Revealing the Molecular Mechanisms of Ozone-Induced Pulmonary Inflammatory Injury: Integrated Analysis of Metabolomics and Transcriptomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and O3 Exposure
2.2. Pulmonary Function Testing
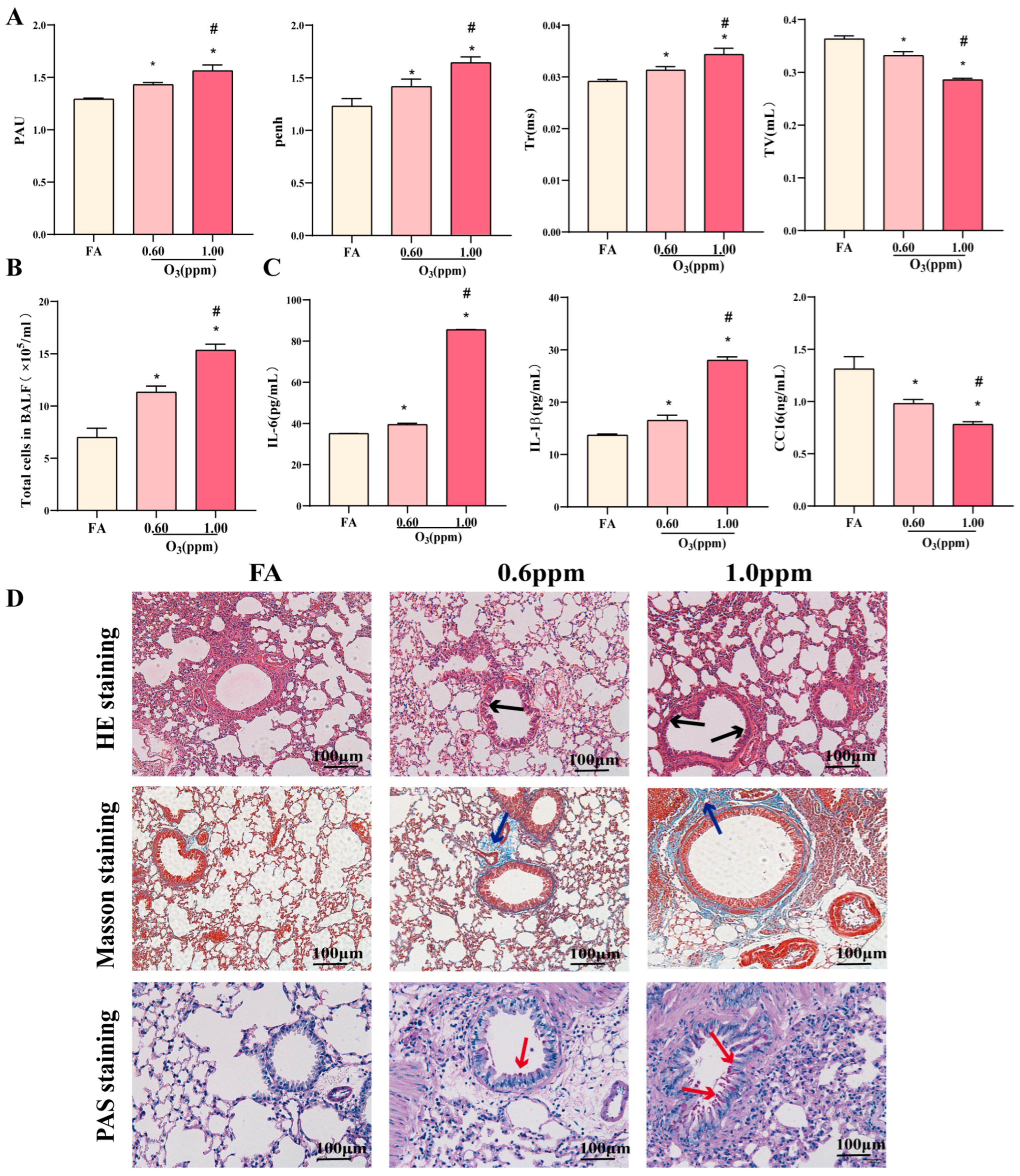
2.3. Measurement of Lung Inflammation
2.3.1. Histopathological Staining of Lung
2.3.2. Collection and Analysis of BALF
2.4. Transcriptomics
Analysis of Differential Expression
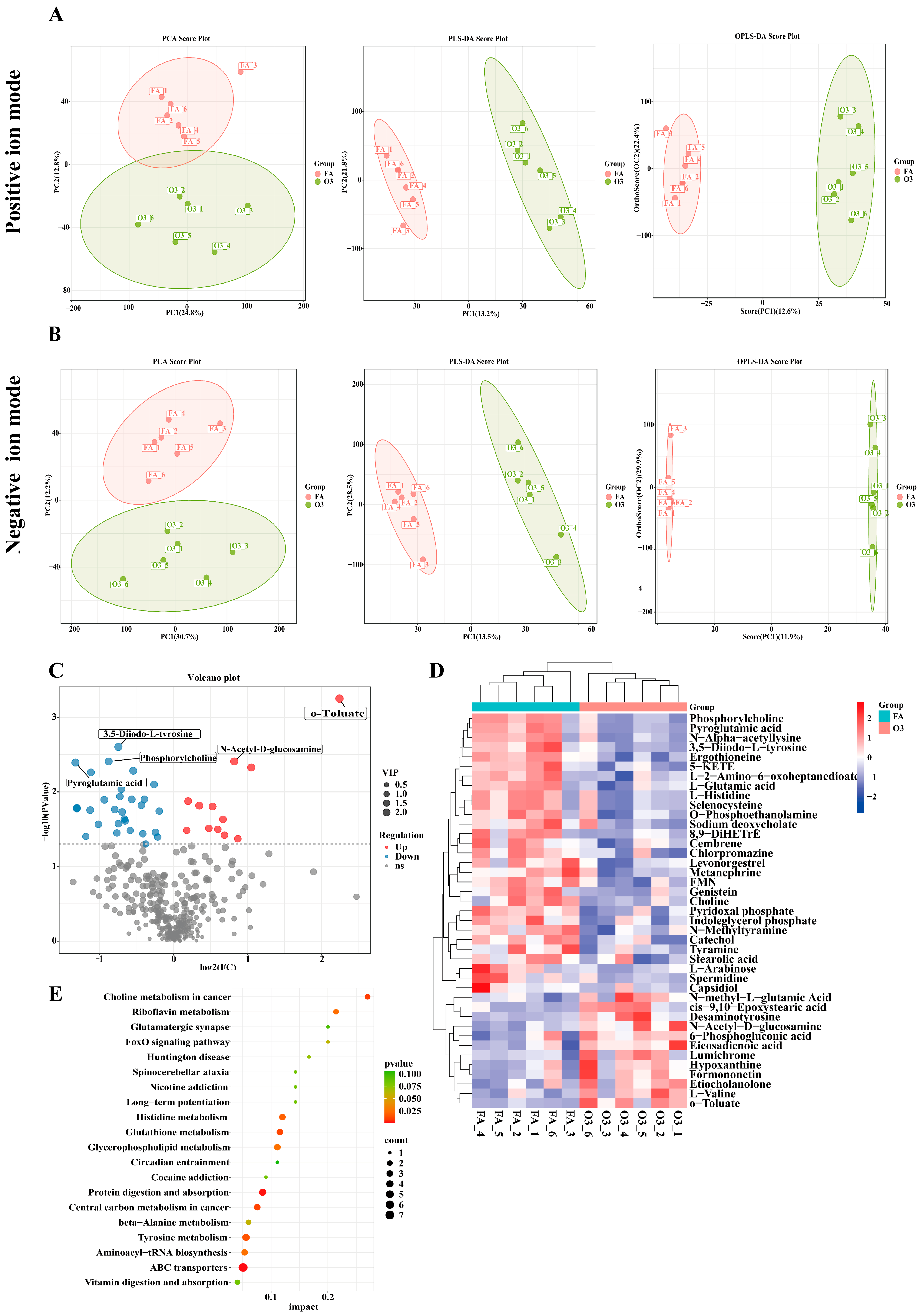
2.5. Untargeted Metabolomics
2.5.1. Sample Extraction
2.5.2. Liquid Chromatography Conditions
2.5.3. Mass Spectrum Conditions
2.5.4. Differential Metabolism Analysis
2.6. Statistical Analyses
3. Results
3.1. O3 Induces Pulmonary Inflammatory Injury in Mice
3.2. Effect of O3 Exposure on Transcriptomics of Lung Tissues in Mice
3.3. Effects of O3 Exposure on Lung Tissue Metabolomics in Mice
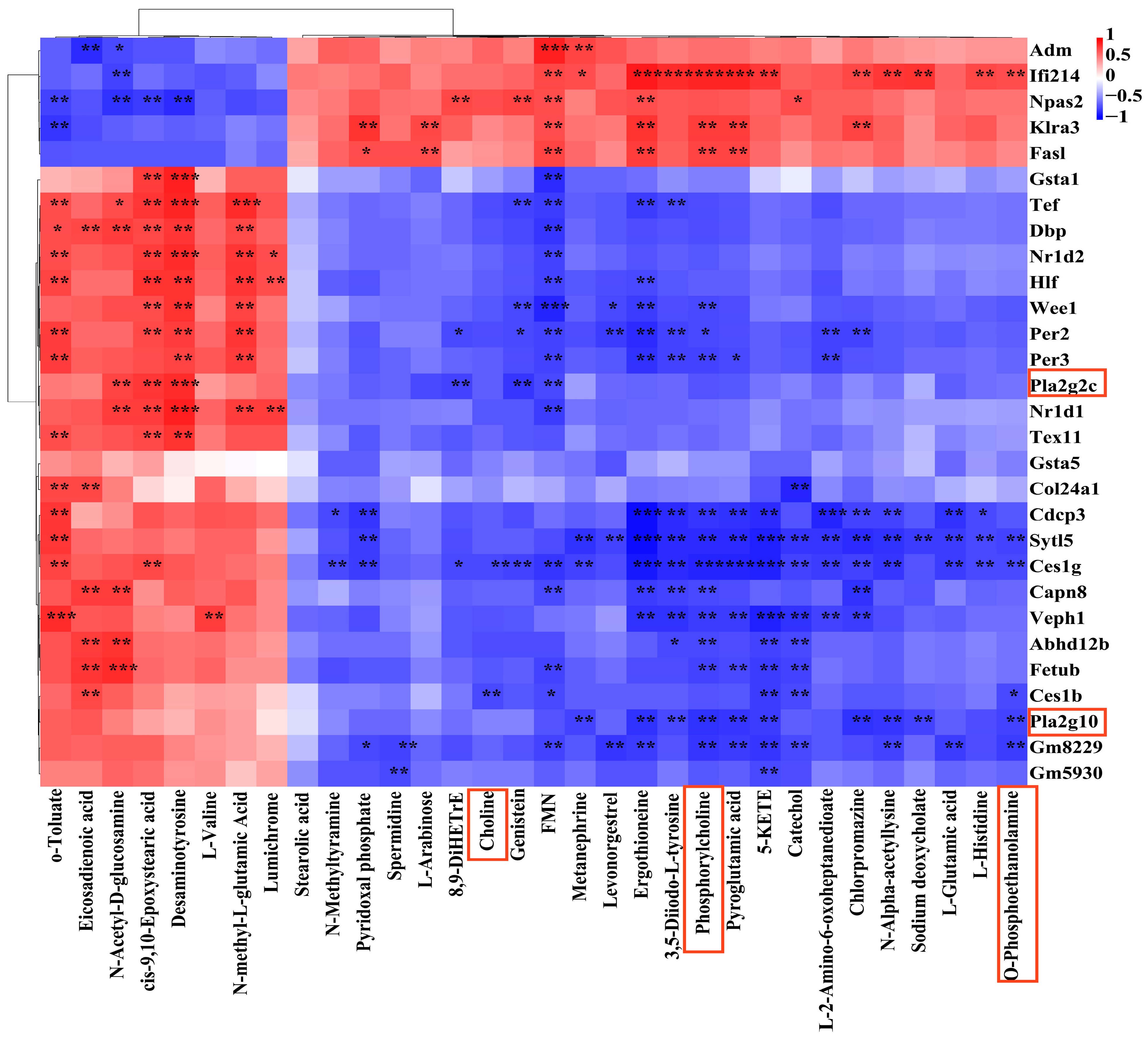
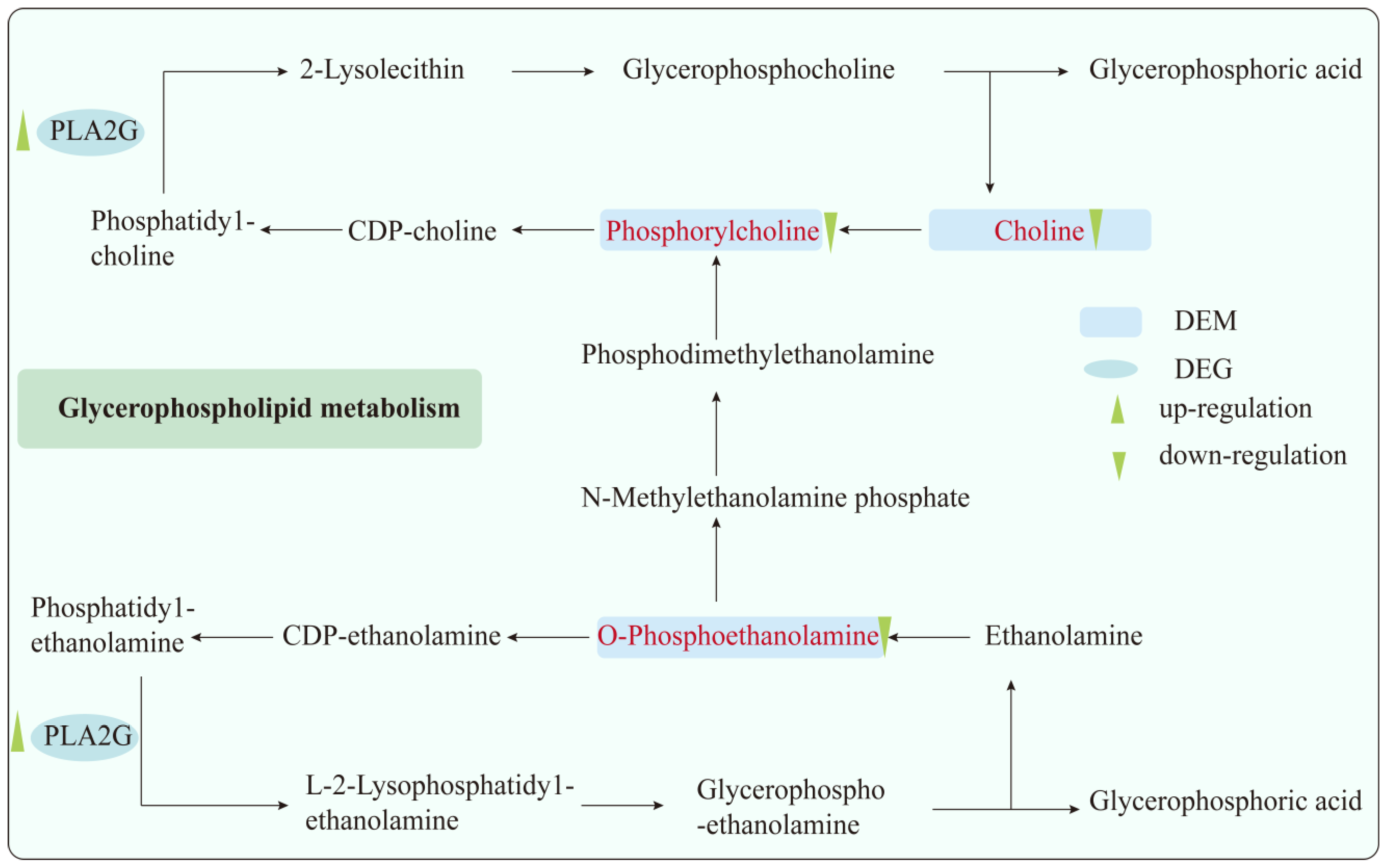
3.4. Combined Analysis of Transcriptomics and Metabolomics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHR | Airway hyperresponsiveness |
| BALF | Bronchoalveolar Lavage Fluid |
| CC16 | Clara Cell Secretory Protein 16 |
| DEGs | Differentially Expressed Genes |
| DEMs | Differential metabolites |
| FA | Filtered Air (Control Group) |
| GO | Gene Ontology |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| O-PEA | O-Phosphoethanolamine |
| PAU | Pause |
| PC | Phosphorylcholine |
| Penh | Enhanced Pause |
| Pla2g10 | Phospholipase A2 Group X |
| PLS-DA | Partial Least Squares-Discriminant Analysis |
| OPLS-DA | Orthogonal PLS-DA |
| SPF | Specific Pathogen-Free |
| Tr | Relaxation Time |
| TV | Tidal Volume |
| VIP | Variable Importance in Projection |
| WBP | Whole Body Plethysmography |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| Pla2g2c | Phospholipase A2 group IIC |
| HMDB | Human Metabolome Database |
| ELISA | Enzyme-linked immunosorbent assay |
References
- Guo, Z.; Jing, X.; Ling, Y.; Yang, Y.; Jing, N.; Yuan, R.; Liu, Y. Optimized air quality management based on air quality index prediction and air pollutants identification in representative cities in China. Sci. Rep. 2024, 14, 17923. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Li, G.; Wang, W.; Wang, K.; Wang, J.; Wei, C.; Li, Y.; Deng, F.; Baccarelli, A.A.; et al. Ozone pollution and hospital admissions for cardiovascular events. Eur. Heart J. 2023, 44, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Bontinck, A.; Maes, T.; Joos, G. Asthma and air pollution: Recent insights in pathogenesis and clinical implications. Curr. Opin. Pulm. Med. 2020, 26, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, P.; Atkinson, R. Long-term exposure to NO(2) and O(3) and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2020, 144, 105998. [Google Scholar] [CrossRef]
- Yang, L.; Xu, F.; Zhao, S.; Zeng, Y.; Wu, Q.; Zhang, L.; Shi, S.; Zhang, F.; Li, J.; An, Z.; et al. Airway microbiota dysbiosis and metabolic disorder in ozone and PM(2.5) co-exposure induced lung inflammatory injury in mice. Ecotox. Environ. Saf. 2025, 290, 117626. [Google Scholar] [CrossRef]
- Wright, N.; Newell, K.; Chan, K.H.; Gilbert, S.; Hacker, A.; Lu, Y.; Guo, Y.; Pei, P.; Yu, C.; Lv, J.; et al. Long-term ambient air pollution exposure and cardio-respiratory disease in China: Findings from a prospective cohort study. Environ. Health-Glob. 2023, 22, 30. [Google Scholar] [CrossRef]
- Radbel, J.; Meshanni, J.A.; Vayas, K.N.; Le-Hoang, O.; Abramova, E.; Zhou, P.; Joseph, L.B.; Laskin, J.D.; Gow, A.J.; Laskin, D.L. Effects of ozone exposure on lung injury, inflammation, and oxidative stress in a murine model of non-pneumonic endotoxemia. Toxicol. Sci. 2024, 200, 299–311. [Google Scholar] [CrossRef]
- Liu, J.; Ma, S.; Deng, D.; Yang, Y.; Li, J.; Zhang, Y.; Yin, P.; Shang, D. Multi-Omics Profiling Reveals Glycerolipid Metabolism-Associated Molecular Subtypes and Identifies ALDH2 as a Prognostic Biomarker in Pancreatic Cancer. Metabolites 2025, 15, 207. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, L.; Yan, R.; Liu, L.; Guo, P.; Liu, S.; Chen, Y.; Yuan, Z.; Gong, W.; Ji, J. Identification of Candidate Lung Function-Related Plasma Proteins to Pinpoint Drug Targets for Common Pulmonary Diseases: A Comprehensive Multi-Omics Integration Analysis. Curr. Issues Mol. Biol. 2025, 47, 167. [Google Scholar] [CrossRef]
- Sundar, I.K.; Duraisamy, S.K.; Choudhary, I.; Saini, Y.; Silveyra, P. Acute and Repeated Ozone Exposures Differentially Affect Circadian Clock Gene Expression in Mice. Adv. Biol.-Ger. 2023, 7, e2300045. [Google Scholar] [CrossRef]
- Li, J.; Wei, H.; Wang, N.; Chen, J.; Zhang, Y.; An, Z.; Song, J.; Niu, T.; Wu, W. Ozone-Induced Lung Injury are Mediated Via PPAR-Mediated Ferroptosis in Mice. Biol. Trace Elem. Res. 2024, 1–13. [Google Scholar] [CrossRef]
- Aderemi, A.V.; Ayeleso, A.O.; Oyedapo, O.O.; Mukwevho, E. Metabolomics: A Scoping Review of Its Role as a Tool for Disease Biomarker Discovery in Selected Non-Communicable Diseases. Metabolites 2021, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, J.; Hu, X.; He, L.; Lin, Y.; Zhang, J.; Meng, X.; Zhang, Y.; Mo, J.; Day, D.B.; et al. Glycerophospholipid metabolism changes association with ozone exposure. J. Hazard. Mater. 2024, 475, 134870. [Google Scholar] [CrossRef]
- Kilburg-Basnyat, B.; Reece, S.W.; Crouch, M.J.; Luo, B.; Boone, A.D.; Yaeger, M.; Hodge, M.; Psaltis, C.; Hannan, J.L.; Manke, J.; et al. Specialized Pro-Resolving Lipid Mediators Regulate Ozone-Induced Pulmonary and Systemic Inflammation. Toxicol. Sci. 2018, 163, 466–477. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, P.; Gong, F.; Tan, Y.; Han, J.; Tian, L.; Yan, J.; Li, K.; Xi, Z.; Liu, X. Altered lipidomic profiles in lung and serum of rat after sub-chronic exposure to ozone. Sci. Total Environ. 2022, 806, 150630. [Google Scholar] [CrossRef] [PubMed]
- Plopper, C.G.; Hyde, D.M. The non-human primate as a model for studying COPD and asthma. Pulm. Pharmacol. Ther. 2008, 21, 755–766. [Google Scholar] [CrossRef]
- Hatch, G.E.; Slade, R.; Harris, L.P.; McDonnell, W.F.; Devlin, R.B.; Koren, H.S.; Costa, D.L.; McKee, J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am. J. Resp. Crit. Care 1994, 150, 676–683. [Google Scholar] [CrossRef]
- Mumaw, C.L.; Levesque, S.; McGraw, C.; Robertson, S.; Lucas, S.; Stafflinger, J.E.; Campen, M.J.; Hall, P.; Norenberg, J.P.; Anderson, T.; et al. Microglial priming through the lung-brain axis: The role of air pollution-induced circulating factors. Faseb J. 2016, 30, 1880–1891. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Yuan, Q.; Li, T.; Zhou, Y.; Zong, L.; Wang, M.; Xie, Z.; Ho, H.C.; Gao, M.; et al. Substantially underestimated global health risks of current ozone pollution. Nat. Commun. 2025, 16, 102. [Google Scholar] [CrossRef]
- Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; Hill, D.P.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef] [PubMed]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Li, M.; Kong, X.; Jian, X.; Bo, Y.; Miao, X.; Chen, H.; Shang, P.; Zhou, X.; Wang, L.; Zhang, Q.; et al. Fatty acids metabolism in ozone-induced pulmonary inflammatory injury: Evidence, mechanism and prevention. Sci. Total Environ. 2024, 933, 173222. [Google Scholar] [CrossRef]
- Papagiannakopoulos, T.; Bauer, M.R.; Davidson, S.M.; Heimann, M.; Subbaraj, L.; Bhutkar, A.; Bartlebaugh, J.; Vander, H.M.; Jacks, T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016, 24, 324–331. [Google Scholar] [CrossRef]
- Andersen, M.E.; Cruzan, G.; Black, M.B.; Pendse, S.N.; Dodd, D.; Bus, J.S.; Sarang, S.S.; Banton, M.I.; Waites, R.; McMullen, P.D. Assessing molecular initiating events (MIEs), key events (KEs) and modulating factors (MFs) for styrene responses in mouse lungs using whole genome gene expression profiling following 1-day and multi-week exposures. Toxicol. Appl. Pharm. 2017, 335, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Chen, S.; Wei, B.; Gao, Y.; Huang, L.; Liu, C.; Huang, T.; Yu, M.; Zhao, S.H.; et al. Ammonia exposure causes lung injuries and disturbs pulmonary circadian clock gene network in a pig study. Ecotox. Environ. Saf. 2020, 205, 111050. [Google Scholar] [CrossRef]
- Song, P.; Li, Z.; Li, X.; Yang, L.; Zhang, L.; Li, N.; Guo, C.; Lu, S.; Wei, Y. Transcriptome Profiling of the Lungs Reveals Molecular Clock Genes Expression Changes after Chronic Exposure to Ambient Air Particles. Int. J. Env. Res. Public Health 2017, 14, 90. [Google Scholar] [CrossRef]
- Tang, W.; Peng, W.; Zhang, H.; Zhang, Y.; Li, B.; Duan, C. Period 3, a tumor suppressor in non-small cell lung cancer, is silenced by hypermethylation. Int. J. Clin. Exp. Pathol. 2018, 11, 120–128. [Google Scholar]
- Wang, Q.; Liu, H.; Wang, Z.; Chen, Y.; Zhou, S.; Hu, X.; Xu, Y.; Zhang, X.; Wang, Y.; Gao, Y.; et al. Circadian gene Per3 promotes astroblastoma progression through the P53/BCL2/BAX signalling pathway. Gene 2024, 895, 147978. [Google Scholar] [CrossRef]
- Course, C.W.; Lewis, P.A.; Kotecha, S.J.; Cousins, M.; Hart, K.; Heesom, K.J.; Watkins, W.J.; Kotecha, S. Evidence of abnormality in glutathione metabolism in the airways of preterm born children with a history of bronchopulmonary dysplasia. Sci. Rep. 2023, 13, 19465. [Google Scholar] [CrossRef]
- Chounlamountry, K.; Boyer, B.; Penalba, V.; François-Bellan, A.M.; Bosler, O.; Kessler, J.P.; Strube, C. Remodeling of glial coverage of glutamatergic synapses in the rat nucleus tractus solitarii after ozone inhalation. J. Neurochem. 2015, 134, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Cao, L.; Sun, X.; Zhou, S.; Zhu, T.; Zheng, J.; Liu, S.; Liu, H. Metabolomic profile of plasma approach to investigate the mechanism of Poria cocos oligosaccharides attenuated LPS-induced acute lung injury in mice. J. Pharmaceut Biomed. 2024, 247, 116262. [Google Scholar] [CrossRef]
- Baek, A.R.; Hong, J.; Song, K.S.; Jang, A.S.; Kim, D.J.; Chin, S.S.; Park, S.W. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp. Mol. Med. 2020, 52, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Massett, M.P. Effect of Spermidine on Endothelial Function in Systemic Lupus Erythematosus Mice. Int. J. Mol. Sci. 2024, 25, 9920. [Google Scholar] [CrossRef]
- Wang, X.; Chen, B.; Chen, C. Identification of biomarkers and candidate small-molecule drugs in lipopolysaccharide (LPS)-induced acute lung injury by bioinformatics analysis. Allergol. Immunopath 2023, 51, 44–53. [Google Scholar] [CrossRef]
- Ma, P.; Miao, X.; Li, M.; Kong, X.; Jiang, Y.; Wang, P.; Zhang, P.; Shang, P.; Chen, Y.; Zhou, X.; et al. Lung proteomics combined with metabolomics reveals molecular characteristics of inflammation-related lung tumorigenesis induced by B(a)P and LPS. Environ. Toxicol. 2023, 38, 2915–2925. [Google Scholar] [CrossRef]
- Sasset, L.; Di Lorenzo, A. Sphingolipid Metabolism and Signaling in Endothelial Cell Functions. Adv. Exp. Med. Biol. 2022, 1372, 87–117. [Google Scholar] [CrossRef]
- Kobayashi, A.; Nagashima, K.; Hu, A.; Harada, Y.; Kobayashi, H. Effectiveness and safety of kamikihito, a traditional Japanese medicine, in managing anxiety among female patients with intractable chronic constipation. Complement. Ther. Clin. 2022, 46, 101526. [Google Scholar] [CrossRef]
- Yang, H.; Lin, Y.; Ma, Y.; Li, J.; Li, J.; Huo, Z.; Yang, P.; Zhang, C. Screening Probiotics for Anti-Helicobacter pylori and Investigating the Effect of Probiotics on Patients with Helicobacter pylori Infection. Foods 2024, 13, 1851. [Google Scholar] [CrossRef] [PubMed]
- Sajja, V.S.; Galloway, M.P.; Ghoddoussi, F.; Thiruthalinathan, D.; Kepsel, A.; Hay, K.; Bir, C.A.; VandeVord, P.J. Blast-induced neurotrauma leads to neurochemical changes and neuronal degeneration in the rat hippocampus. Nmr Biomed. 2012, 25, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, G.; Shao, Z.; Xu, E.; Yuan, H.; Liu, J.; Gao, L. Toxicology of tramadol following chronic exposure based on metabolomics of the cerebrum in mice. Sci. Rep. 2020, 10, 11130. [Google Scholar] [CrossRef]
- Smith, L.C.; Gow, A.J.; Abramova, E.; Vayas, K.; Guo, C.; Noto, J.; Lyman, J.; Rodriquez, J.; Gelfand-Titiyevskiy, B.; Malcolm, C.; et al. Role of PPARγ in dyslipidemia and altered pulmonary functioning in mice following ozone exposure. Toxicol. Sci. 2023, 194, 109–119. [Google Scholar] [CrossRef]
- Sakai, A.; Nishiumi, S.; Shiomi, Y.; Kobayashi, T.; Izumi, Y.; Kutsumi, H.; Hayakumo, T.; Azuma, T.; Yoshida, M. Metabolomic analysis to discover candidate therapeutic agents against acute pancreatitis. Arch. Biochem. Biophys. 2012, 522, 107–120. [Google Scholar] [CrossRef]
- Böckmann, K.A.; Franz, A.R.; Shunova, A.; Minarski, M.; Wiechers, C.; Poets, C.F.; Bernhard, W. Different choline supplement metabolism in adults using deuterium labelling. Eur. J. Nutr. 2023, 62, 1795–1807. [Google Scholar] [CrossRef]
- Mehta, A.K.; Singh, B.P.; Arora, N.; Gaur, S.N. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology 2010, 215, 527–534. [Google Scholar] [CrossRef]
- Chang, K.H.; Cheng, M.L.; Tang, H.Y.; Lin, C.Y.; Chen, C.M. Dysregulation of choline metabolism and therapeutic potential of citicoline in Huntington’s disease. Aging Cell 2024, 23, e14302. [Google Scholar] [CrossRef]
- Kafoury, R.M.; Pryor, W.A.; Squadrito, G.L.; Salgo, M.G.; Zou, X.; Friedman, M. Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am. J. Resp. Crit. Care 1999, 160, 1934–1942. [Google Scholar] [CrossRef]
- Frostegård, J. Antibodies against Phosphorylcholine-Implications for Chronic Inflammatory Diseases. Metabolites 2023, 13, 720. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Zhu, L.; Chen, Y.; Chen, Y.J.; Zhu, Z.; Feng, J.; Qi, Z.; Yu, J.Z.; Yang, Z.; et al. Phosphocholine-induced energy source shift alleviates mitochondrial dysfunction in lung cells caused by geospecific PM(2.5) components. Proc. Natl. Acad. Sci. USA 2024, 121, e1977393175. [Google Scholar] [CrossRef]
- Letsiou, E.; Htwe, Y.M.; Dudek, S.M. Secretory Phospholipase A(2) Enzymes in Acute Lung Injury. Cell Biochem. Biophys. 2021, 79, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Rehman, R.; Dutta, J.; Singh, S.; Ray, A.; Shridhar, M.; Jaisankar, J.; Bhatt, M.; Khandelwal, D.; Sahoo, B.; et al. Cellular Distribution of Secreted Phospholipase A2 in Lungs of IPF Patients and Its Inhibition in Bleomycin-Induced Pulmonary Fibrosis in Mice. Cells 2023, 12, 1044. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, X.; Lou, C.; Zhou, C.; Zhao, X.; Li, N.; Tian, H.; Meng, X. PLA2G10 incorporated in exosomes could be diagnostic and prognostic biomarker for non-small cell lung cancer. Clin. Chim. Acta 2022, 530, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ogden, H.L.; Lai, Y.; Nolin, J.D.; An, D.; Frevert, C.W.; Gelb, M.H.; Altemeier, W.A.; Hallstrand, T.S. Secreted Phospholipase A(2) Group X Acts as an Adjuvant for Type 2 Inflammation, Leading to an Allergen-Specific Immune Response in the Lung. J. Immunol. 2020, 204, 3097–3107. [Google Scholar] [CrossRef]
- Bickford, J.S.; Mueller, C.; Newsom, K.J.; Barilovits, S.J.; Beachy, D.E.; Herlihy, J.D.; Keeler, B.; Flotte, T.R.; Nick, H.S. Effect of allergy and inflammation on eicosanoid gene expression in CFTR deficiency. J. Cyst. Fibros. 2013, 12, 258–265. [Google Scholar] [CrossRef][Green Version]


| Pathway | Differential Metabolites | Differential Gene | p Value |
|---|---|---|---|
| Protein digestion and absorption | L-Glutamic acid; L-Histidine; L-Valine; Tyramine | Col24a1 | 0.0024 |
| Glutathione metabolism | L-Glutamic acid; Spermidine; Pyroglutamic acid | Gsta1; Gsta5 | 0.0109 |
| Glycerophospholipid metabolism | O-Phosphoethanolamine; Phosphorylcholine; Choline; | Pla2g10; Pla2g2c | 0.0255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Guo, Y.; Jian, X.; Miao, X.; Wang, P.; Wang, X.; Wang, L.; Chen, H.; Feng, F. Revealing the Molecular Mechanisms of Ozone-Induced Pulmonary Inflammatory Injury: Integrated Analysis of Metabolomics and Transcriptomics. Toxics 2025, 13, 271. https://doi.org/10.3390/toxics13040271
Zhou X, Guo Y, Jian X, Miao X, Wang P, Wang X, Wang L, Chen H, Feng F. Revealing the Molecular Mechanisms of Ozone-Induced Pulmonary Inflammatory Injury: Integrated Analysis of Metabolomics and Transcriptomics. Toxics. 2025; 13(4):271. https://doi.org/10.3390/toxics13040271
Chicago/Turabian StyleZhou, Xiaolei, Yunnian Guo, Xiaotong Jian, Xinyi Miao, Pengpeng Wang, Xiaoke Wang, Ling Wang, Huaiyong Chen, and Feifei Feng. 2025. "Revealing the Molecular Mechanisms of Ozone-Induced Pulmonary Inflammatory Injury: Integrated Analysis of Metabolomics and Transcriptomics" Toxics 13, no. 4: 271. https://doi.org/10.3390/toxics13040271
APA StyleZhou, X., Guo, Y., Jian, X., Miao, X., Wang, P., Wang, X., Wang, L., Chen, H., & Feng, F. (2025). Revealing the Molecular Mechanisms of Ozone-Induced Pulmonary Inflammatory Injury: Integrated Analysis of Metabolomics and Transcriptomics. Toxics, 13(4), 271. https://doi.org/10.3390/toxics13040271








