Association Between Heavy Metal Exposure and Central Nervous System Tumors: A Case-Control Study Using Single and Multi-Metal Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Urinary Metals Measurement
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Other Tests in Cases and Controls Group
3.3. Comparison of Detection Limits for 47 Urinary Metals
3.4. Urinary Metals Concentrations
3.5. Correlation of Urinary Metals with Risk of CNS Tumors in Single Metal Model
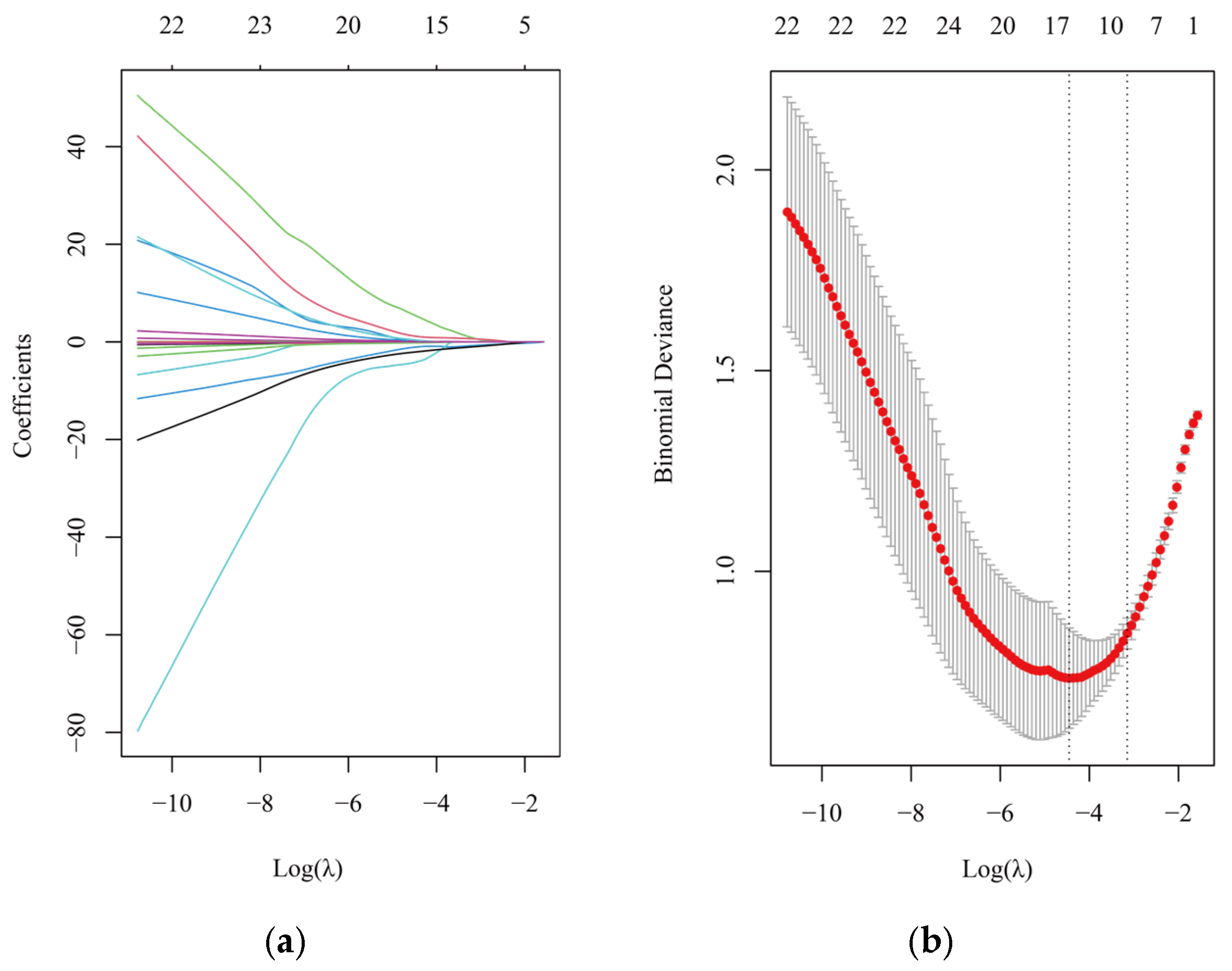
3.6. Multiple Metals Exposure Model for CNS Tumors
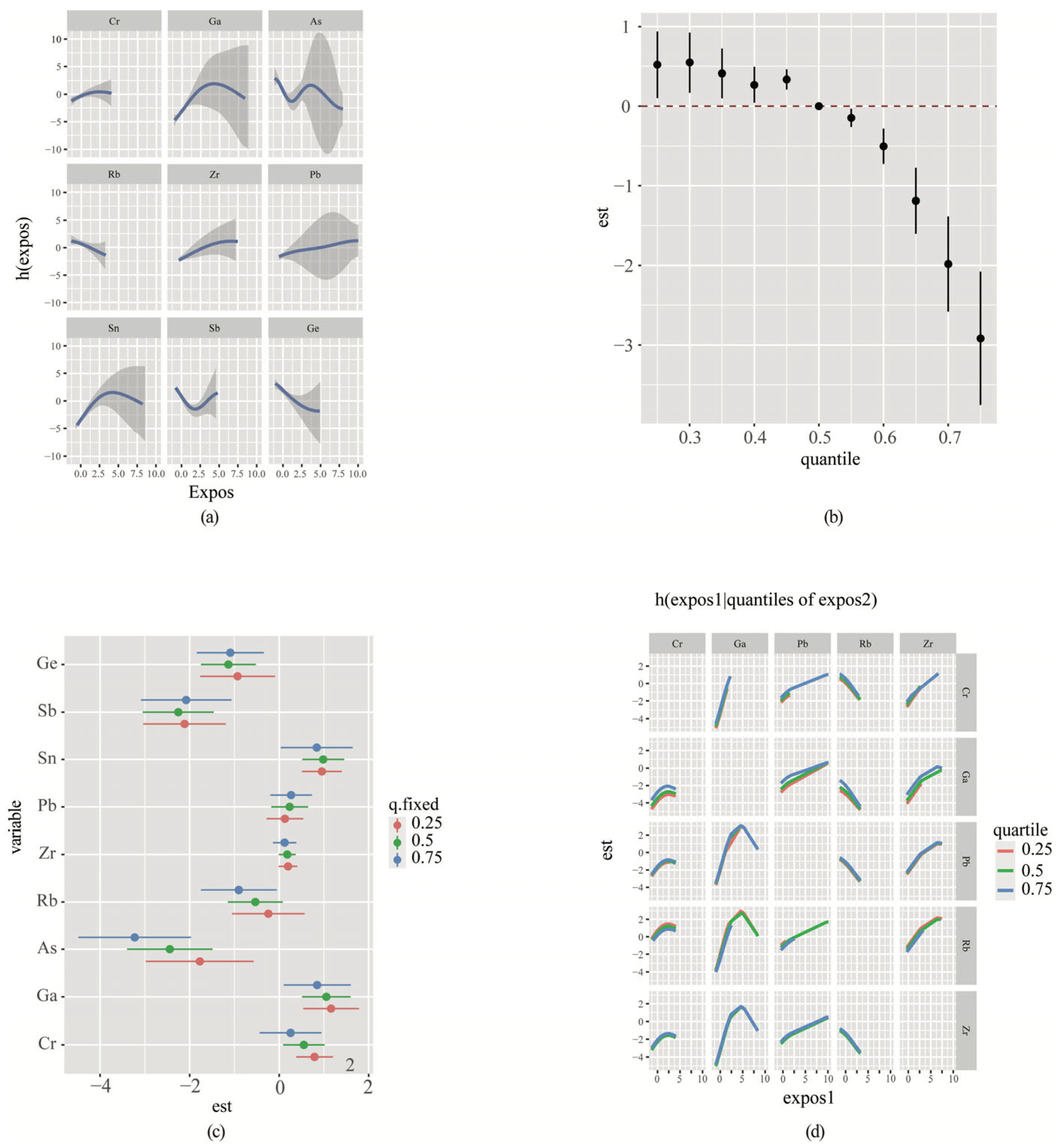
3.7. Bayesian Kernel Machine Regression (BKMR) Analyses
3.8. Correlation of Urinary Metals with Risk of Different Types of CNS Tumors in the Single Metal Model
4. Discussion
4.1. The Sources of the Metals
4.2. Ga Concentrations and Health
4.3. V Concentrations and Health
4.4. Cr Concentrations and Health
4.5. Pb Concentrations and Health
4.6. As Concentrations and Health
4.7. Other Metal and Health
4.8. Multi-Metal and Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Brain and Other CNS Cancer Collaborators. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Cocco, P.; Dosemeci, M.; Heineman, E.F. Brain cancer and occupational exposure to lead. J. Occup. Environ. Med. 1998, 40, 937–942. [Google Scholar] [CrossRef]
- Cocco, P.; Heineman, E.F.; Dosemeci, M. Occupational risk factors for cancer of the central nervous system (CNS) among US women. Am. J. Ind. Med. 1999, 36, 70–74. [Google Scholar] [CrossRef]
- Ahn, J.; Park, M.Y.; Kang, M.Y.; Shin, I.S.; An, S.; Kim, H.R. Occupational Lead Exposure and Brain Tumors: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2020, 17, 3975. [Google Scholar] [CrossRef] [PubMed]
- Anttila, A.; Heikkilä, P.; Nykyri, E.; Kauppinen, T.; Pukkala, E.; Hernberg, S.; Hemminki, K. Risk of nervous system cancer among workers exposed to lead. J. Occup. Environ. Med. 1996, 38, 131–136. [Google Scholar] [CrossRef]
- Lam, T.V.; Agovino, P.; Niu, X.; Roché, L. Linkage study of cancer risk among lead-exposed workers in New Jersey. Sci. Total Environ. 2007, 372, 455–462. [Google Scholar] [CrossRef]
- Van Wijngaarden, E.; Dosemeci, M. Brain cancer mortality and potential occupational exposure to lead: Findings from the National Longitudinal Mortality Study, 1979–1989. Int. J. Cancer 2006, 119, 1136–1144. [Google Scholar] [CrossRef]
- Cilliers, K.; Muller, C.J.F.; Page, B.J. Trace Element Concentration Changes in Brain Tumors: A Review. Anat. Rec. 2020, 303, 1293–1299. [Google Scholar] [CrossRef]
- Gaman, L.; Radoi, M.P.; Delia, C.E.; Luzardo, O.P.; Zumbado, M.; Rodríguez-Hernández, Á.; Stoian, I.; Gilca, M.; Boada, L.D.; Henríquez-Hernández, L.A. Concentration of heavy metals and rare earth elements in patients with brain tumours: Analysis in tumour tissue, non-tumour tissue, and blood. Int. J. Environ. Health Res. 2021, 31, 741–754. [Google Scholar] [CrossRef]
- Martinez-Morata, I.; Sobel, M.; Tellez-Plaza, M.; Navas-Acien, A.; Howe, C.G.; Sanchez, T.R. A State-of-the-Science Review on Metal Biomarkers. Curr. Environ. Health Rep. 2023, 10, 215–249. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, Z.; Shen, J.; Wu, Y.; Fang, L.; Xu, S.; Ma, Y.; Zhao, H.; Pan, F. Associations of exposure to blood and urinary heavy metal mixtures with psoriasis risk among U.S. adults: A cross-sectional study. Sci. Total Environ. 2023, 887, 164133. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Plaza, M.; Guallar, E.; Howard, B.V.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Silbergeld, E.K.; Devereux, R.B.; Navas-Acien, A. Cadmium exposure and incident cardiovascular disease. Epidemiology 2013, 24, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef]
- Song, S.; Liu, N.; Wang, G.; Wang, Y.; Zhang, X.; Zhao, X.; Chang, H.; Yu, Z.; Liu, X. Sex Specificity in the Mixed Effects of Blood Heavy Metals and Cognitive Function on Elderly: Evidence from NHANES. Nutrients 2023, 15, 2874. [Google Scholar] [CrossRef]
- Haupt, S.; Caramia, F.; Klein, S.L.; Rubin, J.B.; Haupt, Y. Sex disparities matter in cancer development and therapy. Nat. Rev. Cancer 2021, 21, 393–407. [Google Scholar] [CrossRef]
- Shobeiri, P.; Seyedmirzaei, H.; Kalantari, A.; Mohammadi, E.; Rezaei, N.; Hanaei, S. The Epidemiology of Brain and Spinal Cord Tumors. In Human Brain and Spinal Cord Tumors: From Bench to Bedside. Volume 1: Neuroimmunology and Neurogenetics; Rezaei, N., Hanaei, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 19–39. [Google Scholar]
- García-Pérez, J.; Morales-Piga, A.; Gómez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; Fernández-Navarro, P.; López-Abente, G.; Ramis, R. Risk of neuroblastoma and residential proximity to industrial and urban sites: A case-control study. Environ. Int. 2016, 92–93, 269–275. [Google Scholar] [CrossRef]
- Afshar, N.; English, D.R.; Milne, R.L. Rural-urban residence and cancer survival in high-income countries: A systematic review. Cancer 2019, 125, 2172–2184. [Google Scholar] [CrossRef]
- Lange, C.N.; Freire, B.M.; Monteiro, L.R.; de Jesus, T.A.; Dos Reis, R.A.; Nakazato, G.; Kobayashi, R.K.T.; Batista, B.L. Multiple potentially toxic elements in urban gardens from a Brazilian industrialized city. Environ. Geochem. Health 2024, 46, 36. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Feng, X.; Guo, Y. The Distribution Characteristics and Source Analysis of Heavy Metals in Arable Soil of Xingyang City, Henan Province. J. Rock. Miner. Test. 2024, 43, 330–343. [Google Scholar] [CrossRef]
- Yuan, X.; Xue, N.; Han, Z. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef]
- David, M.; Qurat Ul, A.; Afzal, M.; Shoaib, M.; Aman, F.; Cloete, K.J.; Turi, N.; Jahan, S. Study of occupational exposure to brick kiln emissions on heavy metal burden, biochemical profile, cortisol level and reproductive health risks among female workers at Rawat, Pakistan. Environ. Sci. Pollut. Res. Int. 2020, 27, 44073–44088. [Google Scholar] [CrossRef]
- Wang, X.; Jin, P.; Zhou, Q.; Liu, S.; Wang, F.; Xi, S. Metal Biomonitoring and Comparative Assessment in Urine of Workers in Lead-Zinc and Steel-Iron Mining and Smelting. Biol. Trace Elem. Res. 2019, 189, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Hwang, L.C.; Kao, J.S.; Yiin, S.J.; Lin, S.F.; Lin, C.H.; Lin, Y.C.; Aw, T.C. Lipid peroxidation in workers exposed to aluminium, gallium, indium, arsenic, and antimony in the optoelectronic industry. J. Occup. Environ. Med. 2006, 48, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Cindrić, M.; Jakovčević, A.; Žarković, K.; Žarković, N. Lipid peroxidation in brain tumors. Neurochem. Int. 2021, 149, 105118. [Google Scholar] [CrossRef]
- Dąbroś, W.; Adamczyk, A.; Ciurkot, K.; Kordowiak, A.M. Vanadium compounds affect growth and morphology of human rhabdomyosarcoma cell line. Pol. J. Pathol. 2011, 62, 262–268. [Google Scholar] [PubMed]
- Gruzewska, K.; Michno, A.; Pawelczyk, T.; Bielarczyk, H. Essentiality and toxicity of vanadium supplements in health and pathology. J. Physiol. Pharmacol. 2014, 65, 603–611. [Google Scholar]
- Holko, P.; Ligeza, J.; Kisielewska, J.; Kordowiak, A.M.; Klein, A. The effect of vanadyl sulphate (VOSO4) on autocrine growth of human epithelial cancer cell lines. Pol. J. Pathol. 2008, 59, 3–8. [Google Scholar]
- Hara, T.; Hoshuyama, T.; Takahashi, K.; Delgermaa, V.; Sorahan, T. Cancer risk among Japanese chromium platers, 1976–2003. Scand. J. Work Environ. Health 2010, 36, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; Garzaro, G.; Pira, E.; Boffetta, P. Mortality and cancer morbidity among cement production workers: A meta-analysis. Int. Arch. Occup. Environ. Health 2016, 89, 1155–1168. [Google Scholar] [CrossRef]
- Iaia, T.E.; Bartoli, D.; Calzoni, P.; Comba, P.; De Santis, M.; Dini, F.; Farina, G.A.; Valiani, M.; Pirastu, R. A cohort mortality study of leather tanners in Tuscany, Italy. Am. J. Ind. Med. 2006, 49, 452–459. [Google Scholar] [CrossRef]
- Yatera, K.; Morimoto, Y.; Ueno, S.; Noguchi, S.; Kawaguchi, T.; Tanaka, F.; Suzuki, H.; Higashi, T. Cancer Risks of Hexavalent Chromium in the Respiratory Tract. J. Uoeh 2018, 40, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.; Men, Y.; Wang, W.; Zhang, W. Heavy metals interfere with plasma metabolites, including lipids and amino acids, in patients with breast cancer. Oncol. Lett. 2020, 19, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Benderli Cihan, Y.; Sözen, S.; Oztürk Yıldırım, S. Trace elements and heavy metals in hair of stage III breast cancer patients. Biol. Trace Elem. Res. 2011, 144, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Pham, T.H.; Lay, P.A. Binding of Chromium(III) to Transferrin Could Be Involved in Detoxification of Dietary Chromium(III) Rather than Transport of an Essential Trace Element. Angew. Chem. Int. Ed. Engl. 2016, 55, 8104–8107. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, M.; Zhen, H.; Chen, L.; Shi, P.; Huang, Z. Genotoxicity of tri- and hexavalent chromium compounds in vivo and their modes of action on DNA damage in vitro. PLoS ONE 2014, 9, e103194. [Google Scholar] [CrossRef]
- DeLoughery, Z.; Luczak, M.W.; Zhitkovich, A. Monitoring Cr intermediates and reactive oxygen species with fluorescent probes during chromate reduction. Chem. Res. Toxicol. 2014, 27, 843–851. [Google Scholar] [CrossRef]
- Wakeman, T.P.; Yang, A.; Dalal, N.S.; Boohaker, R.J.; Zeng, Q.; Ding, Q.; Xu, B. DNA mismatch repair protein Mlh1 is required for tetravalent chromium intermediate-induced DNA damage. Oncotarget 2017, 8, 83975–83985. [Google Scholar] [CrossRef]
- Quievryn, G.; Peterson, E.; Messer, J.; Zhitkovich, A. Genotoxicity and mutagenicity of chromium(VI)/ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry 2003, 42, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol. Cell Biochem. 2001, 222, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Navarra, G.; Coppola, L.; Savarese, B.; Avilia, G.; Giarra, A.; Pagano, G.; Marano, A.; Trifuoggi, M.; Bifulco, M.; et al. Impacts of Environmental Pollution on Brain Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 5045. [Google Scholar] [CrossRef] [PubMed]
- Shefa, S.T.; Héroux, P. Both physiology and epidemiology support zero tolerable blood lead levels. Toxicol. Lett. 2017, 280, 232–237. [Google Scholar] [CrossRef]
- Chibowska, K.; Baranowska-Bosiacka, I.; Falkowska, A.; Gutowska, I.; Goschorska, M.; Chlubek, D. Effect of Lead (Pb) on Inflammatory Processes in the Brain. Int. J. Mol. Sci. 2016, 17, 2140. [Google Scholar] [CrossRef]
- Bouton, C.M.; Frelin, L.P.; Forde, C.E.; Arnold Godwin, H.; Pevsner, J. Synaptotagmin I is a molecular target for lead. J. Neurochem. 2001, 76, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Chicka, M.C.; Hui, E.; Liu, H.; Chapman, E.R. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+. Nat. Struct. Mol. Biol. 2008, 15, 827–835. [Google Scholar] [CrossRef]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Factor-Litvak, P.; van Geen, A.; Slavkovich, V.; LoIacono, N.J.; Cheng, Z.; Hussain, I.; et al. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2004, 112, 1329–1333. [Google Scholar] [CrossRef]
- Calderón, J.; Navarro, M.E.; Jimenez-Capdeville, M.E.; Santos-Diaz, M.A.; Golden, A.; Rodriguez-Leyva, I.; Borja-Aburto, V.; Díaz-Barriga, F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ. Res. 2001, 85, 69–76. [Google Scholar] [CrossRef]
- Wang, A.; Holladay, S.D.; Wolf, D.C.; Ahmed, S.A.; Robertson, J.L. Reproductive and developmental toxicity of arsenic in rodents: A review. Int. J. Toxicol. 2006, 25, 319–331. [Google Scholar] [CrossRef]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Liao, W.T.; Yu, H.S. Aberrant immune responses in arsenical skin cancers. Kaohsiung J. Med. Sci. 2011, 27, 396–401. [Google Scholar] [CrossRef]
- Dehnhardt, M.; Zoriy, M.V.; Khan, Z.; Reifenberger, G.; Ekström, T.J.; Sabine Becker, J.; Zilles, K.; Bauer, A. Element distribution is altered in a zone surrounding human glioblastoma multiforme. J. Trace Elem. Med. Biol. 2008, 22, 17–23. [Google Scholar] [CrossRef]
- Cross, A.J.; Ferrucci, L.M.; Risch, A.; Graubard, B.I.; Ward, M.H.; Park, Y.; Hollenbeck, A.R.; Schatzkin, A.; Sinha, R. A large prospective study of meat consumption and colorectal cancer risk: An investigation of potential mechanisms underlying this association. Cancer Res. 2010, 70, 2406–2414. [Google Scholar] [CrossRef]
- Pinnix, Z.K.; Miller, L.D.; Wang, W.; D’Agostino, R., Jr.; Kute, T.; Willingham, M.C.; Hatcher, H.; Tesfay, L.; Sui, G.; Di, X.; et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2010, 2, 43ra56. [Google Scholar] [CrossRef]
- Tesfay, L.; Clausen, K.A.; Kim, J.W.; Hegde, P.; Wang, X.; Miller, L.D.; Deng, Z.; Blanchette, N.; Arvedson, T.; Miranti, C.K.; et al. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 2015, 75, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Basuli, D.; Tesfay, L.; Deng, Z.; Paul, B.; Yamamoto, Y.; Ning, G.; Xian, W.; McKeon, F.; Lynch, M.; Crum, C.P.; et al. Iron addiction: A novel therapeutic target in ovarian cancer. Oncogene 2017, 36, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Högemann-Savellano, D.; Bos, E.; Blondet, C.; Sato, F.; Abe, T.; Josephson, L.; Weissleder, R.; Gaudet, J.; Sgroi, D.; Peters, P.J.; et al. The transferrin receptor: A potential molecular imaging marker for human cancer. Neoplasia 2003, 5, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Hughes, S.; Turner, F.E.; Reynolds, G.; Sharma, N.; Ismail, T.; Berx, G.; McKie, A.T.; Hotchin, N.; Anderson, G.J.; et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut 2006, 55, 1449–1460. [Google Scholar] [CrossRef]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between serum heavy metals and prostate cancer risk—A multiple metal analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef]
- Parent, M.E.; Turner, M.C.; Lavoué, J.; Richard, H.; Figuerola, J.; Kincl, L.; Richardson, L.; Benke, G.; Blettner, M.; Fleming, S.; et al. Lifetime occupational exposure to metals and welding fumes, and risk of glioma: A 7-country population-based case-control study. Environ. Health 2017, 16, 90. [Google Scholar] [CrossRef] [PubMed]

| Cases | Controls | p Value | ||
|---|---|---|---|---|
| (n = 63) | (n = 71) | |||
| Age | ~40 | 13 | 18 | |
| 40~60 | 33 | 39 | ||
| 60~ | 15 | 14 | 0.746 | |
| Gender | Male | 34 | 39 | |
| Female | 29 | 32 | 0.911 | |
| Nationality | Han Chinese | 60 | 71 | 1 |
| Others | 1 | 2 | ||
| Residential address | the downtown of Chongqing | 21 | 25 | 0.010 |
| Western Chongqing | 9 | 13 | ||
| Northeast Chongqing | 13 | 9 | ||
| Southeast Chongqing | 3 | 4 | ||
| Other provinces | 16 | 2 | ||
| Smoking | Yes | 7 | 9 | 0.946 |
| No | 50 | 62 | ||
| Underlying diseases | Hypertension | 10 | 11 | 0.367 |
| Diabetes | 4 | 1 | ||
| Hypertension and diabetes | 3 | 5 | ||
| None | 36 | 54 |
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Adjusted p Value | |
|---|---|---|---|---|---|
| V | |||||
| Range | ≤29.34 | ~46.98 | ~63.201 | >63.201 | |
| Case/Control | 25/12 | 19/13 | 14/18 | 5/27 | |
| OR (95%CI) | 1 | 0.499 (0.159, 1.565) | 0.361 (0.118, 1.104) | 0.043 (0.01, 0.181) | 0.001 |
| Cr | |||||
| Range | ≤29.34 | ~46.98 | ~104.44 | >104.44 | |
| Case/Control | 18/14 | 7/25 | 13/19 | 25/7 | |
| OR (95%CI) | 1 | 0.223 (0.069, 0.719) | 0.462 (0.155, 1.373) | 2.889 (0.905, 9.226) | 0.001 |
| Mn | |||||
| Range | ≤2.16 | ~3.73 | ~6.18 | >6.18 | |
| Case/Control | 22/8 | 19/12 | 8/24 | 10/19 | |
| OR (95%CI) | 1 | 0.571 (0.179, 1.817) | 0.075 (0.02, 0.275) | 0.09 (0.024, 0.341) | <0.001 |
| Fe | |||||
| Range | ≤353.84 | ~643.61 | ~1018.81 | >1018.81 | |
| Case/Control | 20/11 | 20/11 | 11/21 | 8/23 | |
| OR (95%CI) | 1 | 1.029 (0.338, 3.132) | 0.308 (0.101, 0.935) | 0.196 (0.06, 0.64) | 0.013 |
| Co | |||||
| Range | ≤0.33 | ~0.44 | ~0.65 | >0.65 | |
| Case/Control | 24/10 | 14/15 | 11/23 | 11/19 | |
| OR (95%CI) | 1 | 0.36 (0.118, 1.095) | 0.17 (0.055, 0.521) | 0.262 (0.086, 0.801) | 0.023 |
| Ni | |||||
| Range | ≤2.24 | ~3.78 | ~5.13 | >5.13 | |
| Case/Control | 16/16 | 14/17 | 16/17 | 12/19 | |
| OR (95%CI) | 1 | 1.143 (0.391, 3.344) | 0.841 (0.291, 2.429) | 0.495 (0.163, 1.502) | 0.509 |
| Cu | |||||
| Range | ≤6.66 | ~10.79 | ~17.24 | >17.24 | |
| Case/Control | 18/11 | 12/17 | 11/18 | 9/20 | |
| OR (95%CI) | 1 | 0.421 (0.136, 1.297) | 0.268 (0.084, 0.858) | 0.193 (0.058, 0.643) | 0.055 |
| Zn | |||||
| Range | ≤170.16 | ~296.8 | ~443.04 | >443.04 | |
| Case/Control | 19/13 | 15/16 | 14/19 | 13/18 | |
| OR (95%CI) | 1 | 0.673 (0.23, 1.974) | 0.361 (0.12, 1.082) | 0.461 (0.155, 1.369) | 0.313 |
| Ga | |||||
| Range | ≤0.2 | ~0.28 | ~0.4 | >0.4 | |
| Case/Control | 15/18 | 10/17 | 15/25 | 22/7 | |
| OR (95%CI) | 1 | 0.724 (0.245, 2.138) | 0.696 (0.261, 1.854) | 2.456 (0.772, 7.812) | 0.166 |
| Ge | |||||
| Range | ≤0.33 | ~0.68 | ~1.09 | >1.09 | |
| Case/Control | 23/9 | 19/13 | 15/17 | 2/30 | |
| OR (95%CI) | 1 | 0.969 (0.302, 3.11) | 0.348 (0.106, 1.142) | 0.005 (0, 0.065) | 0.001 |
| As | |||||
| Range | ≤28.15 | ~47.17 | ~72.94 | >72.94 | |
| Case/Control | 31/2 | 21/10 | 6/26 | 4/28 | |
| OR (95%CI) | 1 | 0.198 (0.038, 1.041) | 0.015 (0.002, 0.088) | 0.009 (0.001, 0.057) | <0.001 |
| Rb | |||||
| Range | ≤716.1 | ~1204.16 | ~1842.18 | >1842.18 | |
| Case/Control | 29/3 | 18/13 | 7/26 | 8/23 | |
| OR (95%CI) | 1 | 0.174 (0.041, 0.73) | 0.037 (0.008, 0.162) | 0.037 (0.008, 0.164) | <0.001 |
| Sr | |||||
| Range | ≤70.79 | ~127.81 | ~191.18 | >191.18 | |
| Case/Control | 28/6 | 13/19 | 13/19 | 9/23 | |
| OR (95%CI) | 1 | 0.138 (0.041, 0.459) | 0.14 (0.042, 0.469) | 0.08 (0.023, 0.28) | 0.001 |
| Zr | |||||
| Range | ≤0.02 | ~0.05 | ~0.14 | >0.14 | |
| Case/Control | 3/21 | 17/6 | 8/15 | 19/4 | |
| OR (95%CI) | 1 | 27.743 (5.204, 147.895) | 2.995 (0.59, 15.209) | 29.864 (5.211, 171.148) | <0.001 |
| Mo | |||||
| Range | ≤19.08 | ~38.66 | ~76.14 | >76.14 | |
| Case/Control | 30/2 | 17/15 | 7/25 | 9/23 | |
| OR (95%CI) | 1 | 0.079 (0.015, 0.406) | 0.022 (0.004, 0.119) | 0.025 (0.005, 0.135) | <0.001 |
| Rh | |||||
| Range | ≤0.1 | ~0.22 | ~0.5 | >0.5 | |
| Case/Control | 18/7 | 19/6 | 7/18 | 1/24 | |
| OR (95%CI) | 1 | 1.093 (0.271, 4.415) | 0.1 (0.023, 0.437) | 0.005 (0, 0.078) | <0.001 |
| Pd | |||||
| Range | ≤0.01 | ~0.03 | ~0.05 | >0.05 | |
| Case/Control | 1/10 | 10/0 | 11/5 | 10/0 | |
| OR (95%CI) | 1 | 1 | 1 | 1 | 0.112 |
| Cd | |||||
| Range | ≤0.58 | ~1.04 | ~1.98 | >1.98 | |
| Case/Control | 21/12 | 13/16 | 12/22 | 16/15 | |
| OR (95%CI) | 1 | 0.425 (0.14, 1.29) | 0.247 (0.083, 0.739) | 0.662 (0.227, 1.93) | 0.100 |
| In | |||||
| Range | ≤0.05 | ~0.11 | ~0.2 | >0.2 | |
| Case/Control | 17/10 | 15/10 | 7/22 | 1/25 | |
| OR (95%CI) | 1 | 0.797 (0.234, 2.715) | 0.105 (0.025, 0.447) | 0.011 (0.001, 0.122) | 0.001 |
| Sn | |||||
| Range | ≤0.48 | ~4.49 | ~10.22 | >10.22 | |
| Case/Control | 14/17 | 9/22 | 10/22 | 27/4 | |
| OR (95%CI) | 1 | 0.464 (0.149, 1.44) | 0.37 (0.116, 1.184) | 6.963 (1.848, 26.241) | 0.001 |
| Sb | |||||
| Range | ≤0.05 | ~0.13 | ~0.55 | >0.55 | |
| Case/Control | 13/16 | 22/13 | 21/13 | 4/27 | |
| OR (95%CI) | 1 | 2.513 (0.834, 7.569) | 2.267 (0.741, 6.932) | 0.142 (0.035, 0.581) | 0.001 |
| Cs | |||||
| Range | ≤3.08 | ~5.34 | ~7.72 | >7.72 | |
| Case/Control | 30/2 | 13/19 | 10/22 | 9/23 | |
| OR (95%CI) | 1 | 0.057 (0.011, 0.288) | 0.042 (0.008, 0.22) | 0.027 (0.005, 0.143) | 0.001 |
| Ba | |||||
| Range | ≤0.7 | ~1.83 | ~4.69 | >4.69 | |
| Case/Control | 7/23 | 14/16 | 14/16 | 27/3 | |
| OR (95%CI) | 1 | 2.556 (0.817, 7.994) | 2.75 (0.885, 8.55) | 21.053 (4.731, 93.684) | 0.002 |
| La | |||||
| Range | ≤0.01 | ~0.06 | ~0.12 | >0.12 | |
| Case/Control | 14/0 | 12/4 | 5/11 | 14/1 | |
| OR (95%CI) | 1 | 1 | 1 | 1 | 0.033 |
| Gd | |||||
| Range | ≤7.4 | ~85.97 | ~337.47 | >337.47 | |
| Case/Control | 6/13 | 19/0 | 19/0 | 19/0 | |
| OR (95%CI) | 1 | 1 | 1 | 1 | 1 |
| W | |||||
| Range | ≤0.21 | ~0.5 | ~0.89 | >0.89 | |
| Case/Control | 24/8 | 19/11 | 7/25 | 8/23 | |
| OR (95%CI) | 1 | 0.629 (0.198, 1.995) | 0.093 (0.027, 0.32) | 0.094 (0.027, 0.33) | <0.001 |
| Re | |||||
| Range | ≤0.04 | ~0.08 | ~0.16 | >0.16 | |
| Case/Control | 25/10 | 5/7 | 12/28 | 7/20 | |
| OR (95%CI) | 1 | 0.269 (0.061, 1.18) | 0.159 (0.054, 0.463) | 0.15 (0.045, 0.496) | 0.005 |
| Hg | |||||
| Range | ≤0.27 | ~0.45 | ~0.8 | >0.8 | |
| Case/Control | 10/18 | 9/20 | 13/17 | 15/12 | |
| OR (95%CI) | 1 | 0.597 (0.182, 1.96) | 0.915 (0.287, 2.921) | 1.665 (0.523, 5.299) | 0.427 |
| Tl | |||||
| Range | ≤0.22 | ~0.41 | ~0.62 | >0.62 | |
| Case/Control | 29/4 | 14/17 | 11/22 | 9/23 | |
| OR (95%CI) | 1 | 0.188 (0.05, 0.704) | 0.102 (0.027, 0.39) | 0.056 (0.014, 0.22) | 0.001 |
| Pb | |||||
| Range | ≤0.26 | ~0.76 | ~1.68 | >1.68 | |
| Case/Control | 12/18 | 12/17 | 11/20 | 19/10 | |
| OR (95%CI) | 1 | 0.923 (0.308, 2.762) | 0.676 (0.226, 2.019) | 2.138 (0.696, 6.564) | 0.274 |
| Bi | |||||
| Range | ≤0.01 | ~0.05 | ~0.2 | >0.2 | |
| Case/Control | 2/24 | 10/9 | 10/17 | 15/8 | |
| OR (95%CI) | 1 | 11.781 (1.948, 71.238) | 9.347 (1.631, 53.559) | 19.818 (3.362, 116.832) | 0.017 |
| U | |||||
| Range | ≤0.02 | ~0.02 | ~0.03 | >0.03 | |
| Case/Control | 30/36 | 0/0 | 0/0 | 20/3 | |
| OR (95%CI) | 1 | 5.289 (1.355, 20.646) | 0.024 |
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Adjusted p Value | ||
|---|---|---|---|---|---|---|
| Gliomas | Cr | |||||
| Range | ≤29.34 | ~46.98 | ~104.44 | >104.44 | ||
| Case/Control | 8/14 | 5/25 | 7/19 | 8/7 | ||
| OR (95%CI) | 1 | 0.372 (0.094, 1.471) | 0.554 (0.144, 2.135) | 2.1 (0.5, 8.821) | 0.146 | |
| Ga | ||||||
| Range | ≤0.2 | ~0.28 | ~0.4 | >0.4 | ||
| Case/Control | 11/18 | 4/17 | 6/25 | 6/7 | ||
| OR (95%CI) | 1 | 0.435 (0.11, 1.719) | 0.394 (0.117, 1.332) | 0.619 (0.134, 2.861) | 0.493 | |
| Ge | ||||||
| Range | ≤0.33 | ~0.68 | ~1.09 | >1.09 | ||
| Case/Control | 7/9 | 7/13 | 9/17 | 2/30 | ||
| OR (95%CI) | 1 | 1.168 (0.244, 5.582) | 0.847 (0.193, 3.706) | 0.018 (0.001, 0.257) | 0.044 | |
| As | ||||||
| Range | ≤28.15 | ~47.17 | ~72.94 | >72.94 | ||
| Case/Control | 12/2 | 7/10 | 4/26 | 4/28 | ||
| OR (95%CI) | 1 | 0.167 (0.026, 1.049) | 0.027 (0.004, 0.193) | 0.026 (0.004, 0.171) | 0.002 | |
| Rb | ||||||
| Range | ≤716.1 | ~1204.16 | ~1842.18 | >1842.18 | ||
| Case/Control | 12/3 | 9/13 | 2/26 | 4/23 | ||
| OR (95%CI) | 1 | 0.171 (0.035, 0.84) | 0.025 (0.004, 0.176) | 0.044 (0.008, 0.244) | 0.002 | |
| Zr | ||||||
| Range | ≤0.02 | ~0.05 | ~0.14 | >0.14 | ||
| Case/Control | 1/21 | 6/6 | 3/15 | 7/4 | ||
| OR (95%CI) | 1 | 28.198 (2.473, 321.548) | 3.315 (0.275, 39.955) | 25.585 (2.154, 303.955) | 0.037 | |
| Sn | ||||||
| Range | ≤0.48 | ~4.49 | ~10.22 | >10.22 | ||
| Case/Control | 8/17 | 6/22 | 4/22 | 7/4 | ||
| OR (95%CI) | 1 | 0.547 (0.151, 1.981) | 0.197 (0.04, 0.97) | 1.616 (0.294, 8.871) | 0.146 | |
| Sb | ||||||
| Range | ≤0.05 | ~0.13 | ~0.55 | >0.55 | ||
| Case/Control | 8/16 | 9/13 | 7/13 | 2/27 | ||
| OR (95%CI) | 1 | 1.583 (0.427, 5.871) | 1.116 (0.27, 4.606) | 0.108 (0.018, 0.657) | 0.061 | |
| Pb | ||||||
| Range | ≤0.26 | ~0.76 | ~1.68 | >1.68 | ||
| Case/Control | 6/18 | 3/17 | 7/20 | 6/10 | ||
| OR (95%CI) | 1 | 0.373 (0.072, 1.927) | 0.78 (0.207, 2.932) | 0.873 (0.181, 4.224) | 0.681 | |
| Meningiomas | Cr | |||||
| Range | ≤29.34 | ~46.98 | ~104.44 | >104.44 | ||
| Case/Control | 4/14 | 2/25 | 1/19 | 8/7 | ||
| OR (95%CI) | 1 | 0.258 (0.04, 1.668) | 0.165 (0.016, 1.708) | 3.316 (0.684, 16.081) | 0.053 | |
| Ga | ||||||
| Range | ≤0.2 | ~0.28 | ~0.4 | >0.4 | ||
| Case/Control | 3/18 | 0/17 | 4/25 | 8/7 | ||
| OR (95%CI) | 1 | 1 | 0.927 (0.183, 4.703) | 5.318 (0.999, 28.302) | 0.143 | |
| Ge | ||||||
| Range | ≤0.33 | ~0.68 | ~1.09 | >1.09 | ||
| Case/Control | 7/9 | 6/13 | 2/17 | 0/30 | ||
| OR (95%CI) | 1 | 1.12 (0.221, 5.691) | 0.114 (0.014, 0.94) | 1 | 0.215 | |
| As | ||||||
| Range | ≤28.15 | ~47.17 | ~72.94 | >72.94 | ||
| Case/Control | 8/2 | 5/10 | 2/26 | 0/28 | ||
| OR (95%CI) | 1 | 0.164 (0.022, 1.247) | 0.01 (0.001, 0.151) | 1 | 0.037 | |
| Rb | ||||||
| Range | ≤716.1 | ~1204.16 | ~1842.18 | >1842.18 | ||
| Case/Control | 8/3 | 6/13 | 1/26 | 0/23 | ||
| OR (95%CI) | 1 | 0.198 (0.036, 1.081) | 0.019 (0.002, 0.211) | 1 | 0.032 | |
| Zr | ||||||
| Range | ≤0.02 | ~0.05 | ~0.14 | >0.14 | ||
| Case/Control | 2/21 | 4/6 | 2/15 | 6/4 | ||
| OR (95%CI) | 1 | 8.094 (1.05, 62.405) | 0.935 (0.101, 8.65) | 15.602 (2.105, 115.63) | 0.026 | |
| Sn | ||||||
| Range | ≤0.48 | ~4.49 | ~10.22 | >10.22 | ||
| Case/Control | 2/17 | 1/22 | 4/22 | 8/4 | ||
| OR (95%CI) | 1 | 0.257 (0.019, 3.518) | 0.894 (0.124, 6.424) | 14.303 (1.963, 104.19) | 0.021 | |
| Sb | ||||||
| Range | ≤0.05 | ~0.13 | ~0.55 | >0.55 | ||
| Case/Control | 2/16 | 7/13 | 5/13 | 1/27 | ||
| OR (95%CI) | 1 | 4.396 (0.707, 27.338) | 3.33 (0.499, 22.245) | 0.248 (0.02, 3.155) | 0.082 | |
| Pb | ||||||
| Range | ≤0.26 | ~0.76 | ~1.68 | >1.68 | ||
| Case/Control | 3/18 | 5/17 | 3/20 | 3/10 | ||
| OR (95%CI) | 1 | 1.7 (0.339, 8.533) | 0.688 (0.115, 4.121) | 1.584 (0.25, 10.048) | 0.702 | |
| Cranial and paraspinal nerve tumors | Cr | |||||
| Range | ≤29.34 | ~46.98 | ~104.44 | >104.44 | ||
| Case/Control | 1/14 | 0/25 | 1/19 | 5/7 | ||
| OR (95%CI) | 1 | 1 | 0.715 (0.039, 13.034) | 6.696 (0.583, 76.948) | 0.496 | |
| Ga | ||||||
| Range | ≤0.2 | ~0.28 | ~0.4 | >0.4 | ||
| Case/Control | 1/18 | 0/17 | 3/25 | 3/7 | ||
| OR (95%CI) | 1 | 1 | 1.962 (0.18, 21.409) | 3.604 (0.262, 49.632) | 0.919 | |
| Ge | ||||||
| Range | ≤0.33 | ~0.68 | ~1.09 | >1.09 | ||
| Case/Control | 6/9 | 0/13 | 1/17 | 0/30 | ||
| OR (95%CI) | 1 | 1 | 0.017 (0, 1.01) | 1 | 0.506 | |
| As | ||||||
| Range | ≤28.15 | ~47.17 | ~72.94 | >72.94 | ||
| Case/Control | 4/2 | 3/10 | 0/26 | 0/28 | ||
| OR (95%CI) | 1 | 3.08 (0.081, 116.495) | 1 | 1 | 0.947 | |
| Rb | ||||||
| Range | ≤716.1 | ~1204.16 | ~1842.18 | >1842.18 | ||
| Case/Control | 2/3 | 1/13 | 2/26 | 2/23 | ||
| OR (95%CI) | 1 | 0.049 (0.002, 1.212) | 0.063 (0.005, 0.859) | 0.042 (0.002, 0.72) | 1 | |
| Zr | ||||||
| Range | ≤0.02 | ~0.05 | ~0.14 | >0.14 | ||
| Case/Control | 0/21 | 1/6 | 2/15 | 2/4 | ||
| OR (95%CI) | 1 | 1 | 1 | 1 | 0.906 | |
| Sn | ||||||
| Range | ≤0.48 | ~4.49 | ~10.22 | >10.22 | ||
| Case/Control | 0/17 | 0/22 | 2/22 | 5/4 | ||
| OR (95%CI) | 1 | 1 | 1 | 1 | 0.573 | |
| Sb | ||||||
| Range | ≤0.05 | ~0.13 | ~0.55 | >0.55 | ||
| Case/Control | 1/16 | 1/13 | 4/13 | 1/27 | ||
| OR (95%CI) | 1 | 0.903 (0.038, 21.505) | 4.274 (0.359, 50.92) | 0.264 (0.012, 5.664) | 0.523 | |
| Pb | ||||||
| Range | ≤0.26 | ~0.76 | ~1.68 | >1.68 | ||
| Case/Control | 1/18 | 1/17 | 0/20 | 4/10 | ||
| OR (95%CI) | 1 | 1.07 (0.058, 19.858) | 1 | 4.471 (0.376, 53.119) | 0.841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Wu, H.; Xiao, F.; Yang, T.; Wang, W.; Du, H.; Su, P. Association Between Heavy Metal Exposure and Central Nervous System Tumors: A Case-Control Study Using Single and Multi-Metal Models. Toxics 2025, 13, 92. https://doi.org/10.3390/toxics13020092
Luo S, Wu H, Xiao F, Yang T, Wang W, Du H, Su P. Association Between Heavy Metal Exposure and Central Nervous System Tumors: A Case-Control Study Using Single and Multi-Metal Models. Toxics. 2025; 13(2):92. https://doi.org/10.3390/toxics13020092
Chicago/Turabian StyleLuo, Sen, Haixia Wu, Fang Xiao, Tianwen Yang, Wei Wang, Hang Du, and Peng Su. 2025. "Association Between Heavy Metal Exposure and Central Nervous System Tumors: A Case-Control Study Using Single and Multi-Metal Models" Toxics 13, no. 2: 92. https://doi.org/10.3390/toxics13020092
APA StyleLuo, S., Wu, H., Xiao, F., Yang, T., Wang, W., Du, H., & Su, P. (2025). Association Between Heavy Metal Exposure and Central Nervous System Tumors: A Case-Control Study Using Single and Multi-Metal Models. Toxics, 13(2), 92. https://doi.org/10.3390/toxics13020092






