Abstract
Arsenic is a highly toxic element, and excessive levels can affect human health. Composites possess a larger specific surface area and better adsorption performance than single-MOF materials. In this paper, a simple novel nanocomposite (MnFe2O4@Fe-UiO-67) was synthesized by the one-pot method for the removal of arsenic from industrial wastewater. The synthesis and adsorption mechanism of the adsorbent were analyzed by a series of characterizations. The results showed that the adsorption behavior of MnFe2O4@Fe-UiO-67 was consistent with the pseudo-secondary kinetics and Langmuir isotherm model, i.e., it is a monomolecular layer chemisorption. Characterization by Fourier transform infrared (FT-IR) and X-ray photoelectron spectroscopy (XPS) showed that the active site formed a strong coordination bond (As-O bond) with As ions to achieve efficient adsorption. At 298 K and pH = 10, the arsenic removal rate can reach 98.43%, and the adsorption capacity is 600.25 mg/g, which is more than most of the existing reported adsorbents. Through thermodynamic analysis, it is found that the adsorption of As ions by the adsorbent is a spontaneous exothermic process. It can exhibit excellent adsorption performance at room temperature without the need for additional energy consumption. This adsorbent has great development prospects in the treatment of wastewater.
1. Introduction
Arsenic exists in nature mainly in the form of trivalent and pentavalent compounds; trivalent compounds are more toxic than pentavalent compounds [1,2]. Arsenic mainly comes from the smelting of non-ferrous metals, the mining of arsenic-containing minerals, and the application of arsenic-containing compounds in industrial production, such as paint, glass, textile, semiconductor, and nitrogen fertilizer [3,4]. Arsenic compounds often exist in the form of by-products, and in the wastewater discharged into the environment, causing harm to the human body, such as neurasthenia, cardiovascular disease, diabetes and so on [5]. Therefore, the World Health Organization (WHO) recommends a maximum concentration limit of 10 μg/L for drinking water [6].
Currently, arsenic removal methods include chemical precipitation [7], adsorption [8], and microbial methods [9]. Each method has its own advantages and disadvantages, but currently, adsorption is considered the most promising method because of its high efficiency, suitability for industrial application, low cost, simple operation, and high regeneration capacity [10,11]. In the past, a variety of adsorbents have been developed and applied for the treatment of arsenic-containing wastewater. For example, Ahmad Sadeghi Chevinli et al. [12] synthesized a Mg-Fe LDH-GO adsorbent with high adsorption capacity for both As (III) and As (V) by the co-precipitation method. Xiaoli Song et al. [13] loaded Fe3O4 on halloysite nanotubes (HNTs), and experiments have shown that the nanocomposites have highly efficient arsenic removal performance and recyclability. Conventional adsorbents such as activated carbon, clay, zeolite, and agricultural wastes exhibit limited arsenic removal efficiency, and thus new adsorbent materials have been developed for the removal of arsenic from industrial wastewater [14,15].
Metal–organic frameworks (MOFs) are composed of metal ions or clusters coordinated with organic linkers, which exhibit an exceptional affinity toward metal ions, and are used as a new type of nanomaterial due to their high specific surface area, large pore size, and good stability [16,17,18]. MOF materials can be used for the removal of various pollutants from water, as shown by Hongfei Ma et al. [19], who investigated the synthesis of La-MOFs in different morphologies by altering the solvothermal temperature and applied them for the removal of fluoride ions (F−) from water. Hossein Molavi et al. [20] prepared a series of Ce-MOF materials by using different solvents and thoroughly investigated the effect of such materials on the organic dyes’ adsorption properties. However, single MOFs have fewer applications in the practical treatment of wastewater due to their poor selectivity and weak capture ability. Modifying MOFs is an inevitable trend aimed at increasing the number of adsorption active sites and enable a wider range of applications. The current methods of MOF modification include the doping of metals [21], changing the organic ligands [22], the generation of induced defects [23], the introduction of functional groups [24], etc. In addition, two different forms of MOF are mixed together to obtain new materials with different structures and properties [25].
Spinel ferrites (MnFe2O4) possess excellent chemical stability and abundant surface functional groups, which are advantageous for removing various toxic pollutants from water [26]. Ting Pan et al. [27] synthesized MnFe2O4@S-ZnO and investigated its performance in removing norfloxacin (NOF). Tao Zhang et al. [28] successfully prepared a composite material, MnFe2O4/rGO, through defect engineering, which demonstrated efficient electrochemical arsenic removal performance along with excellent stability. Rauf Foroutan et al. [29] modified biochar (BCSO) to synthesize BCSO/MnFe2O4@La-MOF for the highly efficient removal of fluoride ions (F−) from water. With the increasing awareness of environmental protection, the development of green and eco-friendly materials has become a hot research topic. For example, Sonia Jemli et al. [30] used sunflower seed husk (SFSH) and β-cyclodextrin (β-CD) to green-synthesize a crosslinked adsorbent (SFSH-β-CD) for the removal of phenol and cyclohexane carboxylic acid. Xiang Chen et al. [31] synthesized an eco-friendly adsorbent, 2D/2D Na+-MXene/LDH composite, for cesium adsorption in salt lakes.
In this paper, the doping of metals and introduction of spinel ferrite (MnFe2O4) are carried out to modify MOF materials. Zirconium-based metal–organic frameworks have demonstrated extensive applications due to their high surface area, good stability, and strong adsorption capacity. MnFe2O4@Fe-UiO-67 composites were used for the adsorption of arsenic from industrial wastewater in this study. The structure and adsorption mechanism of the adsorbent were explored by characterization methods such as X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FT-IR), and X-ray photoelectron spectroscopy (XPS), combined with the use of adsorption kinetics and isotherm modeling. In addition, the adsorption performance of the material was explored in terms of pH value and adsorbent dosage.
2. Experimental Parts
2.1. Experimental Materials
Zirconium chloride (ZrCl4, 98%), acetic acid (CH3COOH, 99.5%), ferric nitrate nine water (Fe(NO3)3·9H2O, 99.99%), potassium permanganate (KMnO4, AR), ferrous sulfate heptahydrate (FeSO4·7H2O, 99%), N,N-dimethylformamide (DMF, 99%), 4-4′-biphenyldicarboxylic acid (4,4′-H2BPDC, 98%), methanol (CH3OH, 99.5%) and ethanol (CH3CH2OH, 75%) were used. All reagents were analytical-grade reagents in this experiment.
The industrial wastewater used in this experiment was from a copper smelting plant and contained Cu, Cd, Ni and other heavy-metal elements; it had a complex composition and high toxicity, as shown in Table 1.

Table 1.
Elemental composition analysis of diluted arsenic-containing wastewater (mg/L).
2.2. Preparation of Adsorbents
2.2.1. Preparation of MnFe2O4
MnFe2O4 was synthesized by dissolving 0.015 mol of KMnO4 and 0.045 mol of FeSO4·7H2O in 200 mL of ultrapure water [32]. The potassium permanganate solution was stirred, and the ferrous sulfate solution was slowly added to the potassium permanganate solution, while 5 M NaOH solution was added to adjust the pH to 10, and after stirring for 3 h, it was static-aged, washed with ultrapure water and dried.
2.2.2. Preparation of MnFe2O4@Fe-UiO-67
The 4,4′-H2BPDC (0.5808 g), ZrCl4 (0.2796 g), and acetic acid (2.28 mL) were added to 90 mL of DMF, and stirred until the sample was completely dissolved. Fe(NO3)3·9H2O (0.4848 g) was added and stirred homogeneously, and then 0.1 g of MnFe2O4 was added and stirred homogeneously. The obtained mixture was transferred to a 200 mL PTFE-lined autoclave and kept at 120 °C for 24 h. After the reaction, the reactor was allowed to cool to room temperature. The solid product was separated by centrifugation, washed with DMF, CH3OH, and CH3CH2OH and dried [33,34].
2.3. Methods of Analysis
The crystal structure of the adsorbents was determined using an X-ray diffractometer (XRD, MiniFlex600, Rigaku Corporation, Akishima, Japan) with a scanning range of 0–90° and a scanning speed of 5°/min; a field emission scanning electron microscope (SEM, Apreo 2S, Thermo Fisher Scientific, Waltham, MA, USA) was used to view the microscopic morphology of the adsorbents and surface elements, and the adsorption–resolution isotherm of N2 was determined using a Micromeritics ASAP 2460 instrument (Norcross, GA, USA), while the specific surface area of the adsorbent was calculated by the Brunauer–Emmett–Teller (BET) method and the pore volume and pore size distribution were analyzed by the Barrett–Joyner–Halenda (BJH) method. Infrared spectra in the range of 400–4000 cm−1 were collected using a Fourier transform infrared spectrometer (FTIR, Thermo Scientific Nicolet iS20, USA) in order to analyze the changes in the chemical bonding of the materials before and after adsorption. X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, USA) was used to analyze the elemental composition and content of the adsorbent, and the changes in the valence. An Inductively Coupled Plasma Atomic Emitter (ICP-OES, PQ9000, Jena, Germany) was used to measure the concentration of arsenic ions in solution.
2.4. Adsorption Experiments
There are many factors affecting the adsorption performance of adsorbents, and the batch experiments focused on investigating the effects of solution pH, adsorption time, adsorbent dosage, and arsenic solution concentration.
The pH of the arsenic solution was adjusted to 2–12, and then 10 mg of adsorbent was added to 50 mL of the arsenic solution. We then determined the As ion concentration after 24 h of reaction. An amount of 10 mg of the adsorbent was added to 50 mL of the arsenic solution at the optimal pH = 10, and vacuum-filtered through a 0.45 μm membrane the solution at a specific point of time, after which we determined the concentration of As ions. Under optimal reaction conditions (arsenic solution pH = 10, reaction time 3 h), a certain amount of adsorbent (0.1–1 g/L) was added to 50 mL of the As solution to determine the maximum arsenic removal rate. Different concentrations of arsenic solutions (100 mg/L, 200 mg/L, 300 mg/L, 400 mg/L, 500 mg/L, 600 mg/L, 700 mg/L, 800 mg/L, 900 mg/L, 1000 mg/L and 1100 mg/L) were configured to investigate the maximum adsorption amount of MnFe2O4@Fe-UiO-67 under the conditions of pH = 10 and 3 h of reaction, and the dosage of the adsorbent was 0.8 g/L. In order to systematically explore the adsorption thermodynamic properties of the adsorbent, MnFe2O4@Fe-UiO-67, this study selected three temperatures (298 K, 308 K, and 318 K) with which to carry out the experiments. Solutions with initial arsenic concentrations of 33 mg/L, 130.5 mg/L, and 210 mg/L were prepared, and the pH value of the solutions was adjusted to 10. Then, 50 mL of the above-mentioned arsenic solution was taken, and 0.8 mg/L of the adsorbent was added to it. The reaction was carried out for 3 h at different temperatures. Finally, the experimental data were collected and their thermodynamic properties are analyzed. In the experiments, to investigate the optimal pH, adsorption time, and adsorbent dosage, arsenic solutions with an initial concentration of 30 mg/L were used. To avoid errors, the above experiments were conducted three times. The As removal rate and adsorption capacity were calculated using Equations (1) and (2):
where R (%) is the arsenic removal rate, C0 (mg/L) is the initial arsenic solution concentration, Ce (mg/L) is the arsenic solution concentration after adsorption, qe (mg/g) is the adsorbed amount, V (L) is the volume of the arsenic solution, and m (g) is the mass of the adsorbent, MnFe2O4@Fe-UiO-67.
2.5. Equations for Adsorption Kinetics, Isotherms and Thermodynamic Analysis
Time is an important factor affecting the adsorption performance of an adsorbent. We used the pseudo-first-order model (PFO), pseudo-second-order model (PSO) and intraparticle diffusion model to study the adsorption process [35]. The equations are as follows:
where qe (mg/g) is the adsorbed amount at the equilibrium adsorption time, and qt (mg/g) is the adsorbed amount at a specific time. k1 (1/min), k2 (mg/g·min), and k3 (mg/g·min0.5) represent the rate constants for PFO and PSO. t is the reaction time (min).
In addition to time, the concentration of the arsenic solution is also an important factor affecting the adsorption performance of MnFe2O4@Fe-UiO-67. To investigate the adsorption isotherms, Langmuir, Freundlich and Temkin models were used to fit the isotherms to the experimental data [36]. The adsorption model equations are given below:
where qe and qm are the adsorption amount at the adsorption equilibrium and the maximum adsorption amount of the adsorbent (mg/g), respectively; KL (L/mg), KF (L/mg), and KT (L/g) are the Langmuir adsorption constant, Freundlich’s constant, and Temkin’s constant, respectively; n is Freundlich’s model coefficient, and R is the gas constant (8.314 J/mol/K).
On the other hand, temperature is a factor that cannot be ignored in the adsorption process. Adsorption thermodynamics -reveals the variations in energy and entropy during the adsorption process, allowing us to make judgments about the direction and extent of the adsorption reaction, and thus enabling a profound understanding of the adsorption mechanism. The relevant thermodynamic formulas are as follows:
Among them, the Gibbs free energy change (ΔG0), enthalpy change (ΔH0), and entropy change (ΔS0) are the core thermodynamic parameters, with units of kJ/mol, kJ/mol, and kJ/mol/K, respectively; K is the adsorption equilibrium constant, qe (mg/g) is the adsorption capacity, Ce (mg/L) is the remaining arsenic concentration after adsorption, R is the gas constant with a value of 8.314 J/mol/K. and T (K) is the adsorption temperature.
3. Results and Discussion
3.1. Characterization of Adsorbents
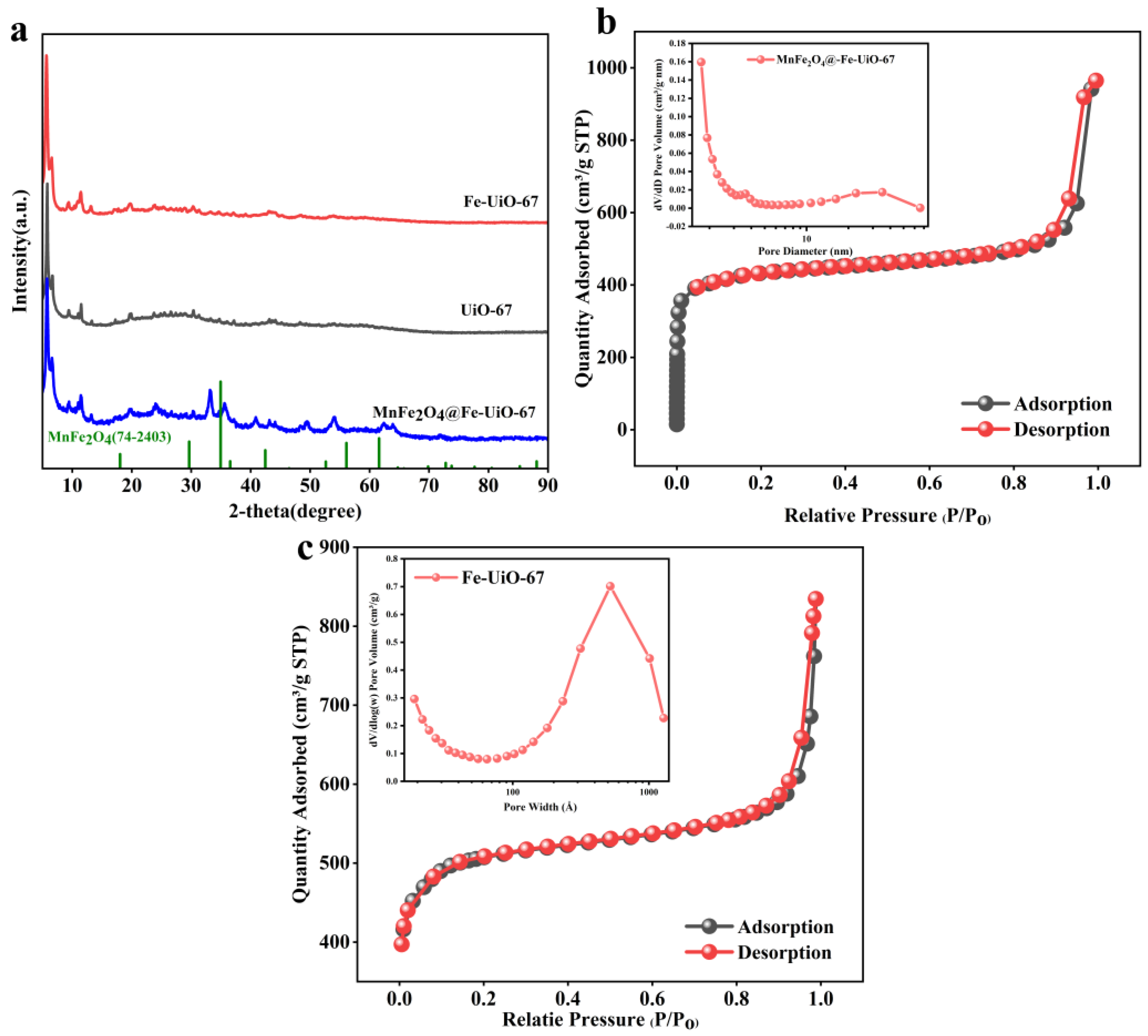
As shown in Figure 1a, the XRD peaks of UiO-67 and Fe-UiO-67 are the same and are in agreement with those in the literature [37], indicating that there is no change in the structure of UiO-67 caused by the doping of iron ions. The characteristic peaks of MnFe2O4 in MnFe2O4@Fe-UiO-67 are shifted when compared with those on the XRD standard card of MnFe2O4 (JCPDS card no.74-2403). Figure 1a shows that the characteristic peaks of MnFe2O4 appear at 33.25°, 35.6°, 43.15°, 54.05°, and 62.4° for MnFe2O4@Fe-UiO-67 [34,38], indicating the successful loading of MnFe2O4 on Fe-UiO-67. Figure 1b,c show the N2 adsorption–desorption curve of the adsorbent, MnFe2O4@Fe-UiO-67, and its precursor, Fe-UiO-67. According to the IUPAC classification, both show typical type IV isotherms accompanied by type H1 hysteresis loops, indicating that they are mesoporous structures. It is worth noting that although the literature reports the pristine UiO-67 as a microporous material [39], the pore structure of the material is significantly changed by Fe3+ doping and composite modification with MnFe2O4. The surface area, pore size, and pore volume of the adsorbent were calculated by the Barrett–Joyner–Halenda (BJH) method (shown in Table 2). It can be seen that the specific surface area and pore size of MnFe2O4@Fe-UiO-67 are larger than those of UiO-67 and Fe-UiO-67, resulting in better adsorption performance. However, the pore volume of MnFe2O4@Fe-UiO-67 is smaller than that of Fe-UiO-67, which may be the reason for partial pore collapse.

Figure 1.
XRD (a) and BET (b) of MnFe2O4@Fe-UiO-67, BET of Fe-UiO-67 (c).

Table 2.
Data related to N2 adsorption–desorption of adsorbents.
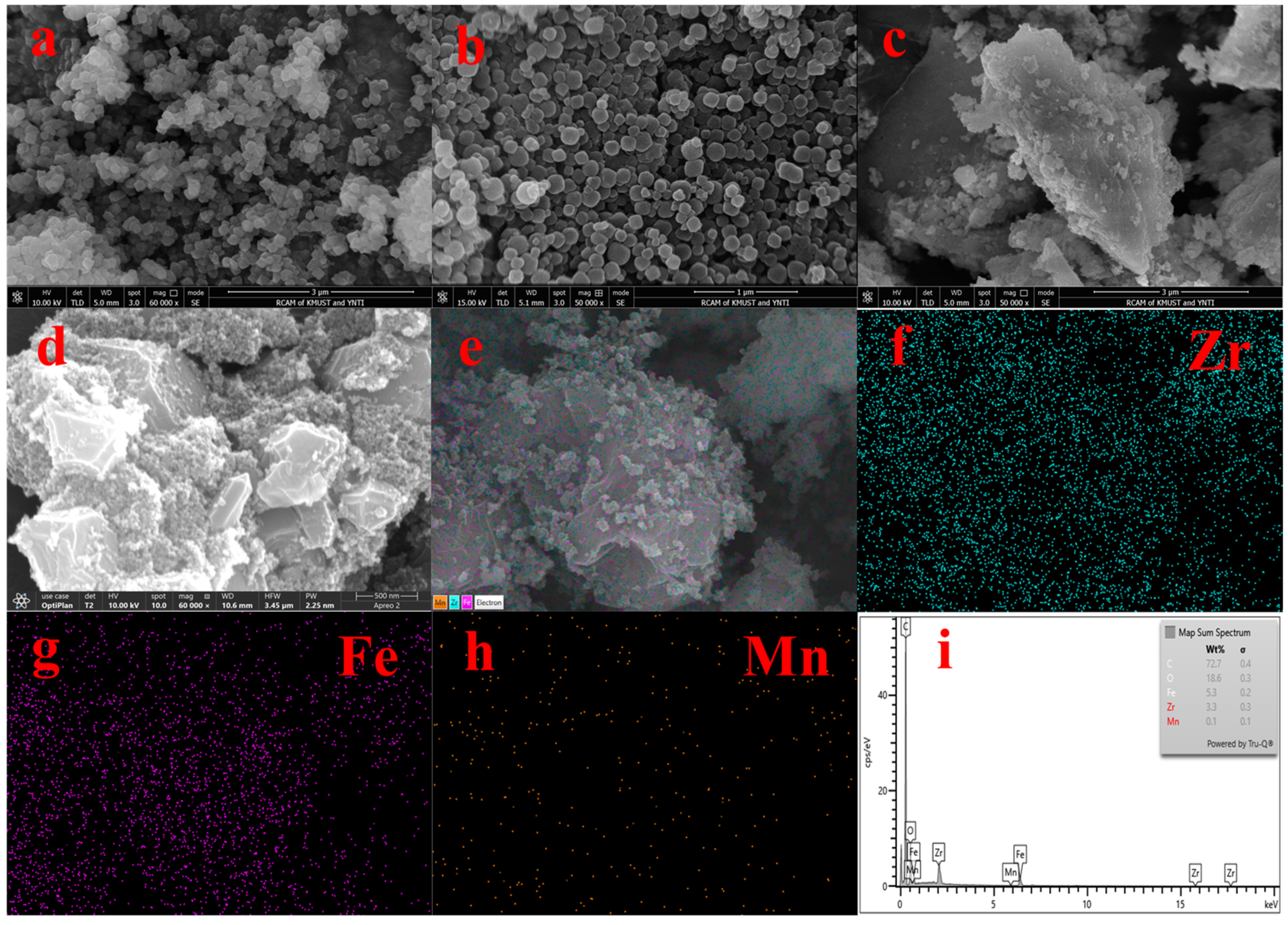
Figure 2 shows the SEM and EDS spectra of the adsorbent. From Figure 2a,b, we can see that UiO-67 and Fe-UiO-67 have the same structure. Figure 2c shows the morphology of MnFe2O4, which large particles with irregular shape. Figure 2d,e show that the morphology MnFe2O4 and Fe ions were successfully doped on UiO-67; the rough small particles are Fe-UiO-67 and the large particles are MnFe2O4 in the SEM image of MnFe2O4@Fe-UiO-67. The EDS (Figure 2f–i) maps have the elements Zr, Fe and Mn, but the content of Mn is lower, so the EDS map of Mn (Figure 2h) is noisy. The content of Mn in the adsorbent is only 0.86%, as measured by ICP.
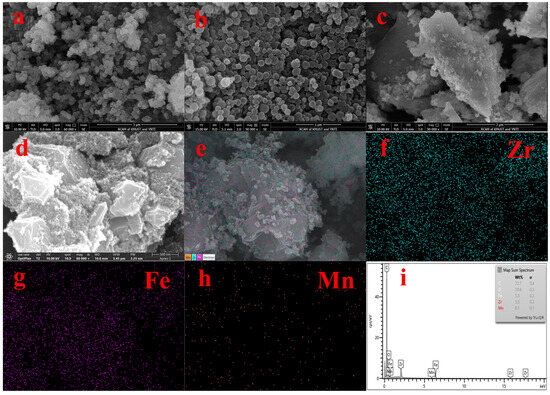
Figure 2.
SEM spectra of adsorbents UiO-67 (a), Fe-UiO-67 (b), MnFe2O4 (c), MnFe2O4@Fe-UiO-67 (d,e) and EDS of MnFe2O4@Fe-UiO-67 (f–i).
3.2. Effect of Initial pH on Adsorbent Performance
The form of arsenic ions in solution depends on the pH range. For example, As (V) exists as H3AsO4 at pH < 2; with increasing pH (2–7), H3AsO4 ionizes an H+ atom to form H2AsO4−. At pH > 7, it exists as HAsO42−; at pH > 12, arsenic exists mainly as AsO43− [40]. The surface charge of the adsorbent is also affected by the pH, so we explored the effect of different pH values on the adsorption performance of the adsorbent and analyzed the zeta potential to understand the adsorption mechanism.
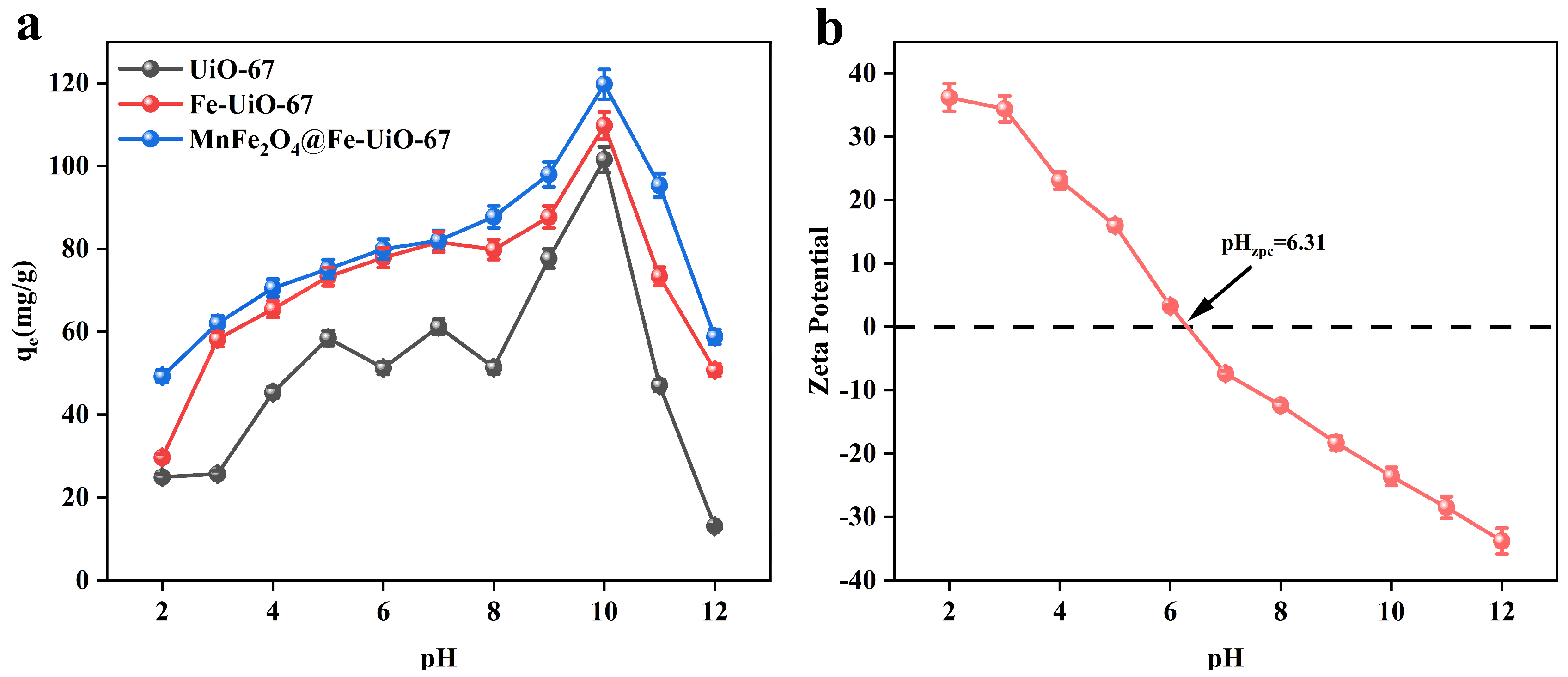
As shown in Figure 3a, the adsorbent, MnFe2O4@Fe-UiO-67, showed the best adsorption effect at pH 10. The adsorption was increasing the whole time when the pH increased from 2 to 10, and decreased at pH > 10. To further investigate the adsorption mechanism, the zeta potential of MnFe2O4@Fe-UiO-67 was detected (Figure 3b). The zeta potential of pHzpc = 6.31; when the pH was <6.31, the surface of the adsorbent Was positively charged, which attracted the negatively charged arsenic ions and had a strong adsorption capacity, and when the pH was >6.31, the surface of the adsorbent was negatively charged, which repelled the arsenic ions and hassled to it having a weakened adsorption capacity. However, the adsorption capacity of the adsorbent was the strongest at pH = 10, indicating that electrostatic adsorption was not the main adsorption mechanism.
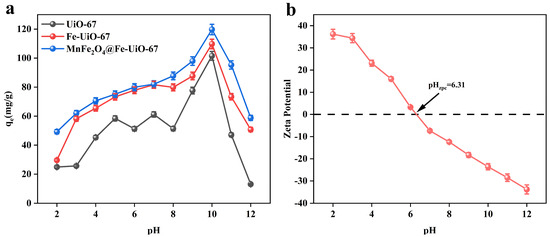
Figure 3.
Effect of pH on three adsorbents (a); zeta potential of MnFe2O4@Fe-UiO-67 (b).
3.3. Exploration of Arsenic Removal Rate and Adsorption Capacity
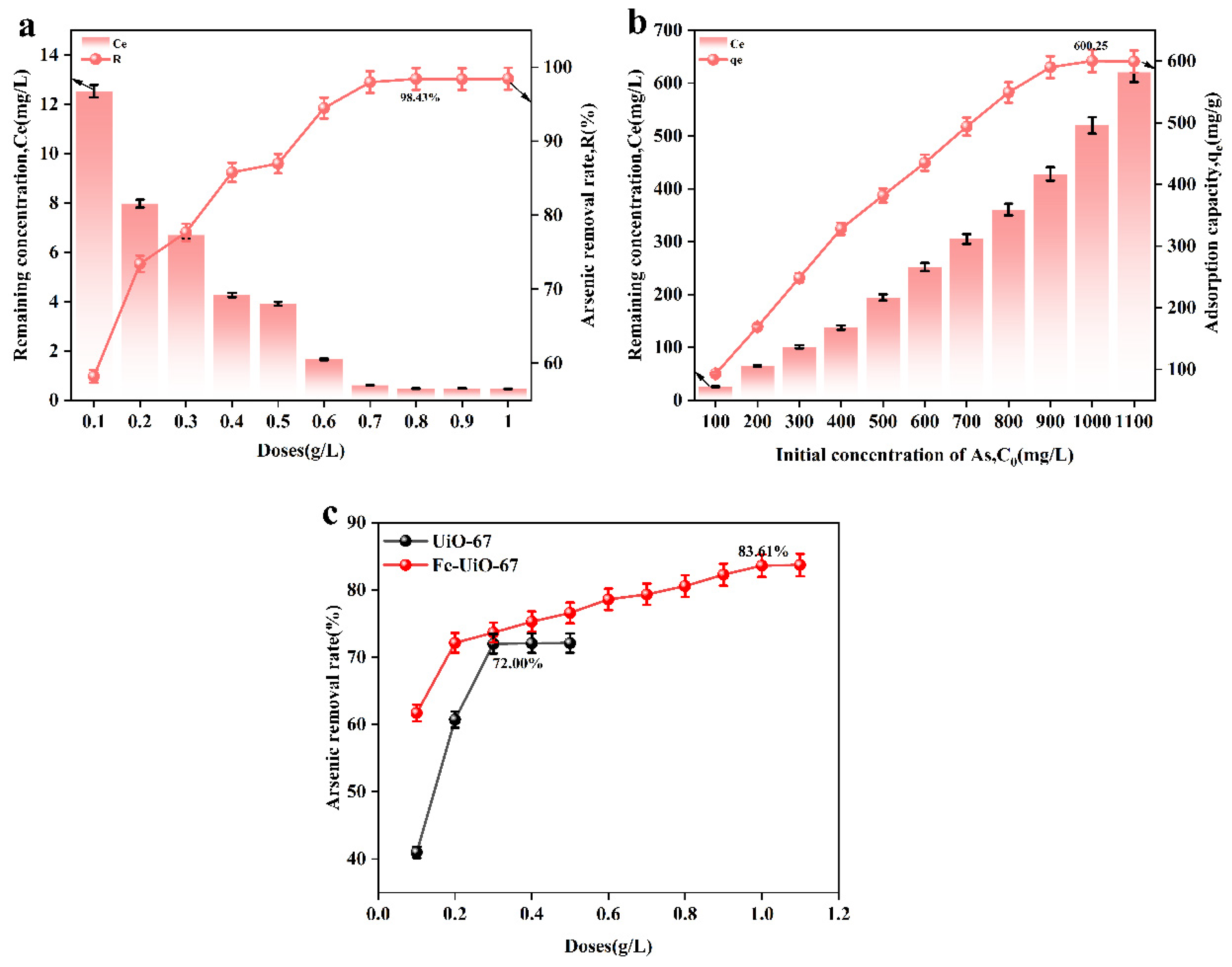
Figure 4a,c show the effect of the adsorbent dosage on adsorbent performance. From Figure 4a, it can be seen that when the adsorbent dosage was 0.8 g/L, the adsorption rate reached a maximum of 98.43%, which is higher than that of UiO-67 and Fe-UiO-67 (Figure 4c). Figure 4b shows the effect of the initial arsenic concentration on adsorbent performance. Prepare an arsenic standard solution with concentrations ranging from 100 to 1000 mg/L using ICP-OES. Transfer 50 mL of the solution into an Erlenmeyer flask, and add the adsorbent at a dosage of 0.8 g/L. When the arsenic solution concentration reached 1000 mg/L, the adsorption capacity of MnFe2O4@Fe-UiO-67 reached the equilibrium, and the maximum adsorption was 600.25 mg/g, which is higher than that of the other adsorbents reported in the literature (shown in Table 3).
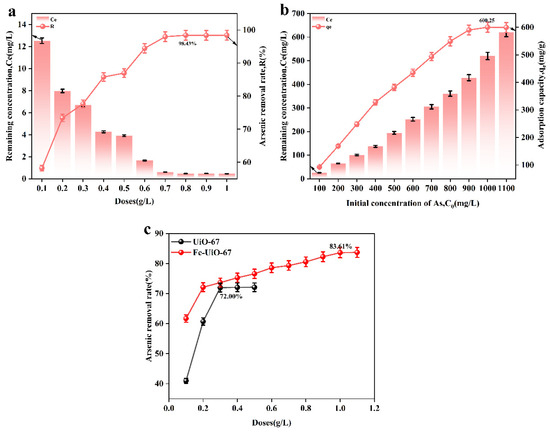
Figure 4.
Effect of different dosages of adsorbent (a,c) and different concentrations of solution on adsorption performance (b).

Table 3.
Adsorption capacity of various adsorbents for arsenic ions in the literature.
3.4. Exploration of Adsorption Kinetics, Isotherms and Thermodynamic
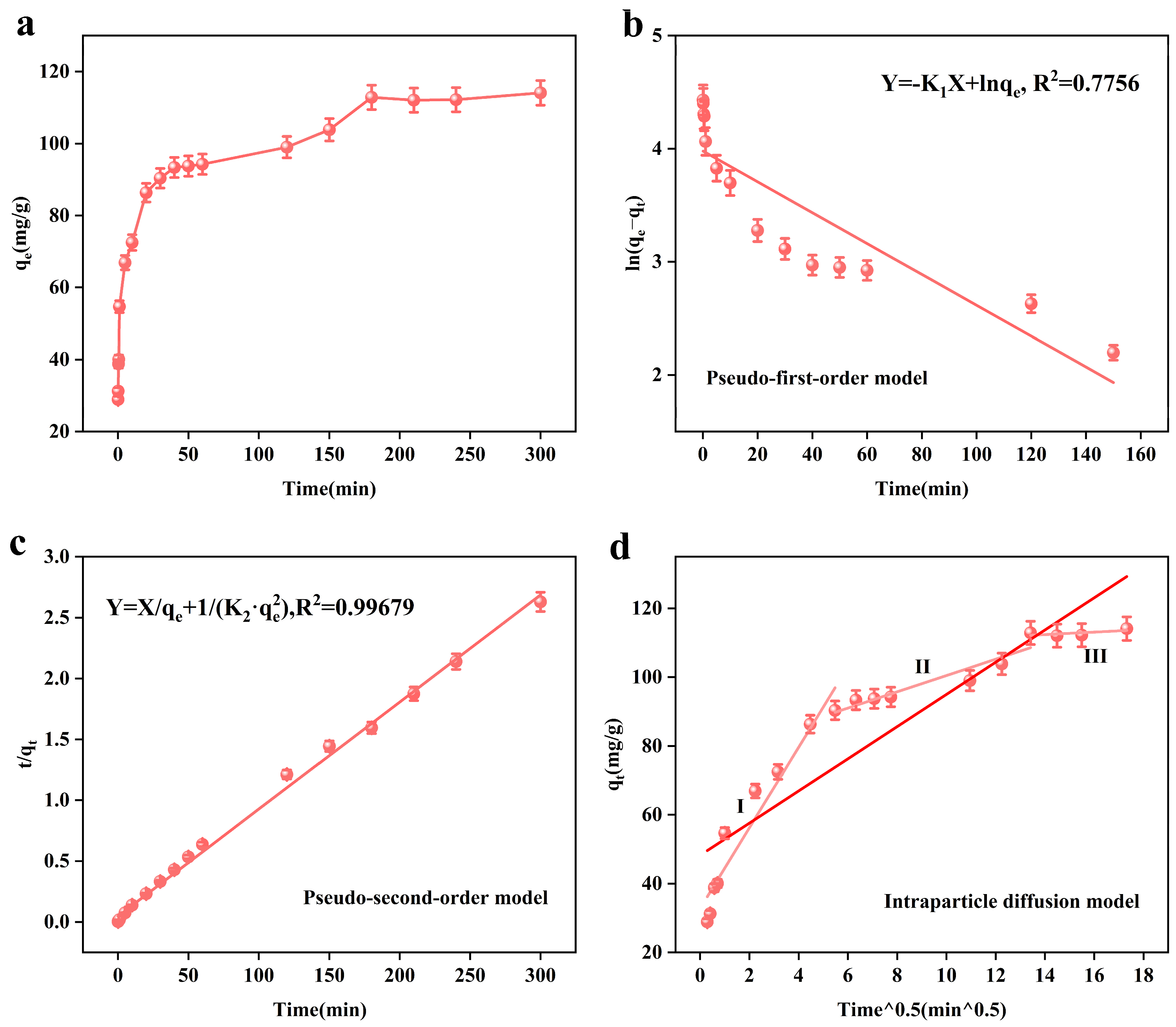
Figure 5a shows the relationship between the adsorption time (t) and adsorption amount (qt). From the figure, we can see that the presence of the adsorbent on the arsenic ions leads to rapid adsorption in the first 40 min, and then the adsorption amount slowly increases until the adsorption equilibrium is reached, after 3 h. This is because at the beginning of the reaction, the adsorbent’s active adsorption sites are more able to quickly bind with the arsenic ions in the solution; after that, the arsenic ions in solution and the adsorption sites of the adsorbent are reduced, resulting in the adsorption rate slowing down and the equilibrium being reached.
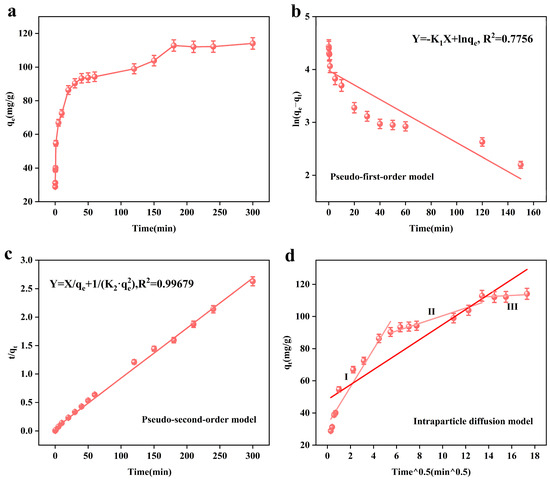
Figure 5.
Effect of time on the adsorption performance of the adsorbent, MnFe2O4@Fe-UiO-67 (a); adsorption kinetic pseudo-first-order model and (b) pseudo-second-order model (c) Intraparticle diffusion model (d).
Figure 5b–d show the kinetic models fitted by us according to the experimental data. Figure 5b,c show the PFO and PSO, respectively. Table 4 and Table 5 show the relevant kinetic model parameters. From Table 4, it can be seen that the pseudo-second-order kinetic model R2 = 0.99679 is larger than the pseudo-first-order kinetic model R2 = 0.7756, so the pseudo-second-order kinetic model can better describe the adsorption process of the adsorbent, which is chemisorption. Figure 5d shows the intraparticle diffusion model, and the related parameters are shown in Table 5. In the first stage, the abundance of arsenic ions and the number of adsorption sites lead to the rapid diffusion of arsenic ions; in the second stage, the number of adsorption active sites are reduced due to occupation by arsenic ions, and the amount of arsenic ions is also reduced, so the adsorption rate slows down until it reaches the equilibrium (the third stage).

Table 4.
Kinetic model parameters associated with MnFe2O4@Fe-UiO-67.

Table 5.
Intraparticle diffusion model parameters.
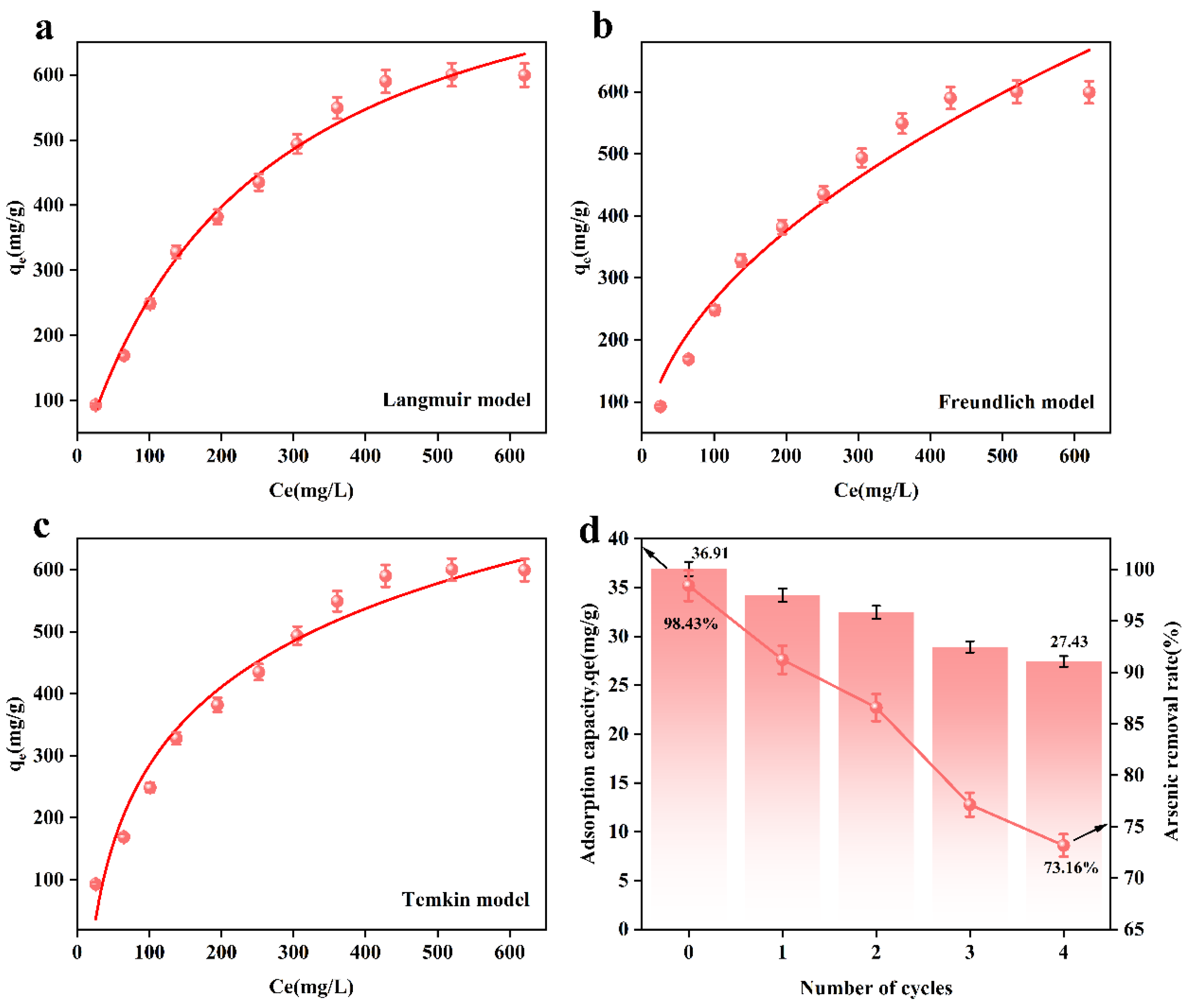
Figure 6a–c show the adsorption isotherm models fitted based on the experimental data. Table 6 shows the isotherm model parameters of the adsorbent. According to Figure 6 and Table 6, the correlation coefficient, R2 = 0.98987, of the Langmuir model of the adsorbent is greater than that of the Freundlich model (R2 = 0.95971) and Temkin model (R2 = 0.96847). This result indicates that the adsorption process is more consistent with the Langmuir model, i.e., the Langmuir model better describes the adsorption behavior of arsenic ions on the adsorbent, indicating that the form of adsorption tends to be monolayer adsorption.
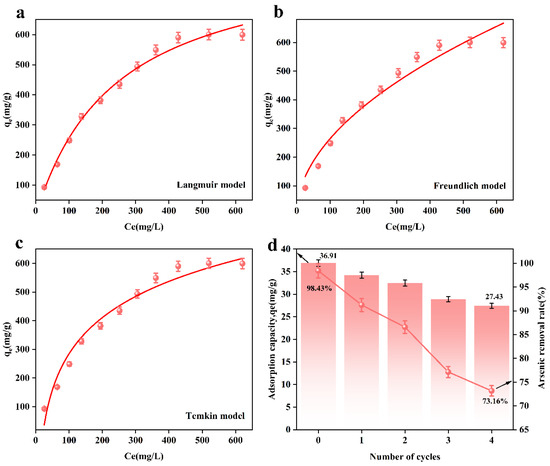
Figure 6.
Adsorption isotherm model for MnFe2O4@Fe-UiO-67; (a) Langmuir, (b) Freundlich, (c) Temkin and adsorption–resolution cycle experiments (d).

Table 6.
Adsorption isotherm model parameters.
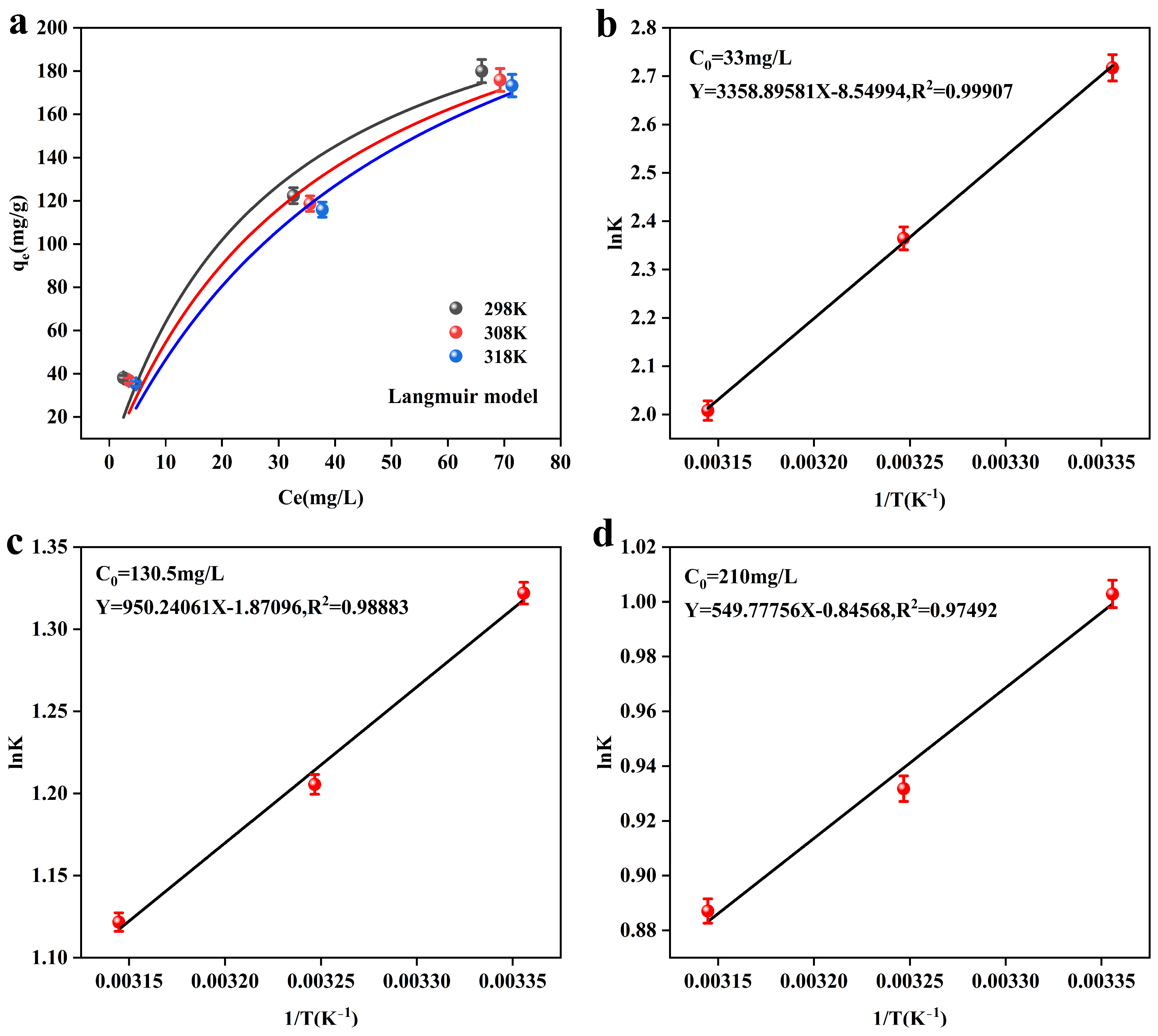
Figure 7 shows the thermodynamic fitting results of the adsorbent, MnFe2O4@Fe-UiO-67, and the corresponding thermodynamic parameters are listed in Table 7. Figure 7a shows the Langmuir model of the adsorbent at different temperatures, which shows a decreasing trend in the adsorption capacity (qe) with increasing reaction temperatures (298 K–318 K), indicating that the adsorbent has the best adsorption performance at room temperature (298 K). Figure 7a–c are the Van’t Hoff curves of MnFe2O4@Fe-UiO-67, and the data fitting shows a good result. From the thermodynamic parameters in Table 7, it can be seen that ΔG0 < 0, indicating that the adsorption reaction is spontaneous and that ΔG0 increases with increasing reaction temperature at the same initial concentration, indicating that a proper decrease in temperature is favorable for the reaction. ΔH0 < 0 indicates that the adsorption reaction is exothermic, and lowering the temperature is favorable for the reaction. ΔS0 represents the degree of disorder of the reaction system; ΔS0 < 0 indicates that the disorder in the adsorption process is reduced, which may be due to the fact that arsenic ions are adsorbed on the MnFe2O4@Fe-UiO-67 surface. In conclusion, the thermodynamic results indicate that the adsorption process is spontaneous and exothermic and tends to occur at room temperature or lower.
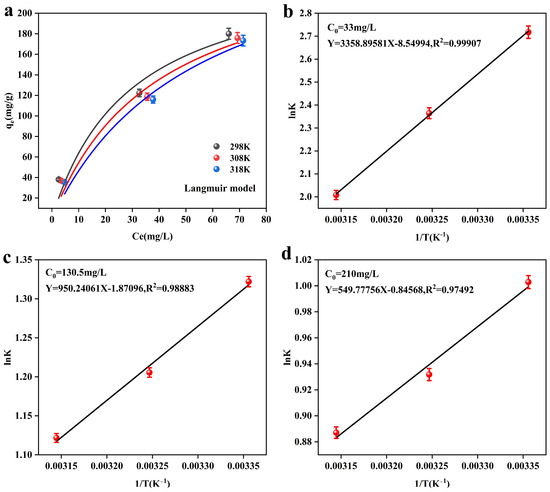
Figure 7.
The Langmuir isotherm of the adsorbent at different temperatures (a), and Van’t Hoff curves for different initial arsenic concentrations (b–d).

Table 7.
Thermodynamic parameters of As adsorption by MnFe2O4@Fe-UiO-67.
3.5. Cyclic Regeneration Capacity of Adsorbent
In order to evaluate the regenerative capacity of the adsorbent, we conducted an adsorption–resolution cycle experiment. The adsorbed MnFe2O4@Fe-UiO-67 was added to 1 M NaOH solution for arsenic removal. After 12 h, and after arsenic removal, the adsorbent was washed with deionized water until it was neutral. The obtained adsorbent was dried in a desiccator for 2 h, and then the adsorption experiment was performed again. After that, the above operation was repeated.
From Figure 6d, it can be observed that after 4 cycles, the arsenic removal efficiency of MnFe2O4@Fe-UiO-67 decreased from 98.43% to 73.16%, and the adsorption capacity decreased from 36.91 mg/g to 27.43 mg/g. This may have been due to incomplete desorption, where a small amount of As ions remained in the pores of the adsorbent, affecting its adsorption efficiency. Nevertheless, even after four cycles, the adsorbent still maintained a relatively high arsenic removal efficiency, indicating its excellent regeneration capability and long-term application potential.
4. Reaction Mechanisms
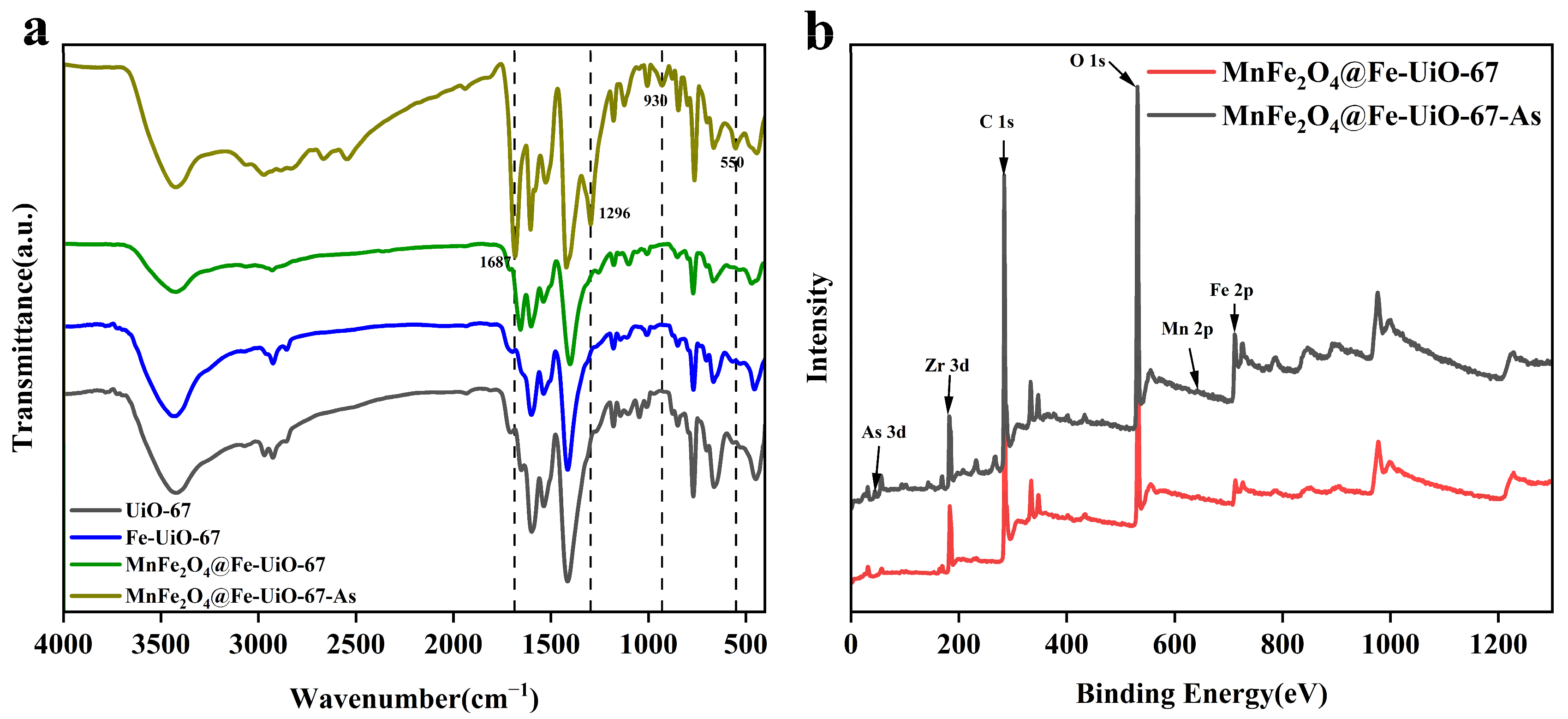
As shown in Figure 8a, the IR spectra of UiO-67 and Fe-UiO-67 are the same, indicating that they are structurally the same. The four extra bonds after the adsorption of MnFe2O4@Fe-UiO-67 are Fe-O bonds (550 cm−1) [34]; the peaks at 930 cm−1 correspond to the As-O bond [46], indicating that the active sites on the adsorbent form coordination bonds with As ions, thereby enabling the removal of arsenic. The peaks observed at 1296 cm⁻¹ and 1687 cm⁻¹ may emerge due to structural modifications induced by the adsorption process. Figure 8b shows the total XPS spectra before and after adsorption, in which the content of the element Fe increased after adsorption and As appeared, indicating that the structure of MnFe2O4@Fe-UiO-67 changed after adsorption and arsenic ions were adsorbed on it, and that there was no obvious peak for Mn due to the trace amounts of it.
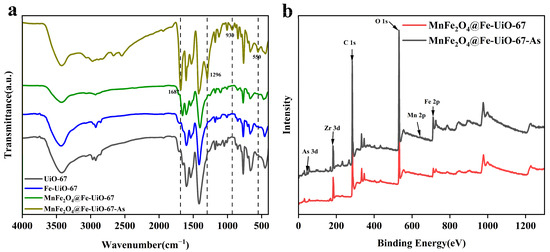
Figure 8.
FT-IR (a) and XPS gross spectra of adsorbents (b).
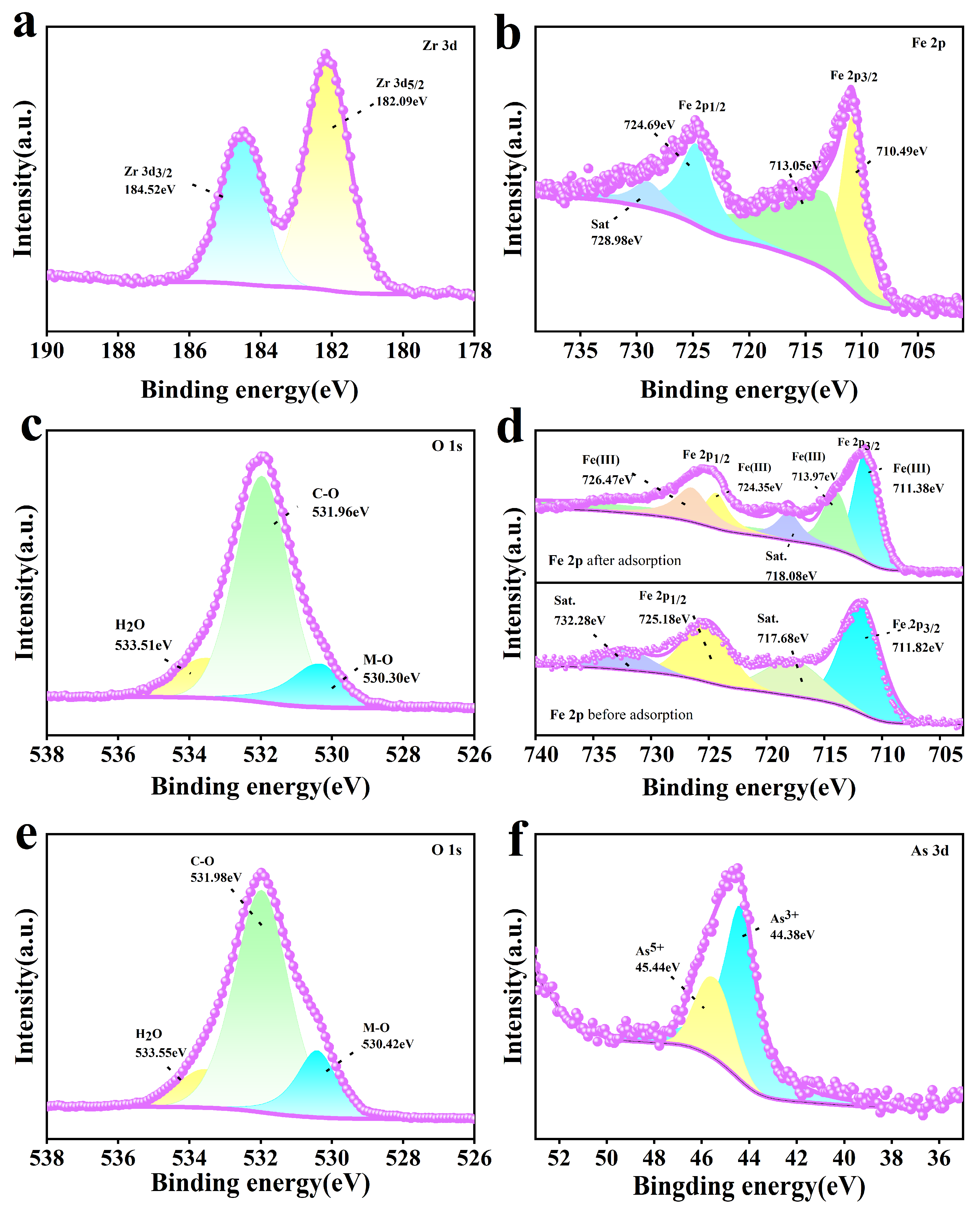
Figure 9 shows the XPS spectra of the adsorbent. The Zr 3d and Fe 2p spectra of Fe-UiO-67 are shown in Figure 9a and b, respectively. In the Zr 3d spectrum, two characteristic peaks are shown at 182.09 eV and 184.52 eV, attributed to Zr 3d5/2 and Zr 3d3/2, respectively [47,48]. In the Fe 2p spectra, the characteristic peaks at 710.49 eV and 724.69 eV are attributed to Fe 2p3/2 and Fe 2p1/2, and the appearance of the characteristic peaks at 713.05 eV and 728.98 eV can be assigned to the satellite peaks, confirming the successful doping of Fe3+ on UiO-67 [49,50]. According to the O 1s spectra of MnFe2O4@Fe-UiO-67 before and after adsorption (Figure 9c,e), the peak area of the M–O bond after adsorption was significantly increased, which, in combination with the FT-IR spectra (Figure 8a), indicates the generation of an As–O bond and Fe–O bond. Figure 9d shows the Fe 2p mapping; the peaks at 711.82 eV and 725.81 eV before adsorption can be attributed to Fe 2p3/2 and Fe 2p1/2, while 717.68 eV and 732.28 eV are satellite peaks [49]. After adsorption, the characteristic peaks of the Fe 2p spectra were at 711.38 eV/713.97 eV and 724.35 eV/726.47 eV, and the satellite peaks were 718.08 eV and 726.47 eV. The peaks were significantly changed compared to those before adsorption, which indicates that a chemical reaction occurred in the system. Figure 9f shows the As 3D spectrum with peaks at 44.38 eV and 45.44 eV for As3+ and As5+ respectively [51]. It indicates that arsenic ions were adsorbed onto the MnFe2O4@Fe-UiO-67.
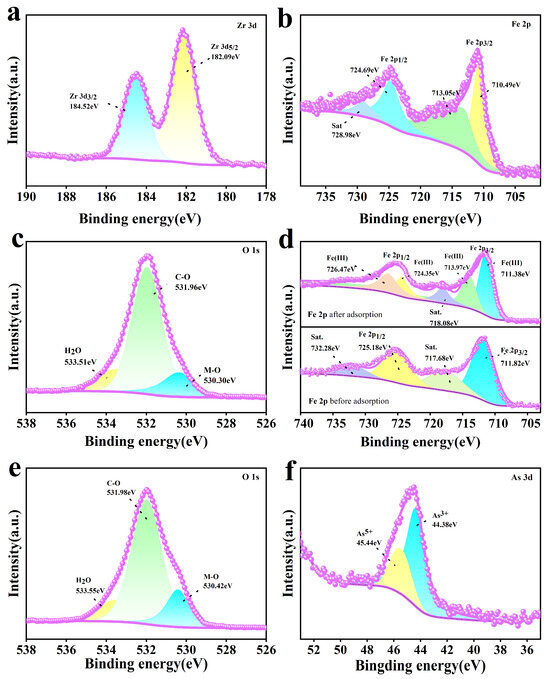
Figure 9.
XPS of adsorbents Fe-UiO-67 and MnFe2O4@Fe-UiO-67. O1s and Fe2p (a,b) of Fe-UiO-67, and O 1s of MnFe2O4@Fe-UiO-67 (c); Fe 2p (d) before and after arsenic adsorption, and O 1s (e) and As 3d (f) after arsenic adsorption of MnFe2O4@Fe-UiO-67.
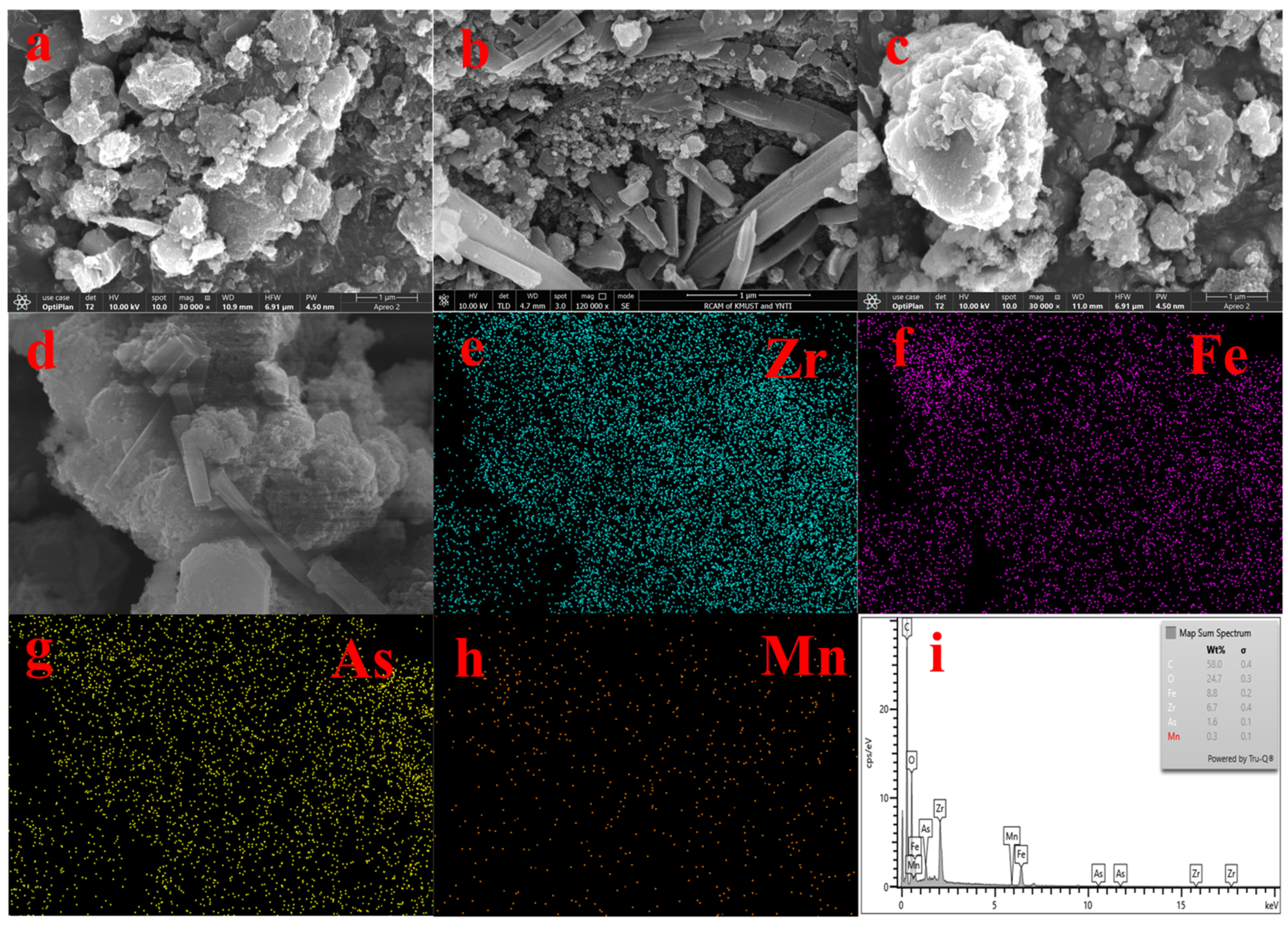
Figure 10 shows the SEM plots (a, b, c, d) of MnFe2O4@Fe-UiO-67 after adsorption. By comparing the morphologies before and after adsorption (Figure 2d,e show the morphology before adsorption), slight changes occurred in the structure of the adsorbent, which confirms the conclusions drawn from Figure 7a,b. The appearance of arsenic elements in the EDS plots (e, f, h, i) suggests that arsenic ions are adsorbed on it.
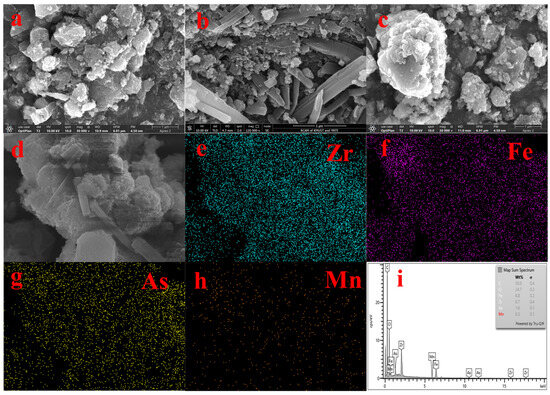
Figure 10.
SEM (a–d) and EDS (e–i) of MnFe2O4@Fe-UiO-67 after adsorption.
5. Environmental Applications Outlook
The adsorbent is synthesized with the help of a low-temperature energy-saving process using green materials as raw materials, which are not only environmentally friendly but also economically feasible. The adsorbent can be recycled many times, and thermodynamic analysis shows that the adsorbent can achieve high adsorption efficiency at room temperature without additional energy consumption, which reduces energy costs. The core component of the adsorbent is Zr-MOF with a fcu topology, which can be introduced into the defective sites by modulators to form more open Zr sites (Lewis acid sites), resulting in superior adsorption performance [52,53]. Meanwhile, both Zr-MOF and MnFe₂O₄ exhibit excellent adsorption performance toward various heavy metal ions (such as Pb2+, Cd2+, Cu2+, etc.) and organic pollutants, which endows them with a broader application scope [54,55,56].
Currently, as a leader in the industry, BASF has taken the lead in overcoming technical challenges and successfully established a commercial production chain for MOF materials [57]. With an annual production capacity of hundreds of tons, it has laid a solid material foundation for the in-depth application and rapid popularization of MOF materials in the field of technology. With the continuous progress of materials science, on the one hand, through the refined improvement of the synthesis process and the exploration of the potential for large-scale production, the preparation cost of MOFs materials has been significantly reduced. This not only broadens the application market and endows the materials with broad application prospects, but also remarkably enhances the cost–performance ratio of MOF adsorbents, making their promotion and application in small and medium-sized enterprises more smooth. On the other hand, these measures have promoted the integration of MOF materials into multiple industries such as environmental protection, chemical engineering, energy, biomedicine, and electronics, helping to solve practical problems. This further highlights the huge development potential of MOF materials and paves the way for a broader future for them.
6. Conclusions
In this study, a simple and green synthesis method was used to synthesize the new composite nanomaterial MnFe2O4@Fe-UiO-67. The material showed excellent adsorption performance, with an arsenic removal rate of 98.43% and an adsorption capacity of 600.25 mg/g. Characterization analyses by XRD and SEM proved that MnFe2O4 and Fe ions were successfully loaded on UiO-67. The experimental results showed that the process of arsenic adsorption on MnFe2O4@Fe-UiO-67 conformed to the Langmuir and pseudo-second-order kinetic models, indicating that the adsorption process was monomolecular-layer chemisorption. Through thermodynamic analysis, we were able to confirm that this adsorption reaction is a spontaneous exothermic reaction, and it exhibits excellent adsorption performance at room temperature without the need for additional energy consumption. Moreover, after four adsorption–desorption cycles, the adsorbent still exhibited high adsorption performance, demonstrating its excellent stability and regeneration capability, which is of significant application value in practical water treatment.
Author Contributions
M.G.: conceptualization, methodology, Formal analysis, writing—review and editing, and visualization. X.Q.: conceptualization, methodology, writing—review and editing, and supervision. J.F.: formal analysis and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (Grant No. 52160011).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank the State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, for the technical and analytical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hu, H.; Xie, B.; Lu, Y.; Zhu, J. Advances in Electrochemical Detection Electrodes for As(III). Nanomaterials 2022, 12, 781. [Google Scholar] [CrossRef]
- Sattar, A.; Xie, S.; Hafeez, M.A.; Wang, X.; Hussain, H.I.; Iqbal, Z.; Pan, Y.; Iqbal, M.; Shabbir, M.A.; Yuan, Z. Metabolism and Toxicity of Arsenicals in Mammals. Environ. Toxicol. Pharmacol. 2016, 48, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Huang, A.-C.; Chen, W.-T.; Wu, C.-Y.; Wan, T.-J. Utilizing Magnetic Nanoparticles Embedded into Polyvinyl Alcohol and Sodium Alginate for the Absorption of Arsenic. J. Loss Prev. Process Ind. 2024, 90, 105348. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, Y.; Zhang, X.; Wang, R.; Guo, T.; Wang, Q.; Zhao, H.; Xing, M. New Insights into Zinc Alleviating Renal Toxicity of Arsenic-Exposed Carp (Cyprinus carpio) through YAP-TFR/ROS Signaling Pathway. Pestic. Biochem. Physiol. 2024, 205, 106153. [Google Scholar] [CrossRef] [PubMed]
- Mng’ong’o, M.E.; Mabagala, F.S. Arsenic and Cadmium Availability and Its Removal in Paddy Farming Areas. J. Environ. Manag. 2024, 360, 121190. [Google Scholar] [CrossRef] [PubMed]
- Adier, M.; Jurdyc, A.-M.; Hurel, C.; Goutaland, F.; Michalon, J.-Y.; Merlen, A.; Dussardier, B.; Vouagner, D. Toward Surface-Enhanced Raman Scattering Using Electroless Substrate for Trace Arsenic Detection and Speciation. J. Appl. Phys. 2023, 133, 073103. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Lo, S.-C.; Mou, J.-L.; Huang, W.-C.; Huang, B.-H.; Yen, H.-Y.; Wang, J.-H.; Wu, P.-W.; Lai, C.-H. Comparative Adsorption Study of As3+ and Se4+ by Different Crystalline Phases of Copper Ferrite with Experiments and DFT Calculation. Appl. Surf. Sci. 2025, 687, 162297. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, A.; Liu, J.; Yang, Y. Gaseous Arsenic Capture and Immobilization from Incineration Flue Gas by MFe2O4 (M = Ca, Mn, Co, Ni, Cu and Zn) Spinel Sorbents. Fuel 2025, 386, 134238. [Google Scholar] [CrossRef]
- William, V.U.; Magpantay, H.D. Arsenic and Microorganisms: Genes, Molecular Mechanisms, and Recent Advances in Microbial Arsenic Bioremediation. Microorganisms 2024, 12, 74. [Google Scholar] [CrossRef]
- Ding, W.-Q.; Labiadh, L.; Xu, L.; Li, X.-Y.; Chen, C.; Fu, M.-L.; Yuan, B. Current Advances in the Detection and Removal of Organic Arsenic by Metal-Organic Frameworks. Chemosphere 2023, 339, 139687. [Google Scholar] [CrossRef]
- Xi, C.; Wang, R.; Rao, P.; Zhang, W.; Yan, L.; Li, G.; Chai, F.; Cai, Y.; Luo, T.; Zhou, X. The Fabrication and Arsenic Removal Performance of Cellulose Nanocrystal-Containing Absorbents Based on the “Bridge Joint” Effect of Iron Ions. Carbohydr. Polym. 2020, 237, 116129. [Google Scholar] [CrossRef] [PubMed]
- Chevinli, A.S.; Rahmatinejad, J.; Hmidi, N.; Rodrigue, D.; Ye, Z. MgFe Layered Double Hydroxide-Graphene Oxide Nanocomposite Adsorbents for Arsenic Removal. J. Water Process Eng. 2024, 59, 105017. [Google Scholar] [CrossRef]
- Song, X.; Zhou, L.; Zhang, Y.; Chen, P.; Yang, Z. A Novel Cactus-like Fe3O4/Halloysite Nanocomposite for Arsenite and Arsenate Removal from Water. J. Clean. Prod. 2019, 224, 573–582. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of Heavy Metals on Conventional and Nanostructured Materials for Wastewater Treatment Purposes: A Review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Qi, Z.; Li, X.; Zhu, Y.; Li, L.; Liu, Y. Construction and Evaluation of pH-Responsive Nitrogen-Containing Metal–Organic Frameworks as Oral Drug Carriers. Appl. Organomet. Chem. 2025, 39, e7675. [Google Scholar] [CrossRef]
- Farwa, U.; Sandhu, Z.A.; Kiran, A.; Raza, M.A.; Ashraf, S.; Gulzarab, H.; Fiaz, M.; Malik, A.; Al-Sehemi, A.G. Revolutionizing Environmental Cleanup: The Evolution of MOFs as Catalysts for Pollution Remediation. RSC Adv. 2024, 14, 37164–37195. [Google Scholar] [CrossRef]
- Khan, M.S.; Zhu, S.; Chen, S.B. Metal-Organic Frameworks (MOFs) for Oxo-Anion Removal in Wastewater Treatment: Advancements and Applications. Chem. Eng. J. 2024, 500, 157396. [Google Scholar] [CrossRef]
- Ma, H.; Huang, Y.; Tang, J.; Wang, W.; Liu, B.; Sun, H.; Yang, S.; Han, G. Enhanced Fluoride Removal via Adsorption Flotation Based on La-MOF Carrier Morphology Modulation Strategy. Sep. Purif. Technol. 2025, 365, 132652. [Google Scholar] [CrossRef]
- Molavi, H.; Salimi, M.S. Investigation the Effect of Exchange Solvents on the Adsorption Performances of Ce-MOFs towards Organic Dyes. Sci. Rep. 2025, 15, 7074. [Google Scholar] [CrossRef]
- Ullah, S.; Bustam, M.A.; Sagir, M.; Raza, M.R.; Nawaz, S.; Assiri, M.A.; Al-Sehemi, A.G.; Mukhtar, A.; Feng, C.L.; Ayoub, M. Synthesis and Doping of Alkali Metals on MOF-74 for CO2 and CH4 Pure and Binary Mixtures Adsorption. Gas Sci. Eng. 2025, 134, 205502. [Google Scholar] [CrossRef]
- Assoualaye, G.; Tom, A.; Djongyang, N. Monte Carlo Study of Hydrogen Adsorption by MOF-5 Doped with Cobalt at Ambient Temperature and Pressure. SN Appl. Sci. 2020, 2, 1815. [Google Scholar] [CrossRef]
- Su, M.; Yang, L. Fe/Mn-MOFs with Monocarboxylic Acid-Induced Defects Enhances the Catalytic Oxidation of Calcium Sulfite in Desulfurization Ash. Sep. Purif. Technol. 2025, 353, 128300. [Google Scholar] [CrossRef]
- He, W.; Lv, D.; Guan, Y.; Yu, S. Post-Synthesis Modification of Metal–Organic Frameworks: Synthesis, Characteristics, and Applications. J. Mater. Chem. A 2023, 11, 24519–24550. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, Y.; Li, L.; Chen, W.; Li, F.; Kitagawa, S. Controllable Modular Growth of Hierarchical MOF-on-MOF Architectures. Angew. Chem. Int. Ed. 2017, 56, 15658–15662. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Y.; Gu, X.; Zhao, Y. Adsorption Behavior and Mechanism of Different Arsenic Species on Mesoporous MnFe2O4 Magnetic Nanoparticles. Chemosphere 2017, 181, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Wang, D.; Song, Y.; Liu, Y.; Nghiem, L.D.; Duan, J.; Che, C.; Sun, X.; Cai, Z. The Promoted Fenton Degradation of Norfloxacin by a S-ZnO Modified MnFe2O4 with Micro-Acidic Environment. Chem. Eng. J. 2025, 506, 159898. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, S.; Chen, D.; Wu, Q.; Pan, L.; Xu, J.; Zhao, Y. Boosting Arsenic Removal with Metastable Fe2+/Mn3+ Redox Process in MnFe2O4/rGO Composites for High Capacity and Stability. J. Hazard. Mater. 2025, 489, 137652. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Razeghi, J.; Ahmadi, M.; Ramavandi, B. Amendment of Sargassum oligocystum Bio-Char with MnFe2O4 and Lanthanum MOF Obtained from PET Waste for Fluoride Removal: A Comparative Study. Environ. Res. 2024, 251, 118641. [Google Scholar] [CrossRef]
- Jemli, S.; Vieira, Y.; Chamtouri, F.; Silva, L.F.O.; Oliveira, M.L.S.; Ben Amara, F.; Bejar, S.; Vizzotto, B.B.; Wahab, R.; Irshad, S.; et al. Development of Sunflower Seed Hulls Crosslinked β-Cyclodextrin (SFSH-β-CD) Composite Materials for Green Adsorption of Phenol and Naphthenic Acid. J. Environ. Chem. Eng. 2025, 13, 115419. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Liu, B.; Gao, L.; Qiao, L.; Xiong, C.; Qiao, L.; Li, Y.; Zhang, P.; Zhu, D.; et al. Ecologically Friendly 2D/2D Na+-MXene/LDH for Cesium Adsorption in Salt Lakes: A Comprehensive Study on Adsorption Performance, Mechanisms, and Environmental Impact. Desalination 2025, 602, 118632. [Google Scholar] [CrossRef]
- Wen, Z.; Xi, J.; Lu, J.; Zhang, Y.; Cheng, G.; Zhang, Y.; Chen, R. Porous Biochar-Supported MnFe2O4 Magnetic Nanocomposite as an Excellent Adsorbent for Simultaneous and Effective Removal of Organic/Inorganic Arsenic from Water. J. Hazard. Mater. 2021, 411, 124909. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lin, Y.; Ma, Y.; Zhao, L. Ce-Doped UiO-67 Nanocrystals with Improved Adsorption Property for Removal of Organic Dyes. RSC Adv. 2019, 9, 27674–27683. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Qi, X.; Wang, H.; Shi, J. Magnetic MnFe2O4-MIL-53 (Fe) Composite as an Effective Adsorbent for As(V) Adsorption in Wastewater. Microporous Mesoporous Mater. 2022, 346, 112290. [Google Scholar] [CrossRef]
- Chen, C.; He, E.; Jiang, X.; Xia, S.; Yu, L. Efficient Removal of Direct Dyes and Heavy Metal Ion by Sodium Alginate-Based Hydrogel Microspheres: Equilibrium Isotherms, Kinetics and Regeneration Performance Study. Int. J. Biol. Macromol. 2025, 294, 139294. [Google Scholar] [CrossRef]
- Jimenez-Paz, J.; Lozada-Castro, J.J.; Lester, E.; Williams, O.; Stevens, L.; Barraza-Burgos, J. Solutions to Hazardous Wastes Issues in the Leather Industry: Adsorption of Chromium Iii and vi from Leather Industry Wastewaters Using Activated Carbons Produced from Leather Industry Solid Wastes. J. Environ. Chem. Eng. 2023, 11, 109715. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, S.; Xu, H.; Liu, Q.; Li, N.; Yang, C.; Amirkhanian, S. Molecular Dynamics-Based Targeted Adsorption of Hazardous Substances from Rubberized Asphalt VOCs by UiO-67. J. Clean. Prod. 2024, 476, 143762. [Google Scholar] [CrossRef]
- Deivatamil, D.; Martin Mark, J.A.; Raghavan, T.; Jesuraj, J.P. Fabrication of MnFe2O4 and Ni: MnFe2O4 Nanoparticles for Ammonia Gas Sensor Application. Inorg. Chem. Commun. 2021, 123, 108355. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, X.; Zhang, Y.; Zhou, C.; Wu, X.; Li, M.; Hua, Y. Stability and Adsorption Performance of UiO-67 for Uranium(VI) in Solution. J. Radioanal. Nucl. Chem. 2024, 333, 305–315. [Google Scholar] [CrossRef]
- Ke, X.; Shao, H.; Liu, H.; Liu, W. How Fe-La-CS Microspheres Have Selective Adsorption Capacity for As(V) over a Wide pH Range: Coupling Molecular Scale Interpretation with Experiments. Chem. Eng. J. 2024, 497, 154768. [Google Scholar] [CrossRef]
- Zhi, G.; Qi, X.; Li, Y.; Wang, J.; Wang, J. Efficient Treatment of Smelting Wastewater: 3D Nickel Foam @MOF Shatters the Previous Limitation, Enabling High-Throughput Selective Capture of Arsenic to Form Non-Homogeneous Nuclei. Sep. Purif. Technol. 2024, 328, 124927. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; et al. Arsenic Removal from Aqueous Solutions by Adsorption Using Novel MIL-53(Fe) as a Highly Efficient Adsorbent. RSC Adv. 2014, 5, 5261–5268. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, J.; Ou, X.; Wan, J.; Cai, Y.; Lin, Z.; Dang, Z.; Xing, B. Enhanced Adsorption of P-Arsanilic Acid from Water by Amine-Modified UiO-67 as Examined Using Extended X-Ray Absorption Fine Structure, X-Ray Photoelectron Spectroscopy, and Density Functional Theory Calculations. Environ. Sci. Technol. 2018, 52, 3466–3475. [Google Scholar] [CrossRef]
- Yang, Y.; Mo, W.; Wei, C.; Islahah, M.N.L.; Huang, Y.; Yang, J.; Feng, J.; Su, X.; Ma, S. Fe/Mn-MOF-Driven Rapid Arsenic Decontamination: Mechanistic Elucidation of Adsorption Processes and Performance Optimization. J. Water Process Eng. 2025, 69, 106691. [Google Scholar] [CrossRef]
- Sahu, U.K.; Sahu, S.; Mahapatra, S.S.; Patel, R.K. Cigarette Soot Activated Carbon Modified with Fe3O4 Nanoparticles as an Effective Adsorbent for As(III) and As(V): Material Preparation, Characterization and Adsorption Mechanism Study. J. Mol. Liq. 2017, 243, 395–405. [Google Scholar] [CrossRef]
- Ta, K.M.; Wisdom, D.O.; Gillie, L.J.; Cooke, D.J.; Zhu, R.; Gonçalves, M.A.; Parker, S.C.; Molinari, M. Sorption of Arsenate on Cerium Oxide: A Simulated Infrared and Raman Spectroscopic Identification. Environ. Sci. Nano 2025, 12, 1896–1907. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, M.; Shang, H.; Cheng, Y.; Ramella, D.; Zhu, K.; Luan, Y. UiO-67 Metal–Organic Framework Immobilized Fe3+ Catalyst for Efficient Morita–Baylis–Hillman Reaction. New J. Chem. 2022, 46, 3199–3206. [Google Scholar] [CrossRef]
- Jia, S.; Lin, P.; Li, Y.; Teng, Y.; Wang, J.; Yang, T.; Li, L.; Wang, C.; Li, X. Flexible UiO-67(Zr)@cyclodextrin-Based Nanofiber Membrane for Efficient Removal of Ibuprofen. Sep. Purif. Technol. 2024, 333, 125850. [Google Scholar] [CrossRef]
- Byun, A.; Moon, D.; Lee, B.; Park, J. Amorphous Porous Fe-BTC Prepared via the Post-Synthetic Metal-Ion Metathesis of HKUST-1. J. Mater. Chem. A 2023, 11, 24591–24597. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Y.; Shi, J.; Zhong, Y.; Zhu, W.; El-Shall, M.S. Polyoxometalates Confined in the Mesoporous Cages of Metal–Organic Framework MIL-100(Fe): Efficient Heterogeneous Catalysts for Esterification and Acetalization Reactions. Chem. Eng. J. 2015, 269, 236–244. [Google Scholar] [CrossRef]
- Zhao, P.; Guan, J.; Liu, Q.; Wu, J.; Ling, Y.; He, P.; Yao, H.; Luo, G.; Hong, J.; Xie, X. Microscopic Spherical α-Fe2O3 for Highly Efficient Gaseous Arsenic Capture in Simulated Flue Gas Under a Wide Temperature Range. Energy Fuels 2021, 35, 19581–19591. [Google Scholar] [CrossRef]
- Fang, F.; Lv, Q.; Li, P.; Tao, Y.; Zhang, Y.; Zhou, Y.; Li, X.; Li, J. Screening of Hierarchical Porous UiO-67 for Efficient Removal of Glyphosate from Aqueous Solution. J. Environ. Chem. Eng. 2022, 10, 107824. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, B.; Wang, F.; Yan, Y.; Hong, X.; Xu, H.; Xia, M.; Wang, F. Green Synthesis of MOF-808 with Modulation of Particle Sizes and Defects for Efficient Phosphate Sequestration. Sep. Purif. Technol. 2022, 300, 121825. [Google Scholar] [CrossRef]
- Tsukada, A.; Konno, H. Simultaneous Removal of Various Organic Dyes from Aqueous Solutions Using a UiO-67-Type Metal–Organic Framework. Colloids Surf. A 2024, 686, 133330. [Google Scholar] [CrossRef]
- Du, M.; Cao, Y.; Luo, X.; Yang, W.; Lin, W.; Wang, Y.; Tang, W.; Li, Z. Shapeable Sodium Alginate Aerogel Beads Incorporated with L-Cysteine-Modified Defective UiO-67 for Heavy Metal Ions Removal. Chem. Eng. J. 2023, 475, 146289. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, J.; Zhu, W.; Hou, R.; Zhang, B.; Ishag, A. The Recent Advances of MnFe2O4-Based Nanoparticles in Environmental Application: A Review. Sci. Total Environ. 2024, 954, 176378. [Google Scholar] [CrossRef]
- Frameworks for Commercial Success. Nat. Chem. 2016, 8, 987. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).