Chlorella vulgaris Supplementation Attenuates Lead Accumulation, Oxidative Stress, and Memory Impairment in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Study Protocol
2.3. T-Maze
2.4. Lipid Peroxidation
2.5. Antioxidant Activity
2.6. Lead Determination
2.7. Data Analysis
3. Results
3.1. Body Weight
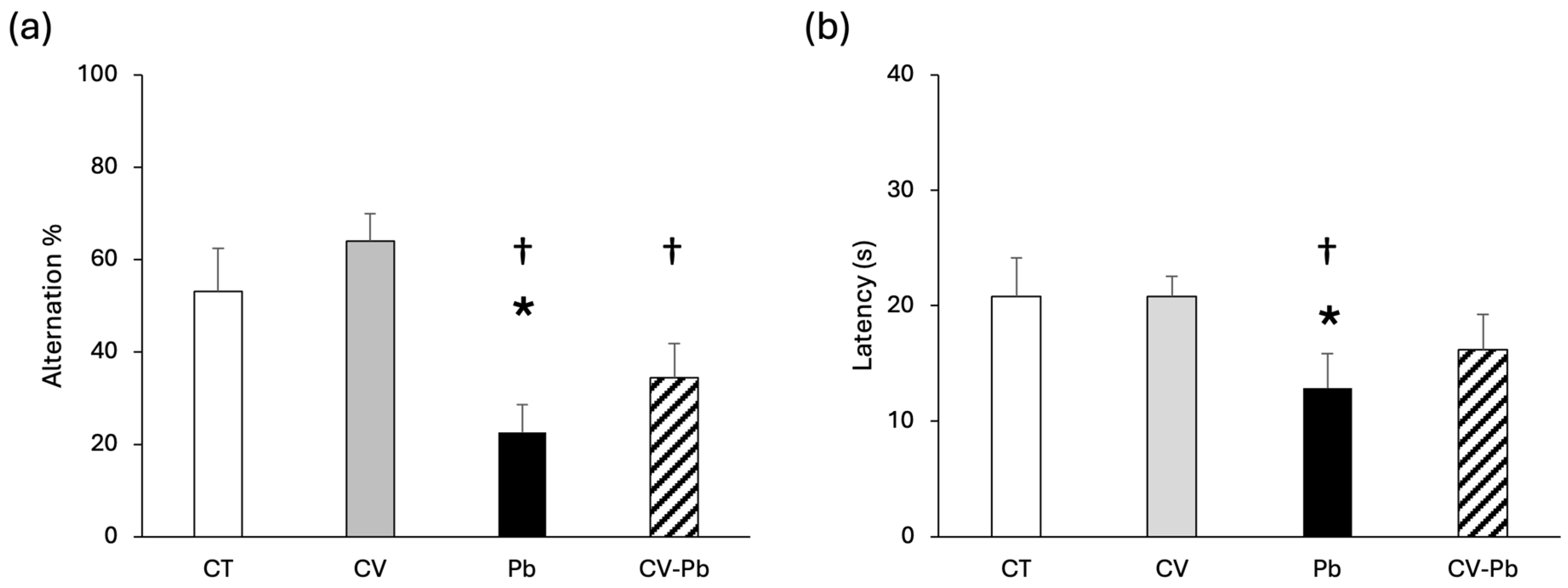
3.2. Effect of Lead Exposure on Memory
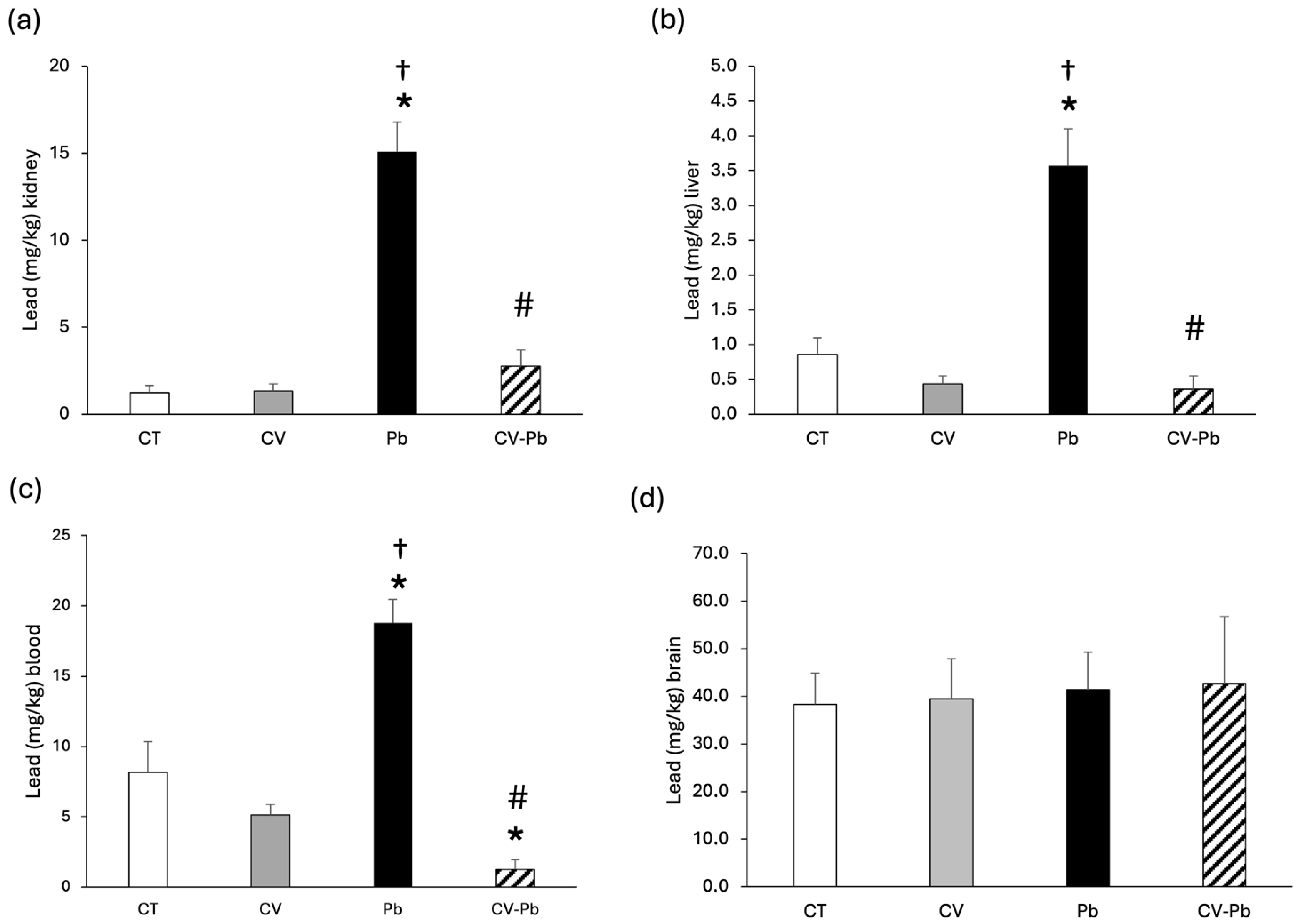
3.3. Lead Concentrations in Blood and Tissue
3.4. Lipid Peroxidation
3.5. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Eizadi-Mood, N.; Hassanian-Moghaddam, H.; Etemad, L.; Moshiri, M.; Vahabzadeh, M.; Sadeghi, M. Recent advances in the clinical management of intoxication by five heavy metals: Mercury, lead, chromium, cadmium and arsenic. Heliyon 2025, 11, e42696. [Google Scholar] [CrossRef]
- Jedličková, A.; Kristeková, D.; Husáková, Z.; Coufalík, P.; Vrlíková, L.; Smutná, T.; Capandová, M.; Alexa, L.; Lusková, D.; Křůmal, K.; et al. Inhaled lead nanoparticles enter the brain through the olfactory pathway and induce neurodegenerative changes resembling tauopathies. ACS Nano 2025, 19, 12799–12826. [Google Scholar] [CrossRef]
- Mitra, P.; Sharma, S.; Purohit, P.; Sharma, P. Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 506–528. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Ortega, D.R.; Esquivel, D.F.G.; Ayala, T.B.; Pineda, B.; Manzo, S.G.; Quino, J.M.; Mora, P.C.; De La Cruz, V.P. Cognitive impairment induced by lead exposure during lifespan: Mechanisms of lead neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Sipos, P.; Szentmihályi, K.; Fehér, E.; Abaza, M.; Szilágyi, M.; Blázovics, A. Some effects of lead contamination on liver and gallbladder bile. Acta Biol. Szeged. 2003, 47, 139–142. [Google Scholar]
- Takeuchi, H.; Taki, Y.; Nouchi, R.; Yokoyama, R.; Kotozaki, Y.; Nakagawa, S.; Sekiguchi, A.; Iizuka, K.; Hanawa, S.; Araki, T.; et al. Lead exposure is associated with functional and microstructural changes in the healthy human brain. Commun. Biol. 2021, 4, 912. [Google Scholar] [CrossRef]
- Saleh, S.R.; Agwah, R.G.; Elblehi, S.S.; Ghareeb, A.Z.; Ghareeb, D.A.; Maher, A.M. Combination of 10-hydroxy-decanoic acid and ZnO nanoparticles abrogates lead acetate-induced nephrotoxicity in rats: Targeting oxidative stress and inflammatory signalling. BMC Pharmacol. Toxicol. 2025, 26, 69. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative stress in health and disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, Q.; Gao, W.; Xiao, Y.; Shi, L.; Lin, F.; Xiong, Y.; Zhang, Y.; Xu, Q.; Wang, L.; et al. Synergistic microglial modulation by laminarin-based platinum nanozymes for potential intracerebral hemorrhage therapy. Biomaterials 2025, 319, 123212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gao, M.; Hu, X.; Wang, P.; Cheng, Y.; Wei, H.; Fu, G.; Ge, J.; Li, H.; Zhang, W.; et al. Biomimetic peroxisome targets myocardial injury and promotes heart repair and regeneration. Biomaterials 2025, 319, 123214. [Google Scholar] [CrossRef]
- Saikiran, G.; Mitra, P.; Sharma, S.; Kumar, P.K.; Sharma, P. Selenium, oxidative stress and inflammatory markers in handicraft workers occupationally exposed to lead. Arch. Environ. Occup. Health 2021, 77, 561–567. [Google Scholar] [CrossRef] [PubMed]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Dobrakowski, M.; Pawlas, N.; Kasperczyk, A.; Kozłowska, A.; Olewińska, E.; Machoń-Grecka, A.; Kasperczyk, S. Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum. Exp. Toxicol. 2017, 36, 744–754. [Google Scholar] [CrossRef]
- Ahamed, M.; Fareed, M.; Kumar, A.; Siddiqui, W.A.; Siddiqui, M.K. Oxidative stress and neurological disorders in relation to blood lead levels in children. Redox Rep. 2008, 13, 117–122. [Google Scholar] [CrossRef]
- Aaseth, J.; Skaug, M.A.; Cao, Y.; Andersen, O. Chelation in metal intoxication—Principles and paradigms. J. Trace Elem. Med. Biol. 2015, 31, 260–266. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Bai, Y.; Ji, B. Advances in responses of microalgal-bacterial symbiosis to emerging pollutants in wastewater. World J. Microbiol. Biotechnol. 2023, 40, 40. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghamarchehreh, M.E.; Beiraghdar, F.; Zare, R.; Jalalian, H.R.; Sahebkar, A. Investigation of the effects of Chlorella vulgaris supplementation in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Hepatogastroenterology 2012, 59, 2099–2103. [Google Scholar] [CrossRef]
- Barghchi, H.; Dehnavi, Z.; Nattagh-Eshtivani, E.; Alwaily, E.R.; Almulla, A.F.; Kareem, A.K.; Barati, M.; Ranjbar, G.; Mohammadzadeh, A.; Rahimi, P.; et al. The effects of Chlorella vulgaris on cardiovascular risk factors: A comprehensive review on putative molecular mechanisms. Biomed. Pharmacother. 2023, 162, 114624. [Google Scholar] [CrossRef] [PubMed]
- Vecina, J.F.; Oliveira, A.G.; Araujo, T.G.; Baggio, S.R.; Torello, C.O.; Saad, M.J.; Queiroz, M.L. Chlorella modulates insulin signaling pathway and prevents high-fat diet-induced insulin resistance in mice. Life Sci. 2014, 95, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Taga, C.; Nishiga, M.; Fujiwara, M.; Konishi, F.; Tanaka, K.; Kamei, C. Effect of docosahexaenoic acid-fortified Chlorella vulgaris strain CK22 on the radial maze performance in aged mice. Biol. Pharm. Bull. 2002, 25, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tu, R.; Wang, Y.; Hu, Y.; Li, X.; Cheng, X.; Yin, Y.; Li, W.; Huang, H. Early-life exposure to lead induces cognitive impairment in elder mice targeting SIRT1 phosphorylation and oxidative alterations. Front. Physiol. 2017, 8, 446. [Google Scholar] [CrossRef]
- Arab, H.H.; Eid, A.H.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.K.; Darwish, H.W.; Sabry, F.M. Targeting autophagy, apoptosis, and oxidative perturbations with dapagliflozin mitigates cadmium-induced cognitive dysfunction in rats. Biomedicines 2023, 11, 3000. [Google Scholar] [CrossRef]
- Cervantes, G.I.V.; Esquivel, D.F.G.; Ortega, D.R.; Ayala, T.B.; Chávez, L.A.R.; López-López, H.E.; Salazar, A.; Flores, I.; Pineda, B.; Gómez-Manzo, S.; et al. Mechanisms associated with cognitive and behavioral impairment induced by arsenic exposure. Cells 2023, 12, 2537. [Google Scholar] [CrossRef]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef]
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef]
- Almeida, H.N.; Calixto, G.Q.; Chagas, B.M.E.; Melo, D.M.A.; Resende, F.M.; Melo, M.A.F.; Braga, R.M. Characterization and pyrolysis of Chlorella vulgaris and Arthrospira platensis: Potential of bio-oil and chemical production by Py-GC/MS analysis. Environ. Sci. Pollut. Res. Int. 2017, 24, 14142–14150. [Google Scholar] [CrossRef]
- Hickman, D.L.; Johnson, S.W. Evaluation of the aesthetics of physical methods of euthanasia of anesthetized rats. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 695–701. [Google Scholar]
- Deacon, R.M.; Rawlins, J.N. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7–12. [Google Scholar] [CrossRef] [PubMed]
- D’Isa, R.; Comi, G.; Leocani, L. Apparatus design and behavioural testing protocol for the evaluation of spatial working memory in mice through the spontaneous alternation T-maze. Sci. Rep. 2021, 11, 21177. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.; Onyeaka, H.; Miri, T.; Soltani, F. Chlorella vulgaris as a food substitute: Applications and benefits in the food industry. J. Food Sci. 2024, 89, 8231–8247. [Google Scholar] [CrossRef] [PubMed]
- Sikiru, A.; Arangasamy, A.; Comfort, A.; Ijaiya, A.; Acheneje, E.; Rao, S.B.N. In vitro evaluation of antioxidant properties of Chlorella vulgaris and its derivatives for use as antioxidant supplements in animal production. Indian J. Anim. Sci. 2024, 94, 88–91. [Google Scholar] [CrossRef]
- Dantas, D.M.D.M.; Costa, R.M.P.B.; Carneiro-Da-Cunha, M.G.; Galvez, A.O.; Drummond, A.R.; Bezerra, R.S.; Bezerra, R.S. Bioproduction, antimicrobial and antioxidant activities of compounds from Chlorella vulgaris. Res. Rev. J. Bot. 2015, 4, 3–9. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Khan, M.H.; Parvez, S. Hesperidin ameliorates heavy metal induced toxicity mediated by oxidative stress in brain of Wistar rats. J. Trace Elem. Med. Biol. 2015, 31, 53–60. [Google Scholar] [CrossRef]
- Villeda-Hernández, J.; Barroso-Moguel, R.; Méndez-Armenta, M.; Nava-Ruíz, C.; Huerta-Romero, R.; Ríos, C. Enhanced brain regional lipid peroxidation in developing rats exposed to low level lead acetate. Brain Res. Bull. 2001, 55, 247–251. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Omobowale, T.O.; Akinrinde, A.S.; Saba, A.B.; Ogunpolu, B.S.; Daramola, O. Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ. Toxicol. 2015, 30, 1235–1243. [Google Scholar] [CrossRef]
- Ozkaya, A.; Sahin, Z.; Dag, U.; Ozkaraca, M. Effects of naringenin on oxidative stress and histopathological changes in the liver of lead acetate administered rats. J. Biochem. Mol. Toxicol. 2016, 30, 243–248. [Google Scholar] [CrossRef]
- Saxena, G.; Flora, S.J. Lead-induced oxidative stress and hematological alterations and their response to combined administration of calcium disodium EDTA with a thiol chelator in rats. J. Biochem. Mol. Toxicol. 2004, 18, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.C.; Zucki, F.; Duarte, J.L.; Iano, F.G.; Ximenes, V.F.; Buzalaf, M.A.; Oliveira, R.C. Influence of iron on modulation of the antioxidant system in rat brains exposed to lead. Environ. Toxicol. 2017, 32, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Pande, M.; Flora, S.J. Lead induced oxidative damage and its response to combined administration of alpha-lipoic acid and succimers in rats. Toxicology 2002, 177, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ben-Azu, B.; Adebayo, O.G.; Wopara, I.; Aduema, W.; Onyeleonu, I.; Umoren, E.B.; Kolawole, T.A.; Ebo, O.T.; Akpotu, A.E.; Ajibo, D.N.; et al. Lead acetate induces hippocampal pyramidal neuron degeneration in mice via up-regulation of executioner caspase-3, oxido-inflammatory stress expression and decreased BDNF and cholinergic activity: Reversal effects of Gingko biloba supplement. J. Trace Elem. Med. Biol. 2022, 71, 126919. [Google Scholar] [CrossRef]
- Lamtai, M.; Chaibat, J.; Ouakki, S.; Berkiks, I.; Rifi, E.H.; Hessni, A.; Mesfioui, A.; Hbibi, A.; Ahyayauch, H.; Essamri, A.; et al. Effect of chronic administration of cadmium on anxiety-like, depression-like and memory deficits in male and female rats: Possible involvement of oxidative stress mechanism. J. Behav. Brain Sci. 2018, 08, 240–268. [Google Scholar] [CrossRef]
- Wang, T.; Guan, R.L.; Liu, M.C.; Shen, X.F.; Chen, J.Y.; Zhao, M.G.; Luo, W.J. Lead exposure impairs hippocampus related learning and memory by altering synaptic plasticity and morphology during juvenile period. Mol. Neurobiol. 2016, 53, 3740–3752. [Google Scholar] [CrossRef]
- Flora, S.J.; Saxena, G.; Gautam, P.; Kaur, P.; Gill, K.D. Response of lead-induced oxidative stress and alterations in biogenic amines in different rat brain regions to combined administration of DMSA and MiADMSA. Chem. Biol. Interact. 2007, 170, 209–220. [Google Scholar] [CrossRef]
- Moorhouse, S.R.; Carden, S.; Drewitt, P.N.; Eley, B.P.; Hargreaves, R.J.; Pelling, D. The effect of chronic low level lead exposure on blood-brain barrier function in the developing rat. Biochem. Pharmacol. 1988, 37, 4539–4547. [Google Scholar] [CrossRef]
- Shalan, M.G. Mitigating lead acetate-induced histopathologic and physiologic disorders in rats receiving vitamin C and glutathione supplement. Heliyon 2024, 11, e41256. [Google Scholar] [CrossRef]
- P’an, A.Y.; Kennedy, C. Lead distribution in rats repeatedly treated with low doses of lead acetate. Environ. Res. 1989, 48, 238–247. [Google Scholar] [CrossRef]
- Guimarães, D.; Carvalho, M.L.; Geraldes, V.; Rocha, I.; Alves, L.C.; Santos, J.P. Lead in liver and kidney of exposed rats: Aging accumulation study. J. Trace Elem. Med. Biol. 2012, 26, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.L.; Rodrigues, A.P.; Bincoletto, C.; Figueirêdo, C.A.; Malacrida, S. Protective effects of Chlorella vulgaris in lead-exposed mice infected with Listeria monocytogenes. Int. Immunopharmacol. 2003, 3, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, M.H.; Rashki, O.; Shahri, E. Application of modified Spirulina platensis and Chlorella vulgaris powder on the adsorption of heavy metals from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103169. [Google Scholar] [CrossRef]
- Metwally, E.S.A.M.; Negm, F.A.; El-Din, R.A.S.; Nabil, E.M. Anatomical and histological study of the effect of lead on hepatocytes of albino rats. Int. J. Biomed. Mater. Res. 2015, 3, 34–45. [Google Scholar] [CrossRef]
- Haouas, Z.; Sallem, A.; Zidi, I.; Hichri, H.; Mzali, I.; Mehdi, M. Hepatotoxic effects of lead acetate in rats: Histopathological and cytotoxic studies. J. Cytol. Histol. 2014, 5, 256. [Google Scholar] [CrossRef]
- Hirsch, G.H. Effect of chronic lead treatment on renal function. Toxicol. Appl. Pharmacol. 1973, 25, 84–93. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Y.; Wang, F.; Song, L.; Qiao, M.; Wang, T.; Huang, X. Folic acid alleviates lead acetate-mediated cardiotoxicity by down-regulating the expression levels of Nrf2, HO-1, GRP78, and CHOP proteins. Environ. Sci. Pollut. Res. 2022, 29, 55916–55927. [Google Scholar] [CrossRef]
- Ibrahim, N.M.; Eweis, E.A.; El-Beltagi, H.S.; Abdel-Mobdy, Y.E. Effect of lead acetate toxicity on experimental male albino rat. Asian Pac. J. Trop. Biomed. 2012, 2, 41–46. [Google Scholar] [CrossRef]
- Mphele, S.B.M.; Balogun, S.K.; Tlhabano, K.N. Effect of chronic administration of lead (Pb) on feeding behavior and weight gain among Wistar albino rats. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 3, 17–20. [Google Scholar] [CrossRef]
- Yang, J.L.; Juhasz, A.L.; Li, M.Y.; Ding, J.; Xue, X.M.; Zhou, D.; Ma, L.Q.; Li, H.B. Chronic exposure to drinking water As, Pb, and Cd at provisional guideline values reduces weight gain in male mice via gut microflora alterations and intestinal inflammation. Environ. Sci. Technol. 2023, 57, 12981–12990. [Google Scholar] [CrossRef]
- Shih, T.M.; Hanin, I. Effects of chronic lead exposure on levels of acetylcholine and choline and on acetylcholine turnover rate in rat brain areas in vivo. Psychopharmacology 1978, 58, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Luo, L.; Zhu, D.; Wang, M.; Luo, Y.; Wang, H.; Ruan, D.Y. Muscarinic cholinergic modulation of synaptic transmission and plasticity in rat hippocampus following chronic lead exposure. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Velankanni, P.; Go, S.H.; Jin, J.B.; Park, J.S.; Park, S.; Lee, S.B.; Kwon, H.K.; Pan, C.H.; Cha, K.H.; Lee, C.G. Chlorella vulgaris modulates gut microbiota and induces regulatory T cells to alleviate colitis in mice. Nutrients 2023, 15, 3293. [Google Scholar] [CrossRef] [PubMed]


| Group | BW, Day 0 | BW, Day 30 |
|---|---|---|
| CT | 552.50 ± 9.41 | 582.30 ± 9.05 * |
| CV | 558.15 ± 7.89 | 598.40 ± 9.22 * |
| Pb | 557.20 ± 11.89 | 577.60 ± 12.17 |
| CV–Pb | 577.30 ± 12.18 | 599.50 ± 10.83 * |
| Organ | CT | CV | Pb | CV–Pb |
|---|---|---|---|---|
| Liver | 21.83 ± 0.80 | 22.23 ± 0.87 | 16.55 ± 0.61 *† | 17.65 ± 0.72 *† |
| Kidney | 4.41 ± 0.14 | 4.29 ± 0.14 | 3.87 ± 0.14 *† | 4.14 ± 0.10 |
| Brain | 1.54 ± 0.03 | 1.45 ± 0.04 | 1.51 ± 0.09 | 1.49 ± 0.02 |
| Heart | 1.56 ± 0.02 | 1.59 ± 0.03 | 1.39 ± 0.05 *† | 1.53 ± 0.03 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz, J.P.; Pena, E.; El Alam, S.; Matte, C.; Cortés, I.; Figueroa, L.; Siques, P.; Brito, J. Chlorella vulgaris Supplementation Attenuates Lead Accumulation, Oxidative Stress, and Memory Impairment in Rats. Toxics 2025, 13, 313. https://doi.org/10.3390/toxics13040313
Diaz JP, Pena E, El Alam S, Matte C, Cortés I, Figueroa L, Siques P, Brito J. Chlorella vulgaris Supplementation Attenuates Lead Accumulation, Oxidative Stress, and Memory Impairment in Rats. Toxics. 2025; 13(4):313. https://doi.org/10.3390/toxics13040313
Chicago/Turabian StyleDiaz, Juan Pablo, Eduardo Pena, Samia El Alam, Cecilia Matte, Isaac Cortés, Leonardo Figueroa, Patricia Siques, and Julio Brito. 2025. "Chlorella vulgaris Supplementation Attenuates Lead Accumulation, Oxidative Stress, and Memory Impairment in Rats" Toxics 13, no. 4: 313. https://doi.org/10.3390/toxics13040313
APA StyleDiaz, J. P., Pena, E., El Alam, S., Matte, C., Cortés, I., Figueroa, L., Siques, P., & Brito, J. (2025). Chlorella vulgaris Supplementation Attenuates Lead Accumulation, Oxidative Stress, and Memory Impairment in Rats. Toxics, 13(4), 313. https://doi.org/10.3390/toxics13040313











