CuO-NPs Induce Apoptosis and Functional Impairment in BV2 Cells Through the CSF-1R/PLCγ2/ERK/Nrf2 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of CuO-NP Suspension
2.2. Cu2+ Precipitation
2.3. Preparation of Copper Chloride
2.4. Cell Culture and Exposure
2.5. Effects of CuO-NPs on BV2 Cell Activity, Determined Using Cell Counting Kit-8 (CCK8) Method
2.6. Cytoplasmography
2.7. Apoptosis Detection Using Flow Cytometry
2.8. Cell Integrity Detection
2.9. Identification of TNF-α, IL-1β, and IL-6 Levels Using Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Cell Oxidative Stress Level Detection and ROS Determination
2.11. Western Blot (WB)
2.12. Statistical Analysis
3. Results
3.1. CuO-NP Particle Size Characterization
3.2. Cu2+ Level of CuO-NPs Precipitated in Cell Culture Medium
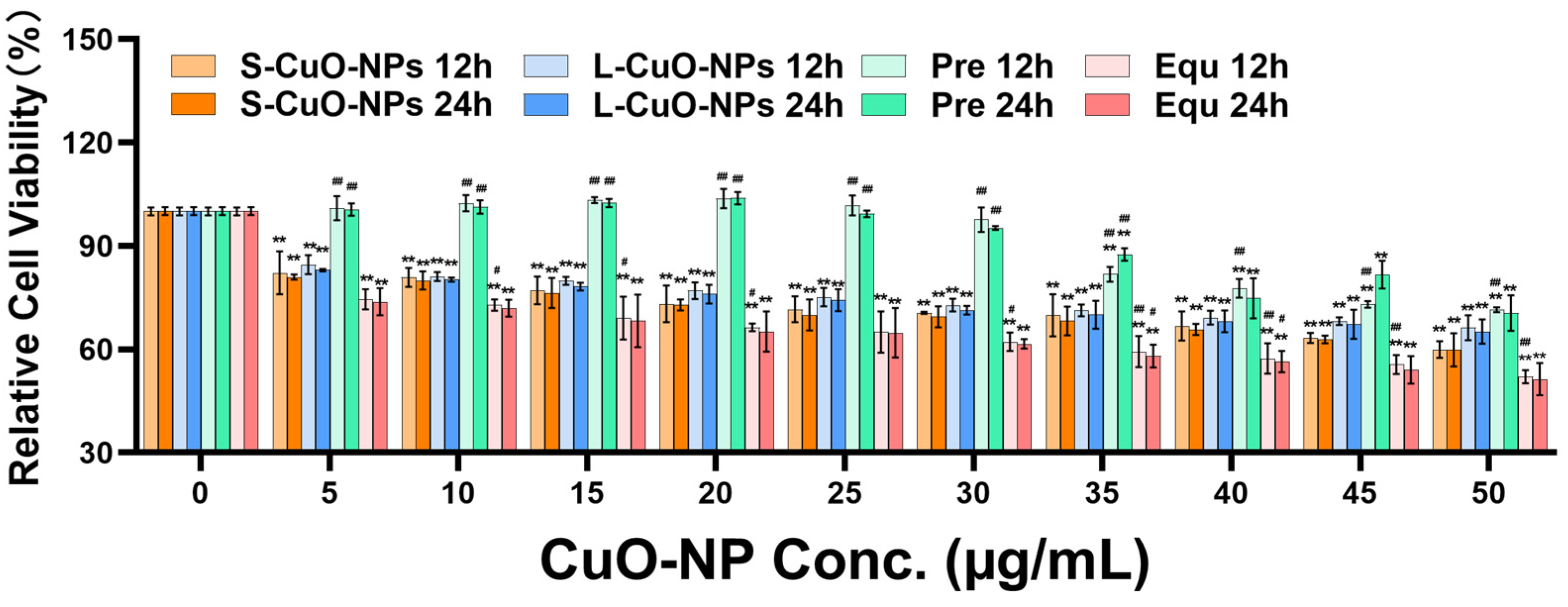
3.3. Cell Activity
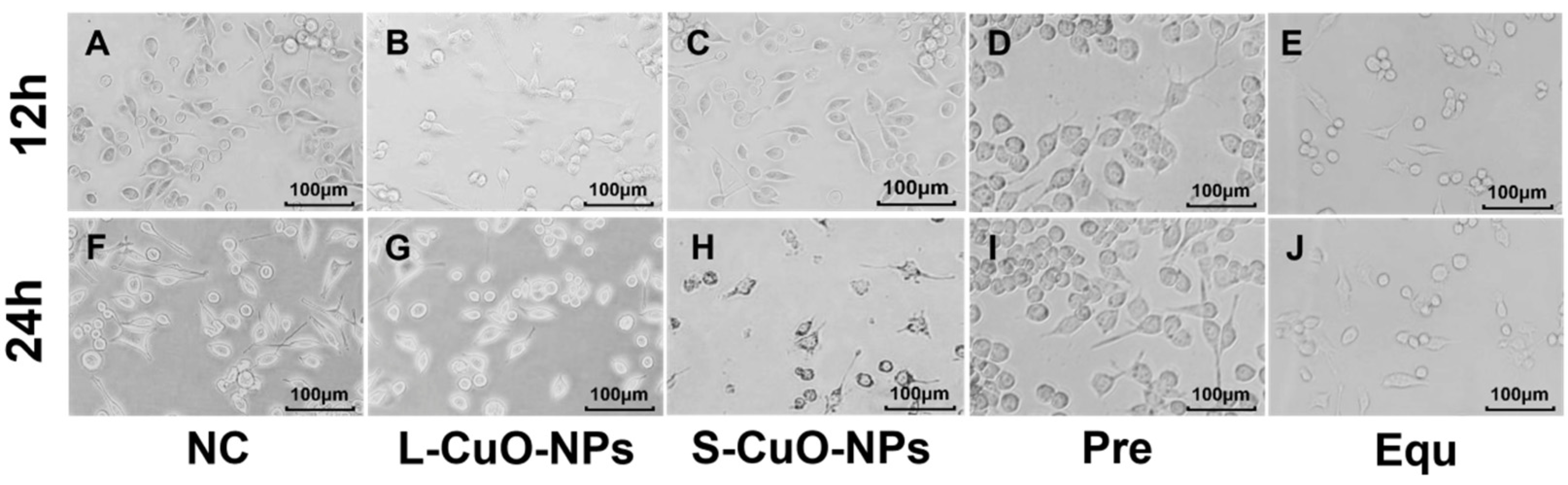
3.4. Cell Morphology Observation
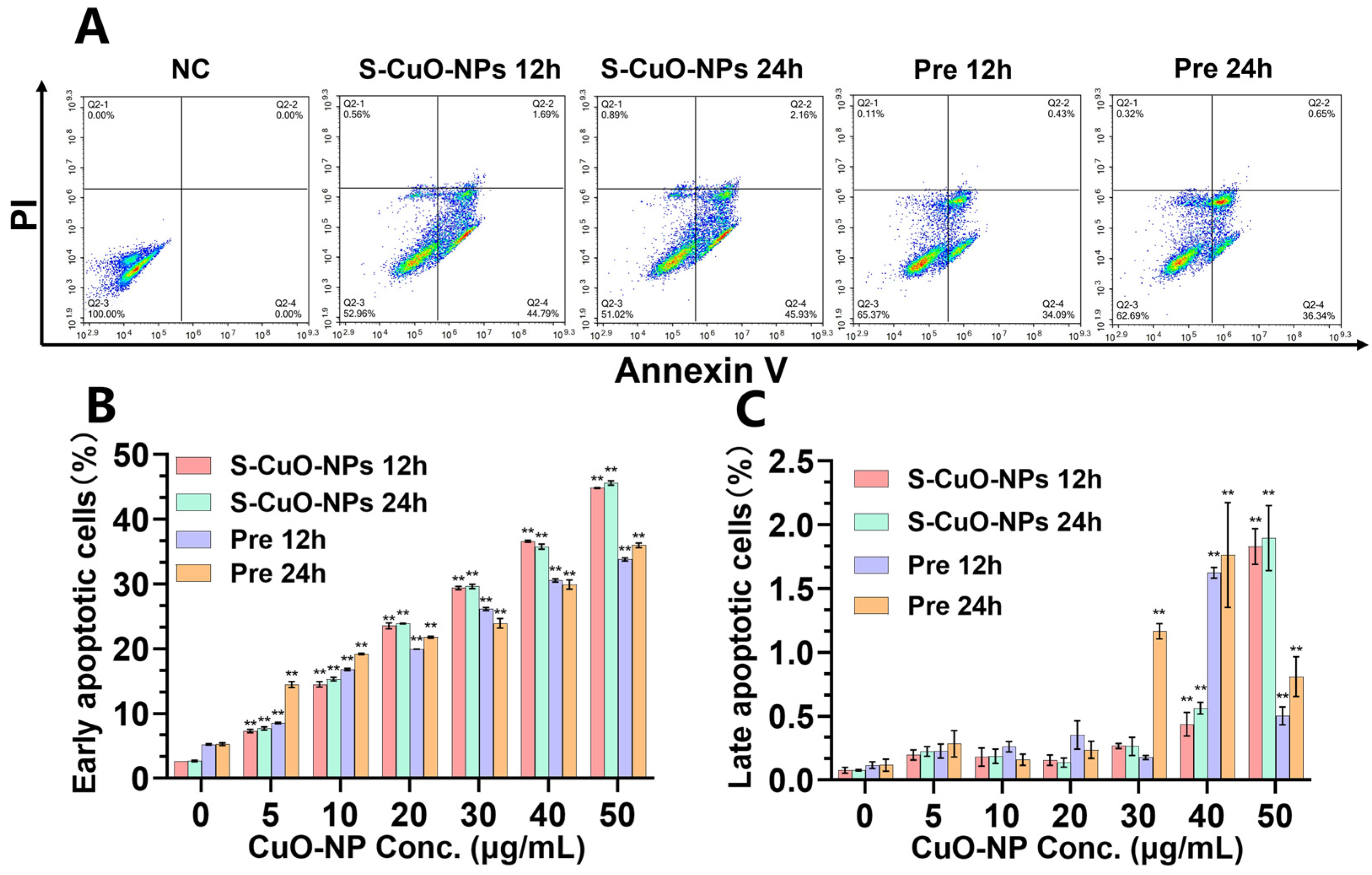
3.5. Apoptosis
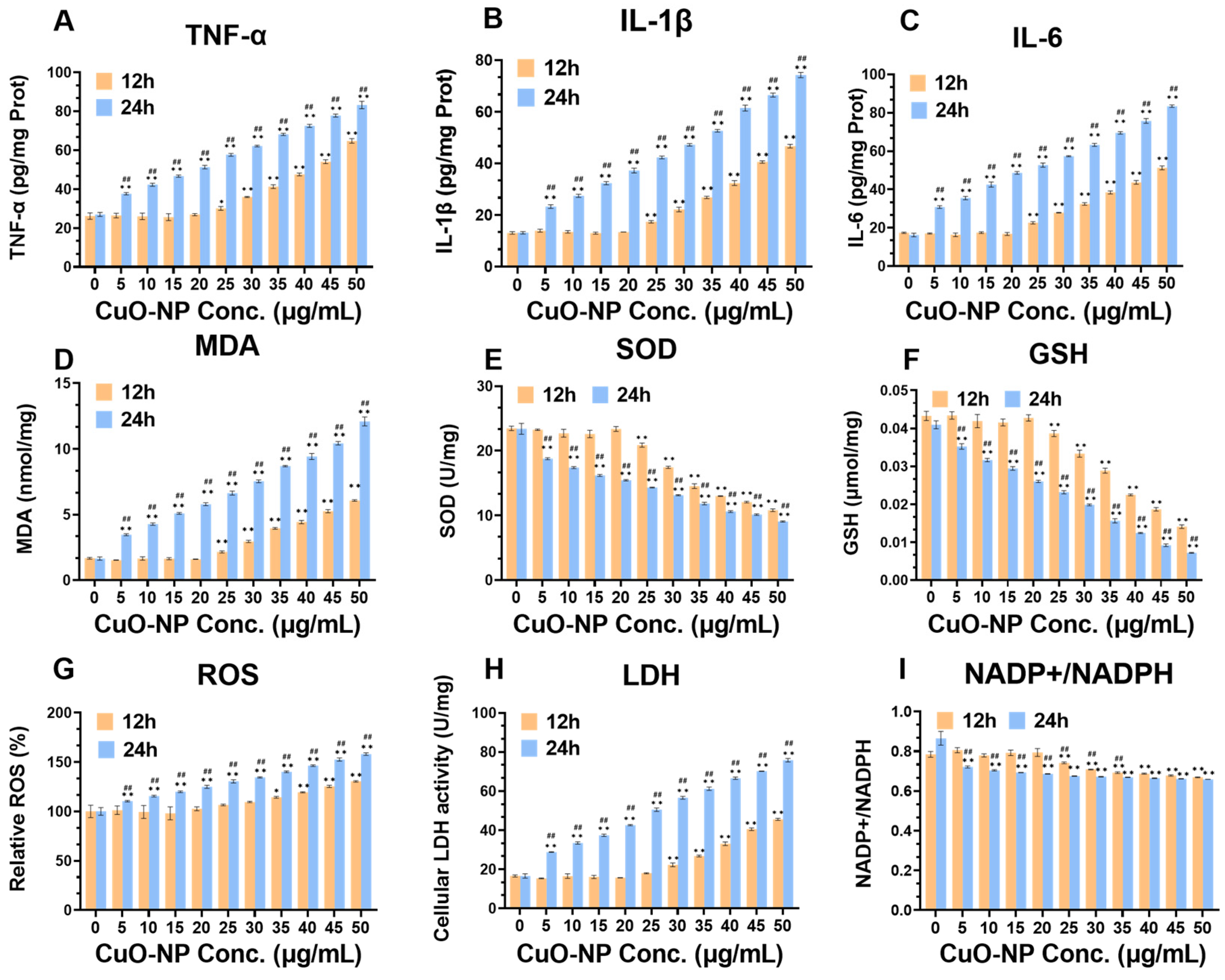
3.6. Extracellular LDH Levels
3.7. Cellular Oxidative Stress and Inflammation Levels
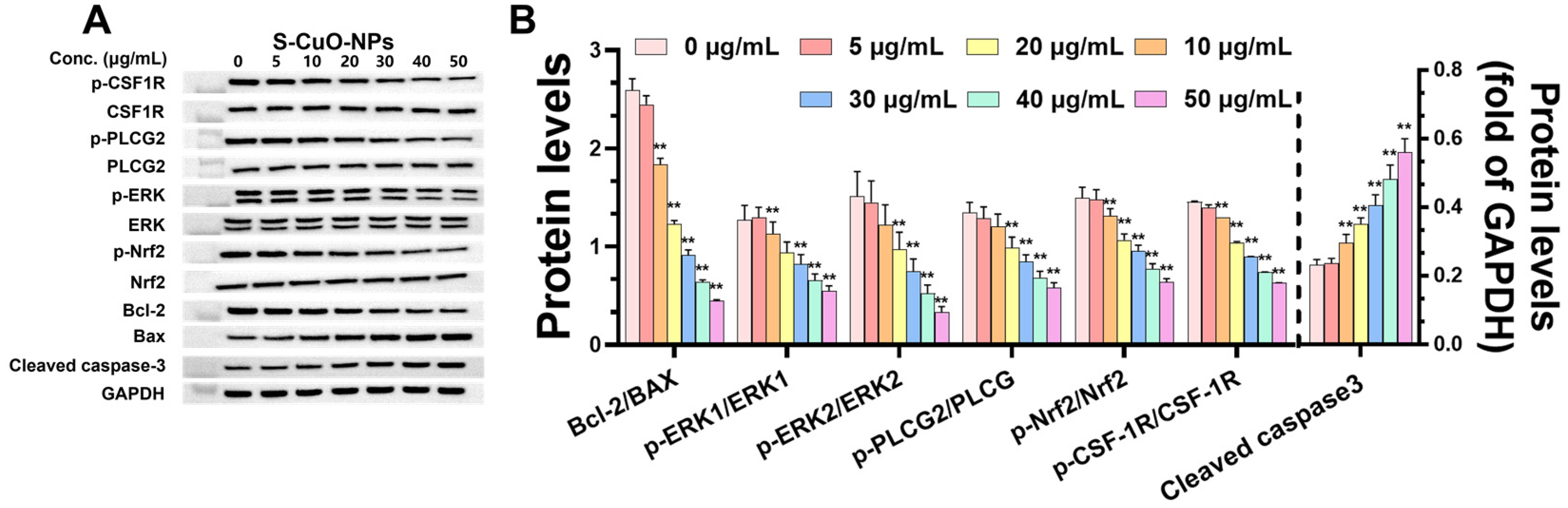
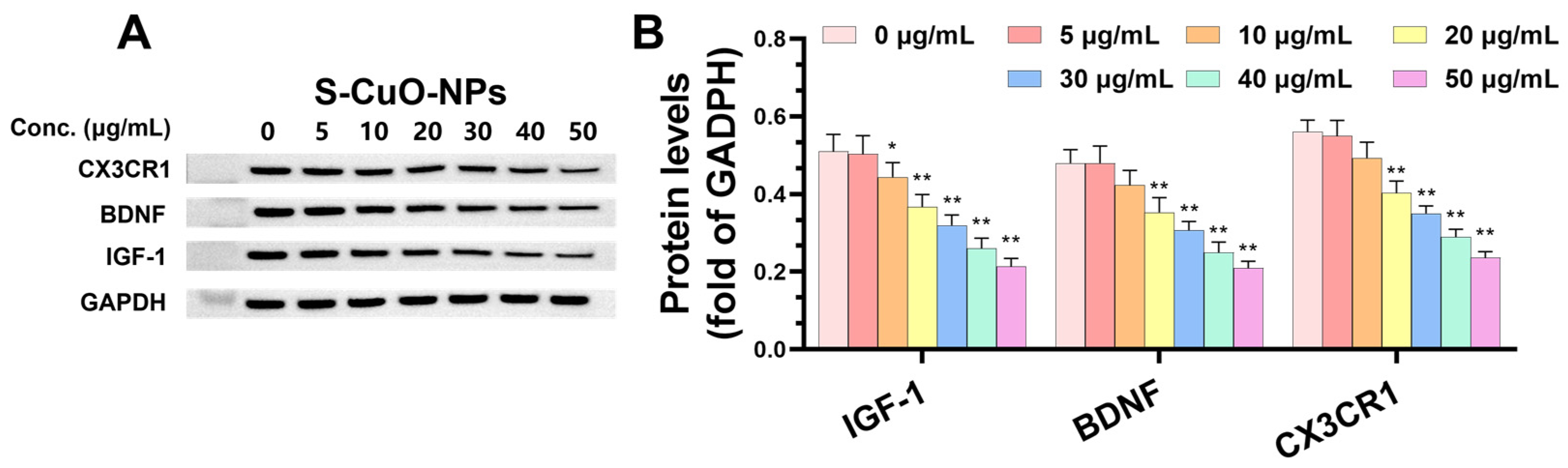
3.8. CuO-NPs Cause Functional Damage to BV2 Cells and Promote BV2 Apoptosis Through CSF-1R/PLCγ2/ERK/Nrf2 Signaling Pathway and Its Effect on Level of Functional Damage Protein and Damage Mechanism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angelé-Martínez, C.; Nguyen, K.V.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive oxygen species generation by copper(II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; McLean, J.E.; Jacobson, A.R.; Britt, D.W. CuO and ZnO Nanoparticles Modify Interkingdom Cell Signaling Processes Relevant to Crop Production. J. Agric. Food. Chem. 2018, 66, 6513–6524. [Google Scholar] [PubMed]
- Pierzynowska, K.; Kamińska, T.; Węgrzyn, G. One drug to treat many diseases: Unlocking the economic trap of rare diseases. Metab. Brain Dis. 2020, 35, 1237–1240. [Google Scholar] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef]
- Arcuri, C.; Mecca, C.; Bianchi, R.; Giambanco, I.; Donato, R. The Pathophysiological Role of Microglia in Dynamic Surveillance, Phagocytosis and Structural Remodeling of the Developing CNS. Front. Mol. Neurosci. 2017, 10, 191. [Google Scholar]
- Liang, X.Y.; Zhang, Z.Q.; Zhang, Y.; Liu, Y.; Wang, X. Effects of zinc oxide nanoparticle on expression of inflammatory factors and phosphorylation of p38MAPK in BV2 microglia. J. Environ. Occup. Med. 2018, 35, 447–451. [Google Scholar]
- Marmiroli, M.; Pagano, L.; Rossi, R.; De La Torre-Roche, R.; Lepore, G.O.; Ruotolo, R.; Gariani, G.; Bonanni, V.; Pollastri, S.; Puri, A.; et al. Copper Oxide Nanomaterial Fate in Plant Tissue: Nanoscale Impacts on Reproductive Tissues. Environ. Sci. Technol. 2021, 55, 10769–10783. [Google Scholar]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 2002, 26, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Wang, H.; Fan, Q.; Chen, L.; Huang, H.; Ran, H. Cystatin F involvement in adenosine A2A receptor-mediated neuroinflammation in BV2 microglial cells. Sci. Rep. 2018, 8, 6820. [Google Scholar]
- Biswas, K. Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation. J. Neuroimmunol. 2023, 383, 578180. [Google Scholar]
- Liu, Z.; Yao, X.; Jiang, W.; Li, W.; Zhu, S.; Liao, C.; Zou, L.; Ding, R.; Chen, J. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. J. Neuroinflamm. 2020, 17, 90. [Google Scholar]
- Pramanik, S.; Devi, M.H.; Chakrabarty, S.; Paylar, B.; Pradhan, A.; Thaker, M.; Ayyadhury, S.; Manavalan, A.; Olsson, P.-E.; Pramanik, G.; et al. Microglia signaling in health and disease—Implications in sex-specific brain development and plasticity. Neurosci. Biobehav. Rev. 2024, 165, 105834. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar]
- Feng-Shiun, S.; Randall, L.W. Manipulation of Microglial Activation as a Therapeutic Strategy in Alzheimers Disease. Curr. Med. Chem. 2007, 14, 2865–2871. [Google Scholar]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar]
- Song, M.F.; Li, Y.S.; Kasai, H.; Kawai, K. Metal nanoparticle-induced micronuclei and oxidative DNA damage in mice. J. Clin. Biochem. Nutr. 2012, 50, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Doudi, M.; Setorki, M. Acute effect of nano-copper on liver tissue and function in rat. Nanomed. J. 2014, 1, 331–338. [Google Scholar]
- Mohammadyari, A.; Razavipour, S.T.; Mohammadbeigi, M.; Negahdary, M.; Ajdary, M. Exploring vivo toxicity assessment of copper oxide nanoparticle in Wistar rats. J. Biol. Today’s World 2014, 3, 123. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Cuillel, M.; Chevallet, M.; Charbonnier, P.; Fauquant, C.; Pignot-Paintrand, I.; Arnaud, J.; Cassio, D.; Michaud-Soret, I.; Mintz, E. Interference of CuO nanoparticles with metal homeostasis in hepatocytes under sub-toxic conditions. Nanoscale 2014, 6, 1707–1715. [Google Scholar]
- Bopp, S.K.; Abicht, H.K.; Knauer, K. Copper-induced oxidative stress in rainbow trout gill cells. Aquat. Toxicol. 2008, 86, 197–204. [Google Scholar]
- Sutton, H.C.; Winterbourn, C.C. On the participation of higher oxidation states of iron and copper in fenton reactions. Free Radical Biol. Med. 1989, 6, 53–60. [Google Scholar]
- Lee, I.C.; Ko, J.W.; Park, S.H.; Lim, J.O.; Shin, I.S.; Moon, C.; Kim, S.H.; Heo, J.D.; Kim, J.C. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomedicine 2016, 11, 2883–2900. [Google Scholar]
- Ganganelli, I.; Galatro, A.; Gergoff Grozeff, G.E.; Bartoli, C.G.; Senn, M.E. 3-Reactive oxygen species (ROS): Chemistry and role in plant physiology. In Oxygen, Nitrogen and Sulfur Species in Post-Harvest Physiology of Horticultural Crops; Ziogas, V., Corpas, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 43–73. [Google Scholar]
- Wang, L.; Huang, X.; Sun, W.; Too, H.Z.; Laserna, A.K.C.; Li, S.F.Y. A global metabolomic insight into the oxidative stress and membrane damage of copper oxide nanoparticles and microparticles on microalga Chlorella vulgaris. Environ. Pollut. 2020, 258, 113647. [Google Scholar]
- Jiang, M.; Tao, X.; Pang, Y.; Qin, Z.; Song, E.; Song, Y. Copper oxide nanoparticles induce cuproptosis and ferroptosis through mitochondrial concatenation. Environ. Sci. Nano 2024, 11, 4089–4101. [Google Scholar]
- Jiang, Y.W.; Gao, G.; Jia, H.R.; Zhang, X.; Zhao, J.; Ma, N.; Liu, J.B.; Liu, P.; Wu, F.G. Copper Oxide Nanoparticles Induce Enhanced Radiosensitizing Effect via Destructive Autophagy. ACS Biomater. Sci. Eng. 2019, 5, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, W.; Xia, X.; Zhang, J.; Wang, M.; Li, Y.; Li, X.; Zheng, Y.; Liu, J.; Zhang, R.; et al. The domino effect in inhaled carbon black nanoparticles triggers bloodbrain barrier disruption via altering circulatory inflammation. Nano Today 2023, 48, 101721. [Google Scholar] [CrossRef]
- Che, X.; Ding, R.; Li, Y.; Zhang, Z.; Gao, H.; Wang, W. Mechanism of long-term toxicity of CuO NPs to microalgae. Nanotoxicology 2018, 12, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Tatsi, K.; Shaw, B.J.; Hutchinson, T.H.; Handy, R.D. Copper accumulation and toxicity in earthworms exposed to CuO nanomaterials: Effects of particle coating and soil ageing. Ecotoxicol. Environ. Saf. 2018, 166, 462–473. [Google Scholar] [CrossRef]
- Yue, L.; Zhao, J.; Yu, X.; Lv, K.; Wang, Z.; Xing, B. Interaction of CuO nanoparticles with duckweed (Lemna minor. L): Uptake, distribution and ROS production sites. Environ. Pollut. 2018, 243 Pt A, 543–552. [Google Scholar] [CrossRef]
- Farshori, N.N.; Siddiqui, M.A.; Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Ahamed, M.; Ahmad, J.; Al-Khedhairy, A.A. Copper Oxide Nanoparticles Exhibit Cell Death Through Oxidative Stress Responses in Human Airway Epithelial Cells: A Mechanistic Study. Biol. Trace Elem. Res. 2022, 200, 5042–5051. [Google Scholar] [CrossRef]
- Sajjad, H.; Sajjad, A.; Haya, R.T.; Khan, M.M.; Zia, M. Copper oxide nanoparticles: In vitro and in vivo toxicity, mechanisms of action and factors influencing their toxicology. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 271, 109682. [Google Scholar] [CrossRef]
- Liu, H.; Lai, W.; Liu, X.; Yang, H.; Fang, Y.; Tian, L.; Li, K.; Nie, H.; Zhang, W.; Shi, Y.; et al. Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J. Hazard. Mater. 2021, 401, 123349. [Google Scholar] [CrossRef]
- Souza, M.R.; Mazaro-Costa, R.; Rocha, T.L. Can nanomaterials induce reproductive toxicity in male mammals? A historical and critical review. Sci. Total Environ. 2021, 769, 144354. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Hayat, T.; Wang, X. Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ. Pollut. 2017, 221, 209–217. [Google Scholar] [CrossRef]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Cytotoxic Origin of Copper(II) Oxide Nanoparticles: Comparative Studies with Micron-Sized Particles, Leachate, and Metal Salts. ACS Nano 2011, 5, 7214–7225. [Google Scholar] [PubMed]
- Dudev, T.; Lim, C. Metal binding affinity and selectivity in metalloproteins: Insights from computational studies. Annu. Rev. Biophys. 2008, 37, 97–116. [Google Scholar] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [PubMed]
- Wang, Y.; Aker, W.G.; Hwang, H.-m.; Yedjou, C.G.; Yu, H.; Tchounwou, P.B. A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci. Total Environ. 2011, 409, 4753–4762. [Google Scholar]
- Siddiqui, S.; Goddard, R.H.; Bielmyer-Fraser, G.K. Comparative effects of dissolved copper and copper oxide nanoparticle exposure to the sea anemone, Exaiptasia pallida. Aquat. Toxicol. 2015, 160, 205–213. [Google Scholar]
- Singla, A.; Chen, Q.; Suzuki, K.; Song, J.; Fedoseienko, A.; Wijers, M.; Lopez, A.; Billadeau, D.D.; van de Sluis, B.; Burstein, E. Regulation of murine copper homeostasis by members of the COMMD protein family. Dis. Model. Mech. 2021, 14, dmm045963. [Google Scholar]
- Prohaska, J.R. Role of copper transporters in copper homeostasis. Am. J. Clin. Nutr. 2008, 88, 826s–829s. [Google Scholar]
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.S.; Mapuskar, K.A.; Spitz, D.R.; Grassian, V.H.; Salem, A.K. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar]
- Han, J.; Chitu, V.; Stanley, E.R.; Wszolek, Z.K.; Karrenbauer, V.D.; Harris, R.A. Inhibition of colony stimulating factor-1 receptor (CSF-1R) as a potential therapeutic strategy for neurodegenerative diseases: Opportunities and challenges. Cell Mol. Life Sci. 2022, 79, 219. [Google Scholar]
- Tarale, P.; Alam, M.M. Colony-stimulating factor 1 receptor signaling in the central nervous system and the potential of its pharmacological inhibitors to halt the progression of neurological disorders. Inflammopharmacology 2022, 30, 821–842. [Google Scholar]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [PubMed]
- Erblich, B.; Zhu, L.; Etgen, A.M.; Dobrenis, K.; Pollard, J.W. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE 2011, 6, e26317. [Google Scholar]
- Adams, R.C.; Carter-Cusack, D.; Llanes, G.T.; Hunter, C.R.; Vinnakota, J.M.; Ruitenberg, M.J.; Vukovic, J.; Bertolino, P.; Chand, K.K.; Wixey, J.A.; et al. CSF1R inhibition promotes neuroinflammation and behavioral deficits during graft-versus-host disease in mice. Blood 2024, 143, 912–929. [Google Scholar] [PubMed]
- Arnò, B.; Grassivaro, F.; Rossi, C.; Bergamaschi, A.; Castiglioni, V.; Furlan, R.; Greter, M.; Favaro, R.; Comi, G.; Becher, B.; et al. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat. Commun. 2014, 5, 5611. [Google Scholar]
- Mickeviciute, G.C.; Valiuskyte, M.; Plattén, M.; Wszolek, Z.K.; Andersen, O.; Danylaité Karrenbauer, V.; Ineichen, B.V.; Granberg, T. Neuroimaging phenotypes of CSF1R-related leukoencephalopathy: Systematic review, meta-analysis, and imaging recommendations. J. Intern. Med. 2022, 291, 269–282. [Google Scholar]
- Konno, T.; Yoshida, K.; Mizuno, T.; Kawarai, T.; Tada, M.; Nozaki, H.; Ikeda, S.I.; Nishizawa, M.; Onodera, O.; Wszolek, Z.K.; et al. Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation. Eur. J. Neurol. 2017, 24, 37–45. [Google Scholar]
- Tsai, A.P.; Dong, C.; Lin, P.B.-C.; Messenger, E.J.; Casali, B.T.; Moutinho, M.; Liu, Y.; Oblak, A.L.; Lamb, B.T.; Landreth, G.E.; et al. PLCG2 is associated with the inflammatory response and is induced by amyloid plaques in Alzheimer’s disease. Genome Med. 2022, 14, 17. [Google Scholar]
- Li, K.; Ran, B.; Wang, Y.; Liu, L.; Li, W. PLCγ2 impacts microglia-related effectors revealing variants and pathways important in Alzheimer’s disease. Front. Cell Dev. Biol. 2022, 10, 999061. [Google Scholar]
- Obba, S.; Hizir, Z.; Boyer, L.; Selimoglu-Buet, D.; Pfeifer, A.; Michel, G.; Hamouda, M.A.; Gonçalvès, D.; Cerezo, M.; Marchetti, S.; et al. The PRKAA1/AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML. Autophagy 2015, 11, 1114–1129. [Google Scholar]
- Ma, D.; Lian, F.; Wang, X. PLCG2 promotes hepatocyte proliferation in vitro via NF-κB and ERK pathway by targeting bcl2, myc and ccnd1. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3786–3792. [Google Scholar]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huang, Z.; Zhang, D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 2009, 4, e6588. [Google Scholar]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, 13. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes. Cells 2011, 16, 123–140. [Google Scholar]
- Bai, Y.; Guo, N.; Xu, Z.; Chen, Y.; Zhang, W.; Chen, Q.; Bi, Z. S100A1 expression is increased in spinal cord injury and promotes inflammation, oxidative stress and apoptosis of PC12 cells induced by LPS via ERK signaling. Mol. Med. Rep. 2023, 27, 30. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, L.; Li, M.; Shen, Y.; Liu, S.; Yang, D. Huanglian Jiedu decoction alleviates neurobehavioral damage in mice with chronic alcohol exposure through the RAS-RAF-MEK-ERK pathway. Heliyon 2024, 10, e29556. [Google Scholar] [CrossRef]
- Lai, Q.; Xie, T.; Huang, Y. Inhibitory effect of miR-27b-3p and Nrf2 regulation on metabolic memory formation in human RPE cells. Chin. J. Exp. Ophthalmol. 2023, 41, 970–979. [Google Scholar]
- Lian, B.; Gu, J.; Zhang, C.; Zou, Z.; Yu, M.; Li, F.; Wu, X.; Zhao, A.Z. Protective effects of isofraxidin against scopolamine-induced cognitive and memory impairments in mice involve modulation of the BDNF-CREB-ERK signaling pathway. Metab. Brain Dis. 2022, 37, 2751–2762. [Google Scholar] [CrossRef]
- Zhang, W.; Geng, X.; Dong, Q.; Li, X.; Ye, P.; Lin, M.; Xu, B.; Jiang, H. Crosstalk between autophagy and the Keap1-Nrf2-ARE pathway regulates realgar-induced neurotoxicity. J. Ethnopharmacol. 2023, 301, 115776. [Google Scholar]
- Zhang, L.; Guo, Y.; Wang, H.; Zhao, L.; Ma, Z.; Li, T.; Liu, J.; Sun, M.; Jian, Y.; Yao, L.; et al. Edaravone reduces Aβ-induced oxidative damage in SH-SY5Y cells by activating the Nrf2/ARE signaling pathway. Life Sci. 2019, 221, 259–266. [Google Scholar]
- Salakou, S.; Kardamakis, D.; Tsamandas, A.C.; Zolota, V.; Apostolakis, E.; Tzelepi, V.; Papathanasopoulos, P.; Bonikos, D.S.; Papapetropoulos, T.; Petsas, T.G.; et al. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo 2007, 21, 123–132. [Google Scholar] [PubMed]
- Lu, X. Structure and Function of Ligand CX3CL1 and its Receptor CX3CR1 in Cancer. Curr. Med. Chem. 2022, 29, 6228–6246. [Google Scholar] [PubMed]
- Subbarayan, M.S.; Joly-Amado, A.; Bickford, P.C.; Nash, K.R. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol. Ther. 2022, 231, 107989. [Google Scholar] [PubMed]
- Liu, Z.; Condello, C.; Schain, A.; Harb, R.; Grutzendler, J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J. Neurosci. 2010, 30, 17091–17101. [Google Scholar]
- Cook, D.N.; Chen, S.C.; Sullivan, L.M.; Manfra, D.J.; Wiekowski, M.T.; Prosser, D.M.; Vassileva, G.; Lira, S.A. Generation and analysis of mice lacking the chemokine fractalkine. Mol. Cell Biol. 2001, 21, 3159–3165. [Google Scholar]
- Jian, Z.; Nonaka, I.; Hattori, S.; Nakamura, S. Activation of ras and protection from apoptotic cell death by BDNF in PC12 cells expressing trkB. Cell. Signal. 1996, 8, 365–370. [Google Scholar]
- Koffie, R.M.; Hyman, B.T.; Spires-Jones, T.L. Alzheimer’s disease: Synapses gone cold. Mol. Neurodegener. 2011, 6, 63. [Google Scholar]

| Size (nm) | Density (g/m3) | SSA (m2/g) | Crystalline Structure | Shape | Composition |
|---|---|---|---|---|---|
| 500 | 6.3–6.49 | 10.1 | Cubic/tetragonal | Nearly spherical | 99.9% |
| 20–40 | 6.3–6.49 | 29 | Monoclinic | Sphere | 99.9% |
| Concentration of S-CuO-NPs (μg/mL) | Concentration of Cu2+ (μg/mL) | CuCl2 with Molar Mass Equal to Cu2 (mM) |
|---|---|---|
| 0 | 0 ± 0 | 0 |
| 0.5 | 0.02 ± 0.03 | 3.15 × 10−4 |
| 1 | 0.06 ± 0.01 | 9.44 × 10−4 |
| 1.5 | 0.09 ± 0.03 | 1.42 × 10−3 |
| 2 | 0.1 ± 0.03 | 1.57 × 10−3 |
| 2.5 | 0.11 ± 0.04 | 1.73 × 10−3 |
| 3 | 0.17 ± 0.04 | 2.68 × 10−3 |
| 3.5 | 0.13 ± 0.02 | 2.05 × 10−3 |
| 4 | 0.35 ± 0.03 | 5.51 × 10−3 |
| 4.5 | 0.33 ± 0.03 | 5.19 × 10−3 |
| 5 | 0.38 ± 0.03 | 5.98 × 10−3 |
| 10 | 0.86 ± 0.04 | 1.35 × 10−2 |
| 15 | 1.26 ± 0.05 | 1.98 × 10−2 |
| 20 | 1.65 ± 0.09 | 2.60 × 10−2 |
| 25 | 2.22 ± 0.04 | 3.49 × 10−2 |
| 30 | 2.7 ± 0.15 | 4.25 × 10−2 |
| 35 | 3.24 ± 0.15 | 5.10 × 10−2 |
| 40 | 3.64 ± 0.07 | 5.73 × 10−2 |
| 45 | 4.19 ± 0.11 | 6.59 × 10−2 |
| 50 | 4.72 ± 0.09 | 7.43 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhu, L.; Lin, B.; Shi, Y.; Lai, W.; Li, K.; Tian, L.; Xi, Z.; Liu, H. CuO-NPs Induce Apoptosis and Functional Impairment in BV2 Cells Through the CSF-1R/PLCγ2/ERK/Nrf2 Pathway. Toxics 2025, 13, 231. https://doi.org/10.3390/toxics13040231
Yang L, Zhu L, Lin B, Shi Y, Lai W, Li K, Tian L, Xi Z, Liu H. CuO-NPs Induce Apoptosis and Functional Impairment in BV2 Cells Through the CSF-1R/PLCγ2/ERK/Nrf2 Pathway. Toxics. 2025; 13(4):231. https://doi.org/10.3390/toxics13040231
Chicago/Turabian StyleYang, Linhui, Lina Zhu, Bencheng Lin, Yue Shi, Wenqing Lai, Kang Li, Lei Tian, Zhuge Xi, and Huanliang Liu. 2025. "CuO-NPs Induce Apoptosis and Functional Impairment in BV2 Cells Through the CSF-1R/PLCγ2/ERK/Nrf2 Pathway" Toxics 13, no. 4: 231. https://doi.org/10.3390/toxics13040231
APA StyleYang, L., Zhu, L., Lin, B., Shi, Y., Lai, W., Li, K., Tian, L., Xi, Z., & Liu, H. (2025). CuO-NPs Induce Apoptosis and Functional Impairment in BV2 Cells Through the CSF-1R/PLCγ2/ERK/Nrf2 Pathway. Toxics, 13(4), 231. https://doi.org/10.3390/toxics13040231







