Screening of Profitable Chrysanthemums for the Phytoremediation of Cadmium-Contaminated Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experiment Design and Treatments
2.3. Sampling and Experiment Analysis
2.4. Evaluation of the Soil Remediation Capacity of Chrysanthemum Plants
2.5. Data Analysis
3. Results and Discussion
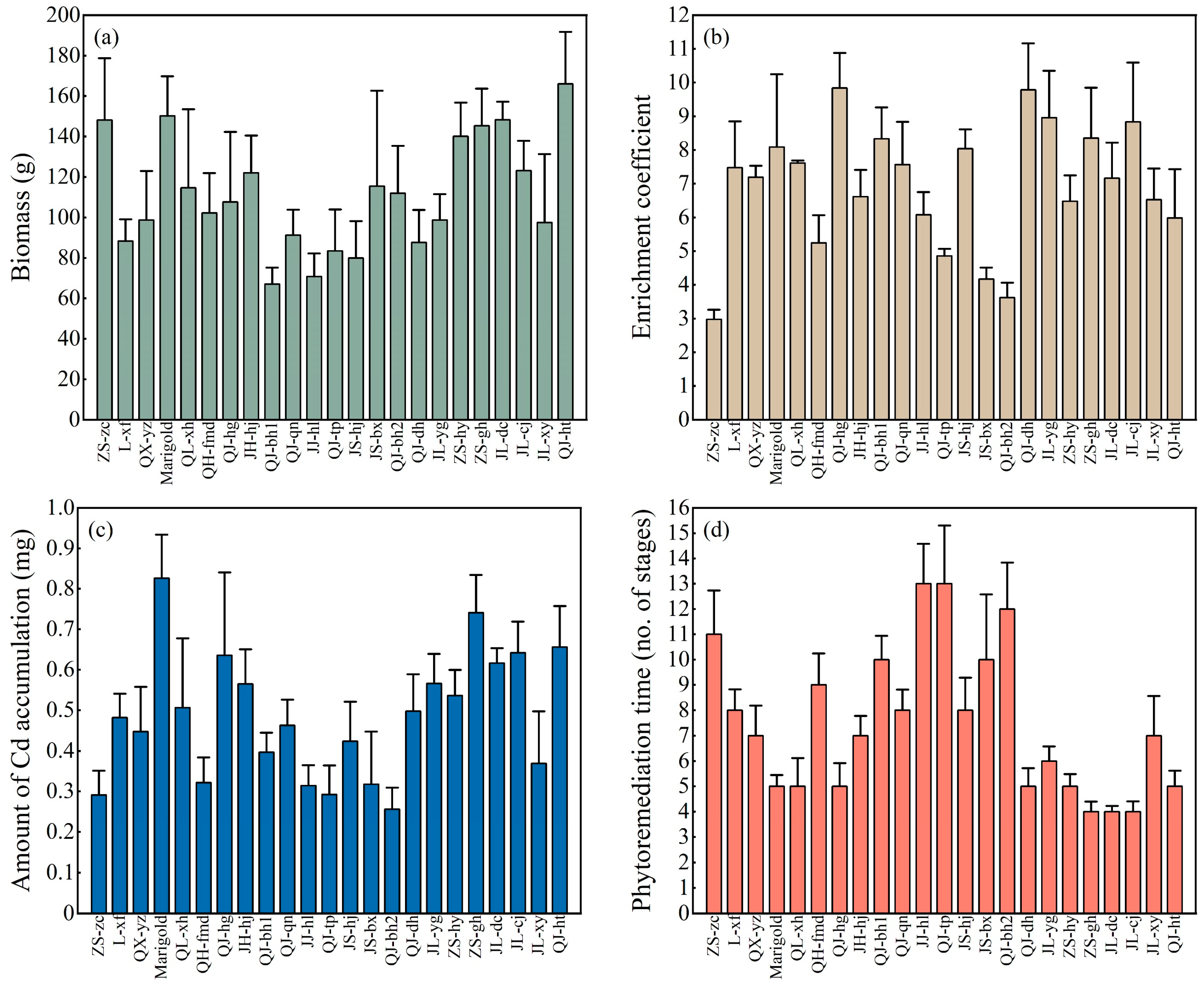
3.1. Cd Cumulative Abilities of 23 Chrysanthemum Cultivars
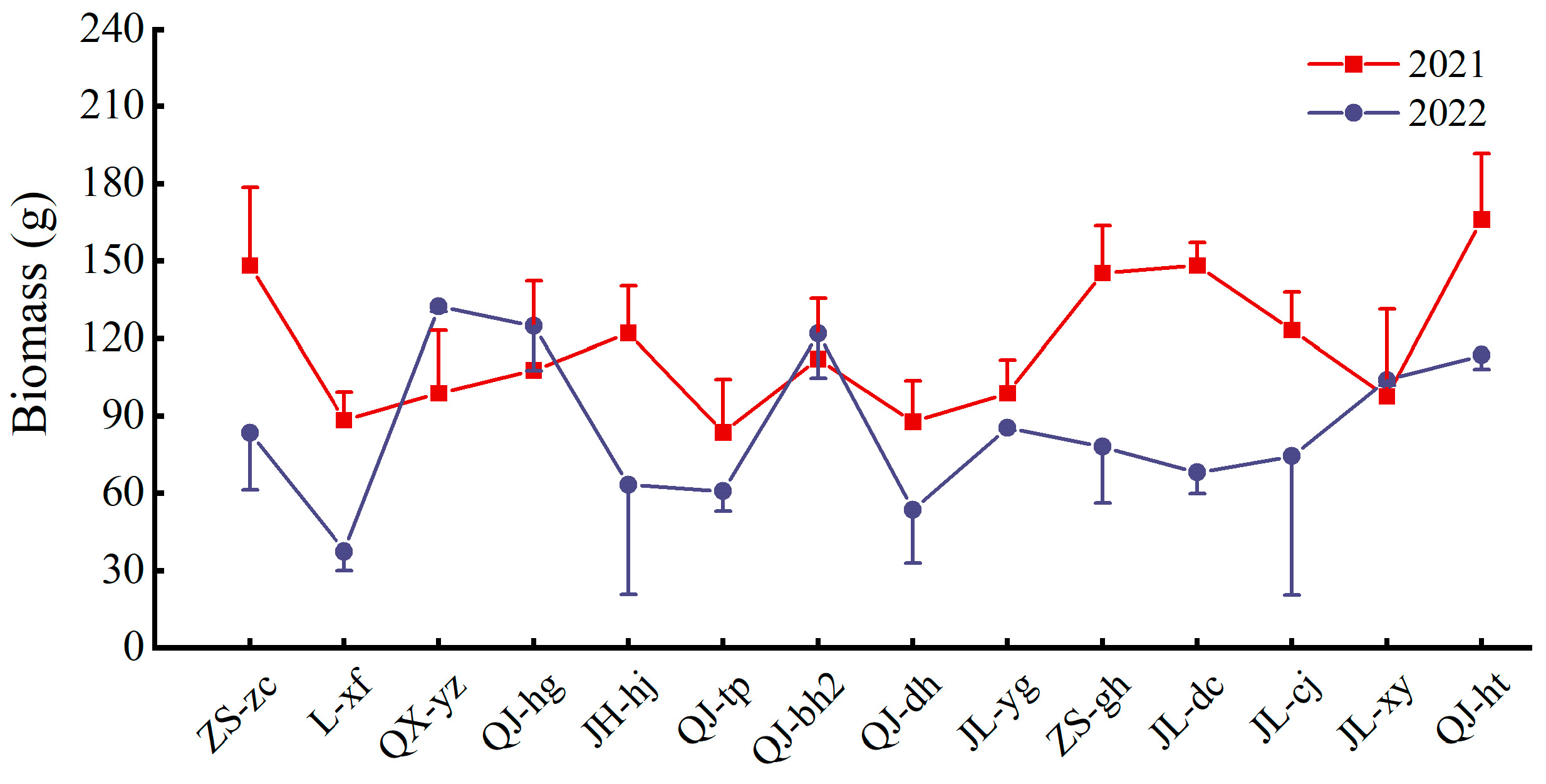
3.2. Heavy Metal Tolerance in Multiple-Cropping Chrysanthemum Cultivars
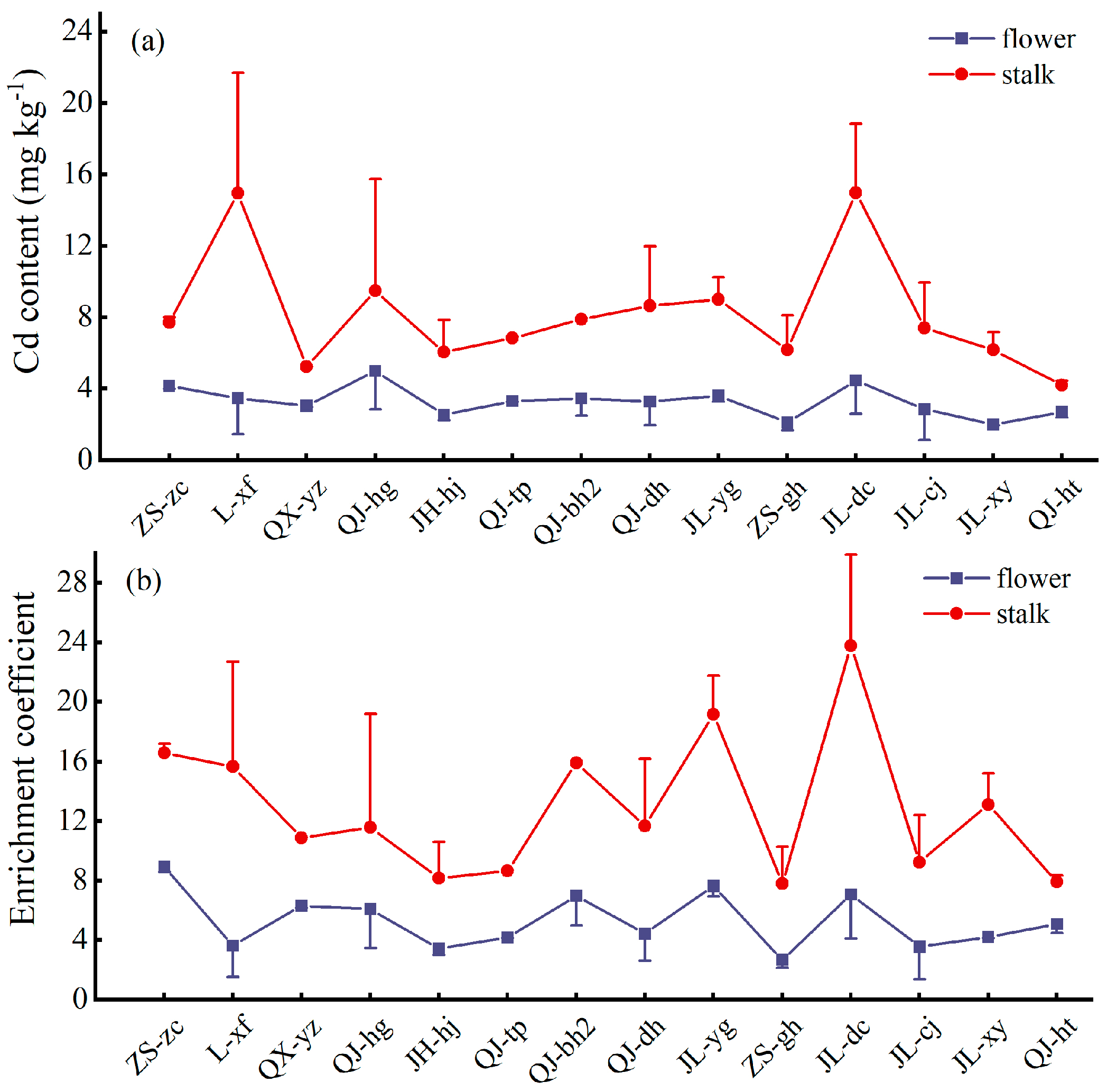
3.3. Enrichment Coefficient to Different Aboveground Parts of Multiple-Cropping Chrysanthemum Cultivars
3.4. Cd Enrichment Efficiency of Chrysanthemum Plants Under Organic Acid and EDTA-Si
3.5. Estimation of the Economic Benefits of Chrysanthemum Remediation for Cd-Contaminated Agricultural Land
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Zhang, Q.; Du, W.; Ai, F.; Yin, Y.; Ji, R.; Guo, H. Microbial communities in the rhizosphere of different willow genotypes affect phytoremediation potential in Cd contaminated soil. Sci. Total Environ. 2021, 769, 145224. [Google Scholar] [CrossRef]
- Xia, X.; Yang, Z.; Yu, T.; Zhang, C.; Hou, Q. Predicting spatial and temporal variation of Cd concentration in rice grains in the Lower Changjiang Plain during 2004–2014 based on soil geochemical survey data with GIS. J. Geochem. Explor. 2018, 200, 276–283. [Google Scholar] [CrossRef]
- Arteaga, J.F.; Gluhar, S.; Kaurin, A.; Lestan, D. Simultaneous removal of arsenic and toxic metals from contaminated soil: Laboratory development and pilot scale demonstration. Environ. Pollut. 2022, 294, 118656. [Google Scholar] [CrossRef]
- Kalyvas, G.; Bilias, F.; Gasparatos, D.; Zafeiriou, I.; Eissa, R.; Karamountzou, E.; Massas, I. Enhanced As, Pb and Zn uptake by Helianthus annuus from a Heavily contaminated mining soil amended with edta and olive mill wastewater due to increased element mobilization, as verified by sequential extraction schemes. Environments 2022, 9, 61. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei, D.; Mi, J.; Shen, Y.; Cui, B.; Han, C. Immobilization of Cu(II) and Zn(II) in simulated polluted soil using sulfurizing agent. Chem. Eng. J. 2015, 277, 312–317. [Google Scholar] [CrossRef]
- Vaxevanidou, K.; Papassiopi, N.; Paspaliaris, I. Removal of heavy metals and arsenic from contaminated soils using bioremediation and chelant extraction techniques. Chemosphere 2008, 70, 1329–1337. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.; Peralta-Videa, J.; de la Rosa, G.; Parsons, J. Phytoremediation of heavy metals and study of the metal coordination by X-ray absorption spectroscopy. Coord. Chem. Rev. 2005, 249, 1797–1810. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Solomou, A.D.; Germani, R.; Proutsos, N.; Petropoulou, M.; Koutroumpilas, P.; Galanis, C.; Maroulis, G.; Kolimenakis, A. Utilizing mediterranean plants to remove contaminants from the soil environment: A short review. Agriculture 2022, 12, 238. [Google Scholar] [CrossRef]
- Wang, J.; Delavar, M.A. Techno-economic analysis of phytoremediation: A strategic rethinking. Sci. Total Environ. 2023, 165949. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land (Trial). Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2018.
- Luo, F.; Hu, X.-F.; Oh, K.; Yan, L.-J.; Lu, X.-Z.; Zhang, W.-J.; Yonekura, T.; Yonemochi, S.; Isobe, Y. Using profitable chrysanthemums for phytoremediation of Cd-and Zn-contaminated soils in the suburb of Shanghai. J. Soils Sediments 2020, 20, 4011–4022. [Google Scholar] [CrossRef]
- Beiyuan, J.; Lau, A.Y.T.; Tsang, D.C.W.; Zhang, W.; Kao, C.-M.; Baek, K.; Ok, Y.S.; Li, X.-D. Chelant-enhanced washing of CCA-contaminated soil: Coupled with selective dissolution or soil stabilization. Sci. Total Environ. 2018, 612, 1463–1472. [Google Scholar] [CrossRef]
- Fabbricino, M.; Ferraro, A.; Luongo, V.; Pontoni, L.; Race, M. Soil Washing Optimization, Recycling of the Solution, and Ecotoxicity Assessment for the Remediation of Pb-Contaminated Sites Using EDDS. Sustainability 2018, 10, 636. [Google Scholar] [CrossRef]
- Jani, Y.; Hogland, W. Chemical extraction of trace elements from hazardous fine fraction at an old glasswork dump. Chemosphere 2017, 195, 825–830. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Zhong, Q.; Zhang, C.; Jia, Y.; Li, T.; Deng, O.; Li, Y. Heavy metal removal by GLDA washing: Optimization, redistribution, recycling, and changes in soil fertility. Sci. Total Environ. 2016, 569–570, 557–568. [Google Scholar] [CrossRef]
- López-Rayo, S.; Sanchis, I.; Ferreira, C.; Lucena, J. [S,S ]-EDDS/Fe: A new chelate for the environmentally sustainable correction of iron chlorosis in calcareous soil. Sci. Total Environ. 2018, 647, 1508–1517. [Google Scholar] [CrossRef]
- Sinhal, V.; Srivastava, A.; Singh, V. EDTA and citric acid mediated phytoextraction of Zn, Cu, Pb and Cd through marigold (Tagetes erecta). J. Environ. Biol. / Acad. Environ. Biol. 2010, 31, 255–259. [Google Scholar]
- DD 2005-03; Technical Requirements for Sample Analysis in Ecological Geochemical Evaluation (Trial). China Geological Survey: Beijing, China, 2005.
- DZ/T 0279-2016; Analysis Methods for Regional Geochemical Sample. Ministry of Land and Resources of China: Beijing, China, 2016.
- Hu, P.; Zhou, X.; Qiu, R.; Tang, Y.; Ying, R. Cadmium tolerance and accumulation features of Zn-hyperaccumulator Potentilla griffithii var. velutina. J. Agro-Environ. Sci. 2007, 26, 2221–2224. [Google Scholar]
- Liu, K.; Zhou, Z.; Yu, F.; Chen, M.; Chen, C.; Zhu, J.; Jiang, Y. A newly found cadmium hyperaccumulator-centella asiatica linn. Environ. Bull. 2016, 2668. [Google Scholar]
- Liu, W.; Shu, W.; Lan, C. Viola baoshanensis, a plant that hyperaccumulates cadmium. Chin. Sci. Bull. 2004, 49, 29–32. [Google Scholar] [CrossRef]
- Liu, X.; Peng, K.; Wang, A.; Lian, C.; Shen, Z. Cadmium accumulation and distribution in populations of Phytolacca americana L. and the role of transpiration. Chemosphere 2010, 78, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qiu, R.; Zeng, X.; Ying, R.; Yu, F.; Zhou, X. Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ. Exp. Bot. 2009, 66, 126–134. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Deng, L.; Gong, G.; Jia, Y.; Xu, X.; Li, T.; Li, Y.; Chen, H. Cadmium tolerance and accumulation characteristics of Siegesbeckia orientalis L. Ecol. Eng. 2013, 51, 133–139. [Google Scholar] [CrossRef]
- Li, J.; Liao, B.; Lan, C.; Ye, Z.; Baker, A.; Shu, W. Cadmium Tolerance and Accumulation in Cultivars of a High-Biomass Tropical Tree (Averrhoa carambola) and Its Potential for Phytoextraction. Agric. Water Manag. 2010, 39, 1262–1268. [Google Scholar] [CrossRef]
- Tiwari, K.; Dwivedi, S.; Mishra, S.; Srivastava, S.; Tripathi, R.; Singh, N.; Chakraborty, S. Phytoremediation efficiency of Portulaca tuberosa rox and Portulaca oleracea L. naturally growing in an industrial effluent irrigated area in Vadodra, Gujrat, India. Environ. Monit. Assess. 2008, 147, 15–22. [Google Scholar] [CrossRef]
- Yang, X.; Long, X.; Ye, H.; He, Z.; Calvert, D.V.; Stoffella, P.J. Cadmium Tolerance and Hyperaccumulation in a New Zn-Hyperaccumulating Plant Species (Sedum alfredii Hance). Plant Soil 2004, 259, 181–189. [Google Scholar] [CrossRef]
- Pulford, I.; Watson, C. Phytoremediation of Heavy Metal-Contaminated Land by Trees—A Review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Seregin, I.; Kozhevnikova, A. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008, 55, 1–22. [Google Scholar] [CrossRef]
- Lal, K.; Minhas, P.S.; Chaturvedi, R.K.; Yadav, R. Extraction of cadmium and tolerance of three annual cut flowers on Cd-contaminated soils. Bioresour. Technol. 2008, 99, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Blaylock, M.J.; Salt, D.E.; Dushenkov, S.; Zakharova, O.; Gussman, C.; Kapulnik, Y.; Ensley, B.D.; Raskin, I.J. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997, 31, 860–865. [Google Scholar] [CrossRef]
- Pasternak, C. A novel role of Ca2+ and Zn2+: Protection of cells against membrane damage. Biosci. Rep. 1988, 8, 579–583. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, D.; Wang, Q.L. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef]
- Chintakovid, W.; Visoottiviseth, P.; Khokiattiwong, S.; Lauengsuchonkul, S. Potential of the hybrid marigolds for arsenic phytoremediation and income generation of remediators in RonPhibun District, Thailand. Chemosphere 2008, 70, 1532–1537. [Google Scholar] [CrossRef]


| Code | Variety | Code | Variety | Code | Variety |
|---|---|---|---|---|---|
| ZS-zc | Zhongshanzichen | QJ-bh1 | Qingjianbaihuang1 | JL-yg | Jinlingyuegui |
| L-xf | Lüxuanfeng | QJ-qn | Qingjianqingniao | ZS-hy | Zhongshanhongyun |
| QX-yz | Qianxiuyinzhen | JJ-hl | Jinjihongling | ZS-gh | Zhongshanguanghui |
| Marigold | Marigold | QJ-tp | Qingjiantaiping | JL-dc | Jinlingdianchun |
| QL-xh | Qunlongxihai | JS-hj | Jinsihuangju | JL-cj | Jinlingcuiju |
| QH-fmd | Qinhuaifenmudan | JS-bx | Jinsibaoxiao | JL-xy | Jinlingxiaoye |
| QJ-hg | Qingjianhuanggai | QJ-bh2 | Qingjianbaihuang2 | QJ-ht | Qingjianhuangtianzan |
| JH-hj | Jiuhuahongjing | QJ-dh | Qingjiandahuang |
| Preparation | Application | |
|---|---|---|
| Control (CK) | Purified water | 1000 mL |
| Treatment 1 | Diluted 15 mL of bamboo vinegar with purified water to 4500 mL | 1500 mL |
| Treatment 2 | Diluted 15 mL of bamboo vinegar with purified water to 3000 mL | 1000 mL |
| Treatment 3 | Dissolved 15 mg EDTA-Si in purified water and diluted it to 3000 mL | 1000 mL |
| Treatment 4 | Dissolved 15 mL of bamboo vinegar and 15 mg EDTA-Si in purified water and diluted it to 6000 mL | 2000 mL |
| Chrysanthemum Varieties | Flower Count (hm−2 year−1) | Net Income (RMB hm−2 year−1) |
|---|---|---|
| QX-yz | 3.96 × 106 ± 0.42 × 106 | 61.67 × 104 ± 9.26 × 104 |
| QJ-hg | 3.89 × 106 ± 0.72 × 106 | 60.46 × 104 ± 8.38 × 104 |
| QJ-bh2 | 4.02 × 106 ± 0.86 × 106 | 62.69 × 104 ± 11.34 × 104 |
| JL-xy | 3.69 × 106 ± 0.41 × 106 | 57.03 × 104 ± 7.87 × 104 |
| JL-yg | 4.16 × 106 ± 0.58 × 106 | 65.09 × 104 ± 10.43 × 104 |
| Average | 5.31 × 106 ± 1.00 × 106 | 60.46 × 104 ± 8.63 × 104 |
| Marigold | 16.88 × 104~55.70 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Chen, Y.; Song, J.; Bao, J.; Dai, C.; Sun, R.; Liu, J.; Jin, C.; Zhong, N.; Huang, C.; et al. Screening of Profitable Chrysanthemums for the Phytoremediation of Cadmium-Contaminated Soils. Toxics 2025, 13, 360. https://doi.org/10.3390/toxics13050360
Lu X, Chen Y, Song J, Bao J, Dai C, Sun R, Liu J, Jin C, Zhong N, Huang C, et al. Screening of Profitable Chrysanthemums for the Phytoremediation of Cadmium-Contaminated Soils. Toxics. 2025; 13(5):360. https://doi.org/10.3390/toxics13050360
Chicago/Turabian StyleLu, Xinzhe, Yanfang Chen, Jinqiu Song, Jiayu Bao, Chunzheng Dai, Rui Sun, Jiacheng Liu, Chenjiang Jin, Nanchong Zhong, Chunlei Huang, and et al. 2025. "Screening of Profitable Chrysanthemums for the Phytoremediation of Cadmium-Contaminated Soils" Toxics 13, no. 5: 360. https://doi.org/10.3390/toxics13050360
APA StyleLu, X., Chen, Y., Song, J., Bao, J., Dai, C., Sun, R., Liu, J., Jin, C., Zhong, N., Huang, C., & Oh, K. (2025). Screening of Profitable Chrysanthemums for the Phytoremediation of Cadmium-Contaminated Soils. Toxics, 13(5), 360. https://doi.org/10.3390/toxics13050360







