Bioaccumulation of Metals in Fish Collected from Macapá Urban Aquatic Environments (Brazilian Amazon) and the Risks to Human Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Fish Sampling
2.3. Preparation of Fish Muscle Samples and Determination of Metals
2.4. Risk Assessment for Human Health from Fish Consumption and Estimated Daily Intake (EDI)
3. Results
3.1. Metal Concentrations in Fish Species and Their Compliance with Legal Limits
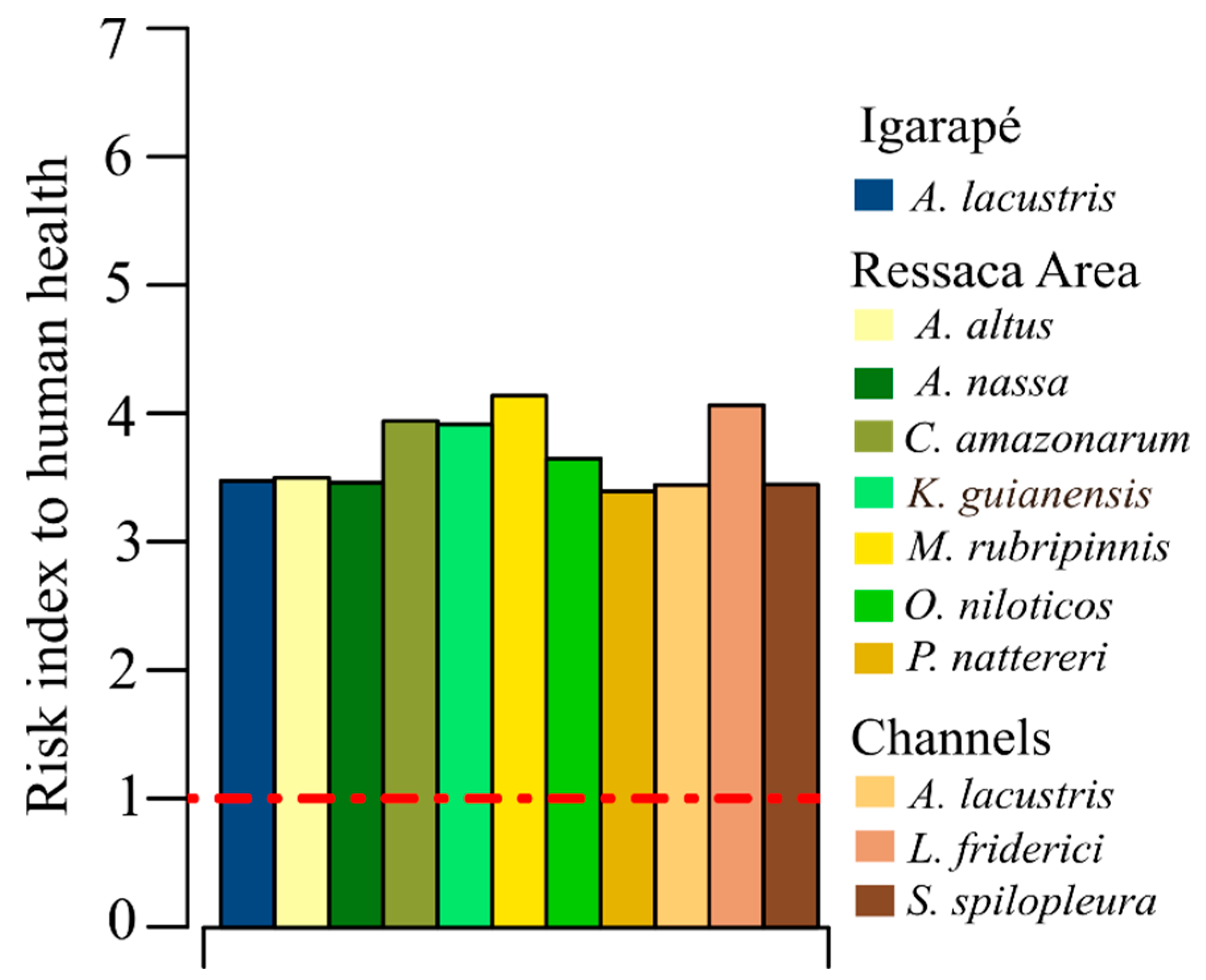
3.2. Human Health Risk Assessment from Fish Consumption
3.3. Estimation of Daily Intake (EDI)
4. Discussion
Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Instituto Brasileiro de Geografia e Estatística (IBGE). Population Estimates, Macapá 2021. Available online: https://www.ibge.gov.br/cidades-e-estados/ap/macapa.html (accessed on 15 February 2022).
- Santos, C.M.B.; Nery, C.H.S. Análise do atual sistema de esgotamento sanitário da cidade de Macapá em conjuntura com realização de estudo de caso do sistema de esgoto encontrado no bairro central. Rev. Mult. CEAP 2022, 4, 9. [Google Scholar]
- Rodrigues, C.C.S.; Santos, L.G.G.V.; Santos, E.; Damasceno, F.C.; Corrêa, J.A.M. Polycyclic aromatic hydrocarbons in sediments of the Amazon River Estuary (Amapá, Northern Brazil): Distribution, sources and potential ecological risk. Mar. Pollut. Bull. 2018, 135, 769–775. [Google Scholar] [CrossRef]
- Sousa, T.S.; Viegas, C.J.T.; Cunha, H.F.A.; Cunha, A.C.D. Drainage and preliminary risk of flooding in an urban zone of Eastern Amazon. GEP 2023, 11, 1–16. [Google Scholar] [CrossRef]
- Costa, P.C.; Samora, P. Formas urbanas para áreas de conflito socioambiental em APP’s: Modelos para os desafios das áreas de ressaca de Macapá-AP. Rev. Morfol. Urbana 2023, 11, 297. [Google Scholar] [CrossRef]
- Takiyama, L.R.; Silva, U.R.L.; Jimenez, E.A.; Pereira, R.A. Zoneamento ecológico-econômico urbano das áreas úmidas de Macapá e Santana, Estado do Amapá. OLAM—CiÊNcia Tecnol. 2013, 1, 129–158. [Google Scholar]
- Flores, C.A.R.; Cunha, A.C.; Cunha, H.F.A. Modelagem de lixiviados e compostos gerados em sistema de drenagem de aterro controlado de Macapá/Brasil, Rev. Ibero-Am. CiÊNc. Ambient. 2022, 12, 568–583. [Google Scholar] [CrossRef]
- Bega, J.M.M.; Zanetoni Filho, J.A.; Albertin, L.L.; Oliveira, J.N.D. Temporal changes in the water quality of urban tropical streams: An approach to daily variation in seasonality. Integr. Environ. Assess. Manag. 2022, 18, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Carim, M.D.J.V.; Torres, A.M.; Takyiama, L.R.; Silva Junior, O.M.D.; Souza, M.O.D.; Souto, F.A.F.; Baia, M.; Barata, J.B.; Souza, A.J.B.D.; Correa, P.R.D.S. Impactos da disposição de resíduos sólidos urbanos no solo e água nos municípios de Macapá e Santana—Amapá. RSD 2022, 11, e37111528211. [Google Scholar] [CrossRef]
- Albuquerque, F.E.A.; Herrero-Latorre, C.; Miranda, M.; Barrêto Júnior, R.A.; Oliveira, F.L.C.; Sucupira, M.C.A.; Ortolani, E.L.; Minervino, A.H.H.; López-Alonso, M. Fish tissues for biomonitoring toxic and essential trace elements in the lower Amazon. Environ. Pollut. 2021, 283, 117024. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.D.S.; Fontes, M.P.F.; Pacheco, A.A.; Lima, H.N.; Santos, J.Z.L. Risk assessment of trace elements pollution of manaus urban rivers. Sci. Total Environ. 2020, 709, 134471. [Google Scholar] [CrossRef]
- Yuan, G.; Dai, S.; Yin, Z.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Zhao, X. Toxicological assessment of combined lead and cadmium: Acute and sub-chronic toxicity study in rats. Food Chem. Toxicol. 2014, 65, 260–268. [Google Scholar] [CrossRef]
- Viana, L.F.; Súarez, Y.R.; Cardoso, C.A.L.; Crispim, B.D.A.; Grisolia, A.B.; Lima-Junior, S.E. Mutagenic and genotoxic effects and metal contaminations in fish of the Amambai River, upper Paraná River, Brazil. Environ. Sci. Pollut. Res. 2017, 24, 27104–27112. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. risk to human health. Environ. Chem. Lett. 2018, 16, 903–917. [Google Scholar] [CrossRef]
- Dagosta, F.C.P.; Pinna, M.D. The fishes of the Amazon: Distribution and biogeographical patterns, with a comprehensive list of species. Bull. Am. Mus. Nat. Hist. 2019, 1, 1–163. [Google Scholar] [CrossRef]
- Albuquerque, F.E.A.; Minervino, A.H.H.; Miranda, M.; Herrero-Latorre, C.; Barrêto Júnior, R.A.; Oliveira, F.L.C.; Sucupira, M.C.A.; Ortolani, E.L.; López-Alonso, M. Toxic and essential trace element concentrations in fish species in the lower Amazon, Brazil. Sci. Total Environ. 2020, 732, 138983. [Google Scholar] [CrossRef] [PubMed]
- Rivero, S.L.M.; Almeida, O.T.D.; Torres, P.C.; De Moraes, A.; Chacón-Montalván, E.; Parry, L. Urban Amazonians use fishing as a strategy for coping with food insecurity. JDS 2022, 58, 2544–2565. [Google Scholar] [CrossRef]
- Hacon, S.D.S.; Oliveira-da-Costa, M.; Gama, C.D.S.; Ferreira, R.; Basta, P.C.; Schramm, A.; Yokota, D. Mercury exposure through fish consumption in traditional communities in the Brazilian northern amazon. Int. J. Environ. Res. Public Health 2020, 17, 5269. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.F.; Kummrow, F.; Cardoso, C.A.L.; Lima, N.A.; Solórzano, J.C.J.; Crispim, B.A.; Barufatti, A.; Florentino, A.C. High concentrations of metals in the waters from Araguari River lower section (Amazon Biome): Relationship with land use and cover, ecotoxicological effects and risks to aquatic biota. Chemosphere 2021, 285, 131451. [Google Scholar] [CrossRef]
- Costa, M.S.; Viana, L.F.; Cardoso, C.A.L.; Isacksson, E.D.G.S.; Silva, J.C.; Florentino, A.C. Landscape composition and inorganic contaminants in water and muscle tissue of Plagioscion squamosissimus in the Araguari River (Amazon, Brazil). Environ. Res. 2022, 208, 112691. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.F.; Kummrow, F.; Cardoso, C.A.L.; Lima, N.A.; Crispim, B.A.; Barufatti, A.; Florentino, A.C. Metal bioaccumulation in fish from the Araguari River (Amazon Biome) and human health risks from fish consumption. Environ. Sci. Pollut. Res. 2023, 30, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.F.; Cardoso, C.A.L.; Oliveira, M.S.B.; Lima-Junior, S.E.; Kummrow, F.; Florentino, A.C. Metals bioaccumulation in fish captured from Araguari River upper section (Amazon Biome), and risk assessment to human health resulting from their consumption. J. Trace Elem. Min. 2024, 7, 100111. [Google Scholar] [CrossRef]
- Martoredjo, I.; Calvão Santos, L.B.; Vilhena, J.C.E.; Rodrigues, A.B.L.; De Almeida, A.; Sousa Passos, C.J.; Florentino, A.C. Trends in mercury contamination distribution among human and animal populations in the amazon region. Toxics 2024, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.; Juras, A.A.; Mérona, B.; Jégue, M. Peixes do Baixo rio Tocantins. 20 anos Depois da Usina Hidrelétrica Tucuruí; Eletronorte: Brasília, Brasil, 2004. [Google Scholar]
- Sleen, P.V.; Albert, J.S. Field Guide to the Fishes of the Amazon, Orinoco, and Guianas; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar]
- Viana, L.F.; Cardoso, C.A.L.; Lima-Junior, S.E.; Súarez, Y.R.; Florentino, A.C. Bioaccumulation of metal in liver tissue of fish in response to water toxicity of the Araguari-Amazon River, Brazil. Environ. Monit. Assess. 2020, 192, 781. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, P.; Pla, A.; Hernández, A.F.; Barbier, F.; Ayouni, L.; Gil, F. Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples: Risk assessment for the consumers. Environ. Int. 2013, 59, 63–72. [Google Scholar] [CrossRef]
- Morgano, M.A.; Gomes, P.C.; Mantovani, D.M.B.; Perrone, A.A.M.; Santos, T.F. Níveis de mercúrio total em peixes de água doce de pisciculturas paulistas. CiÊNc. Tecnol. Aliment. 2005, 25, 250–253. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária do Brasil (ANVISA). Portaria nº 685 de 27 de Agosto de 1998, Brasília. 1998. Available online: https://www.univates.br/unianalises/media/imagens/Anexo_XI_61948_11.pdf (accessed on 12 May 2022).
- Agência Nacional de Vigilância Sanitária do Brasil (ANVISA). Legislação Brasileira, Resolução nº 42 de 29 de Agosto de 2013, Brasília. 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2013/rdc0042_29_08_2013.html (accessed on 10 June 2022).
- Ullah, A.K.M.A.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Quraishi, S.B. Development and validation of a gf-aas method and its application for the trace level determination of Pb, Cd, and Cr in fish feed samples commonly used in the hatcheries of Bangladesh. J. Anal. Sci. Technol. 2017, 8, 15. [Google Scholar] [CrossRef]
- USEPA. Risk Based Concentration Table; United States Environmental Protection Agency: Philadelphia, PA, USA; Washington, DC, USA, 2000. [Google Scholar]
- Duffus, J.H.; Duffus, J.H.; Nordberg, M.; Templeton, D.M. Glossary of terms used in toxicology, 2nd edition (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1153–1344. [Google Scholar] [CrossRef]
- Isaac, V.J.; Almeida, M.C.; Giarrizzo, T.; Deus, C.P.; Vale, R.; Klein, G.; Begossi, A. Food Consumption as an Indicator of the Conservation of Natural Resources in Riverine Communities of the Brazilian Amazon. An. Acad. Bras. CiÊNc. 2015, 87, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Souza-Araujo, J.D.; Hussey, N.E.; Hauser-Davis, R.A.; Rosa, A.H.; Lima, M.D.O.; Giarrizzo, T. Human risk assessment of toxic elements (As, Cd, Hg, Pb) in marine fish from the Amazon. Chemosphere 2022, 301, 134575. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilância Sanitária do Brasil (ANVISA). Nota Técnica nº 8/2019/SEI/GEARE/GGALI/DIRE2/ ANVISA. Processo nº 25351.918291/2019–53, Avaliação de Risco: Consumo de Pescado Proveniente de Regiões Afetadas Pelo Rompimento da Barragem do Fundão/MG. 2019. Available online: https://sanityconsultoria.com/wp-content/uploads/2019/06/nota-tecnica-anvisa-pescado-rio-doce-junho-2019.pdf (accessed on 15 June 2022).
- FAO/WHO. Evaluation of Certain Food Additives and Contaminants. Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Musarrat, M.; Ullah, A.K.M.A.; Moushumi, N.S.; Akon, S.; Nahar, Q.; Saliheen Sultana, S.S.; Quraishi, S.B. Assessment of heavy metal(loid)s in selected small indigenous species of industrial area origin freshwater fish and potential human health risk implications in Bangladesh. LWT 2021, 150, 112041. [Google Scholar] [CrossRef]
- Naka, K.S.; Mendes, L.C.S.; Queiroz, T.K.L.; Costa, B.N.S.; Jesus, I.M.; Câmara, V.M.; Lima, M.O. A comparative study of cadmium levels in blood from exposed populations in an industrial area of the Amazon, Brazil. Sci. Total Environ. 2020, 698, 134309. [Google Scholar] [CrossRef]
- Rico, A.; Oliveira, R.; Nunes, G.S.S.; Rizzi, C.; Villa, S.; López-Heras, I.; Vighi, M.; Waichman, A.V. Pharmaceuticals and other urban contaminants threaten Amazonian freshwater ecosystems. Environ. Int. 2021, 155, 106702. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Masunaga, S. Assessment of trace metals in fish species of urban rivers in Bangladesh and health implications. Environ. Toxicol. Pharmacol. 2015, 39, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, K.M.; Thirukumaran, V.; Suresh, M. Assessment and source identification of heavy metal contamination of groundwater using geospatial technology in Gadilam River Basin, Tamil Nadu, India. Appl. Water Sci. 2021, 11, 102. [Google Scholar] [CrossRef]
- Dhanakumar, S.; Solaraj, G.; Mohanraj, R. Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of River Cauvery Delta Region, India. Ecotoxicol. Environ. Saf. 2015, 113, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, P.; Zhao, F.-J. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 939–963. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Anyanwu, B.; Ezejiofor, A.; Igweze, Z.; Orisakwe, O. Heavy metal mixture exposure and effects in developing nations: An update. Toxics 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Loewe, S.; Muischnek, H. Über kombinationswirkungen: Mitteilung: Hilfsmittel der fragestellung. Archiv. F. Exp. Pathol. U. Pharmakol. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- Martin, O.; Scholze, M.; Ermler, S.; McPhie, J.; Bopp, S.K.; Kienzler, A.; Parissis, N.; Kortenkamp, A. Ten years of research on synergisms and antagonisms in chemical mixtures: A systematic review and quantitative reappraisal of mixture studies. Environ. Int. 2021, 146, 106206. [Google Scholar] [CrossRef] [PubMed]
- Bureš, M.S.; Cvetnić, M.; Miloloža, M.; Kučić Grgić, D.; Markić, M.; Kušić, H.; Bolanča, T.; Rogošić, M.; Ukić, Š. Modeling the toxicity of pollutants mixtures for risk assessment: A review. Environ. Chem. Lett. 2021, 19, 1629–1655. [Google Scholar] [CrossRef]
- Silva, S.F.; De Oliveira Lima, M.O. Mercury in fish marketed in the Amazon Triple Frontier and health risk assessment. Chemosphere 2020, 248, 125989. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Levy, I.E.; Van Damme, P.A.; Carvajal-Vallejos, F.M.; Bervoets, L. Trace element accumulation in different edible fish species from the Bolivian Amazon and the risk for human consumption. Heliyon 2022, 8, e11649. [Google Scholar] [CrossRef] [PubMed]
- Waichman, A.V.; Nunes, G.S.S.; Oliveira, R.; Isabel López-Heras, I.; Rico, A. Human health risks associated to trace elements and metals in commercial fish from the Brazilian Amazon. J. Environ. Sci. 2025, 148, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, S.M.; Nascimento, L.S.; Silva, L.O.; Almeida, M.G.; Azevedo, L.S.; Constantino, W.D.; Bastos, W.R.; Pestana, I.A. Flood pulse as a driving force of Pb variation in four fish guilds from Puruzinho Lake (western Amazon). Environ. Sci. Pollut. Res. 2023, 30, 38728–38737. [Google Scholar] [CrossRef] [PubMed]


| Fish Species | Igarapé | Ressaca Areas | Channels | Standard Length (cm) | Total Weight (g) | Feeding Habits | Habitats | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | |||||
| A. altus | 0 | 5 | 0 | 0 | 0 | 0 | 11.02 ± 0.72 | 18.18 ± 3.41 | Carnivore | Benthopelagic |
| P. nattereri | 0 | 5 | 0 | 0 | 0 | 0 | 8.84 ± 1.66 | 37.56 ± 20.76 | Carnivore | Pelagic |
| S. spilopleura | 0 | 0 | 0 | 0 | 0 | 4 | 7.25 ± 0.31 | 12.80 ± 2.20 | Carnivore | Benthopelagic |
| A. lacustris | 17 | 0 | 0 | 0 | 5 | 0 | 8.50 ± 0.85 | 20.01 ± 6.75 | Omnivore | Benthopelagic |
| A. nassa | 0 | 0 | 0 | 3 | 0 | 0 | 4.50 ± 1.13 | 6.00 ± 4.24 | Omnivore | Benthopelagic |
| C. amazonarum | 0 | 0 | 7 | 0 | 0 | 0 | 10.10 ± 0.88 | 61.62 ± 27.58 | Omnivore | Benthopelagic |
| K. guianensis | 0 | 0 | 20 | 0 | 0 | 0 | 11.16 ± 0.99 | 63.80 ± 40.54 | Omnivore | Benthopelagic |
| L. friderici | 0 | 0 | 0 | 0 | 0 | 9 | 9.02 ± 2.67 | 23.87 ± 14.82 | Omnivore | Benthopelagic |
| M. rubripinnis | 0 | 10 | 0 | 0 | 0 | 0 | 9.55 ± 1.66 | 42.21 ± 15.40 | Omnivore | Benthopelagic |
| O. niloticus | 0 | 0 | 7 | 0 | 0 | 0 | 23.28 ± 2.46 | 512.57 ± 166.44 | Omnivore | Benthopelagic |
| Total | 17 | 20 | 34 | 3 | 5 | 14 | ||||
| Risk Quotient (RQ) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fish Species | Sites | Cd | Pb | Cr | Ni | Hg | Cu | Zn |
| A. lacustris | Igarapé | 1.22 | 0.61 | 0.54 | 0.04 | 0.69 | 0.48 | 0.49 |
| A. altus | Ressaca areas | 1.37 | 0.60 | 0.84 | 0.04 | 0.47 | 0.53 | 0.58 |
| A. nassa | Ressaca areas | 1.88 | 0.68 | 0.48 | 0.02 | 0.47 | 0.24 | 0.25 |
| C. amazonarum | Ressaca areas | 1.26 | 0.62 | 0.84 | 0.05 | 0.91 | 0.56 | 0.58 |
| K. guianensis | Ressaca areas | 1.25 | 0.60 | 0.88 | 0.05 | 0.89 | 0.56 | 0.57 |
| M. rubripinnis | Ressaca areas | 1.12 | 0.92 | 0.90 | 0.05 | 0.79 | 0.64 | 0.66 |
| O. niloticus | Ressaca areas | 1.05 | 0.79 | 0.81 | 0.04 | 0.71 | 0.58 | 0.50 |
| P. nattereri | Ressaca areas | 1.33 | 0.60 | 0.90 | 0.04 | 0.45 | 0.52 | 0.54 |
| A. lacustris | Channels | 1.20 | 0.61 | 0.54 | 0.04 | 0.69 | 0.49 | 0.49 |
| L. friderici | Channels | 1.35 | 0.60 | 0.82 | 0.04 | 0.96 | 0.57 | 0.60 |
| S. spilopleura | Channels | 1.35 | 0.57 | 0.90 | 0.04 | 0.47 | 0.55 | 0.54 |
| Fish Species | Sites | Estimated Daily Intake (EDI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Cr | Ni | Fe | Hg | Mn | Cu | Zn | ||
| A. lacustris | Igarapé | 0.42 | 1.28 | 0.37 | 1.43 | 304.34 | 2.41 | 3.41 | 99.75 | 171.59 |
| A. altus | Ressaca areas | 0.47 | 1.26 | 0.58 | 1.58 | 346.72 | 3.25 | 3.36 | 111.07 | 201.17 |
| A. nassa | Ressaca areas | 0.65 | 1.41 | 0.33 | 0.80 | 158.80 | 1.64 | 2.28 | 49.86 | 87.75 |
| C. amazonarum | Ressaca areas | 0.44 | 1.29 | 0.58 | 1.87 | 329.23 | 3.17 | 4.78 | 116.48 | 202.67 |
| K. guianensis | Ressaca areas | 0.43 | 1.25 | 0.61 | 1.70 | 339.20 | 3.09 | 4.80 | 117.90 | 200.12 |
| M. rubripinnis | Ressaca areas | 0.39 | 1.92 | 9.22 | 1.84 | 307.36 | 2.74 | 3.37 | 134.44 | 229.37 |
| O. niloticus | Ressaca areas | 0.36 | 1.65 | 0.56 | 1.48 | 301.30 | 2.48 | 3.48 | 120.93 | 172.91 |
| P. nattereri | Ressaca areas | 0.46 | 1.25 | 0.62 | 1.48 | 291.54 | 3.15 | 4.44 | 108.93 | 186.86 |
| A. lacustris | Channels | 0.41 | 1.27 | 0.37 | 1.41 | 298.72 | 2.39 | 3.36 | 101.47 | 171.37 |
| L. friderici | Channels | 0.47 | 1.25 | 0.57 | 1.45 | 347.55 | 3.32 | 5.70 | 119.45 | 210.41 |
| S. spilopleura | Channels | 0.47 | 1.20 | 0.62 | 1.44 | 297.96 | 3.24 | 4.20 | 114.98 | 186.63 |
| RfD | 0.83 a | 1.2 b | 45.00 a | 1000.00 a | 3470.00 a | 0.57 a | 2300.00 a | 6935.00 a | 23,500.00 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, D.C.D.d.; Viana, L.F.; Kummrow, F.; Cardoso, C.A.L.; Lima, N.A.d.; Lacerda, I.A.R.; Crispim, B.d.A.; Barufatti, A.; Dias, L.A.V.; Florentino, A.C. Bioaccumulation of Metals in Fish Collected from Macapá Urban Aquatic Environments (Brazilian Amazon) and the Risks to Human Health. Toxics 2025, 13, 67. https://doi.org/10.3390/toxics13020067
Souza DCDd, Viana LF, Kummrow F, Cardoso CAL, Lima NAd, Lacerda IAR, Crispim BdA, Barufatti A, Dias LAV, Florentino AC. Bioaccumulation of Metals in Fish Collected from Macapá Urban Aquatic Environments (Brazilian Amazon) and the Risks to Human Health. Toxics. 2025; 13(2):67. https://doi.org/10.3390/toxics13020067
Chicago/Turabian StyleSouza, Debora Cristina Damasceno de, Lucilene Finoto Viana, Fábio Kummrow, Claudia Andrea Lima Cardoso, Nathalya Alice de Lima, Izabelle Alexandra Rodrigues Lacerda, Bruno do Amaral Crispim, Alexeia Barufatti, Lúcio André Viana Dias, and Alexandro Cezar Florentino. 2025. "Bioaccumulation of Metals in Fish Collected from Macapá Urban Aquatic Environments (Brazilian Amazon) and the Risks to Human Health" Toxics 13, no. 2: 67. https://doi.org/10.3390/toxics13020067
APA StyleSouza, D. C. D. d., Viana, L. F., Kummrow, F., Cardoso, C. A. L., Lima, N. A. d., Lacerda, I. A. R., Crispim, B. d. A., Barufatti, A., Dias, L. A. V., & Florentino, A. C. (2025). Bioaccumulation of Metals in Fish Collected from Macapá Urban Aquatic Environments (Brazilian Amazon) and the Risks to Human Health. Toxics, 13(2), 67. https://doi.org/10.3390/toxics13020067










