Neurotransmitter Systems Affected by PBDE Exposure: Insights from In Vivo and In Vitro Neurotoxicity Studies
Abstract
:1. Overview of Polybrominated Diphenyl Ethers
2. Methodology
3. Toxicity and Metabolism of PBDEs
| Measured BPDEs | Topic | Population/Exposure | Neurological Finding | Reference |
|---|---|---|---|---|
| Children | ||||
| BDE-47, -99, -100, and -153 | Autism spectrum disorder | 154 (36 months of age), Philadelphia, PA; Baltimore, MD San Francisco Bay Area, CA; and Sacramento, CA, USA—Pregnancy at 2rd trimester or the 3rd trimester. | BDE-47 (5.9–19.2 ng/g in umbilical cord) was associated with greater deficits in social reciprocity (β = 6.39, 95% CI: 1.12, 11.65). | [39] |
| BDE-28, -47, -99, -100, -153, -154, and -183 | Executive functions in adolescents | 115, (12–18-year-old, 53 male and 62 female), Green Bay, Wisconsin area, USA—Two weeks after the neuropsychological assessment. | BDE-47 and BDE-153 (median serum total PBDE 29.14 ng/g of serum lipid) were associated with poorer cognitive flexibility | [40] |
| BDE-47, -99, -100, and -153 | Cognitive and psychomotor development | 355 (6–8 years of age), Quebec, Canada—Early pregnancy (12 weeks of gestation) and at delivery. | Decrease in spatial perception and reasoning was associated with higher BDE-100 (0.019 +/− 0.052 in blood) concentration at delivery. | [41] |
| BDE-47 | Placental epigenetic and neurodevelopment | 260 pregnant women with 10 to 13.14 weeks, from 12 clinic sites within the USA—First trimester of pregnancy. | BDE-47 (3.60, 16.67 ng/g lipid) chance-methylated CpG sites in pathways related to brain size and brain morphology and with birth weight (r = −0.16, p value = 0.01) and head circumference (r = −0.16, p value = 0.01). | [42] |
| BDE-17, -28, -47, -66, -85, -99, -100, -153, -154, -183, and -209 | Intrinsic functional network organization | 34, (5-year-old children), New York City—First half of pregnancy (12.2 weeks gestation, SD = 2.8 weeks). | PBDE serum concentrations correlated with higher global efficiency of brain areas involved in visual attention (VA); VA was associated with more executive functioning problems (β’s = 0.01, FDR-corrected p’s < 0.05). | [43] |
| BDE-28, -47, -99, -100, -153, -154, and -183 | Preschool maturity | 91, (6-year-olds), Eastern Slovakia—6-years old. | Negative associations of BDE-153 (p = 0.002, b = −29.8) and WPPSI-III composite score. Adverse effects on preschool maturity and neuropsychological development. | [44] |
| BDE-15, -17, -25, -28, -33, -47, -99, -100, and -153 | Frustration in infancy | 333, (6 to 7 months), Canada—First trimester of pregnancy. | Predisposition to frustration and lack of habituation and BDE-47 (7.32–727.3 ng/g lipid) was associated with negative vocalizations (adjusted Relative Risk [aRR] = 1.04, 95% CI: 1.00, 1.09). | [45] |
| BDE-47, -99, -100, -153, -154, and HBCD | Development at adolescence | 101, (55 boys and 46 girl), Western European—Second and/or third trimester of pregnancy. | BDE-154 was negatively associated with verbal memory recall (−0.303 p = 0.07) and delayed recognition (−0.348 p = 0.041) impairment. BDE-153 was negatively associated with auditory attention (−0.379 p = 0.03). | [46] |
| BDE-17, -28, -47, -66, -85, -99, -100, -153, -183, and -209 | Visual spatial abilities | 199 (8 years), Cincinnati area (OH, USA)—16 ± 3 weeks gestation, 1 year, 2 years, 3 years, 5 years, and 8 years. | Impairments in visual spatial learning with early childhood BDE-153. | [47] |
| BDE-17, -28, -47, -66, -85, -99, -100, -153, -154, and -183 | Intelligence Quotient and externalizing behavior problems | 239, (8 years old), Cincinnati, OH, USA—16 ± 3 weeks of gestation | Associated with the score for externalizing behavior problems (β: 3.5, 95% CI: −0.1, 7.2) at age 8 years. | [48] |
| BDE-28, -47, -99, -100, -153, -154, -183 and -209 | Neurodevelopment | 246 (6-year-old child), Brittany region, France—On partum. | Verbal comprehension scores were lower in children from homes with higher concentrations of BDE-99 or -209. | [49] |
| BDE-47, -85, -99, -100, -153, -154, and -183 | Early childhood attention problems | 210, (3 and 7 years), downtown New York City—At the time of delivery. | Attention problems were associated with BDE-47 (1.21, 95% CI: 1.00, 1.47) and BDE-153 (1.18, 95% CI: 1.00, 1.39) at age 4 years. | [50] |
| BDE-47, -99, -100, and -153 | Attention and executive function | 301, (9 to 12 years old), Salinas Valley CA, USA—~26 weeks gestation (M = 26.7, SD = 2.6 weeks gestation) or upon delivery. | Poorer response consistency on the Conners’ Continuous Performance Test II (β = 2.9; 95% CI: 0.9, 4.8) and poorer working memory on the Behavioral Rating Inventory of Executive Function (β = 2.5; 95% CI: 0.5, 4.4). | [51] |
| BDE-47, -99, -100, and -153 | Neurodevelopmental measure (motor, language, adaptive, and social domains) | 132, and 149 (12 and 24 months), Shandong province, northern China—At partum. | BDE-99 levels were associated with a 2.16-point decrease [95% confidence interval (CI): −4.52, −0.20] in language domain DQs. BDE-47 levels were associated with a 1.89-point decrease (95% CI: −3.75, −0.03) in social domain developmental quotients at 24 months of age | [52] |
| BDE-17, -28, -47, -66, -85, -99, -100, -153, -154, and -183 | Cognitive abilities and hyperactivity behaviors | 309, (1, 2, 3, 4, and 5 years of age), Cincinnati, OH, USA—16 weeks of gestation. | Prenatal BDE-47 decrease of 4.5-points in Full-Scale IQ (95% CI: −8.8, −0.1) and a 3.3-point increase (95% CI: 0.3, 6.3) in the hyperactivity score at age 5 years. | [53] |
| BDE-17, -28, -47, -66, -85, -99, -100, -153, -154, and -183 | Children’s attention, motor functioning, and cognition | 551, (5 and 7 years), Salinas Valley, CA, USA—Pregnancy (mean = 26.7 ± 2.6 weeks gestation, n = 219) or at delivery (n = 60), and from children at the 7-year visit (n = 272). | PBDE concentrations were associated with impaired attention as measured at 5 and 7 years of age, with poorer fine motor coordination, and with decrements in Verbal and Full-Scale IQ at 7 years. | [54] |
| BDE-47, -99, -100, 153, -154, -183, and -209 | Mental and psychomotor development | 290, (12–18 months of age), Gipuzkoa, Basque Country; and Sabadell, Catalonia—In the first 48–96 h postpartum. | BDE-209 (0.04 to 6.49 ng/g lipid) association with mental development score became slightly weaker (β = −2.10, 95% CI: −4.66, 0.46). | [55] |
| BDE-47, -99, -100, -153, -154, and HBCD | Motor, cognitive, and behavioral outcome | 62, (5–6 years old), northern provinces of the Netherlands—35th week of pregnancy. | Brominated flame retardants correlated with worse fine manipulative abilities, worse attention, better coordination, better visual perception, and better behavior. | [56] |
| Adults | ||||
| DE-47 | Post-partum depression (PPD) | 367 asymptomatic pregnant women (29- to 33-year-olds), Southern California, USA—First trimester. | Exposure in the first trimester increase the PPD by 22% (OR = 1.22, 95% CI: 1.03, 1.47). | [57] |
| BDE-28, -47, -66, -85, -99, -100, -138, -153, and -154 | Neuropsychological effects and synergy effects with PCBs | 144 (67 men and 77 women of 55–74 years of age), New York, USA. | PBDEs (∑PBDEs 4.72 to 1590 ng/g) and PCBs may interact to affect verbal learning and memory. | [58] |
4. Neuronal Oxidative Stress by PBDE Exposure
4.1. In Vivo PBDE Exposure and Oxidative Injuries
4.2. In Vitro PBDE Exposure and Oxidative Injuries
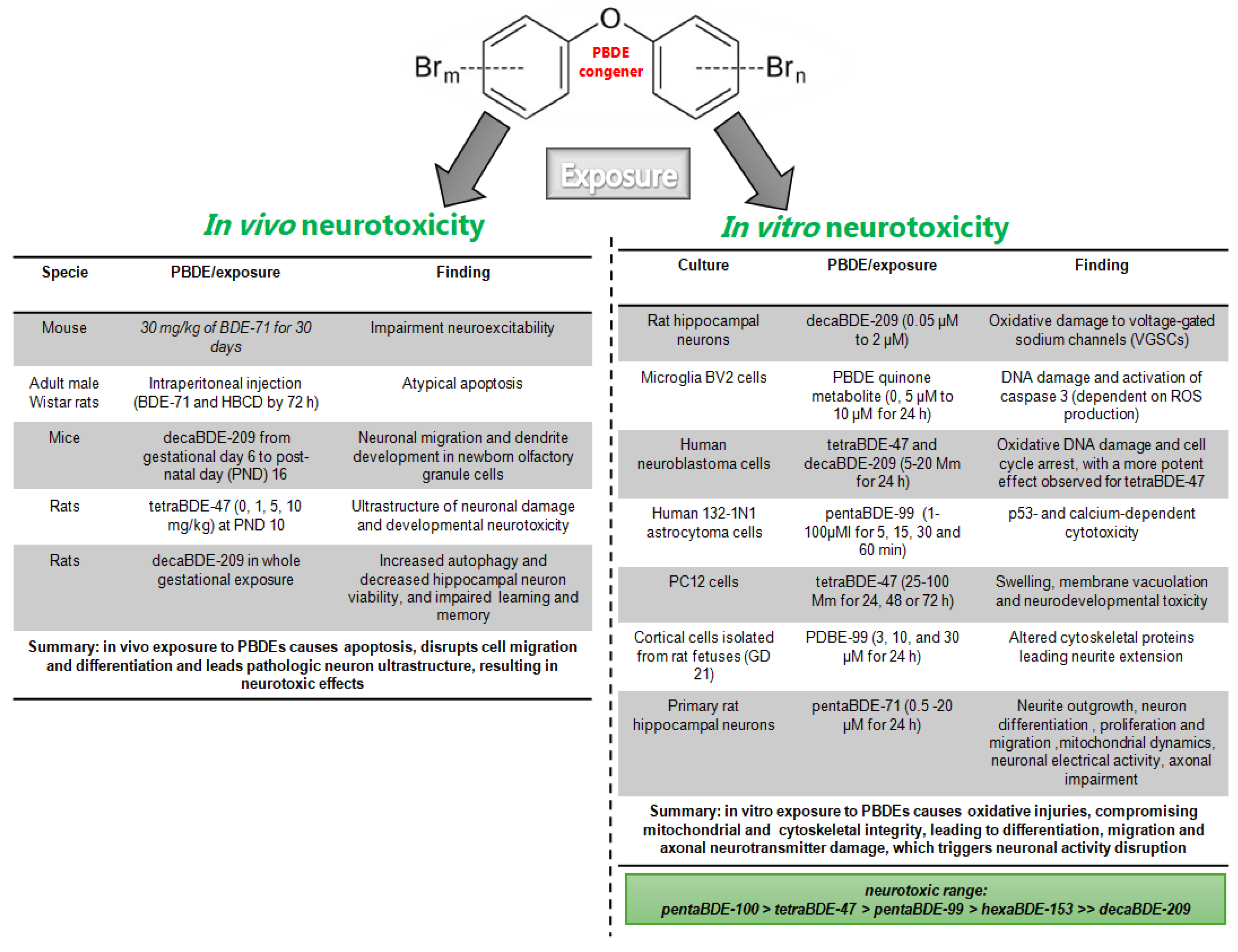
5. Neurotoxicity from PBDE Exposure
5.1. In Vivo PBDE Exposure and Neurotoxicity
5.2. In Vitro PBDE Exposure and Neurotoxicity
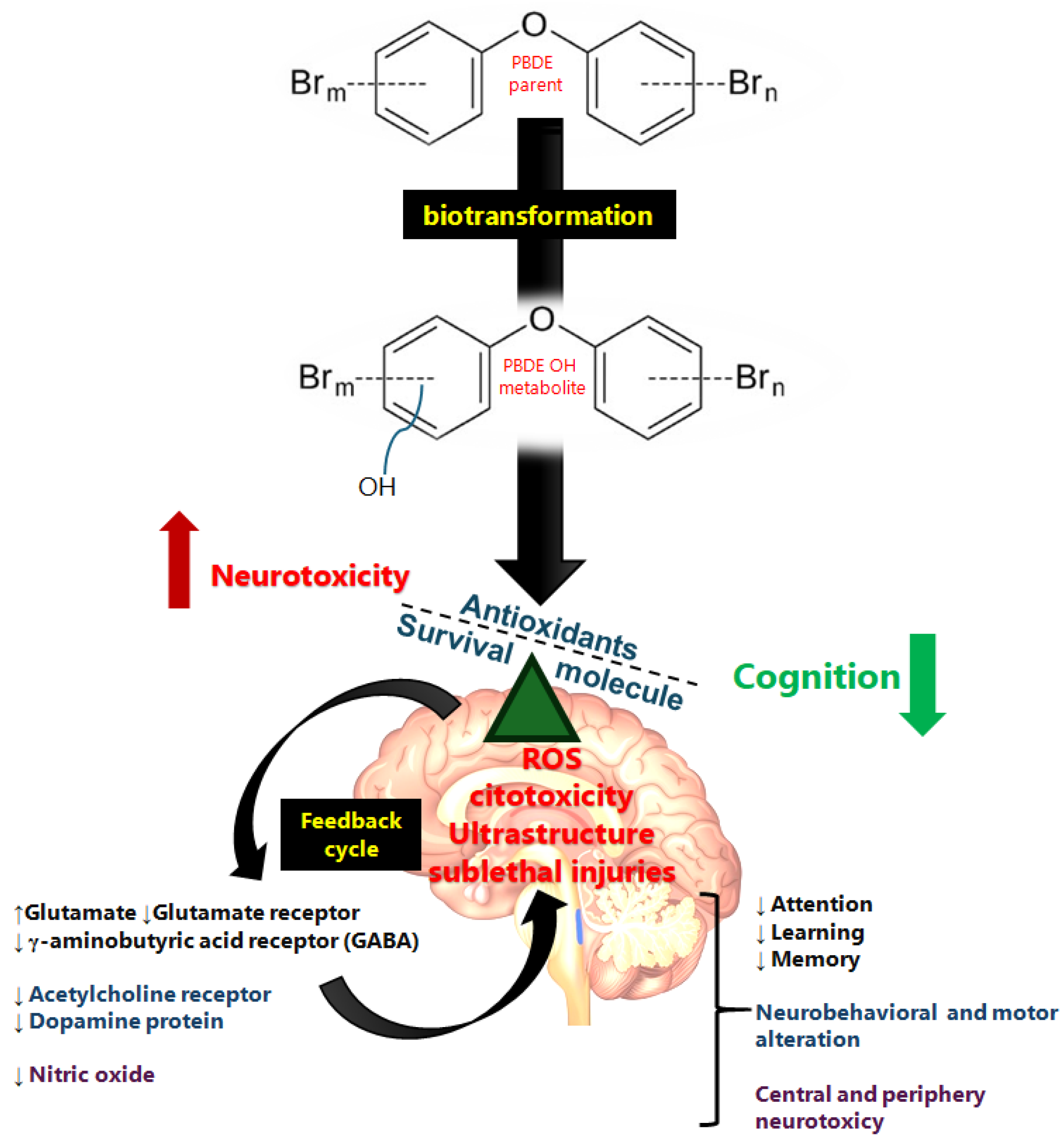
5.3. Pro-Survival Pathway Activated by PBDE Exposure
6. Brain Epigenetic Effects Associated with PBDEs in Brain Injuries
7. Neurological Effects of PBDEs
8. Neurotransmitters Altered by Exposure to PBDEs
8.1. Glutamatergic Impairment by Exposure to BPDEs
8.2. GABAergic Impairment by BPDE Exposure
8.3. Acetylcholine Impairment by Exposure to PBDEs
8.4. Dopaminergic Impairment by PBDE Exposure
8.5. Nitric Oxide Altered by Exposure to PBDEs
9. Conclusions Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PBDEs | Polybrominated diphenyl ethers |
| BDE | Brominated diphenyl ethers |
| Br | Bromine |
| BDE-17 | 2,2′,4-Tribromodiphenyl ether |
| BDE-28 | 2,4,4′-Tribromodiphenyl ether |
| BDE-47 | 2,2′,4,4′-Tetrabromodiphenyl ether |
| BDE-66 | 2,3′,4,4′-Tetrabromodiphenyl ether |
| BDE-77 | 3,3′,4,4′-Tetrabromodiphenyl ether |
| BDE-85 | 2,2′,3,4,4′-Pentabromodiphenyl ether |
| BDE-99 | 2,2′4,4′,5-Pentabromodiphenyl ether |
| BDE-100 | 2,2′,4,4′,6-Pentabromodiphenyl ether |
| BDE-138 | 2,2′,3,4,4′,5′-Hexabromodiphenyl ether |
| BDE-153 | 2,2′,4,4′,5,5′-Hexabromodiphenyl ether |
| HBCD | 1,2,5,6,9,10-Hexabromocyclododecane |
| BDE-154 | 2,2′,4,4′,5,6′-Hexabromodiphenyl ether |
| BDE-183 | 2,2′,3,4,4′,5′,6-Heptabromodiphenyl ether |
| BDE-209 | 2,2′,3,3′,4,4′,5,5′,6,6′-Decabromodiphenyl ether |
References
- Hooper, K.; McDonald, T.A. The PBDEs: An emerging environmental challenge and another reason for breast-milk monitoring programs. Environ. Health Perspect. 2000, 108, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 2007, 28, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O.; Eriksen, G.S.; Jóhannesson, T.; Larsen, P.B.; Viluksela, M. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001, 109 (Suppl. 1), 49–68. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polybrominated Biphenyl and Polybrominated Diphenyl Ethers; US Department of Health and Human Services: Atlanta, GA, USA, 2017; pp. 1–599.
- WHO. International Programme on Chemical Safety—Environmental Health Criteria 162: Brominated Biphenyl Ethers; WHO: Geneva, Switzerland, 1994; p. 162. [Google Scholar]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Decabromodiphenyl Oxide (CAS No. 1163-19-5) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1986, 309, 1–242. [Google Scholar]
- Stapleton, H.M.; Dodder, N.G. Photodegradation of decabromodiphenyl ether in house dust by natural sunlight. Environ. Toxicol. Chem. 2008, 27, 306–312. [Google Scholar] [CrossRef]
- Gerecke, A.C.; Hartmann, P.C.; Heeb, N.V.; Kohler, H.P.; Giger, W.; Schmid, P.; Zennegg, M.; Kohler, M. Anaerobic degradation of decabromodiphenyl ether. Environ. Sci. Technol. 2005, 39, 1078–1083. [Google Scholar] [CrossRef]
- U.S. EPA. An Exposure Assessment of Polybrominated Biphenyl Ethers (PBDE) (Final); EPA/600/R-08/086F; US Environmental Protection Agency: Washington, DC, USA, 2010.
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Polybrominated diphenyl ethers in the environmental systems: A review. J. Environ. Health Sci. Eng. 2021, 19, 1229–1247. [Google Scholar] [CrossRef]
- Trudel, D.; Scheringer, M.; von Goetz, N.; Hungerbühler, K. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ. Sci. Technol. 2011, 45, 2391–2397. [Google Scholar] [CrossRef]
- de Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Watanabe, I.; Sakai, S. Environmental release and behavior of brominated flame retardants. Environ. Int. 2003, 29, 665–682. [Google Scholar] [CrossRef]
- Sjödin, A.; Mueller, J.F.; Jones, R.; Schütze, A.; Wong, L.Y.; Caudill, S.P.; Harden, F.A.; Webster, T.F.; Toms, L.M. Serum elimination half-lives adjusted for ongoing exposure of tri-to hexabrominated diphenyl ethers: Determined in persons moving from North America to Australia. Chemosphere 2020, 248, 125905. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Lebetkin, E.H.; Sanders, J.M.; Burka, L.T. Metabolism and disposition of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica 2006, 36, 515–534. [Google Scholar] [CrossRef]
- Abbasi, G.; Buser, A.M.; Soehl, A.; Murray, M.W.; Diamond, M.L. Stocks and flows of PBDEs in products from use to waste in the U.S. and Canada from 1970 to 2020. Environ. Sci. Technol. 2015, 49, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, G.; Li, L.; Breivik, K. Global Historical Stocks and Emissions of PBDEs. Environ. Sci. Technol. 2019, 53, 6330–6340. [Google Scholar] [CrossRef]
- Domingo, J.L.; Bocio, A.; Falcó, G.; Llobett, J.M. Exposure to PBDEs and PCDEs associated with the consumption of edible marine species. Environ. Sci. Technol. 2006, 40, 4394–4399. [Google Scholar] [CrossRef] [PubMed]
- Turyk, M.E.; Persky, V.W.; Imm, P.; Knobeloch, L.; Chatterton, R.; Anderson, H.A. Hormone disruption by PBDEs in adult male sport fish consumers. Environ. Health Perspect. 2008, 116, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Klinčić, D.; Dvoršćak, M.; Jagić, K.; Mendaš, G.; Herceg Romanić, S. Levels and distribution of polybrominated diphenyl ethers in humans and environmental compartments: A comprehensive review of the last five years of research. Environ. Sci. Pollut. Res. Int. 2020, 27, 5744–5758. [Google Scholar] [CrossRef]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health 2009, 212, 109–134. [Google Scholar] [CrossRef]
- Wu, Z.; He, C.; Han, W.; Song, J.; Li, H.; Zhang, Y.; Jing, X.; Wu, W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res. 2020, 187, 109531. [Google Scholar] [CrossRef]
- Bi, X.; Thomas, G.O.; Jones, K.C.; Qu, W.; Sheng, G.; Martin, F.L.; Fu, J. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ. Sci. Technol. 2007, 41, 5647–5653. [Google Scholar] [CrossRef]
- Qu, W.; Bi, X.; Sheng, G.; Lu, S.; Fu, J.; Yuan, J.; Li, L. Exposure to polybrominated diphenyl ethers among workers at an electronic waste dismantling region in Guangdong, China. Environ. Int. 2007, 33, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Czerska, M.; Zieliński, M.; Kamińska, J.; Ligocka, D. Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int. J. Occup. Med. Environ. Health 2013, 26, 498–510. [Google Scholar] [CrossRef]
- Jin, Y.T.; Deng, X.K.; Zhao, Y.Y.; Li, J.L.; Song, Q.; Zhang, Y.H.; Yang, Q.; Chen, S.Q. Concentrations of Polybrominated Diphenyl Ethers in Maternal Blood, Placental Size, and Risk for Fetal Growth Restriction: A Nested Case-control Study. Biomed. Environ. Sci. 2020, 33, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Ji, H.; Miao, M.; Liang, H.; Wang, Z.; Chen, Y.; Chen, A.; Cao, W.; Yuan, W. Association between prenatal exposure to polybrominated diphenyl ethers and anogenital distance in girls at ages 0-4 years. Int. J. Hyg. Environ. Health 2021, 233, 113706. [Google Scholar] [CrossRef]
- Azar, N.; Booij, L.; Muckle, G.; Arbuckle, T.E.; Séguin, J.R.; Asztalos, E.; Fraser, W.D.; Lanphear, B.P.; Bouchard, M.F. Prenatal exposure to polybrominated diphenyl ethers (PBDEs) and cognitive ability in early childhood. Environ. Int. 2021, 146, 106296. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, M.M.; van den Berg, M.; Westerink, R.H. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ. Health Perspect. 2011, 119, 900–907. [Google Scholar] [CrossRef]
- Sanders, J.M.; Lebetkin, E.H.; Chen, L.J.; Burka, L.T. Disposition of 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE153) and its interaction with other polybrominated diphenyl ethers (PBDEs) in rodents. Xenobiotica 2006, 36, 824–837. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Kelly, S.M.; Pei, R.; Letcher, R.J.; Gunsch, C. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ. Health Perspect. 2009, 117, 197–202. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Peng, L.; Fang, C.; Qin, Q.; Lv, X.; Liu, Z.; Yang, B.; Song, E.; Song, Y. Polybrominated diphenyl ethers quinone-induced intracellular protein oxidative damage triggers ubiquitin-proteasome and autophagy-lysosomal system activation in LO2 cells. Chemosphere 2021, 275, 130034. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Q.; Song, E.; Song, Y. Polybrominated diphenyl ethers quinone exhibits neurotoxicity by inducing DNA damage, cell cycle arrest, apoptosis and p53-driven adaptive response in microglia BV2 cells. Toxicology 2021, 457, 152807. [Google Scholar] [CrossRef]
- Dong, W.; Yang, B.; Wang, Y.; Yuan, J.; Fan, Y.; Song, E.; Song, Y. Polybrominated Diphenyl Ethers Quinone Induced Parthanatos-like Cell Death through a Reactive Oxygen Species-Associated Poly(ADP-ribose) Polymerase 1 Signaling. Chem. Res. Toxicol. 2018, 31, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, H.; Yin, H.; Liu, X.; Peng, H.; Lu, G.; Dang, Z.; He, C. Effect of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) and its metabolites on cell viability, oxidative stress, and apoptosis of HepG2. Chemosphere 2018, 193, 978–988. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Li, S.; Zhong, Y.; Wang, Y.; Zhen, K.; Zhang, X.; Wu, M.; Yu, Z.; Sheng, G.; Fu, J.; et al. The cytotoxic effects of synthetic 6-hydroxylated and 6-methoxylated polybrominated diphenyl ether 47 (BDE47). Environ. Toxicol. 2011, 26, 591–599. [Google Scholar] [CrossRef]
- Song, R.; Duarte, T.L.; Almeida, G.M.; Farmer, P.B.; Cooke, M.S.; Zhang, W.; Sheng, G.; Fu, J.; Jones, G.D. Cytotoxicity and gene expression profiling of two hydroxylated polybrominated diphenyl ethers in human H295R adrenocortical carcinoma cells. Toxicol. Lett. 2009, 185, 23–31. [Google Scholar] [CrossRef]
- Hartley, K.; MacDougall, M.C.; Terrizzi, B.; Xu, Y.; Cecil, K.M.; Chen, A.; Braun, J.M.; Lanphear, B.P.; Newman, N.C.; Vuong, A.M.; et al. Gestational exposure to polybrominated diphenyl ethers and social skills and problem behaviors in adolescents: The HOME study. Environ. Int. 2022, 159, 107036. [Google Scholar] [CrossRef]
- Song, A.Y.; Kauffman, E.M.; Hamra, G.B.; Dickerson, A.S.; Croen, L.A.; Hertz-Picciotto, I.; Schmidt, R.J.; Newschaffer, C.J.; Fallin, M.D.; Lyall, K.; et al. Associations of prenatal exposure to a mixture of persistent organic pollutants with social traits and cognitive and adaptive function in early childhood: Findings from the EARLI study. Environ. Res. 2023, 229, 115978. [Google Scholar] [CrossRef]
- Sprowles, J.L.N.; Monaikul, S.; Aguiar, A.; Gardiner, J.; Monaikul, N.; Kostyniak, P.; Schantz, S.L. Associations of concurrent PCB and PBDE serum concentrations with executive functioning in adolescents. Neurotoxicol. Teratol. 2022, 92, 107092. [Google Scholar] [CrossRef]
- Solazzo, G.; Wu, H.; Laue, H.E.; Brennan, K.; Knox, J.M.; Gillet, V.; Bovin, A.; Abdelouahab, N.; Posner, J.; Raffanello, E.; et al. The association between prenatal concentrations of polybrominated diphenyl ether and child cognitive and psychomotor function. Environ. Epidemiol. 2021, 5, e156. [Google Scholar] [CrossRef]
- Ouidir, M.; Mendola, P.; Buck Louis, G.M.; Kannan, K.; Zhang, C.; Tekola-Ayele, F. Concentrations of persistent organic pollutants in maternal plasma and epigenome-wide placental DNA methylation. Clin. Epigenet. 2020, 12, 103. [Google Scholar] [CrossRef]
- de Water, E.; Curtin, P.; Zilverstand, A.; Sjödin, A.; Bonilla, A.; Herbstman, J.B.; Ramirez, J.; Margolis, A.E.; Bansal, R.; Whyatt, R.M.; et al. A preliminary study on prenatal polybrominated diphenyl ether serum concentrations and intrinsic functional network organization and executive functioning in childhood. J. Child. Psychol. Psychiatry 2019, 60, 1010–1020. [Google Scholar] [CrossRef]
- Drobná, B.; Fabišiková, A.; Čonka, K.; Gago, F.; Oravcová, P.; Wimmerová, S.; Oktapodas Feiler, M.; Šovčíková, E. PBDE serum concentration and preschool maturity of children from Slovakia. Chemosphere 2019, 233, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; Tremblay, É.; Arbuckle, T.E.; Fraser, W.D.; Lemelin, J.P.; Séguin, J.R.; Ouellet, E.; Forget-Dubois, N.; Ayotte, P.; Boivin, M.; et al. Prenatal exposure to polybrominated diphenyl ethers and predisposition to frustration at 7 months: Results from the MIREC study. Environ. Int. 2018, 119, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, S.A.; Van Braeckel, K.N.J.A.; Sauer, P.J.J.; Bos, A.F. Prenatal exposure to persistent organic pollutants and cognition and motor performance in adolescence. Environ. Int. 2018, 121, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Vuong, A.M.; Braun, J.M.; Yolton, K.; Xie, C.; Webster, G.M.; Sjödin, A.; Dietrich, K.N.; Lanphear, B.P.; Chen, A. Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environ. Res. 2017, 153, 83–92. [Google Scholar] [CrossRef]
- Zhang, H.; Yolton, K.; Webster, G.M.; Sjödin, A.; Calafat, A.M.; Dietrich, K.N.; Xu, Y.; Xie, C.; Braun, J.M.; Lanphear, B.P.; et al. Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Environ. Health Perspect. 2017, 125, 746–752. [Google Scholar] [CrossRef]
- Chevrier, C.; Warembourg, C.; Le Maner-Idrissi, G.; Lacroix, A.; Dardier, V.; Le Sourn-Bissaoui, S.; Rouget, F.; Monfort, C.; Gaudreau, E.; Mercier, F.; et al. Childhood exposure to polybrominated diphenyl ethers and neurodevelopment at six years of age. Neurotoxicology 2016, 54, 81–88. [Google Scholar] [CrossRef]
- Cowell, W.J.; Lederman, S.A.; Sjödin, A.; Jones, R.; Wang, S.; Perera, F.P.; Wang, R.; Rauh, V.A.; Herbstman, J.B. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3–7 years. Neurotoxicol. Teratol. 2015, 52, 143–150. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Kogut, K.; Gaspar, F.W.; Gunier, R.B.; Harley, K.G.; Parra, K.; Villaseñor, D.; Bradman, A.; Holland, N.; Eskenazi, B. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicol. Teratol. 2015, 52, 151–161. [Google Scholar] [CrossRef]
- Ding, G.; Yu, J.; Cui, C.; Chen, L.; Gao, Y.; Wang, C.; Zhou, Y.; Tian, Y. Association between prenatal exposure to polybrominated diphenyl ethers and young children’s neurodevelopment in China. Environ. Res. 2015, 142, 104–111. [Google Scholar] [CrossRef]
- Chen, A.; Yolton, K.; Rauch, S.A.; Webster, G.M.; Hornung, R.; Sjödin, A.; Dietrich, K.N.; Lanphear, B.P. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: The HOME study. Environ. Health Perspect. 2014, 122, 856–862. [Google Scholar] [CrossRef]
- Eskenazi, B.; Chevrier, J.; Rauch, S.A.; Kogut, K.; Harley, K.G.; Johnson, C.; Trujillo, C.; Sjödin, A.; Bradman, A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ. Health Perspect. 2013, 121, 257–262. [Google Scholar] [CrossRef]
- Gascon, M.; Fort, M.; Martínez, D.; Carsin, A.E.; Forns, J.; Grimalt, J.O.; Santa Marina, L.; Lertxundi, N.; Sunyer, J.; Vrijheid, M. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ. Health Perspect. 2012, 120, 1760–1765. [Google Scholar] [CrossRef]
- Roze, E.; Meijer, L.; Bakker, A.; Van Braeckel, K.N.; Sauer, P.J.; Bos, A.F. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 2009, 117, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Peltier, M.R.; Fassett, M.J.; Arita, Y.; Chiu, V.Y.; Takhar, H.S.; Getahun, D. Exposure to polybrominated diphenyl ether-47 increases the risk of post-partum depression. J. Matern. Fetal Neonatal Med. 2022, 35, 8350–8354. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.F.; Shrestha, S.; Gomez, M.I.; McCaffrey, R.J.; Zimmerman, E.A.; Kannan, K.; Hwang, S.A. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and neuropsychological status among older adults in New York. Neurotoxicology 2012, 33, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Pellacani, C.; Dao, K.; Kavanagh, T.J.; Roque, P.J. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. Neurotoxicology 2015, 48, 68–76. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Xue, J.; Xiao, Q.; Zhang, M.; Li, D.; Wang, X. Toxic Effects and Mechanisms of Polybrominated Diphenyl Ethers. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Zhong, Y.F.; Wang, L.L.; Yin, L.L.; An, J.; Hou, M.L.; Zheng, K.W.; Zhang, X.Y.; Wu, M.H.; Yu, Z.Q.; Sheng, G.Y.; et al. Cytotoxic effects and oxidative stress response of six PBDE metabolites on human L02 cells. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2011, 46, 1320–1327. [Google Scholar] [CrossRef]
- Costa, L.G.; de Laat, R.; Tagliaferri, S.; Pellacani, C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett. 2014, 230, 282–294. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, X.H.; Zou, L.W.; Ding, S.S.; Zhai, J.X. Oxidative stress of decabromodiphenylether in mice brain tissue. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2010, 28, 900–903. [Google Scholar] [PubMed]
- Bellés, M.; Alonso, V.; Linares, V.; Albina, M.L.; Sirvent, J.J.; Domingo, J.L.; Sánchez, D.J. Behavioral effects and oxidative status in brain regions of adult rats exposed to BDE-99. Toxicol. Lett. 2010, 194, 1–7. [Google Scholar] [CrossRef]
- Alonso, V.; Linares, V.; Bellés, M.; Albina, M.L.; Pujol, A.; Domingo, J.L.; Sánchez, D.J. Effects of BDE-99 on hormone homeostasis and biochemical parameters in adult male rats. Food Chem. Toxicol. 2010, 48, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Vagula, M.C.; Kubeldis, N.; Nelatury, C.F. Effects of BDE-85 on the oxidative status and nerve conduction in rodents. Int. J. Toxicol. 2011, 30, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Kavanagh, T.J.; Costa, L.G. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol. Appl. Pharmacol. 2008, 232, 161–168. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Y.; Jiang, Y.; Cheng, J.; Xu, S.; Zhang, J. Cellular metabolomics reveals glutamate and pyrimidine metabolism pathway alterations induced by BDE-47 in human neuroblastoma SK-N-SH cells. Ecotoxicol. Environ. Saf. 2019, 182, 109427. [Google Scholar] [CrossRef]
- Giordano, G.; Kavanagh, T.J.; Costa, L.G. Mouse cerebellar astrocytes protect cerebellar granule neurons against toxicity of the polybrominated diphenyl ether (PBDE) mixture DE-71. Neurotoxicology 2009, 30, 326–329. [Google Scholar] [CrossRef]
- He, P.; He, W.; Wang, A.; Xia, T.; Xu, B.; Zhang, M.; Chen, X. PBDE-47-induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology 2008, 29, 124–129. [Google Scholar] [CrossRef]
- Chen, J.; Liufu, C.; Sun, W.; Sun, X.; Chen, D. Assessment of the neurotoxic mechanisms of decabrominated diphenyl ether (PBDE-209) in primary cultured neonatal rat hippocampal neurons includes alterations in second messenger signaling and oxidative stress. Toxicol. Lett. 2010, 192, 431–439. [Google Scholar] [CrossRef]
- Lin, H.; Dai, R.; Li, J.; Li, Y.; Tang, J.; Zhai, J. The mechanisms of hippocampal neurons exposed to PBDE-209 induce oxidative stress and apoptosis. Wei Sheng Yan Jiu 2016, 45, 977–983. [Google Scholar]
- Raldúa, D.; Padrós, F.; Solé, M.; Eljarrat, E.; Barceló, D.; Riva, M.C.; Barata, C. First evidence of polybrominated diphenyl ether (flame retardants) effects in feral barbel from the Ebro River basin (NE, Spain). Chemosphere 2008, 73, 56–64. [Google Scholar] [CrossRef]
- Pereira, L.C.; de Souza, A.O.; Dorta, D.J. Polybrominated diphenyl ether congener (BDE-100) induces mitochondrial impairment. Basic Clin. Pharmacol. Toxicol. 2013, 112, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hao, Z.; Wang, Y.; Yan, D.; Meng, J.; Ma, H. Melatonin alleviates BDE-209-induced cognitive impairment and hippocampal neuroinflammation by modulating microglia polarization via SIRT1-mediated HMGB1/TLR4/NF-κB pathway. Food Chem. Toxicol. 2023, 172, 113561. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhai, J. Protective effects of n-acetylcysteine against decabromodiphenyl ether-induced brain oxidative injury in mice. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2014, 32, 674–678. [Google Scholar] [PubMed]
- Zhou, Z.; Zhou, B.; Chen, H.; Lu, K.; Wang, Y. Oxidative stress activates the Nrf2-mediated antioxidant response and P38 MAPK pathway: A possible apoptotic mechanism induced by BDE-47 in rainbow trout (Oncorhynchus mykiss) gonadal RTG-2 cells. Environ. Pollut. 2021, 287, 117341. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.; Bulygina, E.; Makhro, A. Glutamate receptors modulate oxidative stress in neuronal cells. A mini-review. Neurotox. Res. 2004, 6, 581–587. [Google Scholar] [CrossRef]
- Costa, L.G.; Tagliaferri, S.; Roqué, P.J.; Pellacani, C. Role of glutamate receptors in tetrabrominated diphenyl ether (BDE-47) neurotoxicity in mouse cerebellar granule neurons. Toxicol. Lett. 2016, 241, 159–166. [Google Scholar] [CrossRef]
- Bradner, J.M.; Suragh, T.A.; Caudle, W.M. Alterations to the circuitry of the frontal cortex following exposure to the polybrominated diphenyl ether mixture, DE-71. Toxicology 2013, 312, 48–55. [Google Scholar] [CrossRef]
- Reistad, T.; Fonnum, F.; Mariussen, E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch. Toxicol. 2006, 80, 785–796. [Google Scholar] [CrossRef]
- Xu, M.; Huang, Y.; Li, K.; Cheng, X.; Li, G.; Liu, M.; Nie, Y.; Geng, S.; Zhao, S. Developmental exposure of decabromodiphenyl ether impairs subventricular zone neurogenesis and morphology of granule cells in mouse olfactory bulb. Arch. Toxicol. 2018, 92, 529–539. [Google Scholar] [CrossRef]
- He, P.; Wang, A.G.; Xia, T.; Gao, P.; Niu, Q.; Guo, L.J.; Chen, X.M. Mechanisms underlying the developmental neurotoxic effect of PBDE-47 and the enhanced toxicity associated with its combination with PCB153 in rats. Neurotoxicology 2009, 30, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Du, L.; Tang, W.; Kuang, L.; Du, P.; Chen, J.; Chen, D. PBDE-209 exposure damages learning and memory ability in rats potentially through increased autophagy and apoptosis in the hippocampus neuron. Environ. Toxicol. Pharmacol. 2017, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Giordano, G.; Costa, L.G. Comparative cytotoxicity and intracellular accumulation of five polybrominated diphenyl ether congeners in mouse cerebellar granule neurons. Toxicol. Sci. 2010, 114, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.R.; Yong, W.; Chen, L.; Tang, M.L.; Wang, M.; Chen, J.T.; Ruan, D.Y. Effects of decabrominated diphenyl ether (PBDE 209) on voltage-gated sodium channels in primary cultured rat hippocampal neurons. Environ. Toxicol. 2010, 25, 400–408. [Google Scholar] [CrossRef]
- Pellacani, C.; Buschini, A.; Galati, S.; Mussi, F.; Franzoni, S.; Costa, L.G. Evaluation of DNA damage induced by 2 polybrominated diphenyl ether flame retardants (BDE-47 and BDE-209) in SK-N-MC cells. Int. J. Toxicol. 2012, 31, 372–379. [Google Scholar] [CrossRef]
- Madia, F.; Giordano, G.; Fattori, V.; Vitalone, A.; Branchi, I.; Capone, F.; Costa, L.G. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol. Lett. 2004, 154, 11–21. [Google Scholar] [CrossRef]
- Liu, D.; Xue, D.; Lu, W.; Yang, Z.; Li, L.; Xia, B.; Wei, J.; Chen, X.; Yang, Y.; Wang, X.; et al. BDE-47 induced PC-12 cell differentiation via TrkA downstream pathways and caused the loss of hippocampal neurons in BALB/c mice. J. Hazard. Mater. 2022, 422, 126850. [Google Scholar] [CrossRef]
- Alm, H.; Scholz, B.; Kultima, K.; Nilsson, A.; Andrén, P.E.; Savitski, M.M.; Bergman, A.; Stigson, M.; Fex-Svenningsen, A.; Dencker, L. In vitro neurotoxicity of PBDE-99: Immediate and concentration-dependent effects on protein expression in cerebral cortex cells. J. Proteome Res. 2010, 9, 1226–1235. [Google Scholar] [CrossRef]
- Alm, H.; Kultima, K.; Scholz, B.; Nilsson, A.; Andrén, P.E.; Fex-Svenningsen, A.; Dencker, L.; Stigson, M. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal protein expression in the neonatal mouse cerebral cortex. Neurotoxicology 2008, 29, 628–637. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Chen, D. Role of brominated diphenly ether-209 in the differentiation of neural stem cells in vitro. Int. J. Dev. Neurosci. 2010, 28, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T.; Gassmann, K.; Götz, C.; Hübenthal, U.; Moors, M.; Krause, G.; Merk, H.F.; Nguyen, N.H.; Scanlan, T.S.; Abel, J.; et al. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: Evidence for endocrine disruption. Environ. Health Perspect. 2010, 118, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, P.; Yang, K.; Liu, L.; Gao, H.; Zhou, G.; Zhao, Q.; Xia, T.; Wang, A.; Zhang, S. Promotion of mitochondrial fusion protects against developmental PBDE-47 neurotoxicity by restoring mitochondrial homeostasis and suppressing excessive apoptosis. Theranostics 2020, 10, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Poston, R.G.; Murphy, L.; Rejepova, A.; Ghaninejad-Esfahani, M.; Segales, J.; Mulligan, K.; Saha, R.N. Certain ortho-hydroxylated brominated ethers are promiscuous kinase inhibitors that impair neuronal signaling and neurodevelopmental processes. J. Biol. Chem. 2020, 295, 6120–6137. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, K.; Schreiber, T.; Dingemans, M.M.; Krause, G.; Roderigo, C.; Giersiefer, S.; Schuwald, J.; Moors, M.; Unfried, K.; Bergman, Å.; et al. BDE-47 and 6-OH-BDE-47 modulate calcium homeostasis in primary fetal human neural progenitor cells via ryanodine receptor-independent mechanisms. Arch. Toxicol. 2014, 88, 1537–1548. [Google Scholar] [CrossRef]
- Fan, C.Y.; Besas, J.; Kodavanti, P.R. Changes in mitogen-activated protein kinase in cerebellar granule neurons by polybrominated diphenyl ethers and polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 2010, 245, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Li, X.; Zhang, Z.; Hou, L.; Wang, Z.; Niu, Q.; Wang, T. Neurotrophins and cholinergic enzyme regulated by calpain-2: New insights into neuronal apoptosis induced by polybrominated diphenyl ether-153. Toxicol. Lett. 2018, 291, 29–38. [Google Scholar] [CrossRef]
- Kafitz, K.W.; Rose, C.R.; Thoenen, H.; Konnerth, A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 1999, 401, 918–921. [Google Scholar] [CrossRef]
- Blanco, J.; Mulero, M.; López, M.; Domingo, J.L.; Sánchez, D.J. BDE-99 deregulates BDNF, Bcl-2 and the mRNA expression of thyroid receptor isoforms in rat cerebellar granular neurons. Toxicology 2011, 290, 305–311. [Google Scholar] [CrossRef]
- Kozlova, E.V.; Valdez, M.C.; Denys, M.E.; Bishay, A.E.; Krum, J.M.; Rabbani, K.M.; Carrillo, V.; Gonzalez, G.M.; Lampel, G.; Tran, J.D.; et al. Persistent autism-relevant behavioral phenotype and social neuropeptide alterations in female mice offspring induced by maternal transfer of PBDE congeners in the commercial mixture DE-71. Arch. Toxicol. 2022, 96, 335–365. [Google Scholar] [CrossRef]
- Dorman, D.C.; Chiu, W.; Hales, B.F.; Hauser, R.; Johnson, K.J.; Mantus, E.; Martel, S.; Robinson, K.A.; Rooney, A.A.; Rudel, R.; et al. Polybrominated diphenyl ether (PBDE) neurotoxicity: A systematic review and meta-analysis of animal evidence. J. Toxicol. Environ. Health Part B Crit. Rev. 2018, 21, 269–289. [Google Scholar] [CrossRef]
- Garduño-Gutiérrez, R.; Rodríguez-Manzo, G.; Velázquez-Alvarado, A.; Miller-Pérez, C.; León-Olea, M. The endocrine disruptor DE-79 alters oxytocinergic transmission and sexual behavior expression in male rats. Toxicol. Appl. Pharmacol. 2023, 479, 116723. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Fouse, S.; Fan, G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr. Res. 2007, 61, 58R–63R. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Kannan, M. Epigenetics in the nervous system: An overview of its essential role. Indian J. Hum. Genet. 2013, 19, 384–391. [Google Scholar] [CrossRef]
- Keverne, E.B.; Pfaff, D.W.; Tabansky, I. Epigenetic changes in the developing brain: Effects on behavior. Proc. Natl. Acad. Sci. USA 2015, 112, 6789–6795. [Google Scholar] [CrossRef]
- Poston, R.G.; Saha, R.N. Epigenetic Effects of Polybrominated Diphenyl Ethers on Human Health. Int. J. Environ. Res. Public Health 2019, 16, 2703. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Dong, X.; Zhou, G.; Sang, Y.; Gao, L.; Zhou, X.; Sun, Z. DNA methylation changes induced by BDE-209 are related to DNA damage response and germ cell development in GC-2spd. J. Environ. Sci. 2021, 109, 161–170. [Google Scholar] [CrossRef]
- Ding, Y.C.; Hurley, S.; Park, J.S.; Steele, L.; Rakoff, M.; Zhu, Y.; Zhao, J.; LaBarge, M.; Bernstein, L.; Chen, S.; et al. Methylation biomarkers of polybrominated diphenyl ethers (PBDEs) and association with breast cancer risk at the time of menopause. Environ. Int. 2021, 156, 106772. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Q.; Ge, W.; Jin, Y.; Chen, S.; Xiao, X.; Zhang, Y. Associations between in utero exposure to polybrominated diphenyl ethers, pathophysiological state of fetal growth and placental DNA methylation changes. Environ. Int. 2019, 133, 105255. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Hong, X.; Wang, X.; Tang, W.Y. Aberrant 5′-CpG Methylation of Cord Blood TNFα Associated with Maternal Exposure to Polybrominated Diphenyl Ethers. PLoS ONE 2015, 10, e0138815. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.M.; Benachour, N.; Zalko, D.; Frisardi, M.C.; Colicino, E.; Takser, L.; Baccarelli, A.A. Epigenetic effects of low perinatal doses of flame retardant BDE-47 on mitochondrial and nuclear genes in rat offspring. Toxicology 2015, 328, 152–159. [Google Scholar] [CrossRef]
- Woods, R.; Vallero, R.O.; Golub, M.S.; Suarez, J.K.; Ta, T.A.; Yasui, D.H.; Chi, L.H.; Kostyniak, P.J.; Pessah, I.N.; Berman, R.F.; et al. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum. Mol. Genet. 2012, 21, 2399–2411. [Google Scholar] [CrossRef]
- Pilsner, J.R.; Shershebnev, A.; Wu, H.; Marcho, C.; Dribnokhodova, O.; Shtratnikova, V.; Sergeyev, O.; Suvorov, A. Aging-induced changes in sperm DNA methylation are modified by low dose of perinatal flame retardants. Epigenomics 2021, 13, 285–297. [Google Scholar] [CrossRef]
- Suvorov, A.; Pilsner, J.R.; Naumov, V.; Shtratnikova, V.; Zheludkevich, A.; Gerasimov, E.; Logacheva, M.; Sergeyev, O. Aging Induces Profound Changes in sncRNA in Rat Sperm and These Changes Are Modified by Perinatal Exposure to Environmental Flame Retardant. Int. J. Mol. Sci. 2020, 21, 8252. [Google Scholar] [CrossRef]
- Poston, R.G.; Dunn, C.J.; Sarkar, P.; Saha, R.N. Persistent 6-OH-BDE-47 exposure impairs functional neuronal maturation and alters expression of neurodevelopmentally-relevant chromatin remodelers. Environ. Epigenet. 2018, 4, dvx020. [Google Scholar] [CrossRef]
- Anzalone, G.; Moscato, M.; Montalbano, A.M.; Albano, G.D.; Gagliardo, R.; Marchese, R.; Fucarino, A.; Nigro, C.L.; Drago, G.; Profita, M. PBDEs affect inflammatory and oncosuppressive mechanisms via the EZH2 methyltransferase in airway epithelial cells. Life Sci. 2021, 282, 119827. [Google Scholar] [CrossRef]
- Vuong, A.M.; Yolton, K.; Dietrich, K.N.; Braun, J.M.; Lanphear, B.P.; Chen, A. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Horm. Behav. 2018, 101, 94–104. [Google Scholar] [CrossRef]
- Steil, G.J.; Buzzo, J.L.A.; de Oliveira Ribeiro, C.A.; Filipak Neto, F. Polybrominated diphenyl ethers BDE-47 and BDE-99 modulate murine melanoma cell phenotype in vitro. Environ. Sci. Pollut. Res. Int. 2022, 29, 11291–11303. [Google Scholar] [CrossRef]
- Alvarez-Gonzalez, M.Y.; Sánchez-Islas, E.; Mucio-Ramirez, S.; de Gortari, P.; Amaya, M.I.; Kodavanti, P.R.S.; León-Olea, M. Perinatal exposure to octabromodiphenyl ether mixture, DE-79, alters the vasopressinergic system in adult rats. Toxicol. Appl. Pharmacol. 2020, 391, 114914. [Google Scholar] [CrossRef]
- Martin, O.V.; Evans, R.M.; Faust, M.; Kortenkamp, A. A Human Mixture Risk Assessment for Neurodevelopmental Toxicity Associated with Polybrominated Diphenyl Ethers Used as Flame Retardants. Environ. Health Perspect. 2017, 125, 087016. [Google Scholar] [CrossRef]
- Eriksson, P.; Jakobsson, E.; Fredriksson, A. Brominated flame retardants: A novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 2001, 109, 903–908. [Google Scholar] [CrossRef]
- Cowell, W.J.; Margolis, A.; Rauh, V.A.; Sjödin, A.; Jones, R.; Wang, Y.; Garcia, W.; Perera, F.; Wang, S.; Herbstman, J.B. Associations between prenatal and childhood PBDE exposure and early adolescent visual, verbal and working memory. Environ. Int. 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Chao, H.R.; Tsou, T.C.; Huang, H.L.; Chang-Chien, G.P. Levels of breast milk PBDEs from southern Taiwan and their potential impact on neurodevelopment. Pediatr. Res. 2011, 70, 596–600. [Google Scholar] [CrossRef]
- Gee, J.R.; Moser, V.C. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol. Teratol. 2008, 30, 79–87. [Google Scholar] [CrossRef]
- Xiong, L.; Liyue, H.; Fancai, Z.; Maoting, L.; Ya, L.; Ting, H.; Zhen, Y.; Shanshan, Z.; Wenwen, G.; Yan, T. Effect of decabrominated diphenyl ether exposure on spatial learning and memory, the expression and phosphorylation of hippocampal glutamate receptor subunits in adult Sprague-Dawley rats. J. Toxicol. Sci. 2018, 43, 645–657. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Wang, W.; Yue, C.; Tang, Y. Neonatal exposure to BDE 209 impaired learning and memory, decreased expression of hippocampal core SNAREs and synaptophysin in adult rats. Neurotoxicology 2017, 59, 40–48. [Google Scholar] [CrossRef]
- Dingemans, M.M.; Ramakers, G.M.; Gardoni, F.; van Kleef, R.G.; Bergman, A.; Di Luca, M.; van den Berg, M.; Westerink, R.H.; Vijverberg, H.P. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ. Health Perspect. 2007, 115, 865–870. [Google Scholar] [CrossRef]
- Llansola, M.; Erceg, S.; Monfort, P.; Montoliu, C.; Felipo, V. Prenatal exposure to polybrominated diphenylether 99 enhances the function of the glutamate-nitric oxide-cGMP pathway in brain in vivo and in cultured neurons. Eur. J. Neurosci. 2007, 25, 373–379. [Google Scholar] [CrossRef]
- Qian, B.; Zen, Z.; Zheng, Z.; Wang, C.; Song, J. A preliminary study on the mechanism of the neurosteroid-mediated ionotropic receptor dysfunction in neurodevelopmental toxicity induced by decabromodiphenyl ether. Ecotoxicol. Environ. Saf. 2021, 217, 112198. [Google Scholar] [CrossRef]
- Bauer, M.; Goetz, T.; Glenn, T.; Whybrow, P.C. The thyroid-brain interaction in thyroid disorders and mood disorders. J. Neuroendocrinol. 2008, 20, 1101–1114. [Google Scholar] [CrossRef]
- Verma, P.; Gupta, R.K.; Gandhi, B.S.; Singh, P. CDRI-08 Attenuates REST/NRSF-Mediated Expression of NMDAR1 Gene in PBDE-209-Exposed Mice Brain. Evid. Based Complement. Altern. Med. 2015, 2015, 403840. [Google Scholar] [CrossRef]
- Yan, T.; Xiang, L.; Xuejun, J.; Chengzhi, C.; Youbin, Q.; Xuelan, Y.; Yang, L.; Changyan, P.; Hui, C. Spatial learning and memory deficit of low level polybrominated diphenyl ethers-47 in male adult rat is modulated by intracellular glutamate receptors. J. Toxicol. Sci. 2012, 37, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.S.; Antunes Fernandes, E.C.; Bergman, A.; van den Berg, M.; Westerink, R.H. PCB-47, PBDE-47, and 6-OH-PBDE-47 differentially modulate human GABAA and alpha4beta2 nicotinic acetylcholine receptors. Toxicol. Sci. 2010, 118, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, H.; Yang, Y.; Xie, D.; Dang, Y.; Xiang, M.; Yu, Y. Neurotoxicity of tetrabromobisphenol-A-bis(2,3-dibromopropyl ether) through the GABAergic and serotonergic neurotransmission in Caenorhabditis elegans. Environ. Pollut. 2024, 357, 124392. [Google Scholar] [CrossRef] [PubMed]
- Buratovic, S.; Viberg, H.; Fredriksson, A.; Eriksson, P. Developmental exposure to the polybrominated diphenyl ether PBDE 209: Neurobehavioural and neuroprotein analysis in adult male and female mice. Environ. Toxicol. Pharmacol. 2014, 38, 570–585. [Google Scholar] [CrossRef]
- Viberg, H.; Fredriksson, A.; Eriksson, P. Changes in spontaneous behaviour and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209). Neurotoxicology 2007, 28, 136–142. [Google Scholar] [CrossRef]
- Viberg, H.; Johansson, N.; Fredriksson, A.; Eriksson, J.; Marsh, G.; Eriksson, P. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol. Sci. 2006, 92, 211–218. [Google Scholar] [CrossRef]
- Viberg, H.; Fredriksson, A.; Eriksson, P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 2003, 192, 95–106. [Google Scholar] [CrossRef]
- Viberg, H.; Eriksson, P. Differences in neonatal neurotoxicity of brominated flame retardants, PBDE 99 and TBBPA, in mice. Toxicology 2011, 289, 59–65. [Google Scholar] [CrossRef]
- Pham-Lake, C.; Aronoff, E.B.; Camp, C.R.; Vester, A.; Peters, S.J.; Caudle, W.M. Impairment in the mesohippocampal dopamine circuit following exposure to the brominated flame retardant, HBCDD. Environ. Toxicol. Pharmacol. 2017, 50, 167–174. [Google Scholar] [CrossRef]
- Bradner, J.M.; Suragh, T.A.; Wilson, W.W.; Lazo, C.R.; Stout, K.A.; Kim, H.M.; Wang, M.Z.; Walker, D.I.; Pennell, K.D.; Richardson, J.R.; et al. Exposure to the polybrominated diphenyl ether mixture DE-71 damages the nigrostriatal dopamine system: Role of dopamine handling in neurotoxicity. Exp. Neurol. 2013, 241, 138–147. [Google Scholar] [CrossRef]
- Genskow, K.R.; Bradner, J.M.; Hossain, M.M.; Richardson, J.R.; Caudle, W.M. Selective damage to dopaminergic transporters following exposure to the brominated flame retardant, HBCDD. Neurotoxicol Teratol. 2015, 52, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, M.M.; Heusinkveld, H.J.; de Groot, A.; Bergman, A.; van den Berg, M.; Westerink, R.H. Hexabromocyclododecane inhibits depolarization-induced increase in intracellular calcium levels and neurotransmitter release in PC12 cells. Toxicol. Sci. 2009, 107, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, R.; de Stein, M.L.; Fin, C.; Izquierdo, I.; Medina, J.H. Role of hippocampal NO in the acquisition and consolidation of inhibitory avoidance learning. Neuroreport 1995, 6, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Harooni, H.E.; Naghdi, N.; Sepehri, H.; Rohani, A.H. The role of hippocampal nitric oxide (NO) on learning and immediate, short- and long-term memory retrieval in inhibitory avoidance task in male adult rats. Behav. Brain Res. 2009, 201, 166–172. [Google Scholar] [CrossRef]
- Okere, C.O.; Kaba, H. Increased expression of neuronal nitric oxide synthase mRNA in the accessory olfactory bulb during the formation of olfactory recognition memory in mice. Eur. J. Neurosci. 2000, 12, 4552–4556. [Google Scholar] [CrossRef]
- Paul, V.; Ekambaram, P. Involvement of nitric oxide in learning & memory processes. Indian J. Med. Res. 2011, 133, 471–478. [Google Scholar]
- Currás-Collazo, M.C. Nitric oxide signaling as a common target of organohalogens and other neuroendocrine disruptors. J. Toxicol. Environ. Health Part B Crit. Rev. 2011, 14, 495–536. [Google Scholar] [CrossRef]
- Mucio-Ramírez, S.; Sánchez-Islas, E.; Sánchez-Jaramillo, E.; Currás-Collazo, M.; Juárez-González, V.R.; Álvarez-González, M.Y.; Orser, L.E.; Hou, B.; Pellicer, F.; Kodavanti, P.R.S.; et al. Perinatal exposure to organohalogen pollutants decreases vasopressin content and its mRNA expression in magnocellular neuroendocrine cells activated by osmotic stress in adult rats. Toxicol. Appl. Pharmacol. 2017, 329, 173–189. [Google Scholar] [CrossRef]
- Sánchez-Islas, E.; León-Olea, M. Nitric oxide synthase inhibition during synaptic maturation decreases synapsin I immunoreactivity in rat brain. Nitric Oxide 2004, 10, 141–149. [Google Scholar] [CrossRef]
- Pitsikas, N. The role of nitric oxide in the object recognition memory. Behav. Brain Res. 2015, 285, 200–207. [Google Scholar] [CrossRef]
- Shen, F.; Wang, X.W.; Ge, F.F.; Li, Y.J.; Cui, C.L. Essential role of the NO signaling pathway in the hippocampal CA1 in morphine-associated memory depends on glutaminergic receptors. Neuropharmacology 2016, 102, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Shou, X.J.; Xu, X.J.; Zeng, X.Z.; Liu, Y.; Yuan, H.S.; Xing, Y.; Jia, M.X.; Wei, Q.Y.; Han, S.P.; Zhang, R.; et al. A Volumetric and Functional Connectivity MRI Study of Brain Arginine-Vasopressin Pathways in Autistic Children. Neurosci. Bull. 2017, 33, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Herbstman, J.B.; Sjödin, A.; Kurzon, M.; Lederman, S.A.; Jones, R.S.; Rauh, V.; Needham, L.L.; Tang, D.; Niedzwiecki, M.; Wang, R.Y.; et al. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 2010, 118, 712–719. [Google Scholar] [CrossRef]
- Gump, B.B.; Yun, S.; Kannan, K. Polybrominated diphenyl ether (PBDE) exposure in children: Possible associations with cardiovascular and psychological functions. Environ. Res. 2014, 132, 244–250. [Google Scholar] [CrossRef]
- Shah, A.; Coburn, C.G.; Watson-Siriboe, A.; Whitley, R.; Shahidzadeh, A.; Gillard, E.R.; Nichol, R.; Leon-Olea, M.; Gaertner, M.; Kodavanti, P.R.; et al. Altered cardiovascular reactivity and osmoregulation during hyperosmotic stress in adult rats developmentally exposed to polybrominated diphenyl ethers (PBDEs). Toxicol. Appl. Pharmacol. 2011, 256, 103–113. [Google Scholar] [CrossRef]
- Hou, Y.; Fu, J.; Sun, S.; Jin, Y.; Wang, X.; Zhang, L. BDE-209 induces autophagy and apoptosis via IRE1α/Akt/mTOR signaling pathway in human umbilical vein endothelial cells. Environ. Pollut. 2019, 253, 429–438. [Google Scholar] [CrossRef]
| Family | Water Solubility | Clearance | Mobilization | Stored | Toxicity |
|---|---|---|---|---|---|
| tetraBDE pentaBDE hexaBDE heptaBDE octaBDE decaBDE |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Suastegui, W.A.; Navarro-Mabarak, C.; Silva-Adaya, D.; Dolores-Raymundo, H.G.; Alvarez-Gonzalez, M.Y.; León-Olea, M.; Ramos-Chávez, L.A. Neurotransmitter Systems Affected by PBDE Exposure: Insights from In Vivo and In Vitro Neurotoxicity Studies. Toxics 2025, 13, 316. https://doi.org/10.3390/toxics13040316
García-Suastegui WA, Navarro-Mabarak C, Silva-Adaya D, Dolores-Raymundo HG, Alvarez-Gonzalez MY, León-Olea M, Ramos-Chávez LA. Neurotransmitter Systems Affected by PBDE Exposure: Insights from In Vivo and In Vitro Neurotoxicity Studies. Toxics. 2025; 13(4):316. https://doi.org/10.3390/toxics13040316
Chicago/Turabian StyleGarcía-Suastegui, Wendy Argelia, Cynthia Navarro-Mabarak, Daniela Silva-Adaya, Heidy Galilea Dolores-Raymundo, Mhar Yovavyn Alvarez-Gonzalez, Martha León-Olea, and Lucio Antonio Ramos-Chávez. 2025. "Neurotransmitter Systems Affected by PBDE Exposure: Insights from In Vivo and In Vitro Neurotoxicity Studies" Toxics 13, no. 4: 316. https://doi.org/10.3390/toxics13040316
APA StyleGarcía-Suastegui, W. A., Navarro-Mabarak, C., Silva-Adaya, D., Dolores-Raymundo, H. G., Alvarez-Gonzalez, M. Y., León-Olea, M., & Ramos-Chávez, L. A. (2025). Neurotransmitter Systems Affected by PBDE Exposure: Insights from In Vivo and In Vitro Neurotoxicity Studies. Toxics, 13(4), 316. https://doi.org/10.3390/toxics13040316







