Silicon Speciation and Its Relationship with Carbon and Nitrogen in the Sediments of a Macrophytic Eutrophic Lake
Abstract
1. Introduction
2. Materials and Methods
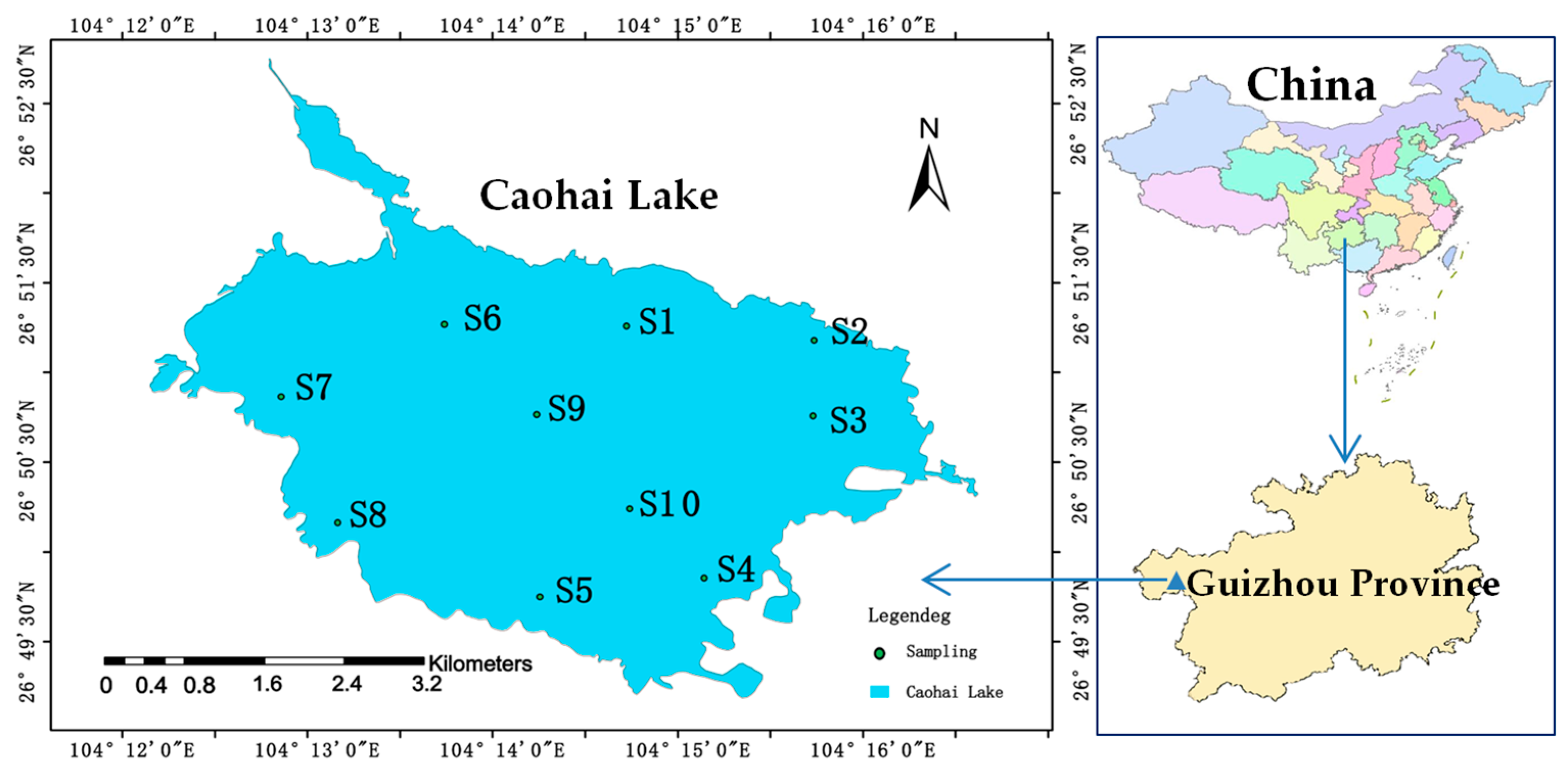
2.1. Research Area and Sample Collection
2.2. Extraction and Determination of Si in Sediments
2.2.1. Sequential Extraction of Different Si Forms
2.2.2. Extraction of BSi
2.3. Determination of TOC, TN, and Chlorophyll-a in Sediments
2.4. Data Analysis
3. Results and Discussions
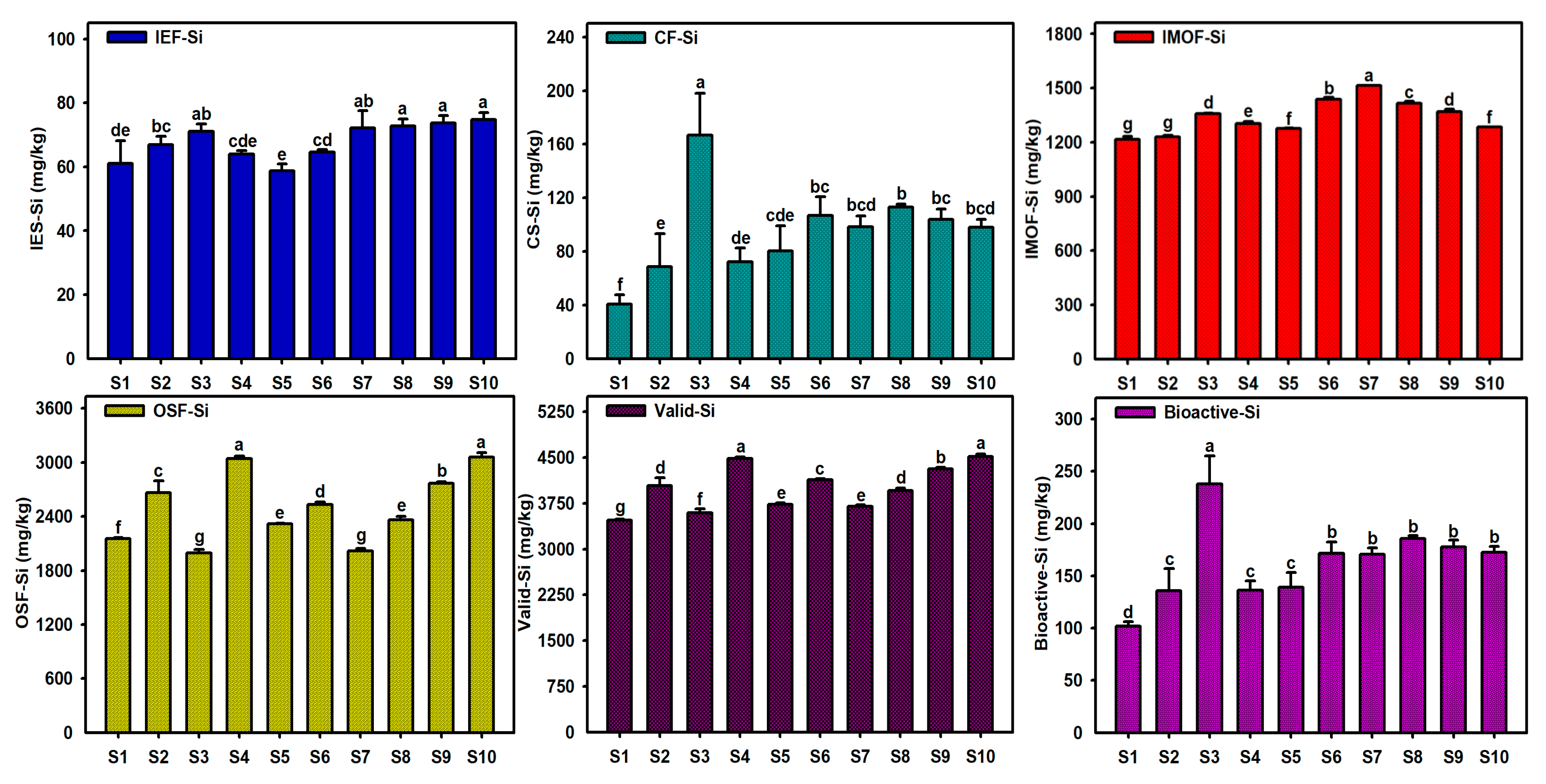
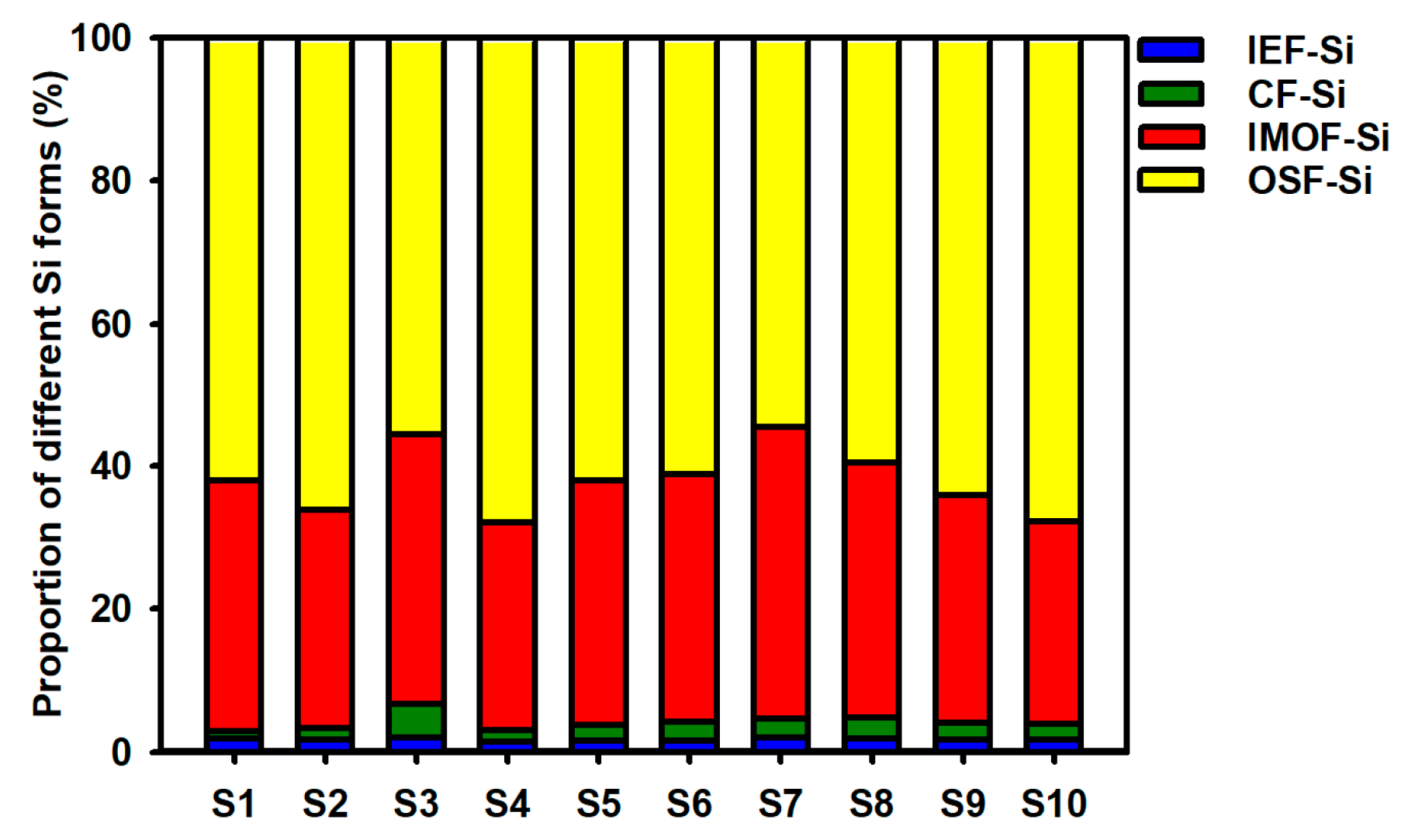
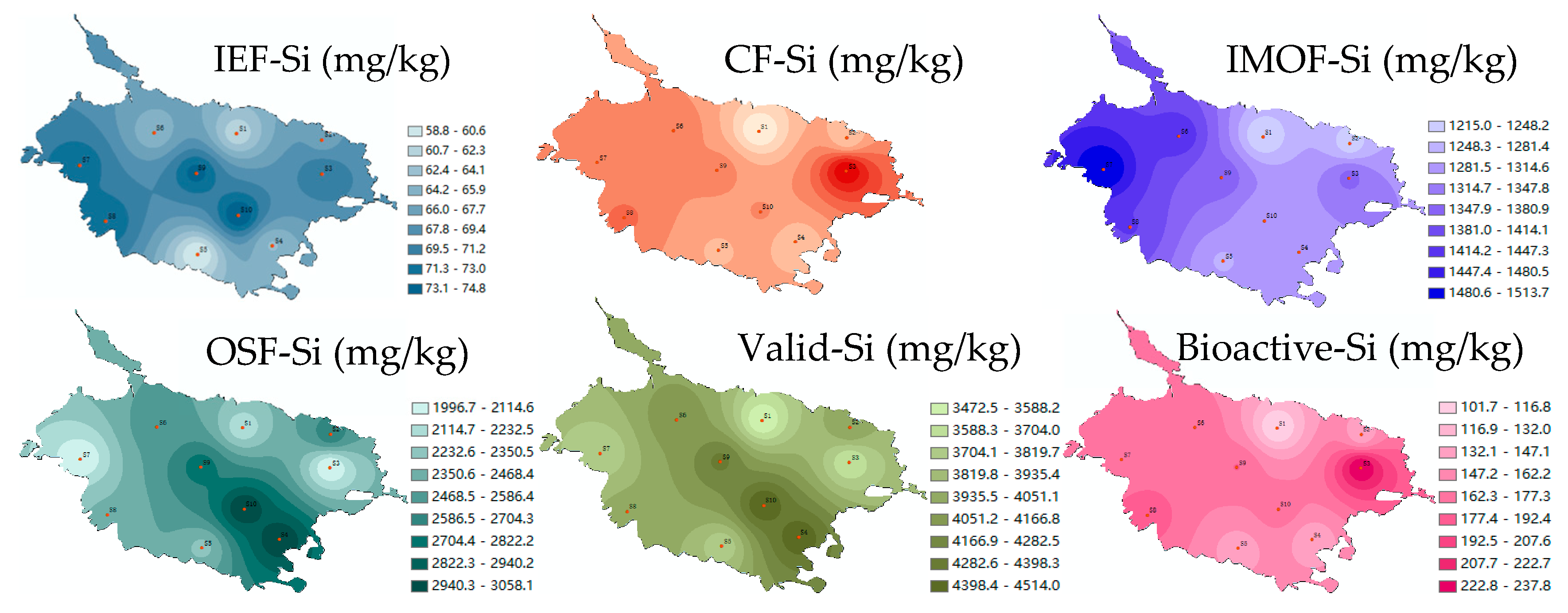
3.1. Content Characteristics of Different Si Forms in Caohai Lake Sediments
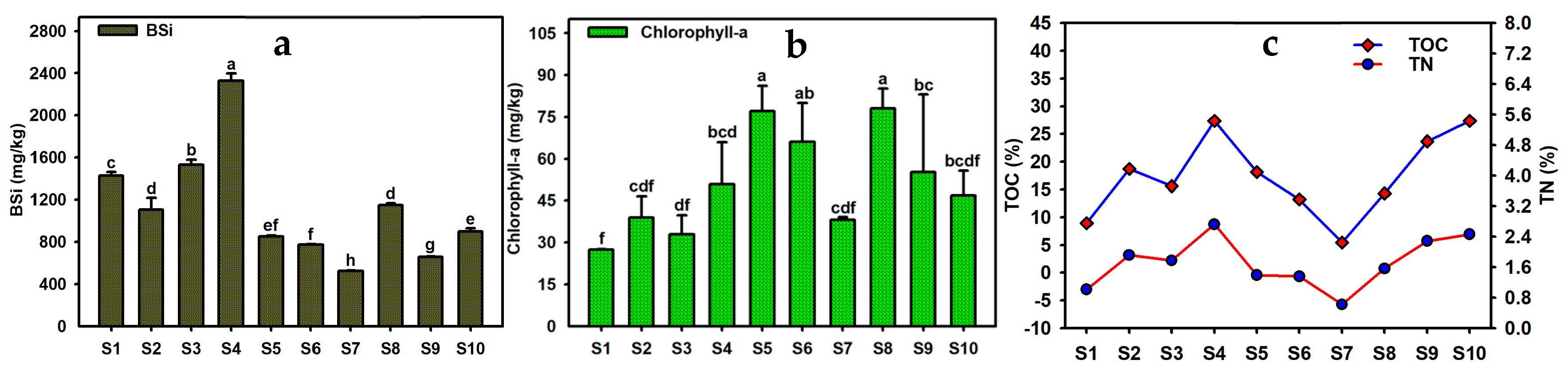
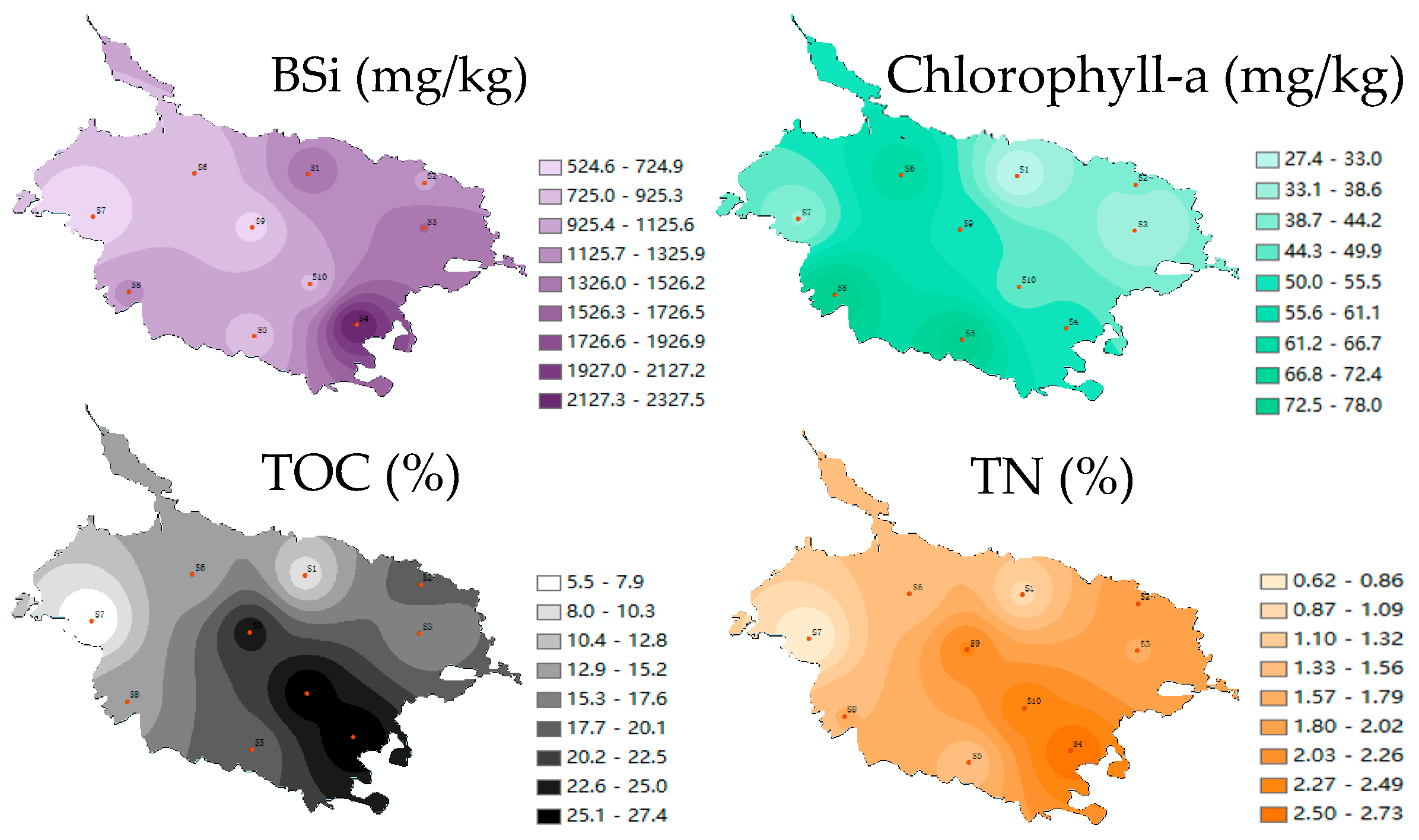
3.2. Content Characteristics of BSi, TOC, TN, and Chlorophyll-a in Sediments of Caohai Lake
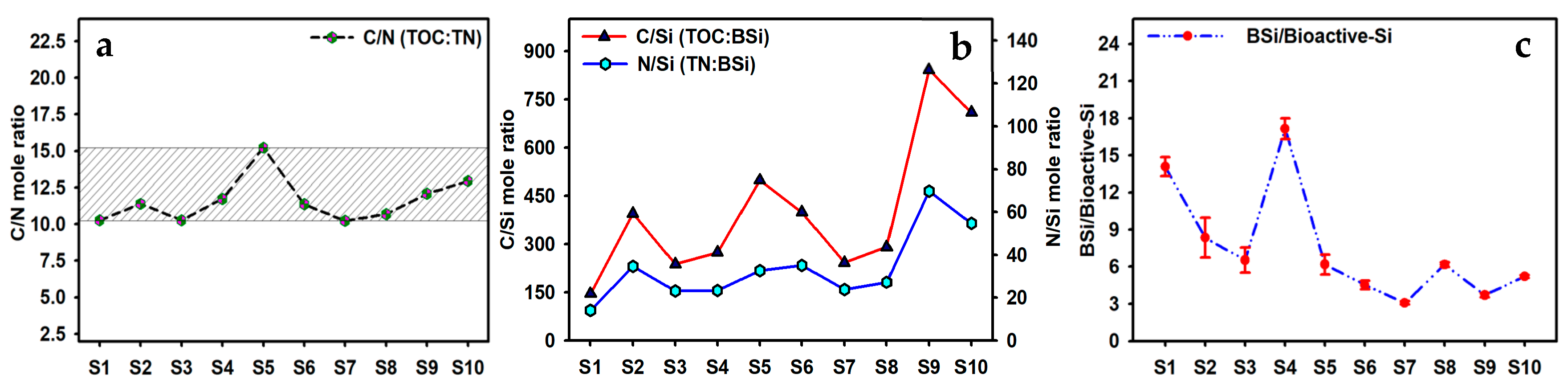
3.3. Indicative Significance of C/N, C/Si, N/Si, and BSi/Bioactive-Si in Caohai Lake Sediments
3.4. Correlations of Different Si Forms with BSi, TOC, TN, and Chlorophyll-a in Caohai Lake Sediments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Wan, H.; Ma, W.; Zhou, K.; Cao, Y.; Liu, X.; Ma, R. Advanced silicon nanostructures derived from natural silicate minerals for energy storage and conversion. Green Energy Environ. 2022, 7, 205–220. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Danilovtseva, E.N.; Pal’shin, V.A.; Verkhozina, O.N.; Zelinskiya, S.N.; Krishnan, U.M. Silicic acid condensation under the influence of water-soluble polymers: From biology to new materials. RSC Adv. 2017, 7, 20995–21027. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; de Mello Prado, R.; da Silveira Sousa Junior, G.; Gratão, P.L.; Felisberto, G.; Viciedo, D.O.; dos Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef]
- Limmer, M.A.; Thomas, J.; Seyfferth, A.L. The effect of silicon on the kinetics of rice root iron plaque formation. Plant Soil 2022, 477, 171–181. [Google Scholar] [CrossRef]
- Brzezinski, M.A.; Dickson, M.L.; Nelson, D.M.; Sambrotto, R. Ratios of Si, C and N uptake by microplankton in the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 619–633. [Google Scholar] [CrossRef]
- Pearson, L.K.; Hendy, C.H.; Hamilton, D.P. Dynamics of silicon in lakes of the Taupo Volcanic Zone, New Zealand, and implications for diatom growth. Inland Waters 2016, 6, 185–198. [Google Scholar] [CrossRef]
- Huang, L.; Parsons, C.T.; Slowinski, S.; Van Cappellen, P. Amorphous silica dissolution kinetics in freshwater environments: Effects of Fe2+ and other solution compositional controls. Sci. Total Environ. 2022, 851 Pt 2, 158239. [Google Scholar] [CrossRef]
- Frings, P.J.; Clymans, W.; Jeppesen, E.; Lauridsen, T.L.; Struyf, E.; Conley, D.J. Lack of steady-state in the global biogeochemical Si cycle: Emerging evidence from lake Si sequestration. Biogeochemistry 2014, 117, 255–277. [Google Scholar] [CrossRef]
- Sutton, J.N.; André, L.; Cardinal, D.; Conley, D.J.; de Souza, G.F.; Dean, J.; Dodd, J.; Ehlert, C.; Ellwood, M.J.; Frings, P.J.; et al. A review of the stable isotope bio-geochemistry of the global silicon cycle and its associated trace elements. Front. Earth Sci. 2018, 5, 112. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, G.; Gao, Q.; Tao, Z. Impacts of damming on the biogeochemical cycles of dissolved silicon in a tributary of the Pearl river in South China. J. Asian Earth Sci. 2023, 250, 105654. [Google Scholar] [CrossRef]
- Isaji, C. Silica fractionation: A method and differences between two Japanese reservoirs. Hydrobiologia 2003, 504, 31–38. [Google Scholar] [CrossRef]
- Lv, C.; Cui, M.; Gao, J.; Zhang, X.; Wan, L.; He, J.; Meng, T.; Bai, F.; Yang, X. Adsorption characteristic and form distribution of silicate in lakes sediments. Environ. Sci. 2012, 33, 135–141. [Google Scholar] [CrossRef]
- Haynes, R.J.; Zhou, Y.F. Silicate sorption and desorption by a Si-deficient soil—Effects of pH and period of contact. Geoderma 2020, 365, 114204. [Google Scholar] [CrossRef]
- Huang, T.H.; Sun, X.; Somelar, P.; Kirsimäe, K.; Pickering, R.A.; Kim, J.H.; Kielman-Schmitt, M.; Hong, W.L. Separating Si phases from diagenetically-modified sediments through sequential leaching. Chem. Geol. 2023, 637, 121681. [Google Scholar] [CrossRef]
- Zhao, X.; Song, Z.; Zwieten, L.V.; Wang, Y.; Ran, X.; Hao, Q.; Zhang, J.; Li, Z.; Sun, J.; Wei, Y.; et al. Silicon fractionations in coastal wetland sediments: Implications for biogeochemical silicon cycling. Sci. Total Environ. 2024, 912, 169206. [Google Scholar] [CrossRef]
- Tallberg, P.; Opfergelt, S.; Cornelis, J.T.; Liljendahl, A.; Weckström, J. High concentrations of amorphous, biogenic Si (BSi) in the sediment of a small high-latitude lake: Implications for biogeochemical Si cycling and for the use of BSi as a paleoproxy. Aquat. Sci. 2015, 77, 293–305. [Google Scholar] [CrossRef]
- Kienel, U.; Kirillin, G.; Brademann, B.; Plessen, B.; Lampe, R.; Braue, A. Effects of spring warming and mixing duration on diatom deposition in deep Tiefer See, NE Germany. J. Paleolimnol. 2017, 57, 37–49. [Google Scholar] [CrossRef]
- Ardila PA, R.; Alonso, R.Á.; Valsero, J.J.D.; García, R.M.; Cabrera, F.Á.; Cosío, E.L.; Laforet, S.D. Assessment of heavy metal pollution in marine sediments from southwest of Mallorca island, Spain. Environ. Sci. Pollut. Res. 2023, 30, 16852–16866. [Google Scholar] [CrossRef]
- Gallinari, M.; Ragueneau, O.; Corrin, L.; DeMaster, D.J.; Treguer, P. The importance of water column processes on the dissolution properties of biogenic silica in deep-sea sediments I. Solubility. Geochim. Cosmochim. Acta 2002, 66, 2701–2717. [Google Scholar] [CrossRef]
- Tian, C.; Yang, H. Distribution characteristics and forms of sediment silicon in the Inner Mongolia stretch of Yellow River. Environ. Pollut. Control 2020, 42, 432–436. (In Chinese) [Google Scholar] [CrossRef]
- Yin, C.; Yang, H.; Wang, J.; Guo, J.; Tang, X.; Chen, J. Combined use of stable nitrogen and oxygen isotopes to constrain the nitrate sources in a karst lake. Agric. Ecosyst. Environ. 2020, 303, 107089. [Google Scholar] [CrossRef]
- Guo, Y.M.; Xu, Y.Z.; Li, R.W.; Zhou, C.Q.; Du, M.P.; Liu, K.Y. Phytoplankton community structure and its relationship with environmental factors in Caohai Lake, Guizhou, China. Asian J. Ecotoxicol. 2024, 19, 306–318. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, X.J.; Yang, L. Evaluation of conservation value of Caohai National Nature Reserve in Guizhou Province. Prot. For. Sci. Technol. 2021, 03, 45–47. (In Chinese) [Google Scholar] [CrossRef]
- Li, A.Y.; Huang, X.F.; Tian, Y.B.; Dong, J.X.; Zheng, F.F.; Xia, P.H. Chlorophyll a variation and its driving factors during phase shift from macrophyte- to phytoplankton-dominated states in Caohai Lake, Guizhou, China. Chin. J. Plant Ecol. 2023, 47, 1171–1181. (In Chinese) [Google Scholar] [CrossRef]
- Wu, J.; Yang, H.; Yu, W.; Yin He, Y.; Zhang, Z.; Xu, D.; Li, Q.; Chen, J. Effect of ecosystem degradation on the source of particulate organic matter in a karst lake: A case study of the Caohai Lake, China. Water 2022, 14, 1867. [Google Scholar] [CrossRef]
- Yu, W.; Yang, H.; Chen, J.; Liao, P.; Chen, Q.; Yang, Y.; Liu, Y. Organic phosphorus mineralization dominates the release of internal phosphorus in a macrophyte-dominated eutrophication lake. Front. Environ. Sci. 2022, 9, 812834. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Cao, X.; Yang, T.; An, S. Increasing trends in heavy metal risks in the Caohai Lake sediments from 2011 to 2022. Arab. J. Chem. 2024, 17, 105543. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xu, G.; Wang, J.; Xu, K.; Jin, Z.; Huang, G. The recycling characteristics of different silicon forms and biogenic silicon in the surface sediments of Dianchi Lake, Southwest China. Water 2024, 16, 1824. [Google Scholar] [CrossRef]
- Mortlock, R.A.; Froelich, P.N. A simple method for the rapid determination of biogenic opal in pelagic marine sediments. Deep Sea Res. Part A Oceanogr. Res. Pap. 1989, 36, 1415–1426. [Google Scholar] [CrossRef]
- Liao, B.; Zhu, J.; Wu, Y.; Yu, L.; Liu, Q. Exploration on the determination method of biosilicon in reservoir bottom mud. J. Neijiang Teach. Coll. 2006, 21, 226–229. (In Chinese) [Google Scholar] [CrossRef]
- Hou, L.; Liu, M.; Yan, H.; Xu, S.; Ou, D.; Lin, X. Distribution of biogenic silica in tidal flat sediments of Yangtze Estuary and its influence factors. China Environ. Sci. 2007, 27, 665–669. (In Chinese) [Google Scholar] [CrossRef]
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D. Silicate, phosphate and carbonate mineral dissolution behaviour in the presence of organic acids: A review. Miner. Eng. 2017, 100, 115–123. [Google Scholar] [CrossRef]
- Pham AL, T.; Doyle, F.M.; Sedlak, D.L. Inhibitory effect of dissolved silica on H2O2 decomposition by iron(III) and manganese(IV) oxides: Implications for H2O2-based in situ chemical oxidation. Environ. Sci. Technol. 2012, 46, 1055–1062. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H. Redox reactions of iron and manganese oxides in complex systems. Front. Environ. Sci. Eng. 2020, 14, 76. [Google Scholar] [CrossRef]
- Yi, C.; Guo, P.; Lu, D.; Chen, J.; Teng, C.; Wang, Y. Distribution characteristics and environmental significance of different silicon forms in surface sediments of Shanmei Reservoir. China Environ. Sci. 2015, 35, 211–217. Available online: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjMxMjI2Eg96Z2hqa3gyMDE1MDEwMjgaCDV1Mjhucndw (accessed on 25 January 2025). (In Chinese).
- Vlasova, N.N.; Sorokin, M.S.; Oborina, E.N. Carbofunctional sulfur-containing organosilicon compounds. Appl. Organomet. Chem. 2016, 31, e3668. [Google Scholar] [CrossRef]
- Yang, N.; Li, W.; Dong, L. Modification of a H-terminated silicon surface by organic sulfide molecules: The mechanism and origin of reactivity. New J. Chem. 2020, 44, 11056–11063. [Google Scholar] [CrossRef]
- Yu, W.; Yang, H.; Guo, J.; Tang, X.; Zhang, Z.; Yang, Y. Hydrochemical characteristics and major ion sources of Lake Caohai in Guizhou province. Earth Environ. 2021, 49, 32–41. (In Chinese) [Google Scholar] [CrossRef]
- Haque, F.; Khalidy, R.; Chiang, Y.W.; Santos, R.M. Constraining the capacity of global croplands to CO2 drawdown via mineral weathering. ACS Earth Space Chem. 2023, 7, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, C.; Hou, J.; Miao, L.; Yao, Y.A. Simulation study on the effect of redox change on the Si in the estuarine sediment. Environ. Chem. 2018, 37, 974–983. (In Chinese) [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Liu, Y.; Wang, Y.; Shuai, J.; Chen, M. Fe/Mn (oxyhydr)oxides reductive dissolution promoted by cyanobacterial algal bloom-derived dissolved organic matter caused sediment W release during an algal bloom in Taihu Lake. Water Res. 2024, 260, 121899. [Google Scholar] [CrossRef]
- Liu, B.; Xu, H.; Lan, J.; Sheng, E.; Che, S.; Zhou, X. Biogenic silica contents of Lake Qinghai sediments and its environmental significance. Front. Earth Sci. 2014, 8, 573–581. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, F.; Lu, D.; Mei, H. Determination of biogenic silica in sediments of reservoirs in the Wujiang River and its environment significance. Acta Mineral. Sin. 2011, 31, 30–35. (In Chinese) [Google Scholar] [CrossRef]
- Li, L.W.; Wu, P.; Cao, X.X.; Yang, S.; Liu, S.; Liao, J. Spatial-temporal distribution of organic matter in surface sediments of Caohai wetland in Weining, Guizhou Province, China. J. Agro-Environ. Sci. 2022, 41, 153–161. (In Chinese) [Google Scholar] [CrossRef]
- Kelly, J.A.; Honeywill, C.; Paterson, D.M. Microscale analysis of chlorophyll-a in cohesive, intertidal sediments: The implications of microphytobenthos distribution. J. Mar. Biol. Assoc. United Kingd. 2001, 81, 151–162. [Google Scholar] [CrossRef]
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry—An overview of indicators of organic matter sources and diagenesis in lake sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, F.; He, Z.; Giesy, J.P.; Feng, W.; Mu, Y.; Feng, C.; Zhao, X.; Liao, H.; Tang, Z. Influence of natural organic matter on the bioavailability and preservation of organic phosphorus in lake sediments. Chem. Geol. 2015, 397, 51–60. [Google Scholar] [CrossRef]
- Wu, B. Distribution, Dissolution and Influencing Factors of Biogenic Silica in Sediments of the Yellow Sea and the East China Sea. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2015. Available online: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCFkyODg0MjYzGgg5ZGVxMm95Nw%3D%3D (accessed on 25 January 2025). (In Chinese).
- Li, S.; Hu, X.; Tang, Y.; Huang, C. Biogenic silica distribution in the sediments from Arrow Bamboo Lake in Jiuzhaigou World Nature Heritage Reserve, China: Environmental implication. J. Montain Sci. 2011, 29, 395–401. (In Chinese) [Google Scholar] [CrossRef]
- DeMaster, D.J. The accumulation and cycling of biogenic silica in the Southern Ocean: Revisiting the marine silica budget. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 3155–3167. [Google Scholar] [CrossRef]
- Brzezinski, M.A. The Si: C: N ratio of marine diatoms: Interspecific variability and the effect of some environmental variables. J. Phycol. 1985, 21, 347–357. [Google Scholar] [CrossRef]
- Liu, W. Geochemical Characteristics of Silicon in Dali-Nor Lake Sediments. Master’s Thesis, Inner Mongolia University, Hohhot, China, 2011. (In Chinese). [Google Scholar] [CrossRef]
- Liao, M.; Jiang, X.; Huang, X.; Xia, P. Organic carbon and nitrogen isotope characteristics and sources of particulate matter and sediment in Caohai Lake, Guizhou Province. J. Hydroecology 2024, 45, 40–47. (In Chinese) [Google Scholar] [CrossRef]
- Pérez, A.; Machado, W.; Gutierrez, D.; Smoak, J.M.; Breithaupt, J.L.; Saldarriaga, M.S.; Sanders, L.; Marotta, H.; Sanders, C.J. Carbon and nutrient accumulation in mangrove sediments affected by multiple environmental changes. J. Soils Sediments 2020, 20, 2504–2509. [Google Scholar] [CrossRef]
- Naidu, S.A.; Kathiresan, K.; Simonson, J.H.; Blanchard, A.L.; Sanders, C.J.; Pérez, A.; Post, R.M.; Subramoniam, T.; Naidu, R.A.; Narender, R. Carbon and nitrogen contents driven by organic matter source within Pichavaram Wetland Sediments. J. Mar. Sci. Eng. 2022, 10, 53. [Google Scholar] [CrossRef]
- Bergström, A.K.; Karlsson, J.; Karlsson, D.; Vrede, T. Contrasting plankton stoichiometry and nutrient regeneration in northern arctic and boreal lakes. Aquat. Sci. 2018, 80, 24. [Google Scholar] [CrossRef]
- Elser, J.J.; Devlin, S.P.; Yu, J.; Baumann, A.; Church, M.J.; Dore, J.E.; Hall, R.O.; Hollar, M.; Johnson, T.; Vick-Majors, T.; et al. Sustained stoichiometric imbalance and its ecological consequences in a large oligotrophic lake. Proc. Natl. Acad. Sci. USA 2022, 119, e2202268119. [Google Scholar] [CrossRef]
- Kelly, P.T.; Taylor, J.M.; Andersen, I.M.; Scott, J.T. Zooplankton densities reduced by increases in resource N:P in hypereutrophic mesocosms. Hydrobiologia 2024, 851, 4077–4089. [Google Scholar] [CrossRef]
- Rosso, A.; Vione, D. Pollutant photodegradation affected by evaporative water concentration in a climate change scenario. Molecules 2024, 29, 2655. [Google Scholar] [CrossRef]
- Ziervogel, K.; Dike, C.; Asper, V.; Montoya, J.; Battles, J.; D’souza, N.; Passow, U.; Diercks, A.; Esch, M.; Joye, S.; et al. Enhanced particle fluxes and heterotrophic bacterial activities in Gulf of Mexico bottom waters following storm-induced sediment resuspension. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 129, 77–88. [Google Scholar] [CrossRef]

| Name | IEF-Si | CF-Si | IMOF-Si | OSF-Si | Valid-Si | Bioactive-Si | BSi | Chlorophyll-a | TN | TOC |
|---|---|---|---|---|---|---|---|---|---|---|
| IEF-Si | 1 | 0.527 * | 0.269 | 0.398 | 0.529 * | 0.671 ** | −0.448 | 0.242 | 0.455 | 0.469 * |
| CF-Si | 0.637 * | 1 | 0.696 ** | 0.341 | 0.618 ** | 0.984 ** | −0.637 ** | 0.708 ** | 0.336 | 0.327 |
| IMOF-Si | 0.402 | 0.755 ** | 1 | −0.324 | 0.011 | 0.664 ** | −0.733 ** | 0.363 | −0.366 | −0.362 |
| OSF-Si | −0.280 | −0.720 * | −0.471 | 1 | 0.942 ** | 0.382 | −0.160 | 0.226 | 0.956 ** | 0.973 ** |
| Valid-Si | −0.166 | −0.596 | −0.316 | 0.983 ** | 1 | 0.651 ** | −0.419 | 0.383 | 0.887 ** | 0.904 ** |
| Bioactive-Si | 0.696 * | 0.997 ** | 0.745 ** | −0.694 * | −0.567 | 1 | −0.651 ** | 0.669 ** | 0.389 | 0.384 |
| BSi | 0.240 | 0.040 | 0.434 | 0.588 | 0.713 * | 0.062 | 1 | −0.115 | −0.073 | −0.120 |
| Chlorophyll-a | −0.786 ** | −0.338 | −0.271 | 0.122 | 0.009 | −0.394 | −0.301 | 1 | 0.270 | 0.218 |
| TN | 0.163 | −0.245 | 0.079 | 0.804 ** | 0.889 ** | −0.212 | 0.924 ** | −0.302 | 1 | 0.994 ** |
| TOC | −0.301 | −0.534 | −0.124 | 0.932 ** | 0.956 ** | −0.528 | 0.766 ** | 0.138 | 0.876 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, G.; Wang, G.; Yang, H.; Liu, J.; Guo, H.; Wu, J.; Jiang, L.; Wang, J. Silicon Speciation and Its Relationship with Carbon and Nitrogen in the Sediments of a Macrophytic Eutrophic Lake. Toxics 2025, 13, 266. https://doi.org/10.3390/toxics13040266
Liu Y, Xu G, Wang G, Yang H, Liu J, Guo H, Wu J, Jiang L, Wang J. Silicon Speciation and Its Relationship with Carbon and Nitrogen in the Sediments of a Macrophytic Eutrophic Lake. Toxics. 2025; 13(4):266. https://doi.org/10.3390/toxics13040266
Chicago/Turabian StyleLiu, Yong, Guoli Xu, Guocheng Wang, Haiquan Yang, Jv Liu, Hai Guo, Jiaxi Wu, Lujia Jiang, and Jingfu Wang. 2025. "Silicon Speciation and Its Relationship with Carbon and Nitrogen in the Sediments of a Macrophytic Eutrophic Lake" Toxics 13, no. 4: 266. https://doi.org/10.3390/toxics13040266
APA StyleLiu, Y., Xu, G., Wang, G., Yang, H., Liu, J., Guo, H., Wu, J., Jiang, L., & Wang, J. (2025). Silicon Speciation and Its Relationship with Carbon and Nitrogen in the Sediments of a Macrophytic Eutrophic Lake. Toxics, 13(4), 266. https://doi.org/10.3390/toxics13040266







