Progestin Pollution in Surface Waters of a Major Southwestern European Estuary: The Douro River Estuary (Iberian Peninsula)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Collection
2.2.1. Sampling Areas at the North Margin (Porto City) of the Douro River Estuary
2.2.2. Sampling Areas at the South Margin (Gaia City) of the Douro River Estuary
2.3. Sample Preparation (Extraction and Cleanup)
2.4. LC-MS/MS Analysis
2.4.1. LC-MS/MS System Requirements
2.4.2. Quality Assurance (QA) and Quality Control (QC)
2.5. Theoretical Bioconcentration and Pharmacological Effects of PGs in Fish Plasma
2.6. Preliminary Risk Assessments for the Environmental PGs
2.7. Statistical Analysis
3. Results
3.1. PGs Levels in the Douro River Estuary Surface Waters
3.2. RQs for Environmental Synthetic PGs
3.3. Predicted Effect of Environmental Concentrations of PGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robledo, A.P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; Durán Valsero, J.J.; Morales García, R.; Lamas-Cosío, E.; Oceguera-Vargas, I.; DelValls, A. Assessment and review of heavy metals pollution in sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]
- Rocha, M.J.; Rocha, E. Synthetic progestins in waste and surface waters: Concentrations, impacts and ecological risk. Toxics 2022, 10, 163. [Google Scholar] [CrossRef]
- Golovko, O.; Šauer, P.; Fedorova, G.; Kroupová, H.K.; Grabic, R. Determination of progestogens in surface and waste water using SPE extraction and LC-APCI/APPI-HRPS. Sci. Total Environ. 2018, 621, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Mordor, I. Progesterone Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/progesterone-market (accessed on 3 March 2025).
- Li, L.; Li, M.; Lu, J.; Ge, X.; Xie, W.; Wang, Z.; Li, X.; Li, C.; Wang, X.; Han, Y. Prenatal Progestin exposure is associated with autism spectrum disorders. Front. Psychiatry 2018, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- NIEHS. National Institute of Environmental Health Sciences. Endocrine Disruptors. U.S. Department of Health and Human Services. Available online: https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm (accessed on 3 March 2025).
- Weizel, A.; Schlüsener, M.P.; Dierkes, G.; Wick, A.; Ternes, T.A. Fate and behavior of progestogens in activated sludge treatment: Kinetics and transformation products. Water Res. 2021, 188, 116515. [Google Scholar] [CrossRef]
- Kumar, V.; Johnson, A.C.; Trubiroha, A.; Tumova, J.; Ihara, M.; Grabic, R.; Kloas, W.; Tanaka, H.; Kroupova, H.K. The challenge presented by progestins in ecotoxicological research: A critical review. Environ. Sci. Technol. 2015, 49, 2625–2638. [Google Scholar] [CrossRef]
- Fent, K. Progestins as endocrine disrupters in aquatic ecosystems: Concentrations, effects and risk assessment. Environ. Int. 2015, 84, 115–130. [Google Scholar] [CrossRef]
- Statistics Portugal. Census 2021: Portugal—Porto and Vila Nova de Gaia. Instituto Nacional de Estatística. 2021. Available online: https://censos.ine.pt/xportal/xmain?xpgid=censos21_main&xpid=CENSOS21&xlang=pt (accessed on 3 March 2025).
- Rocha, M.J.; Silva, F.; Rocha, E. Annual Evaluation of 17 oestrogenic endocrine disruptors and hazard indexes in the Douro River estuary—The Atlantic discharge of the highest-flow river of Southwestern Europe. Water 2022, 14, 2046. [Google Scholar] [CrossRef]
- Chang, H.; Wu, S.; Hu, J.; Asami, M.; Kunikane, S. Trace analysis of androgens and progestogens in environmental waters by ultra-performance liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2008, 1195, 44–51. [Google Scholar] [CrossRef]
- Morais, H.; Cruzeiro, C.; Pardal, M.A.; Cardoso, P.G. Baseline progestins characterization in surface waters of three main Portuguese estuaries. Mar. Pollut. Bull. 2023, 194, 115352. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Rocha, E.; Pardal, M.A.; Rocha, M.J. Uncovering seasonal patterns of 56 pesticides in surface coastal waters of the Ria Formosa lagoon (Portugal), using a GC-MS method. Int. J. Environ. Anal. Chem. 2015, 95, 1370–1384. [Google Scholar] [CrossRef]
- Fick, J.; Lindberg, R.H.; Tysklind, M.; Larsson, D.G.J. Predicted critical environmental concentrations for 500 pharmaceuticals. Regul. Toxicol. Pharmacol. 2010, 58, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Cook, J.C.; Ericson, J.F.; Williams, R.T. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk Assess. Int. J. 2003, 9, 1789–1799. [Google Scholar] [CrossRef]
- von der Ohe, P.C.; Dulio, V.; Slobodnik, J.; De Deckere, E.; Kühne, R.; Ebert, R.-U.; Ginebreda, A.; De Cooman, W.; Schüürmann, G.; Brack, W. A new risk assessment approach for the prioritization of 500 classical and emerging organic microcontaminants as potential river basin specific pollutants under the European Water Framework Directive. Sci. Total Environ. 2011, 409, 2064–2077. [Google Scholar] [CrossRef]
- Sanderson, H.; Johnson, D.J.; Wilson, C.J.; Brain, R.A.; Solomon, K.R. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Wentsel, R.S.; LaPoint, T.W.; Simini, M.; Checkail, R.T.; Ludwig, D.; Brewer, L. Tri-Service Procedural Guidelines for Ecological Risk Assessments. Volume 1. Final Report, January–May 1996. Available online: https://apps.dtic.mil/sti/tr/pdf/ADA297968.pdf (accessed on 3 March 2025).
- Hammer, Ø.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 3 March 2025).
- Wu, J.; Li, P.; Wang, D.; Ren, X.; Wei, M. Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 1603–1621. [Google Scholar] [CrossRef]
- Ratha, D.S.; Venkataraman, G. Application of statistical methods to study seasonal variation in the mine contaminants in soil and groundwater of Goa, India. Environ. Geol. 1997, 29, 253–262. [Google Scholar] [CrossRef]
- Bick, A.J.; Louw-du Toit, R.; Skosana, S.B.; Africander, D.; Hapgood, J.P. Pharmacokinetics, metabolism and serum concentrations of progestins used in contraception. Pharmacol. Ther. 2021, 222, 107789. [Google Scholar] [CrossRef]
- Shen, X.; Chang, H.; Shao, B.; Sun, F.; Wu, F. Occurrence and mass balance of sixty-two progestins in a municipal sewage treatment plant. Water Res. 2019, 165, 114991. [Google Scholar] [CrossRef]
- Liu, S.S.; Ying, G.G.; Liu, Y.S.; Yang, Y.Y.; He, L.Y.; Chen, J.; Liu, W.R.; Zhao, J.L. Occurrence and removal of progestagens in two representative swine farms: Effectiveness of lagoon and digester treatment. Water Res. 2015, 77, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, F.; Pan, Y.F.; Li, H.X.; Lin, L.; Hou, R.; Zhang, L.B.; Zhang, Z.; Liu, S.S.; Xu, X.R.; et al. Contamination and ecological risks of steroid metabolites require more attention in the environment: Evidence from the fishing ports. Sci. Total Environ. 2022, 807, 150814. [Google Scholar] [CrossRef]
- Xu, R.; Liu, S.; Pan, Y.F.; Wu, N.N.; Huang, Q.Y.; Li, H.X.; Lin, L.; Hou, R.; Xu, X.R.; Cheng, Y.Y. Steroid metabolites as overlooked emerging contaminants: Insights from multimedia partitioning and source–sink simulation in an estuarine environment. J. Hazard. Mater. 2024, 461, 132673. [Google Scholar] [CrossRef]
- Sorensen, P.W.; Scott, A.P.; Stacey, N.E.; Bowdin, L. Sulfated 17,20β-Dihydroxy-4-pregnen-3-one Functions as a Potent and Specific Olfactory Stimulant with Pheromonal Actions in the Goldfish. Gen. Comp. Endocrinol. 1995, 100, 128–142. [Google Scholar] [CrossRef]
- Rocha, M.J.; Arukwe, A.; Kapoor, B.G. (Eds.) Fish Reproduction, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Statista. Share of Women Using the Contraceptive Pill in Europe in 2022, by Country. Available online: https://www.statista.com/statistics/1063450/birth-control-pill-use-in-europe/ (accessed on 3 March 2025).
- Amorim, V.E.; Morais, H.; Ferreira, A.C.S.; Pardal, M.A.; Cruzeiro, C.; Cardoso, P.G. Application of a robust analytical method for quantifying progestins in environmental samples from three Portuguese Estuaries. Mar. Pollut. Bull. 2024, 199, 115967. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Y.; Fent, K. Occurrence and ecotoxicological effects of free, conjugated, and halogenated steroids including 17α-hydroxypregnanolone and pregnanediol in Swiss wastewater and surface water. Environ. Sci. Technol. 2017, 51, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Šauer, P.; Stará, A.; Golovko, O.; Valentová, O.; Bořík, A.; Grabic, R.; Kroupová, H.K. Two synthetic progestins and natural progesterone are responsible for most of the progestagenic activities in municipal wastewater treatment plant effluents in the Czech and Slovak republics. Water Res. 2018, 137, 64–71. [Google Scholar] [CrossRef]
- Zhang, K.; Fent, K. Determination of two progestin metabolites (17α-hydroxypregnanolone and pregnanediol) and different classes of steroids (androgens, estrogens, corticosteroids, progestins) in rivers and wastewaters by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Sci. Total Environ. 2018, 610–611, 1164–1172. [Google Scholar] [CrossRef]
- Avar, P.; Maasz, G.; Takács, P.; Lovas, S.; Zrinyi, Z.; Svigruha, R.; Takátsy, A.; Tóth, L.G.; Pirger, Z. HPLC-MS/MS analysis of steroid hormones in environmental water samples. Drug Test. Anal. 2016, 8, 123–127. [Google Scholar] [CrossRef]
- King, O.C.; van de Merwe, J.P.; McDonald, J.A.; Leusch, F.D.L. Concentrations of levonorgestrel and ethinylestradiol in wastewater effluents: Is the progestin also cause for concern? Environ. Toxicol. Chem. 2016, 35, 1378–1385. [Google Scholar] [CrossRef]
- Comtois-Marotte, S.; Chappuis, T.; Vo Duy, S.; Gilbert, N.; Lajeunesse, A.; Taktek, S.; Desrosiers, M.; Veilleux, É.; Sauvé, S. Analysis of emerging contaminants in water and solid samples using high resolution mass spectrometry with a Q Exactive orbital ion trap and estrogenic activity with YES-assay. Chemosphere 2017, 166, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Goeury, K.; Vo Duy, S.; Munoz, G.; Prévost, M.; Sauvé, S. Analysis of Environmental Protection Agency priority endocrine disruptor hormones and bisphenol A in tap, surface and wastewater by online concentration liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2019, 1591, 87–98. [Google Scholar] [CrossRef]
- Tan, E.S.S.; Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Simultaneous extraction and determination of pharmaceuticals and personal care products (PPCPs) in river water and sewage by solid-phase extraction and liquid chromatography-tandem mass spectrometry. Int. J. Environ. Anal. Chem. 2015, 95, 816–832. [Google Scholar] [CrossRef]
- Xu, G.; Ma, S.; Tang, L.; Sun, R.; Xiang, J.; Xu, B.; Bao, Y.; Wu, M. Occurrence, fate, and risk assessment of selected endocrine disrupting chemicals in wastewater treatment plants and receiving river of Shanghai, China. Environ. Sci. Pollut. Res. Int. 2016, 23, 25442–25450. [Google Scholar] [CrossRef] [PubMed]
- EPF. European Contraception Policy Atlas—Portugal. Available online: https://www.epfweb.org/node/746 (accessed on 3 March 2025).
- Liu, S.; Xu, X.-R.; Qi, Z.-H.; Chen, H.; Hao, Q.-W.; Hu, Y.-X.; Zhao, J.-L.; Ying, G.-G. Steroid bioaccumulation profiles in typical freshwater aquaculture environments of South China and their human health risks via fish consumption. Environ. Pollut. 2017, 228, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, G.G.; Gastaldini, M.C. Presence of emerging contaminants in urban water bodies in southern Brazil. J. Water Health 2019, 17, 329–337. [Google Scholar] [CrossRef]
- UN. United Nations, Department of Economic and Social Affairs, Population Division. Model-based Estimates and Projections of Family Planning Indicators 2024, Custom Data Acquired via Website. Available online: https://www.un.org/development/desa/pd/data/family-planning-indicators (accessed on 16 March 2025).
- Shen, X.; Zhang, Q.; Xiang, Q.; Zhao, J.; Cao, Y.; Li, K.; Song, J.; Wang, Z.; Zhao, X.; Chen, Q. Occurrences, source apportionment, and potential risks of 55 progestins in surface water of the Yellow River Delta, China. J. Hazard. Mater. 2024, 480, 136098. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Hu, J. Determination and source apportionment of five classes of steroid hormones in urban rivers. Environ. Sci. Technol. 2009, 43, 7691–7698. [Google Scholar] [CrossRef]
- Manickum, T.; John, W. Occurrence, fate and environmental risk assessment of endocrine disrupting compounds at the wastewater treatment works in Pietermaritzburg (South Africa). Sci. Total Environ. 2014, 468–469, 584–597. [Google Scholar] [CrossRef]
- Liu, S.S.; Ying, G.G.; Liu, S.; Lai, H.J.; Chen, Z.F.; Pan, C.G.; Zhao, J.L.; Chen, J. Analysis of 21 progestagens in various matrices by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) with diverse sample pretreatment. Anal. Bioanal. Chem. 2014, 406, 7299–7311. [Google Scholar] [CrossRef]
- Velicu, M.; Suri, R. Presence of steroid hormones and antibiotics in surface water of agricultural, suburban and mixed-use areas. Environ. Monit. Assess. 2009, 154, 349–359. [Google Scholar] [CrossRef]
- Vulliet, E.; Baugros, J.B.; Flament-Waton, M.M.; Grenier-Loustalot, M.F. Analytical methods for the determination of selected steroid sex hormones and corticosteriods in wastewater. Anal. Bioanal. Chem. 2007, 387, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Kroll, K.J.; Silva-Sanchez, C.; Carriquiriborde, P.; Fernandino, J.I.; Denslow, N.D.; Somoza, G.M. Steroid hormones and estrogenic activity in the wastewater outfall and receiving waters of the Chascomús chained shallow lakes system (Argentina). Sci. Total Environ. 2020, 743, 140401. [Google Scholar] [CrossRef]
- Jenila, J.S.; Issac, P.K.; Lam, S.S.; Oviya, J.C.; Jones, S.; Munusamy-Ramanujam, G.; Chang, S.W.; Ravindran, B.; Mannacharaju, M.; Ghotekar, S.; et al. Deleterious effect of gestagens from wastewater effluent on fish reproduction in aquatic environment: A review. Environ. Res. 2023, 236, 116810. [Google Scholar] [CrossRef]
- Steinbach, C.; Lutz, I.; Šandová, M.; Pech, M.; Šálková, E.; Bořík, A.; Valentová, O.; Kroupová, H.K. Effects of the synthetic progestin levonorgestrel on some aspects of thyroid physiology in common carp (Cyprinus carpio). Chemosphere 2023, 310, 136860. [Google Scholar] [CrossRef]
- Baekelandt, S.; Bouchat, A.; Leroux, N.; Robert, J.-B.; Burattin, L.; Cishibanji, E.; Lambert, J.; Gérard, C.; Delierneux, C.; Kestemont, P. Estetrol/drospirenone versus 17α-ethinylestradiol/drospirenone: An extended one generation test to evaluate the endocrine disruption potential on zebrafish (Danio rerio). Environ. Int. 2024, 187, 108702. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, M.; Lopes, C.; Seabra, R.; Rocha, E. 17α-Ethynylestradiol and Levonorgestrel Exposure of Rainbow Trout RTL-W1 Cells at 18 °C and 21 °C Mainly Reveals Thermal Tolerance, Absence of Estrogenic Effects, and Progestin-Induced Upregulation of Detoxification Genes. Genes 2024, 15, 1189. [Google Scholar] [CrossRef] [PubMed]
- Madureira, T.V.; Barreiro, J.C.; Rocha, M.J.; Rocha, E.; Cass, Q.B.; Tiritan, M.E. Spatiotemporal distribution of pharmaceuticals in the Douro River estuary (Portugal). Sci. Total Environ. 2010, 408, 5513–5520. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Amaral, S.; Rocha, E.; Rocha, M.J. Determination of 54 pesticides in waters of the Iberian Douro River estuary and risk assessment of environmentally relevant mixtures using theoretical approaches and Artemia salina and Daphnia magna bioassays. Ecotoxicol. Environ. Saf. 2017, 145, 126–134. [Google Scholar] [CrossRef]
- Rocha, M.J.; Dores-Sousa, J.L.; Cruzeiro, C.; Rocha, E. PAHs in water and surface sediments from Douro River estuary and Porto Atlantic coast (Portugal)—Impacts on human health. Environ. Monit. Assess. 2017, 189, 425. [Google Scholar] [CrossRef]
- Kroupova, H.K.; Trubiroha, A.; Lorenz, C.; Contardo-Jara, V.; Lutz, I.; Grabic, R.; Kocour, M.; Kloas, W. The progestin levonorgestrel disrupts gonadotropin expression and sex steroid levels in pubertal roach (Rutilus rutilus). Aquat. Toxicol. 2014, 154, 154–162. [Google Scholar] [CrossRef] [PubMed]


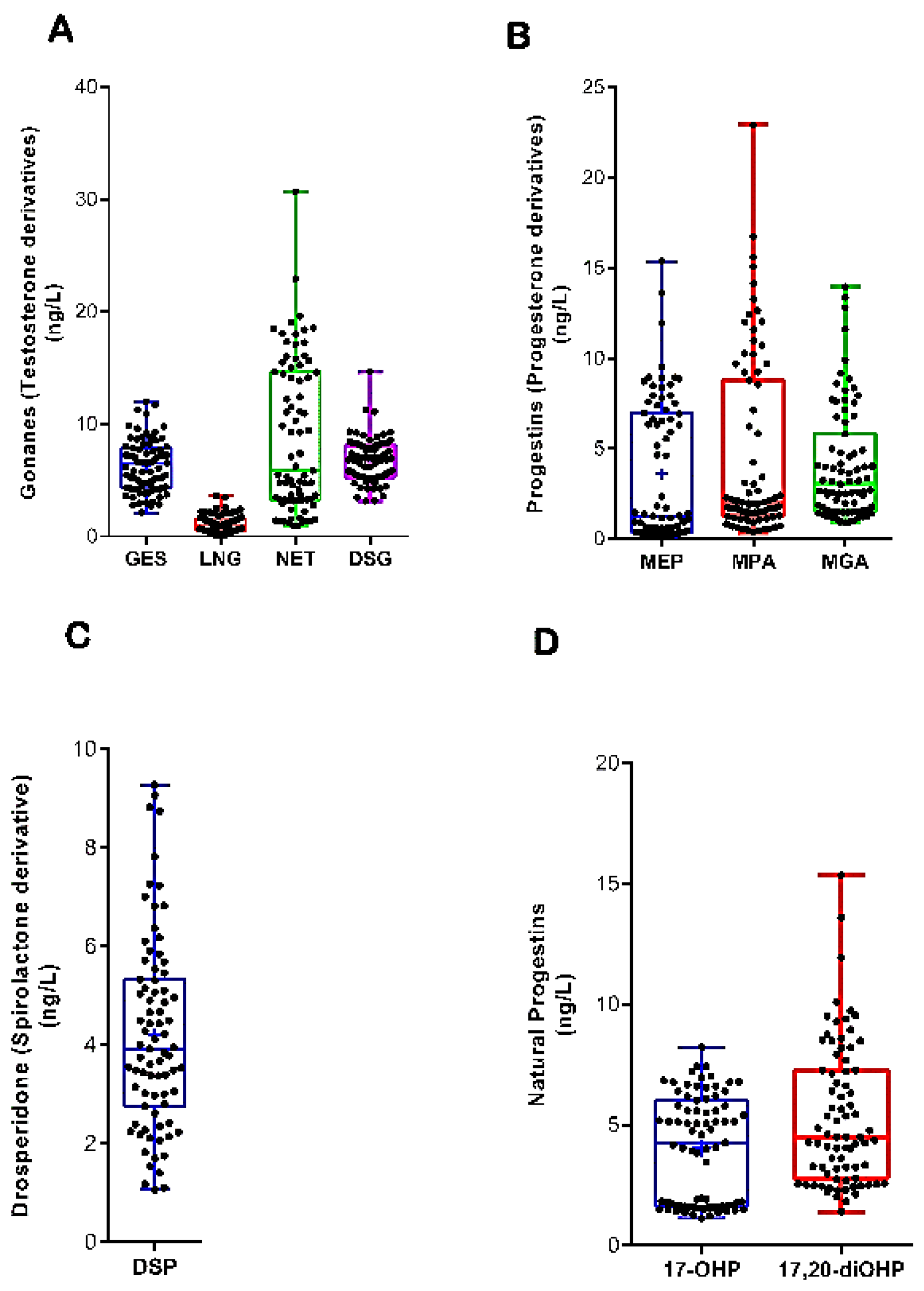
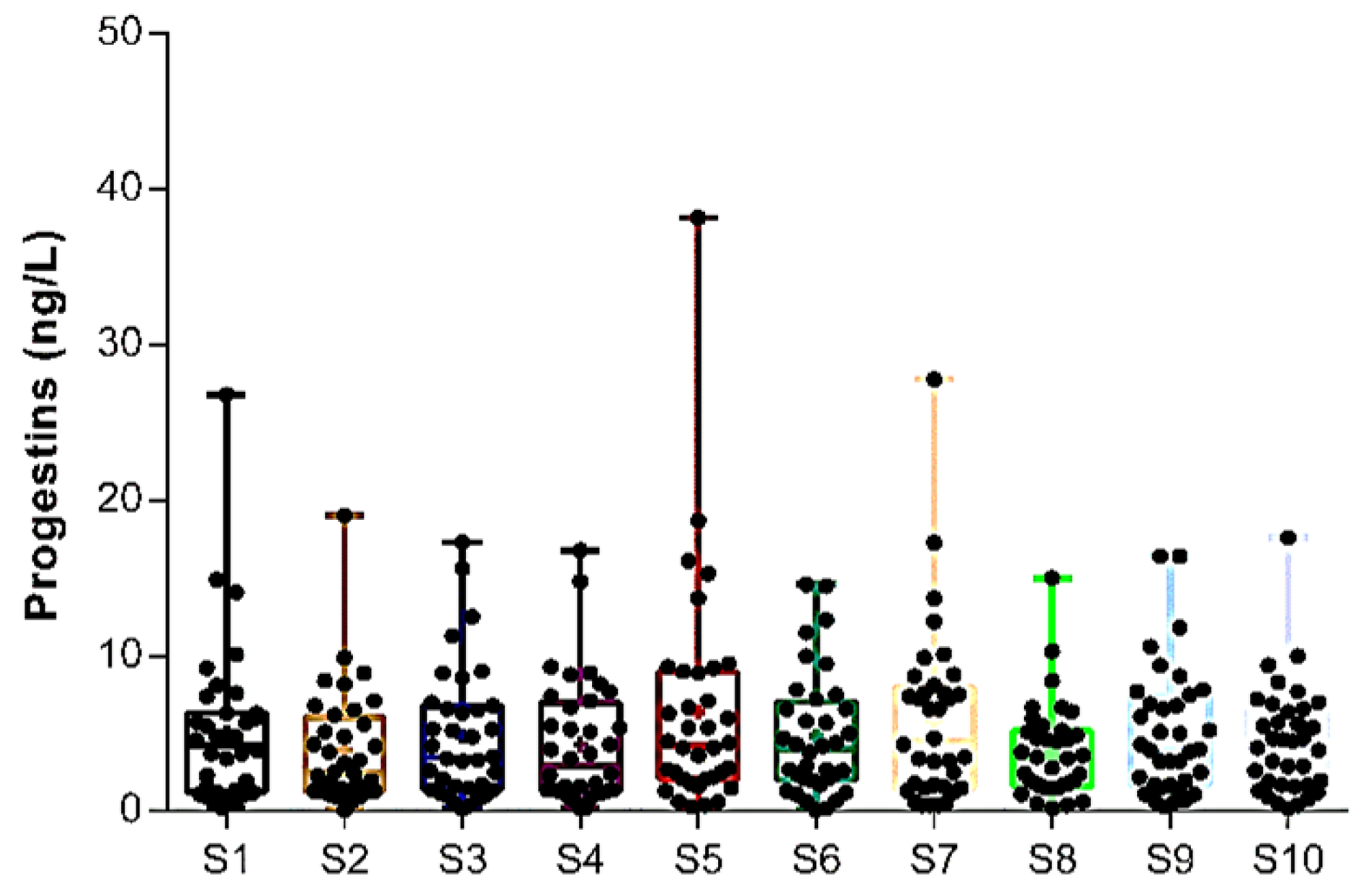

| PGs | MDL | DR (%) | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ng/L) | ||||||||||||
| GES | 0.3 | 79 | 5.6 ± 1.4 | 3.4 ± 0.7 | 3.6 ± 1.9 | 5.9 ± 3.1 | 6.3 ± 3.2 | 6.2 ± 3.4 | 5.7 ± 3.6 | 6.0 ± 3.7 | 4.3 ± 2.9 | 2.9 ± 1.7 |
| LNG | 0.3 | 81 | 5.3 ± 0.6 | 3.3 ± 3.2 | 1.4 ± 1.2 | 2.4 ± 2.1 | 2.7 ± 2.5 | 2.5 ± 2.5 | 1.3 ± 1.0 | 3.0 ± 3.5 | 0.6 ± 0.6 | 0.8 ± 0.5 |
| NTD | 0.7 | 90 | 4.4 ± 0.2 | 5.8 ± 3.9 | 5.5 ± 5.8 | 9.8 ± 5.3 | 11.3 ± 6.7 | 10.8 ± 6.2 | 9.8 ± 7.5 | 14.3 ± 10.6 | 9.4 ± 8.6 | 6.2 ± 4.5 |
| NTDA | 0.2 | 80 | 0.6 ± 0.4 | 5.2 ± 1.2 | 7.0 ± 1.5 | 7.3 ± 0.7 | 6.7 ± 1.7 | 8.0 ± 1.7 | 7.5 ± 1.1 | 8.4 ± 3.0 | 6.9 ± 1.6 | 5.0 ± 0.7 |
| MEP | 0.7 | 78 | 0.7 ± 0.7 | 2.4 ± 3.3 | 2.5 ± 3.8 | 5.6 ± 6.3 | 4.1 ± 4.0 | 4.1 ± 3.9 | 4.6 ± 4.5 | 4.5 ± 4.7 | 3.6 ± 3.7 | 3.5 ± 4.0 |
| MGA | 0.6 | 80 | 1.4 ± 0.1 | 1.9 ± 0.4 | 2.5 ± 1.0 | 7.6 ± 5.0 | 4.9 ± 2.9 | 5.3 ± 3.3 | 6.5 ± 4.8 | 4.3 ± 2.5 | 3.6 ± 2.3 | 2.6 ± 1.2 |
| MPA | 0.3 | 89 | 1.0 ± 0.3 | 1.2 ± 0.7 | 1.6 ± 0.8 | 8.1 ± 5.7 | 6.2 ± 5.4 | 8.3 ± 7.5 | 7.2 ± 5.7 | 4.9 ± 4.2 | 5.8 ± 5.3 | 1.5 ± 0.8 |
| DSP | 0.6 | 98 | 1.6 ± 0.4 | 2.6 ± 0.6 | 2.7 ± 0.7 | 7.6 ± 1.6 | 5.0 ± 0.8 | 5.6 ± 0.9 | 6.2 ± 1.4 | 4.3 ± 1.0 | 3.6 ± 0.7 | 3.3 ± 0.5 |
| 17-OHP | 0.5 | 96 | 2.7 ± 2.5 | 3.2 ± 3.0 | 2.9 ± 2.3 | 4.9 ± 2.7 | 4.5 ± 2.4 | 4.8 ± 2.3 | 4.9 ± 2.3 | 4.4 ± 2.0 | 4.3 ± 1.9 | 3.9 ± 1.8 |
| 17,20-diOHP | 0.2 | 92 | 3.8 ± 2.2 | 5.2 ± 2.9 | 5.7 ± 2.8 | 8.3 ± 4.8 | 4.7 ± 2.3 | 5.5 ± 2.9 | 6.0 ± 3.3 | 5.1 ± 2.7 | 4.1 ± 1.8 | 5.1 ± 0.7 |
| ∑Progestins by site | 27.1 ± 1.9 | 34.2 ± 1.5 | 35.4 ± 1.9 | 67.5 ± 2.1 | 56.4 ± 2.3 | 61.1 ± 2.4 | 59.6 ± 2.2 | 59.2 ± 3.3 | 46.0 ± 2.3 | 34.8 ± 1.6 | ||
| Global amounts of Progestins in Douro River Estuary | 48.1 ± 2.5 | |||||||||||
| PGs | Log Kow | BCFFP (ng/mL) | CFP (ng/L) | PECw (ng/L) | MEC (ng/L) |
|---|---|---|---|---|---|
| GES | 3.3 | 32 | 1.0 | 31 | 5.3 |
| LNG | 3.5 | 46 | 2.4 | 52 | 14.3 |
| NTD | 3.0 | 19 | 9.8 | 6.7 | 6.3 |
| NTDA | 4.0 | 108 | 9.8 | 2.7 | 8.4 |
| MEP | 3.5 | 47 | 1.0 | 21.0 | 5.6 |
| MGA | 4.0 | 110 | - | <10 | 7.6 |
| MPA | 4.1 | 128 | 1.0 | 8.0 | 8.3 |
| DSP | 4.0 | 113 | 30.8 | 273 | 7.6 |
| PGs | Endpoint Value (ng/L) | PNEC (ng/L) | MEC (ng/L) | RQ |
|---|---|---|---|---|
| GES | EC50 = 10 | 0.01 | 6.3 | 630 |
| AF = 1000 | ||||
| LNG | NOEC = 0.42 | 0.01 | 5.3 | 530 |
| AF = 50 | ||||
| NTD | LOEC = 4 | 0.08 | 14.3 | 179 |
| AF = 50 | ||||
| NTDA | NOEC = 816 | 0.80 | 8.4 | 11 |
| AF = 1000 | ||||
| MEP | - | - | 5.6 | - |
| MGA | NOEC = 33 | 0.66 | 7.6 | 12 |
| AF = 50 | ||||
| MPA | NOEC = 342 | 6.84 | 8.3 | 1.2 |
| AF = 50 | ||||
| DSP | NOEC = 100 | 2.00 | 7.6 | 3.8 |
| AF = 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.; Alves, R.F.; Rocha, E.; Rocha, M.J. Progestin Pollution in Surface Waters of a Major Southwestern European Estuary: The Douro River Estuary (Iberian Peninsula). Toxics 2025, 13, 225. https://doi.org/10.3390/toxics13030225
Silva F, Alves RF, Rocha E, Rocha MJ. Progestin Pollution in Surface Waters of a Major Southwestern European Estuary: The Douro River Estuary (Iberian Peninsula). Toxics. 2025; 13(3):225. https://doi.org/10.3390/toxics13030225
Chicago/Turabian StyleSilva, Frederico, Rodrigo F. Alves, Eduardo Rocha, and Maria João Rocha. 2025. "Progestin Pollution in Surface Waters of a Major Southwestern European Estuary: The Douro River Estuary (Iberian Peninsula)" Toxics 13, no. 3: 225. https://doi.org/10.3390/toxics13030225
APA StyleSilva, F., Alves, R. F., Rocha, E., & Rocha, M. J. (2025). Progestin Pollution in Surface Waters of a Major Southwestern European Estuary: The Douro River Estuary (Iberian Peninsula). Toxics, 13(3), 225. https://doi.org/10.3390/toxics13030225








