Development of an In Vitro Method for Assessing the Potential Irritation of Medical Devices and OTC Products Used in the Oral Cavity
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
2.2. Quality Control of the Tissues
2.3. In Vitro Irritation Assay
2.4. MTT Viability Assay
2.5. ET-50 Calculation
3. Results
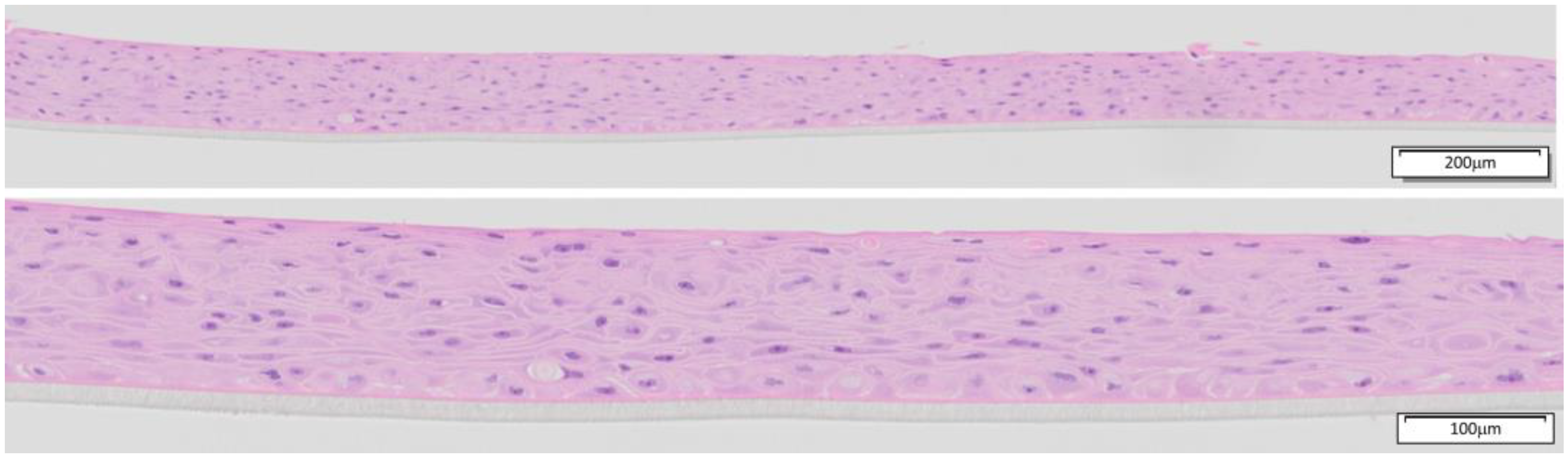
3.1. Quality Control of EpiOral Tissue
3.2. Effect of Concentration and Exposure Time
3.3. ET-50 and Irritant Potency
3.4. In Vivo/In Vitro Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISO 10993-23:2021; Biological Evaluation of Medical Devices—Part 23: Tests for Irritation. International Organization for Standardization: Geneva, Switzerland, 2021.
- Klausner, M.; Handa, Y.; Aizawa, S. In vitro three-dimensional organotypic culture models of the oral mucosa. Vitr. Cell Dev. Biol. Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef]
- Odraska, P.; Mazurova, E.; Dolezalova, L.; Blaha, L. In vitro evaluation of the permeation of cytotoxic drugs through reconstructed human epidermis and oral epithelium. Klin Onkol. 2011, 24, 195–202. Available online: https://www.linkos.cz/files/klinicka-onkologie/162/3793.pdf (accessed on 12 January 2025). [PubMed]
- Giannola, L.I.; De Caro, V.; Giandalia, G.; Siragusa, M.G.; Giuseppina, C.; Florena, A.M.; Ciach, T. Diffusion of naltrexone across reconstituted human oral epithelium and histomorphological features. Eur. J. Pharm. Biopharm. 2007, 65, 238–246. [Google Scholar] [CrossRef]
- Morse, D.J.; Wilson, M.J.; Wei, X.; Lewis, M.A.; Bradshaw, D.J.; Murdoch, C.; Williams, D.W. Denture-associated biofilm infection in three-dimensional oral mucosal tissue models. J. Med. Microbiol. 2018, 67, 364–375. [Google Scholar] [CrossRef]
- Keyser, B.M. Cytotoxicity, oxidative stress, and inflammatory response of smokeless tobacco extracts and cytotoxicity of combustible cigarette whole smoke in a 3D oral organotypic buccal cell model. Toxicol. Mech. Methods 2022, 32, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Klausner, M.; Ayehunie, S.; Breyfogle, B.; Wertz, P.; Bacca, L.; Kubilus, J. Organotypic human oral tissue models for toxicological studies. Toxicol. Vitr. 2007, 21, 938–949. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Piao, Y.-Z.; Kim, K.-M.; Kwon, J.-S. Cytokine Release from Organotypic Human Oral Tissue Following Exposure to Oral Care Product Chemicals. J. Korean Dent. Sci. 2021, 14, 51–60. [Google Scholar] [CrossRef]
- Aizawa, S.; Yoshida, H.; Umeshita, K.; Watanabe, S.; Takahashi, Y.; Sakane, S.; Kataoka, S. Development of an oral mucosal irritation test using a three-dimensional human buccal oral mucosal model. Toxicol. Vitr. 2023, 87, 105519. [Google Scholar] [CrossRef]
- Squier, C.; Kremer, M. Biology of Oral Mucosa and Esophagus. JNCI Monogr. 2001, 2001, 7–15. [Google Scholar] [CrossRef]
- Rispin, A.; Stitzel, K.; Harbell, J.; Klausner, M. Ensuring quality of in vitro alternative test methods: Current practice. Regul. Toxicol. Pharmacol. 2006, 45, 97–103. [Google Scholar] [CrossRef]
- MatTek. MTT Effective Time 50 (ET-50) for Use with EpiDerm Skin Model (EPI-200). 2020. Available online: https://www.mattek.com/wp-content/uploads/EPI-200-MTT-ET-50-Protocol-MK-24-007-0001.pdf (accessed on 31 January 2025).
- Zeng, P.; Rao, A.; Wiedmann, T.; Bowles, W. Solubility Properties of Chlorhexidine Salts. Drug Dev. Ind. Pharm. 2009, 35, 172–176. [Google Scholar] [CrossRef]
- Park, K.; Kim, K.; Kim, B. Condition Setting for Oral Mucosal Irritation Evaluation using Hamster Cheek Pouch. J. Environ. Health Sci. 2015, 41, 405–411. [Google Scholar] [CrossRef]
- ISO 10993-10:2010; Biological Evaluation of Medical Devices—Part 10: Tests for Irritation and Skin Sensitization. International Organization for Standardization: Geneva, Switzerland, 2021.
- McCabe, J.F.; Walls, A.W.G. Endodontic Materials. In Applied Dental Materials, 9th ed.; Blackwell Publishing: Hoboken, NJ, USA, 2008; Volume 3, pp. 289–296. [Google Scholar]
- Dinesh, R.; Viritpon, S.; Janak, S.; Jiaqian, Q.; Krisana, S.; Vilailuck, S. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Sabri, H.; Azarm, A.; Sadighnia, N.; Ghanbari, F.; Kheiri, P.; Deravi, N.; Mokhtari, M. The Yin and Yang of Sodium Lauryl Sulfate Use for Oral and Periodontal Health: A Literature Review. J. Dent. 2023, 24, 262–276. [Google Scholar] [CrossRef]
- Leggat, P.; Kedjarune, U. Toxicity of methyl methacrylate in dentistry. Int. Dent. J. 2003, 53, 126–131. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Ectoxicology and Toxicology of Chemicals (ECETOC). Technical Report No. 66: Skin Irritation and Corrosion: Reference Chemicals Data Bank. 1995. Available online: https://www.ecetoc.org/wp-content/uploads/2014/08/ECETOC-TR-066.pdf (accessed on 31 January 2025).
- Pellevoisin, C.; Videau, C.; Briotet, D.; Grégoire, C.; Tornier, C.; Alonso, A.; Rigaudeau, A.S.; Bouez, C.; Seyler, N. SkinEthic RHE for in vitro evaluation of skin irritation of medical device extracts. Toxicol. Vitr. 2018, 50, 418–425. [Google Scholar] [CrossRef]
- Rooney, J.P.; Choksi, N.Y.; Ceger, P.; Daniel, A.B.; Truax, J.; Allen, D.; Kleinstreuer, N. Analysis of variability in the rabbit skin irritation assay. Regul. Toxicol. Pharmacol. 2021, 122, 104920. [Google Scholar] [CrossRef]
- Luechtefeld, T.; Martens, A.; Russo, D.P.; Rovida, C.; Zhu, H.; Hartung, T. Analysis of Draize eye irritation testing and its prediction by mining publicly available 2008-2014 REACH data. Altex 2016, 33, 123–134. [Google Scholar] [CrossRef]
- Pobis, P.; Milasova, T.; Kandarova, H. Exploring the potential of reconstructed human epithelial tissue models for safety assessment of intraoral medical devices. Toxicol Vitr. 2025, 104, 105956. [Google Scholar] [CrossRef]
- Rostami, A.; Brooks, J. Intraoral chemical burn from use of 3% hydrogen peroxide. Gen. Dent. 2011, 59, 504–506. [Google Scholar]
- Shetty, K. Hydrogen peroxide burn of the oral mucosa. Ann. Pharmacother. 2006, 40, 351. [Google Scholar] [PubMed]
- Gutierrez, R.; Toman, B.; Ma, Y.; Elliott, J.; Petersen, E. Sensitivity analysis and quality indicators for an in vitro oral irritation assay. ALTEX-Altern. Anim. Exp. 2024, 41, 633–646. [Google Scholar] [CrossRef]
- Moghaddam, B.; Yabg, J.; Roohpour, N. Biologic evaluation of devices with chronic exposure using 3D human gingival model. In Proceedings of the 10th World Biomaterials Congress, Montréal, QC, Canada, 17–22 May 2016. [Google Scholar] [CrossRef]
- Vannet, B.; Hanssens, J.-L.; Wehrbein, H. The use of three-dimensional oral mucosa cell cultures to assess the toxicity of soldered and welded wires. Eur. J. Orthod. 2007, 29, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Boulmederat, L.; Bochot, A.; Lesieur, S.; Fattal, E. Evaluation of Buccal Methyl-b-Cyclodextrin Toxicity on Human Oral Epithelial Cell Culture Model. J. Pharm. Sci. 2005, 94, 1300–1309. [Google Scholar] [CrossRef]
| Chemical | CAS No. | Initial Conc | Skin Irritation (GHS) | Manufacturer |
|---|---|---|---|---|
| Sesame oil (SO) | 8008-74-0 | NC | Merck, Darmstadt, Germany | |
| Saline (0.9% NaCl) | 7647-14-5 | ≥99% | NC | Merck, Darmstadt, Germany |
| Lactic acid | 50-21-5 | 90% | 2 | Merck, Darmstadt, Germany |
| Sodium dodecyl sulfate (SDS) | 151-21-3 | 20% | 2 | Merck, Darmstadt, Germany |
| Methyl methacrylate | 80-62-6 | 100% | 2 | Merck, Darmstadt, Germany |
| 1-decanol | 112-30-1 | 98% | 3 | Merck, Darmstadt, Germany |
| Ethanol | 64-17-5 | 100% | 2 | Merck, Darmstadt, Germany |
| Chlorhexidine digluconate | 18472-51-0 | 20% | 2 | Merck, Darmstadt, Germany |
| Hydrogen peroxide | 7722-84-1 | 30% | 2 | Merck, Darmstadt, Germany |
| Sodium hypochlorite | 7681-52-9 | 6–14% | 1 | Merck, Darmstadt, Germany |
| Phosphoric acid | 7664-38-2 | ≥85% | 1 | Merck, Darmstadt, Germany |
| ET-50 (Hours) | Classification |
|---|---|
| ≤1 | Strong irritant/possibly corrosive |
| 1–4 | Moderate irritant |
| 4–18 | Mild irritant |
| ≥18 | Non-irritant |
| MatTek Corporation | MatTek Europe (Start of Production in 2022) | |||||||
|---|---|---|---|---|---|---|---|---|
| Year | ET-50 (min) | H2O (OD) | CV (%) | Tissue Lots | ET-50 (min) | H2O (OD) | CV (%) | Tissue Lots |
| 2024 | 48.5 | 1.507 | 9.1 | 23 | 64.4 | 1.731 | 7.9 | 31 |
| 2023 | 78.8 | 1.548 | 7.5 | 30 | 80.5 | 1.750 | 6.5 | 20 |
| 2022 | 86.7 | 1.503 | 7.8 | 30 | 70.1 | 1.689 | 7.5 | 14 |
| 2021 | 56.0 | 1.570 | 6.9 | 24 | ||||
| 2020 | 58.6 | 1.561 | 6.4 | 24 | ||||
| 2019 | 67.8 | 1.603 | 9.5 | 24 | ||||
| 2018 | 78.3 | 1.559 | 8.5 | 27 | ||||
| 2017 | 88.4 | 1.535 | 8.7 | 23 | ||||
| 2016 | 82.8 | 1.615 | 7.4 | 24 | ||||
| Chemicals | Concentration | Exposure Time | ||
|---|---|---|---|---|
| 1 h | 4 h | 18 h | ||
| Lactic acid | 0.10% | 93.1 | 90.2 | 101.2 |
| 0.50% | 88.2 | 81.1 | 81.6 | |
| 1% | 90.7 | 38.8 | 18.7 | |
| 4% | 11.1 | 9.9 | 9.0 | |
| 5% | 14.7 | 8.9 | 12.3 | |
| SDS | 0.10% | 107.2 | 110.8 | 109.2 |
| 1% | 80.6 | 11.1 | 4.9 | |
| 3% | 50.2 | 7.6 | 3.3 | |
| 5% | 22.2 | 4.7 | 2.8 | |
| Methyl methacrylate | 0.10% | 103.1 | 92.4 | 109.5 |
| 1% | 95.1 | 90.8 | 100.3 | |
| 5% | 102.4 | 101.3 | 94.1 | |
| 25% | 108.9 | 104.7 | 91.6 | |
| 50% | 108.9 | 8.2 | 22.6 | |
| 1-decanol | 1% | 100.4 | 90.5 | 108.9 |
| 5% | 99.9 | 96.3 | 121.7 | |
| 10% | 98.8 | 98.9 | 113.1 | |
| 100% | 131.8 | 96.3 | 8.1 | |
| Ethanol | 25% | 84.2 | 101.9 | 87.9 |
| 50% | 71.1 | 70.0 | 39.1 | |
| 100% | 55.6 | 23.9 | 7.6 | |
| Chlorhexidine digluconate * | 0.2% | 125.5 | 131.1 | 24.9 |
| 1% | 103.2 | 105.4 | 89.7 | |
| 2% | 37.0 | 13.2 | 7.3 | |
| 10% | 16.1 | 10.7 | 8.6 | |
| Hydrogen peroxide | 1% | 75.8 | 73.5 | 68.0 |
| 3% | 60.0 | 52.5 | 29.7 | |
| 10% | 11.1 | 5.7 | 6.4 | |
| Sodium hypochlorite | 0.1% | 108.8 | 99.2 | 84.0 |
| 0.2% | 88.7 | 99.6 | 101.3 | |
| 1% | 4.9 | 4.2 | 6.2 | |
| 2% | 4.9 | 4.2 | 7.8 | |
| 10% | 0.8 | 1.9 | 2.8 | |
| Phosphoric acid | 0.1% | 105.1 | 100.9 | 93.6 |
| 1% | 96.6 | 15.5 | 12.3 | |
| 5% | 15.1 | 14.4 | 15.0 | |
| 10% | 14.7 | 17.6 | 19.8 | |
| 25% | 10.9 | 14.0 | 16.6 | |
| 50% | 1.4 | 4.7 | 9.3 | |
| Chemicals | Concentration (w/v) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.10% | 0.20% | 1% | 2% | 3% | 5% | 10% | 25% | 50% | 100% | |
| Sodium hypochlorite | >18 | >18 | <1 | <1 | - | - | <1 | - | - | - |
| Sodium dodecyl sulfate | >18 | 1.6 | - | 1.0 | <1 | - | - | - | - | |
| Phosphoric acid | >18 | - | 2.2 | - | - | <1 | <1 | <1 | <1 | - |
| Lactic acid | >18 | - | 3.0 | - | - | <1 | - | - | - | - |
| Hydrogen peroxide | - | - | >18 | - | 4.7 | - | <1 | - | - | - |
| Chlorhexidine digluconate | - | 12.6 | - | <1 | - | - | <1 | - | - | - |
| Methyl methacrylate | >18 | - | >18 | - | - | >18 | - | >18 | 2.2 | - |
| Ethanol | - | - | - | - | - | - | - | >18 | 10.6 | 1.3 |
| 1-decanol | - | - | >18 | - | - | >18 | >18 | - | - | 8.8 |
| In Vivo | In Vitro | |||||
|---|---|---|---|---|---|---|
| Test Solutions | Conc. | Park et al. [14] | ET-50 (Hours) | |||
| SDS | 1% | I | Moderate | I | 1.8 | Moderate |
| Triton X-100 | 1% | I | Moderate | I | 0.5–1.8 * | Strong to moderate |
| Hydrogen peroxide | 3% | I | Moderate | I | 4.7 | Mild |
| Ethanol | 100% | I | Moderate | I | 1.3 | Moderate |
| Chlorhexidine | 2% | I | Mild | I | <1 | Strong |
| Chlorhexidine | 0.2% | I | Mild | I | 12.6 | Mild |
| Year | Model | Test Materials | Dosing | Exposure Times | Endpoint | Reference |
|---|---|---|---|---|---|---|
| 2025 | EpiOral | Oral care products | 100 μL | 4, 18 h | Cell viability, IL-1α | Pobis et al., 2025 [24] |
| 2024 | EpiOral | Y-4, RM-C, SDS | 100 µL | 0.33, 1, 6 h | Cell viability | Gutierrez et al., 2024 [27] |
| 2023 | EpiOral | Ingredients oral care and lipsticks, chemical eye irritants | 100 µL | 2 h | Cell viability | Aizawa et al., 2023 [9] |
| 2021 | EpiOral | Oral care, ethanol, SLS, H2O2 | 100 μL | 1.5 h | Cell viability, IL-1α, IL-8 | Yang et al., 2021 [8] |
| 2016 | EpiGingival | Oral care products | 24 h | Cell viability | Moghaddam et al., 2016 [28] | |
| 2007 | EpiOral | Oral care products | 100 μL | 1, 2, 4, 6, 18 h | Cell viability, ET-50 | Klausner et al., 2007 [7] |
| 2007 | SkinEthic RHOE | Orthodontic wires | 1 mm pieces | 24 h | Cell viability | Vannet et al., 2007 [29] |
| 2005 | SkinEthic RHOE | Methylated b-cyclodextrin (RAMEB) | 30 μL | 1, 4, and 24 h | Cell viability, IL-1α, histology | Boulmederat et al., 2005 [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellevoisin, C.; Puskar, M.; Molignano, J.; Coen, K.; Klausner, M.; Letasiova, S. Development of an In Vitro Method for Assessing the Potential Irritation of Medical Devices and OTC Products Used in the Oral Cavity. Toxics 2025, 13, 233. https://doi.org/10.3390/toxics13040233
Pellevoisin C, Puskar M, Molignano J, Coen K, Klausner M, Letasiova S. Development of an In Vitro Method for Assessing the Potential Irritation of Medical Devices and OTC Products Used in the Oral Cavity. Toxics. 2025; 13(4):233. https://doi.org/10.3390/toxics13040233
Chicago/Turabian StylePellevoisin, Christian, Marek Puskar, Jennifer Molignano, Kaitlyn Coen, Mitchell Klausner, and Silvia Letasiova. 2025. "Development of an In Vitro Method for Assessing the Potential Irritation of Medical Devices and OTC Products Used in the Oral Cavity" Toxics 13, no. 4: 233. https://doi.org/10.3390/toxics13040233
APA StylePellevoisin, C., Puskar, M., Molignano, J., Coen, K., Klausner, M., & Letasiova, S. (2025). Development of an In Vitro Method for Assessing the Potential Irritation of Medical Devices and OTC Products Used in the Oral Cavity. Toxics, 13(4), 233. https://doi.org/10.3390/toxics13040233






