Perfluorobutane Sulfonate (PFOS) Accumulation in Tissues of Cherax quadricarinatus and Its Toxicity Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. C. quadricarinatus Culture
2.3. Sample Collection
2.4. Content Detection
2.5. Transcriptomics
2.5.1. Data Quality Control
2.5.2. Sequence Alignment to the Reference Genome
2.5.3. Differential Expression Gene Analysis
2.5.4. Enrichment Analysis
2.6. qRT-PCR Validation Experiments
2.7. Data Analysis
3. Results and Discussion
3.1. The Content of PFOS in C. quadricarinatus
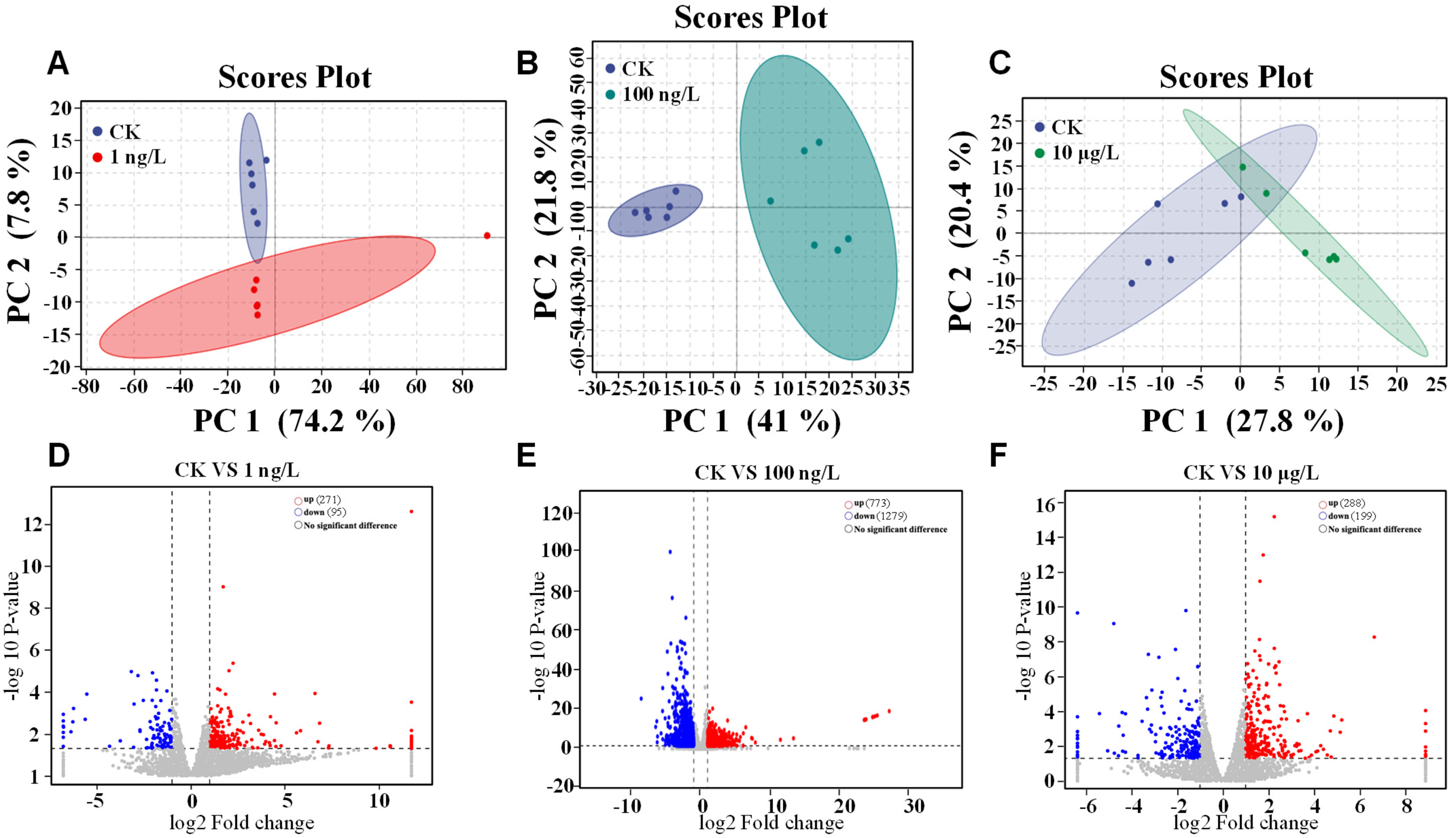
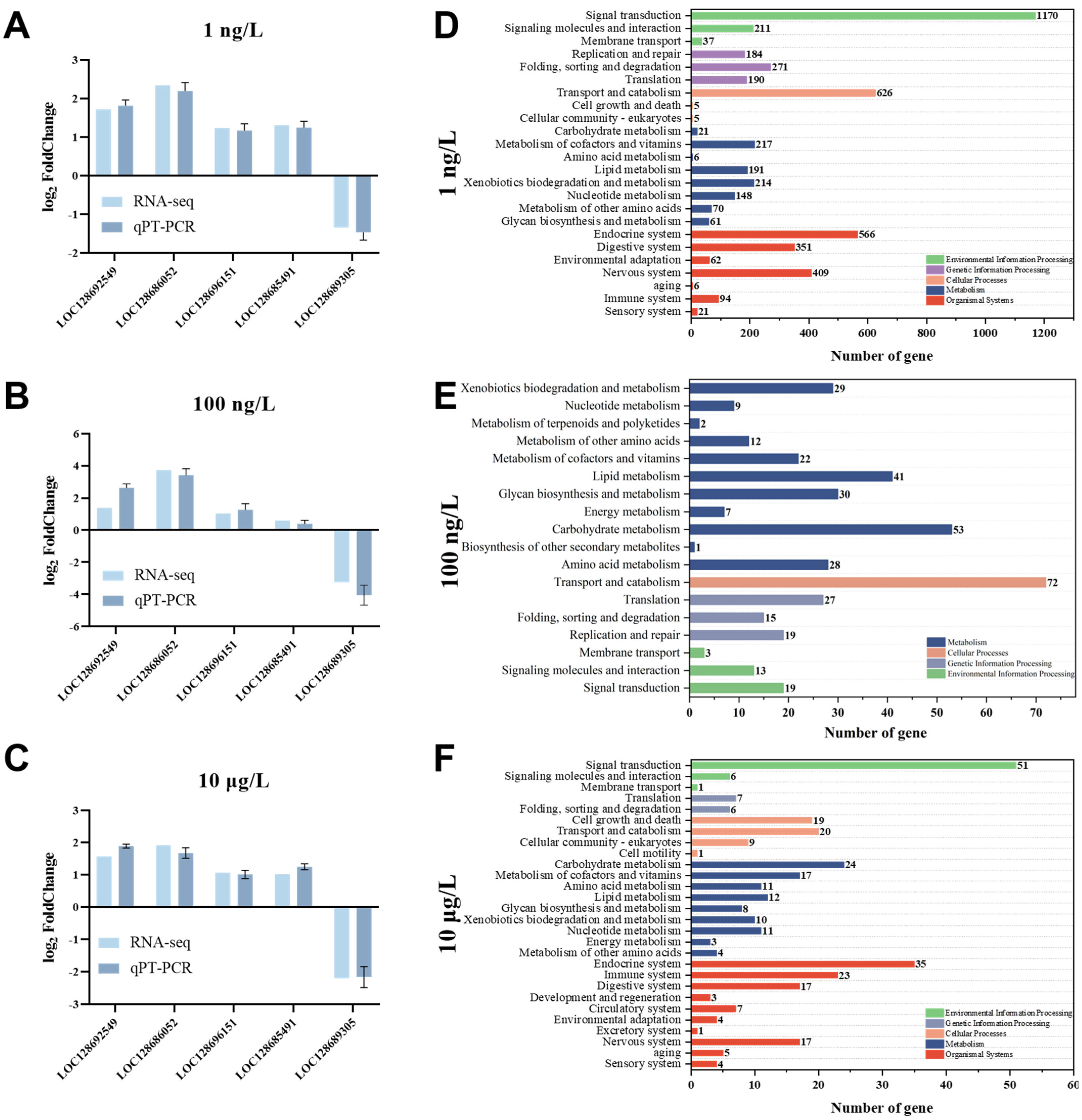
3.2. Effects of PFOS Exposure to Hepatopancreas Gene Expression
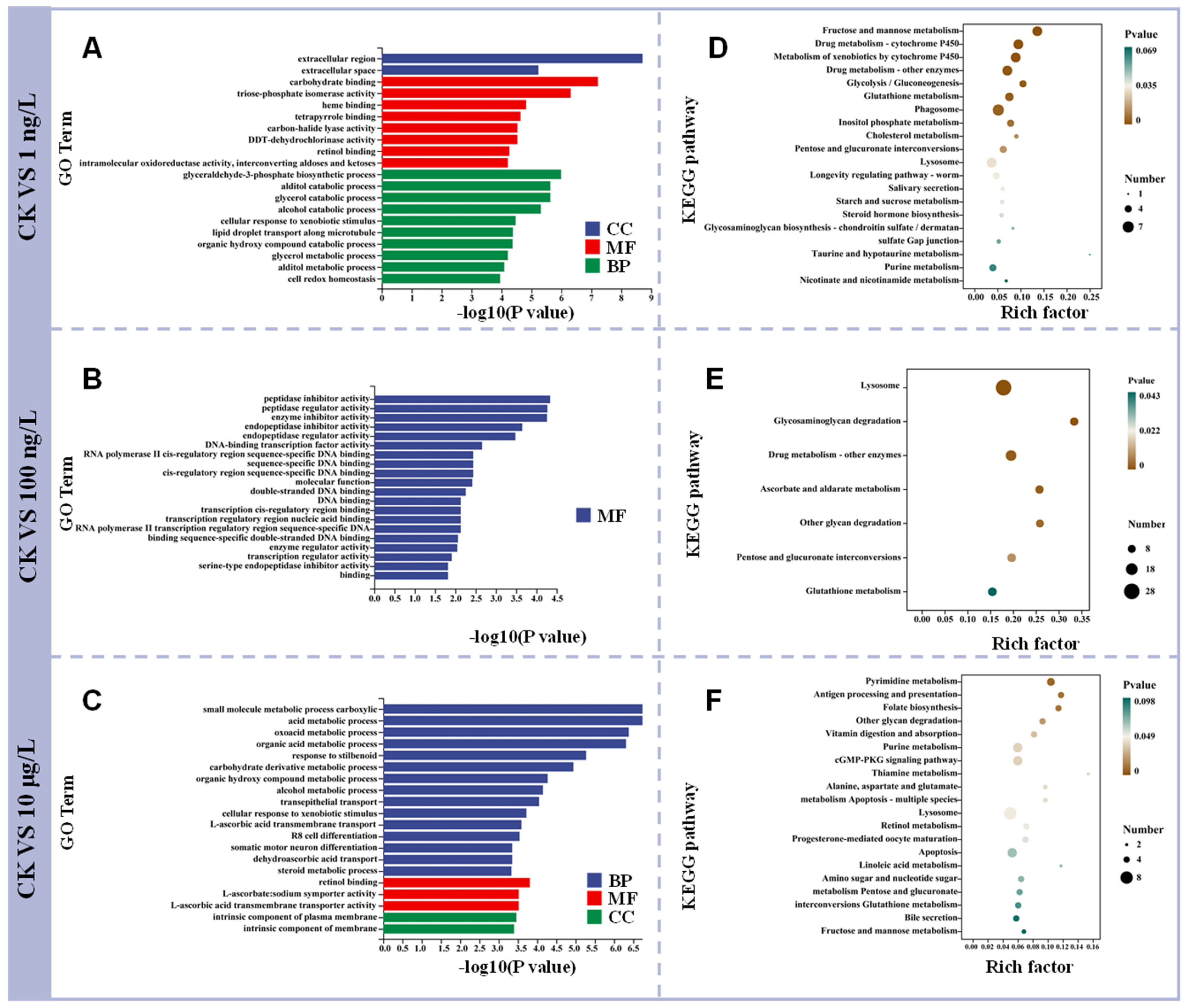
3.3. GO and KEGG Enrichment Analysis After PFOS Exposure
3.4. Validation of Transcriptomic Results
3.5. Changes in Lysosome Signaling Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFOS | Perfluorobutane sulfonate |
| C. quadricarinatus | Cherax quadricarinatus |
| DEGs | Differentially expressed genes |
| PFASs | Poly- and perfluoroalkyl substances |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| qRT-PCR | Quantitative real-time fluorescent quantitative PCR |
| ANOVA | One-way analysis of variance |
Appendix A
| Group | 1 ng/L | 100 ng/L |
|---|---|---|
| MF | 11 | 27 |
| CC | 2 | 1 |
| BP | 12 | 19 |
| total | 25 | 47 |
Appendix B

References
- Tarapore, P.; Ouyang, B. Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility? Int. J. Environ. Res. Public Health 2021, 18, 3794. [Google Scholar] [CrossRef]
- Kang, P.; Zhao, Y.; Zuo, C.; Cai, Y.; Shen, C.; Ji, B.; Wei, T. The Unheeded Inherent Connections and Overlap between Microplastics and Poly- and Perfluoroalkyl Substances: A Comprehensive Review. Sci. Total Environ. 2023, 878, 163028. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Yang, Y.; Liu, W.; Sun, X. Phytotoxic Effects of Perfluorooctane Sulfonate on the Physiological Responses of the Seaweed Gracilaria lemaneiformis. Algal Res. 2024, 83, 103741. [Google Scholar] [CrossRef]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C.G. Biological Monitoring of Polyfluoroalkyl Substances: A Review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- Hu, J.; Yu, J.; Tanaka, S.; Fujii, S. Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Water Environment of Singapore. Water Air Soil Pollut. 2011, 216, 179–191. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and Polyfluoroalkyl Substances in Water and Wastewater: A Critical Review of Their Global Occurrence and Distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Bräunig, J.; Baduel, C.; Heffernan, A.; Rotander, A.; Donaldson, E.; Mueller, J.F. Fate and Redistribution of Perfluoroalkyl Acids through AFFF-Impacted Groundwater. Sci. Total Environ. 2017, 596, 360–368. [Google Scholar] [CrossRef]
- Ding, J.; Dai, Y.; Zhang, J.; Wang, Z.; Zhang, L.; Xu, S.; Tan, R.; Guo, J.; Qi, X.; Chang, X.; et al. Associations of Perfluoroalkyl Substances with Adipocytokines in Umbilical Cord Serum: A Mixtures Approach. Environ. Res. 2023, 216, 114654. [Google Scholar] [CrossRef]
- Xuan, R.; Qiu, X.; Wang, J.; Liu, S.; Magnuson, J.T.; Xu, B.; Qiu, W.; Zheng, C. Hepatotoxic Response of Perfluorooctane Sulfonamide (PFOSA) in Early Life Stage Zebrafish (Danio rerio) Is Greater than Perfluorooctane Sulfonate (PFOS). J. Hazard. Mater. 2024, 461, 132552. [Google Scholar] [CrossRef]
- Kim, W.-K.; Lee, S.-K.; Jung, J. Integrated Assessment of Biomarker Responses in Common Carp (Cyprinus Carpio) Exposed to Perfluorinated Organic Compounds. J. Hazard. Mater. 2010, 180, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gautam, K.; Mir, S.; Anbumani, S. Genotoxicity and Cytotoxicity Assessment of ‘Forever Chemicals’ in Zebrafish (Danio rerio). Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2024, 897, 503788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gu, X.; Mo, L.; Wan, N.; Wu, L.; Liu, S.; Zhang, M.; Li, M.; Liu, X.; Liu, Y. Per- and Polyfluoroalkyl Substances Induce Lipid Metabolic Impairment in Fish: Integration on Field Investigation and Laboratory Study. Environ. Int. 2024, 187, 108687. [Google Scholar] [CrossRef]
- Baudry, T.; Gismondi, E.; Goût, J.-P.; Arqué, A.; Smith-Ravin, J.; Grandjean, F. The Invasive Crayfish Cherax quadricarinatus Facing Chlordecone in Martinique: Bioaccumulation and Depuration Study. Chemosphere 2022, 286, 131926. [Google Scholar] [CrossRef]
- Tierney, L.J.; Wild, C.H.; Furse, J.M. Total Incombustible (Mineral) Content of Cherax quadricarinatus Differs between Feral Populations in Central-Eastern Australia. PeerJ 2019, 7, e6351. [Google Scholar] [CrossRef]
- Chen, Q.; Lv, W.; Jiao, Y.; Liu, Z.; Li, Y.; Cai, M.; Wu, D.; Zhou, W.; Zhao, Y. Effects of Exposure to Waterborne Polystyrene Microspheres on Lipid Metabolism in the Hepatopancreas of Juvenile Redclaw Crayfish, Cherax quadricarinatus. Aquat. Toxicol. 2020, 224, 105497. [Google Scholar] [CrossRef]
- Cheng, H.; Dai, Y.; Ruan, X.; Duan, X.; Zhang, C.; Li, L.; Huang, F.; Shan, J.; Liang, K.; Jia, X.; et al. Effects of Nanoplastic Exposure on the Immunity and Metabolism of Red Crayfish (Cherax quadricarinatus) Based on High-Throughput Sequencing. Ecotoxicol. Environ. Saf. 2022, 245, 114114. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhou, M.; Chen, D.; Yi, C.; Sun, B.; Wang, S.; Ru, Y.; Chen, H.; Wang, H. A New Insight to Characterize Immunomodulation Based on Hepatopancreatic Transcriptome and Humoral Immune Factor Analysis of the Cherax quadricarinatus Infected with Aeromonas veronii. Ecotoxicol. Environ. Saf. 2021, 219, 112347. [Google Scholar] [CrossRef]
- Lu, Y.-P.; Zheng, P.-H.; Zhang, Z.-L.; Zhang, X.-X.; Li, J.-T.; Wang, D.-M.; Xu, J.-R.; Xian, J.-A.; Wang, A.-L. Hepatopancreas Transcriptome Alterations in Red Claw Crayfish (Cherax quadricarinatus) under Microcystin-LR (MC-LR) Stress. Aquac. Rep. 2023, 29, 101478. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, X.; Zhang, T.; Zhang, Q.; Fu, K.; Hua, J.; Zhang, M.; Qi, Q.; Zhao, B.; Zhao, M.; et al. Interactions between Intestinal Microbiota and Metabolites in Zebrafish Larvae Exposed to Polystyrene Nanoplastics: Implications for Intestinal Health and Glycolipid Metabolism. J. Hazard. Mater. 2024, 472, 134478. [Google Scholar] [CrossRef]
- Afshari, C.A.; Hamadeh, H.; Bushel, P.R. The Evolution of Bioinformatics in Toxicology: Advancing Toxicogenomics. Toxicol. Sci. 2011, 120 (Suppl. 1), S225–S237. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster Reproduction Is Affected by Exposure to Polystyrene Microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef]
- Cheng, J.; Lv, S.; Nie, S.; Liu, J.; Tong, S.; Kang, N.; Xiao, Y.; Dong, Q.; Huang, C.; Yang, D. Chronic Perfluorooctane Sulfonate (PFOS) Exposure Induces Hepatic Steatosis in Zebrafish. Aquat. Toxicol. 2016, 176, 45–52. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Ge, X.; Wang, D.; Wang, T.; Zhang, L.; Tanguay, R.L.; Simonich, M.; Huang, C.; Dong, Q. Chronic Perfluorooctanesulphonic Acid (PFOS) Exposure Produces Estrogenic Effects in Zebrafish. Environ. Pollut. 2016, 218, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rao, Q.; Zhang, Q.; Du, P.; Song, W. Determination of 14 perfluoroalkyl substances in Chinese mitten crab by multi-plug filtration cleanup coupled with ultra-performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2023, 41, 1095–1105. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Q.; Lin, C.; He, L.; Wei, L. Integrative Analyses of Gene Expression and Alternative Splicing to Gain Insights into the Effects of Copper on Hepatic Lipid Metabolism in Swamp Eel (Monopterus albus). Aquaculture 2021, 546, 737367. [Google Scholar] [CrossRef]
- Dong, H.; Lu, G.; Yan, Z.; Liu, J.; Yang, H.; Zhang, P.; Jiang, R.; Bao, X.; Nkoom, M. Distribution, Sources and Human Risk of Perfluoroalkyl Acids (PFAAs) in a Receiving Riverine Environment of the Nanjing Urban Area, East China. J. Hazard. Mater. 2020, 381, 120911. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Jin, R.; Qiao, Y.; Mao, J.; Wang, Z. Prevalent Per- and Polyfluoroalkyl Substances (PFASs) Pollution in Freshwater Basins in China: A Short Review. Toxics 2025, 13, 135. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, S.; Jin, H.; Fan, C.; Liao, K.; Zhang, S.; Xue, J. Relationship Between Perfluoroalkyl Acids in Human Serum and Sjogren’s Syndrome: A Case–Control Study of Populations in Hangzhou, China. Toxics 2024, 12, 764. [Google Scholar] [CrossRef]
- Goodrow, S.M.; Ruppel, B.; Lippincott, R.L.; Post, G.B.; Procopio, N.A. Investigation of Levels of Perfluoroalkyl Substances in Surface Water, Sediment and Fish Tissue in New Jersey, USA. Sci. Total Environ. 2020, 729, 138839. [Google Scholar] [CrossRef]
- Khan, E.A.; Zhang, X.; Hanna, E.M.; Yadetie, F.; Jonassen, I.; Goksøyr, A.; Arukwe, A. Application of Quantitative Transcriptomics in Evaluating the Ex Vivo Effects of Per- and Polyfluoroalkyl Substances on Atlantic Cod (Gadus morhua) Ovarian Physiology. Sci. Total Environ. 2021, 755, 142904. [Google Scholar] [CrossRef]
- Khan, E.A.; Zhang, X.; Hanna, E.M.; Bartosova, Z.; Yadetie, F.; Jonassen, I.; Goksøyr, A.; Arukwe, A. Quantitative Transcriptomics, and Lipidomics in Evaluating Ovarian Developmental Effects in Atlantic Cod (Gadus morhua) Caged at a Capped Marine Waste Disposal Site. Environ. Res. 2020, 189, 109906. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.H.; Kraak, G.V.D.; Brown, S.B.; Eales, J.G. Chapter 15 The Biology and Toxicology of Retinoids in Fish. Biochem. Mol. Biol. Fishes 2005, 6, 413–428. [Google Scholar] [CrossRef]
- Lee, W.K.; Lam, T.K.Y.; Tang, H.C.; Ho, T.C.; Wan, H.T.; Wong, C.K.C. PFOS-Elicited Metabolic Perturbation in Liver and Fatty Acid Metabolites in Testis of Adult Mice. Front. Endocrinol. 2023, 14, 1302965. [Google Scholar] [CrossRef] [PubMed]
- Lacaze, É. Cumulative Effects of Municipal Effluent and Parasite Infection in Yellow Perch: A Field Study Using High-Throughput RNA-Sequencing. Sci. Total Environ. 2019, 665, 797–809. [Google Scholar] [CrossRef]
- Qu, M.; Xu, K.; Li, Y.; Wong, G.; Wang, D. Using acs-22 Mutant Caenorhabditis elegans to Detect the Toxicity of Nanopolystyrene Particles. Sci. Total Environ. 2018, 643, 119–126. [Google Scholar] [CrossRef]
- Shi, Z.; Tang, Z.; Wang, C. A Brief Review and Evaluation of Earthworm Biomarkers in Soil Pollution Assessment. Environ. Sci. Pollut. Res. 2017, 24, 13284–13294. [Google Scholar] [CrossRef]
- Chen, D.; Guo, L.; Yi, C.; Wang, S.; Ru, Y.; Wang, H. Hepatopancreatic Transcriptome Analysis and Humoral Immune Factor Assays in Red Claw Crayfish (Cherax quadricarinatus) Provide Insight into Innate Immunomodulation under Vibrio Parahaemolyticus Infection. Ecotoxicol. Environ. Saf. 2021, 217, 112266. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, W.; Chen, H.; Tian, F.; Cai, W. Transcriptome analysis of acute exposure of the Manila clam, Ruditapes philippinarum to perfluorooctane sulfonate (PFOS). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108736. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.-Y.; Zeng, H.-C.; Wei, J.; Wan, Y.-J.; Chen, J.; Xu, S.-Q. MicroRNA Expression Changes during Zebrafish Development Induced by Perfluorooctane Sulfonate. J. Appl. Toxicol. 2011, 31, 210–222. [Google Scholar] [CrossRef]
- Chauhan, N.; Patro, B.S. Emerging Roles of Lysosome Homeostasis (Repair, Lysophagy and Biogenesis) in Cancer Progression and Therapy. Cancer Lett. 2024, 584, 216599. [Google Scholar] [CrossRef] [PubMed]
- de Duve, C.; Pressman, B.C.; Gianetto, R.; Wattiaux, R.; Appelmans, F. Tissue Fractionation Studies. 6. Intracellular Distribution Patterns of Enzymes in Rat-Liver Tissue. Biochem. J. 1955, 60, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, N.; Chen, L.; Shao, Y.; Shi, X.; Zhu, D. Perfluorooctane Sulfonate Induced Toxicity in Embryonic Stem Cell-Derived Cardiomyocytes via Inhibiting Autophagy-Lysosome Pathway. Toxicol. Vitr. 2020, 69, 104988. [Google Scholar] [CrossRef]
- Yao, X.; Cao, J.; Xu, L.; Sun, X.; Kang, J.; Yang, G.; Jiang, L.; Geng, C.; Gao, C.; Zhong, L.; et al. Perfluorooctane Sulfonate Blocked Autophagy Flux and Induced Lysosome Membrane Permeabilization in HepG2 Cells. Food Chem. Toxicol. 2014, 67, 96–104. [Google Scholar] [CrossRef]
- Wang, M.; Song, B.; Song, T.; Sun, K.; He, J.; Deng, J.; Fang, L.; Luan, T.; Lin, L. Efflux Transport Proteins of Tetrahymena Thermophila Play Important Roles in Resistance to Perfluorooctane Sulfonate Exposure. J. Hazard. Mater. 2023, 459, 132287. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Shirazi, S.; Aghvami, M.; Pourahmad, J. Perfluorooctanesulfonate (PFOS) Induces Apoptosis Signaling and Proteolysis in Human Lymphocytes through ROS Mediated Mitochondrial Dysfunction and Lysosomal Membrane Labialization. Iran. J. Pharm. Res. 2018, 17, 995–1007. [Google Scholar] [CrossRef]
- Li, J.; Feng, R.; Yang, W.; Liang, P.; Qiu, T.; Zhang, J.; Sun, X.; Li, Q.; Yang, G.; Yao, X. Lysosomal Iron Accumulation and Subsequent Lysosomes-Mitochondria Iron Transmission Mediate PFOS-Induced Hepatocyte Ferroptosis. Ecotoxicol. Environ. Saf. 2024, 284, 116890. [Google Scholar] [CrossRef]


| Gene | Forward Sequence (5′-3′) | Reserve Sequence (5′-3′) |
|---|---|---|
| LOC128692549 | CCCACACCGTCTACCAAGTC | GGAGTGCCCATAGTCTCGC |
| LOC128686052 | ACGGAGACATGCTCCATCA | GTGGCTCCACTACCACAGTC |
| LOC128696151 | GGTACGAGTCTCAGTGCGTC | CAGGAGACTCCACTGCCTTG |
| LOC128685491 | TGGGGATCACTCCATCACCT | GGTGACGATTTTGACTCAGCA |
| LOC128689305 | GCTTACAGCTTCTGGGGTGT | TGCTTGCCCGAGATTAGACG |
| β-actin | AAATCCTACGAGCTTCCTGACG | TACCACAAGATTCCATGCCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Zhu, T.; Liu, C.; Li, Y.; Song, W.; Wang, X.; Liu, X.; Wang, H.; Li, K.; Cao, X.; et al. Perfluorobutane Sulfonate (PFOS) Accumulation in Tissues of Cherax quadricarinatus and Its Toxicity Mechanism. Toxics 2025, 13, 269. https://doi.org/10.3390/toxics13040269
Hong S, Zhu T, Liu C, Li Y, Song W, Wang X, Liu X, Wang H, Li K, Cao X, et al. Perfluorobutane Sulfonate (PFOS) Accumulation in Tissues of Cherax quadricarinatus and Its Toxicity Mechanism. Toxics. 2025; 13(4):269. https://doi.org/10.3390/toxics13040269
Chicago/Turabian StyleHong, Shuang, Tian Zhu, Chengbin Liu, Yameng Li, Wei Song, Xianli Wang, Xiaoyu Liu, Hongzhuo Wang, Kepiao Li, Xiaolong Cao, and et al. 2025. "Perfluorobutane Sulfonate (PFOS) Accumulation in Tissues of Cherax quadricarinatus and Its Toxicity Mechanism" Toxics 13, no. 4: 269. https://doi.org/10.3390/toxics13040269
APA StyleHong, S., Zhu, T., Liu, C., Li, Y., Song, W., Wang, X., Liu, X., Wang, H., Li, K., Cao, X., Yao, C., & Lv, W. (2025). Perfluorobutane Sulfonate (PFOS) Accumulation in Tissues of Cherax quadricarinatus and Its Toxicity Mechanism. Toxics, 13(4), 269. https://doi.org/10.3390/toxics13040269






