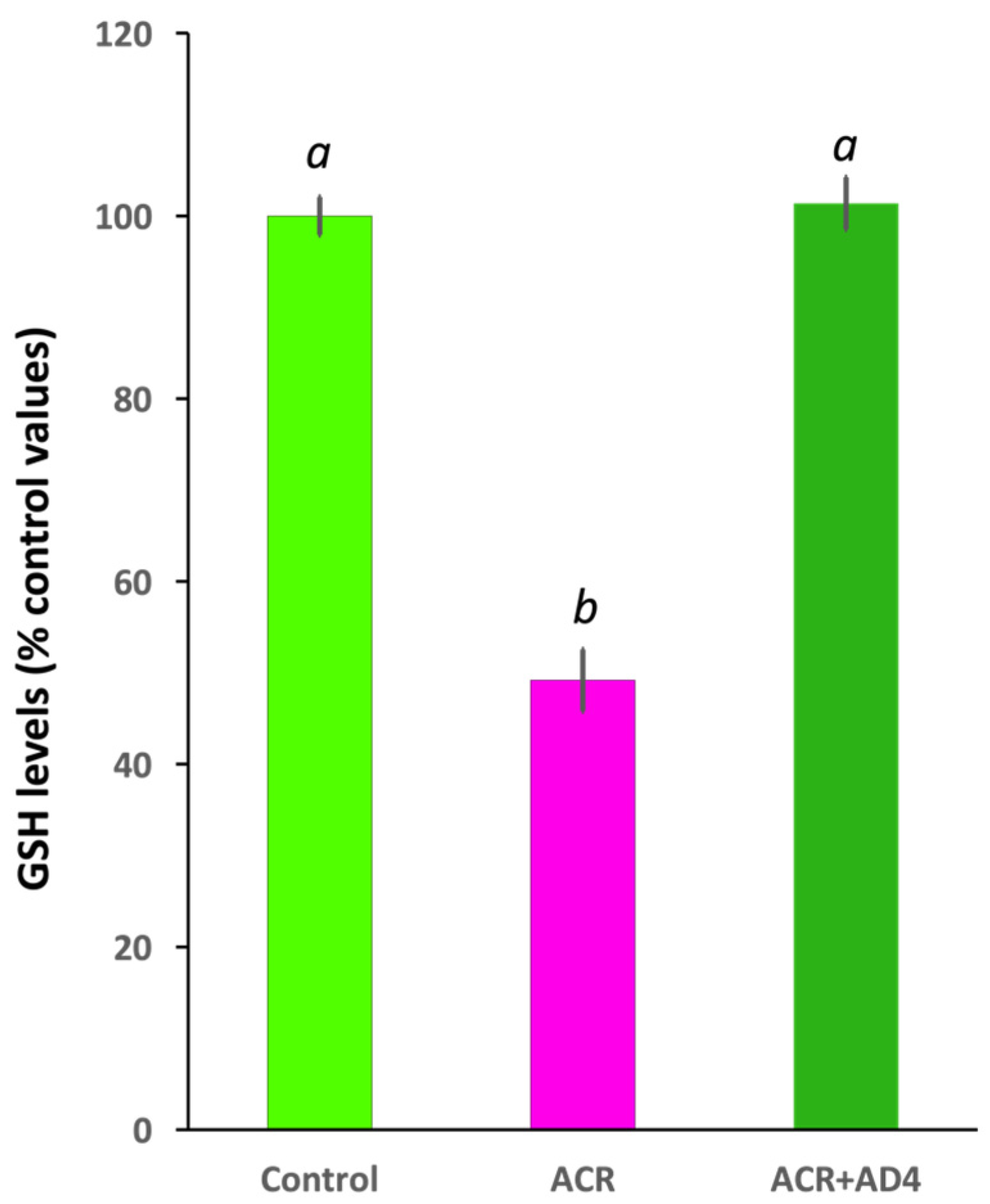
3.2. AD4 Fully Counteracts the Depletion of GSH Stores in the Brain Led by ACR Exposure
3.3. Neurochemical Profile in the Brain of ACR-Injected Zebrafish Remains Unaltered
3.4. Lack of Differential Gene Expression in the Brain of ACR-Injected Zebrafish
3.5. ACR Delays Habituation to a Series of Acoustic Startle Stimuli and This Effect Is Counteracted by AD4
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD4 | N-acetylcysteine-amide |
| ACR | Acrylamide |
| ASR | Acoustic Startle Response |
| GSH | Reduced Glutathione |
| NAC | N-acetylcysteine |
References
- Park, R.M. Preliminary Risk assessment for Acrylamide and Peripheral Neuropathy. Neurotoxicology 2021, 85, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kjuus, H.; Goffeng, L.O.; Heier, M.S.; Sjöholm, H.; Øvrebø, S.; Skaug, V.; Paulsson, B.; Törnqvist, M.; Brudal, S. Effects on the peripheral nervous system of tunnel workers exposed to acrylamide and N-methylolacrylamide. Scand. J. Work. Environ. Heal. 2004, 30, 21–29. [Google Scholar] [CrossRef]
- Buyukdere, Y.; Akyol, A. From a toxin to an obesogen: A review of potential obesogenic roles of acrylamide with a mechanistic approach. Nutr. Rev. 2023, 82, 128–142. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Abdel-Fattah, A.F.M.; Saied, E.M.; El-Seedi, H.R.; Abdel-Daim, M.M. Acrylamide-induced peripheral neuropathy: Manifestations, mechanisms, and potential treatment modalities. Environ. Sci. Pollut. Res. 2021, 28, 13031–13046. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Luo, L.; Li, J.; Huang, H.; Xie, Y. Toxic encephalopathy, vision loss, and memory disorder caused by acute acrylamide exposure. J. Occup. Environ. Hyg. 2024, 21, 152–161. [Google Scholar] [CrossRef]
- Kopańska, M.; Łagowska, A.; Kuduk, B.; Banaś-Ząbczyk, A. Acrylamide Neurotoxicity as a PossibleFactor Responsible for Inflammation in the Cholinergic Nervous System. Int. J. Mol. Sci. 2022, 23, 2030. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, B.; Deng, L. The Mechanism of Acrylamide-Induced Neurotoxicity: Current Status and Future Perspectives. Front. Nutr. 2022, 9, 859189. [Google Scholar] [CrossRef]
- Raldúa, D.; Casado, M.; Prats, E.; Faria, M.; Puig-Castellví, F.; Pérez, Y.; Alfonso, I.; Hsu, C.-Y.; Arick II, M.A.; Garcia-Reyero, N.; et al. Targeting redox metabolism: The perfect storm induced by acrylamide poisoning in the brain. Sci. Rep. 2020, 10, 312. [Google Scholar] [CrossRef]
- Faria, M.; Ziv, T.; Gómez-Canela, C.; Ben-Lulu, S.; Prats, E.; Novoa-Luna, K.A.; Admon, A.; Piña, B.; Tauler, R.; Gómez-Oliván, L.M.; et al. Acrylamide acute neurotoxicity in adult zebrafish. Sci. Rep. 2018, 8, 7918. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T.; Hawkey, A.B.; Levin, E.D. Translating Neurobehavioral Toxicity Across Species from Zebrafish to Rats to Humans: Implications for Risk Assessment. Front. Toxicol. 2021, 3, 629229. [Google Scholar] [CrossRef]
- Firdous, S.M.; Pal, S.; Khanam, S.; Zakir, F. Behavioral neuroscience in zebrafish: Unravelling the complexity of brain-behavior relationships. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 9295–9313. [Google Scholar] [CrossRef] [PubMed]
- Prats, E.; Gómez-Canela, C.; Ben-Lulu, S.; Ziv, T.; Padrós, F.; Tornero, D.; Garcia-Reyero, N.; Tauler, R.; Admon, A.; Raldúa, D. Modelling acrylamide acute neurotoxicity in zebrafish larvae. Sci. Rep. 2017, 7, 13952. [Google Scholar] [CrossRef]
- Faria, M.; Valls, A.; Prats, E.; Bedrossiantz, J.; Orozco, M.; Porta, J.M.; Gómez-Oliván, L.M.; Raldúa, D. Further characterization of the zebrafish model of acrylamide acute neurotoxicity: Gait abnormalities and oxidative stress. Sci. Rep. 2019, 9, 7075. [Google Scholar] [CrossRef]
- Faria, M.; Prats, E.; Gómez-Canela, C.; Hsu, C.-Y.; Arick, M.A.; Bedrossiantz, J.; Orozco, M.; Garcia-Reyero, N.; Ziv, T.; Ben-Lulu, S.; et al. Therapeutic potential of N-acetylcysteine in acrylamide acute neurotoxicity in adult zebrafish. Sci. Rep. 2019, 9, 16467. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.V.; Kolesnikova, T.O.; Galstyan, D.S.; Ilyin, N.P.; de Abreu, M.S.; Petersen, E.V.; Demin, K.A.; Yenkoyan, K.B.; Kalueff, A.V. Current State of Modeling Human Psychiatric Disorders Using Zebrafish. Int. J. Mol. Sci. 2023, 24, 3187. [Google Scholar] [CrossRef]
- Martin, V.; Trus, M.; Atlas, D. Thiol-Based Redox Molecules: Potential Antidotes for Acrylamide Toxicity. Antioxidants 2024, 13, 1431. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wu, X.; Yan, D.; Peng, C.; Rao, C.; Yan, H. Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: Involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol. Lett. 2018, 288, 55–64. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Barhum, Y.; Atlas, D.; Melamed, E.; Offen, D. Analysis of gene expression in MOG-induced experimental autoimmune encephalomyelitis after treatment with a novel brain-penetrating antioxidant. J. Mol. Neurosci. 2005, 27, 125–135. [Google Scholar] [CrossRef]
- Atlas, D. Emerging therapeutic opportunities of novel thiol-amides, NAC-amide (AD4/NACA) and thioredoxin mimetics (TXM-Peptides) for neurodegenerative-related disorders. Free Radic. Biol. Med. 2021, 176, 120–141. [Google Scholar] [CrossRef]
- Maximino, C.; Meinerz, D.L.; Fontana, B.D.; Mezzomo, N.J.; Stefanello, F.V.; Prestes, A.d.S.; Batista, C.B.; Rubin, M.A.; Barbosa, N.V.; Rocha, J.B.T.; et al. Extending the analysis of zebrafish behavioral endophenotypes for modeling psychiatric disorders: Fear conditioning to conspecific alarm response. Behav. Process. 2018, 149, 35–42. [Google Scholar] [CrossRef]
- Lima, M.G.; Silva, R.X.d.C.; Silva, S.d.N.d.S.; Rodrigues, L.d.S.d.S.; Oliveira, K.R.H.M.; Batista, E.d.J.O.; Maximino, C.; Herculano, A.M. Time-dependent sensitization of stress responses in zebrafish: A putative model for post-traumatic stress disorder. Behav. Process. 2016, 128, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Percie Du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Cachat, J.M.; Suciu, C.; Hart, P.C.; Gaikwad, S.; Utterback, E.; Dileo, J.; Kalueff, A.V. Intraperitoneal injection as a method of psychotropic drug delivery in adult zebrafish. Neuromethods 2011, 51, 169–179. [Google Scholar] [CrossRef]
- White, C.C.; Viernes, H.; Krejsa, C.M.; Botta, D.; Kavanagh, T.J. Fluorescence-based microtiter plate assay for glutamate–cysteine ligase activity. Anal. Biochem. 2003, 318, 175–180. [Google Scholar] [CrossRef]
- Mayol-Cabré, M.; Prats, E.; Raldúa, D.; Gómez-Canela, C. Characterization of monoaminergic neurochemicals in the different brain regions of adult zebrafish. Sci. Total Environ. 2020, 745, 141205. [Google Scholar] [CrossRef]
- Faria, M.; Bedrossiantz, J.; Ramírez, J.R.R.; Mayol, M.; García, G.H.; Bellot, M.; Prats, E.; Garcia-Reyero, N.; Gómez-Canela, C.; Gómez-Oliván, L.M.; et al. Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environ. Int. 2021, 146, 106253. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 C T Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Stevanović, M.; Tagkalidou, N.; Multisanti, C.R.; Pujol, S.; Aljabasini, O.; Prats, E.; Faggio, C.; Porta, J.M.; Barata, C.; Raldúa, D. Zebra_K, a kinematic analysis automated platform for assessing sensitivity, habituation and prepulse inhibition of the acoustic startle response in adult zebrafish. Sci. Total Environ. 2025, 958, 178028. [Google Scholar] [CrossRef]
- Wolman, M.A.; Jain, R.A.; Liss, L.; Granato, M. Chemical modulation of memory formation in larval zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 15468–15473. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Sensorimotor gating in larval zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Atlas, D.; Melamed, E.; Ofen, D. Brain Targeted Low Molecular Weight Hydrophobic Antioxidant Compounds. U.S. Patent USOO5874468A, 23 February 1999. [Google Scholar]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Acrylamide-induced nerve terminal damage: Relevance to neurotoxic and neurodegenerative mechanisms. J. Agric. Food Chem. 2008, 56, 5994–6003. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Barber, D.S. Synaptic Cysteine Sulfhydryl Groups as Targets of Electrophilic Neurotoxicants. Toxicol. Sci. 2006, 94, 240–255. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T.; Barber, D.S. Type-2 alkenes mediate synaptotoxicity in neurodegenerative diseases. Neurotoxicology 2008, 29, 871–882. [Google Scholar] [CrossRef]
- Exon, J.H. A Review of the Toxicology of Acrylamide. J. Toxicol. Environ. Health Part B 2006, 9, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Richendrfer, H.; Creton, R. Chlorpyrifos and malathion have opposite effects on behaviors and brain size that are not correlated to changes in AChE activity. Neurotoxicology 2015, 49, 50–58. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular mechanism of acrylamide neurotoxicity: Lessons learned from organic chemistry. Environ. Health Perspect. 2012, 120, 1650. [Google Scholar] [CrossRef]
- Nelson ID, J.C.; Shoenhard, H.; Granato, M.I.; Moens, C. Integration of cooperative and opposing molecular programs drives learning-associated behavioral plasticity. PLoS Genet. 2023, 19, e1010650. [Google Scholar] [CrossRef]
- Kulak, A.; Steullet, P.; Cabungcal, J.H.; Werge, T.; Ingason, A.; Cuenod, M.; Do, K.Q. Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: Insights from animal models. Antioxid. Redox Signal. 2013, 18, 1428–1443. [Google Scholar] [CrossRef]
- Preissmann, D.; Dépré, M.; Schenk, F.; Gisquet-Verrier, P. Anxiety modulates cognitive deficits in a perinatal glutathione deficit animal model of schizophrenia. Brain Res. 2016, 1648, 459–468. [Google Scholar] [CrossRef]
- Lévesque, F.; Seeberger, P.H. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem. Int. Ed. Engl. 2012, 51, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.W.; Blair, D.J.; Burke, M.D. Towards the generalized iterative synthesis of small molecules. Nat. Rev. Chem. 2018, 2, 0115. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Control [Median, (IQR)] | Acrylamide [Median, (IQR)] | U | z | p |
|---|---|---|---|---|---|
| Latency (ms) | 11 (10–12) | 11 (10–12) | 1729 | 0.347 | 0.729 |
| Duration (ms) | 12 (11–14) | 11 (10–13) | 1392 | −1.556 | 0.120 |
| Curvature (°) | 99.3 (86.6–112.0) | 102.5 (77.5–115.8) | 1696 | 0.147 | 0.883 |
| Average Angular Velocity (°/ms) | 8.4 (7.6–9.3) | 8.8 (7.7–10.0) | 1898 | 1.267 | 0.205 |
| Maximal Angular Velocity (°/ms) | 17.6 (15.5–19.7) | 18.4 (16.2–20.9) | 1906 | 1.311 | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagkalidou, N.; Goyenechea-Cunillera, J.; Romero-Alfano, I.; Martí, M.O.; Bedrossiantz, J.; Prats, E.; Gomez-Canela, C.; Raldúa, D. N-Acetylcysteine-Amide Protects Against Acute Acrylamide Neurotoxicity in Adult Zebrafish. Toxics 2025, 13, 362. https://doi.org/10.3390/toxics13050362
Tagkalidou N, Goyenechea-Cunillera J, Romero-Alfano I, Martí MO, Bedrossiantz J, Prats E, Gomez-Canela C, Raldúa D. N-Acetylcysteine-Amide Protects Against Acute Acrylamide Neurotoxicity in Adult Zebrafish. Toxics. 2025; 13(5):362. https://doi.org/10.3390/toxics13050362
Chicago/Turabian StyleTagkalidou, Niki, Júlia Goyenechea-Cunillera, Irene Romero-Alfano, Maria Olivella Martí, Juliette Bedrossiantz, Eva Prats, Cristian Gomez-Canela, and Demetrio Raldúa. 2025. "N-Acetylcysteine-Amide Protects Against Acute Acrylamide Neurotoxicity in Adult Zebrafish" Toxics 13, no. 5: 362. https://doi.org/10.3390/toxics13050362
APA StyleTagkalidou, N., Goyenechea-Cunillera, J., Romero-Alfano, I., Martí, M. O., Bedrossiantz, J., Prats, E., Gomez-Canela, C., & Raldúa, D. (2025). N-Acetylcysteine-Amide Protects Against Acute Acrylamide Neurotoxicity in Adult Zebrafish. Toxics, 13(5), 362. https://doi.org/10.3390/toxics13050362