Acute Toxicity, Neurotoxic, Immunotoxic, and Behavioral Effects of Deltamethrin and Sulfamethoxazole in Adult Zebrafish: Insights into Chemical Interactions and Environmental Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Zebrafish Maintenance and Exposure Design
2.3. Feeding Behavior Assessment
2.4. Assessment of Neurotransmitter and AChE Levels
2.5. Analysis of Oxidative Stress Enzyme Activity
2.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.7. Data Statistics and Analysis
3. Results and Discussion
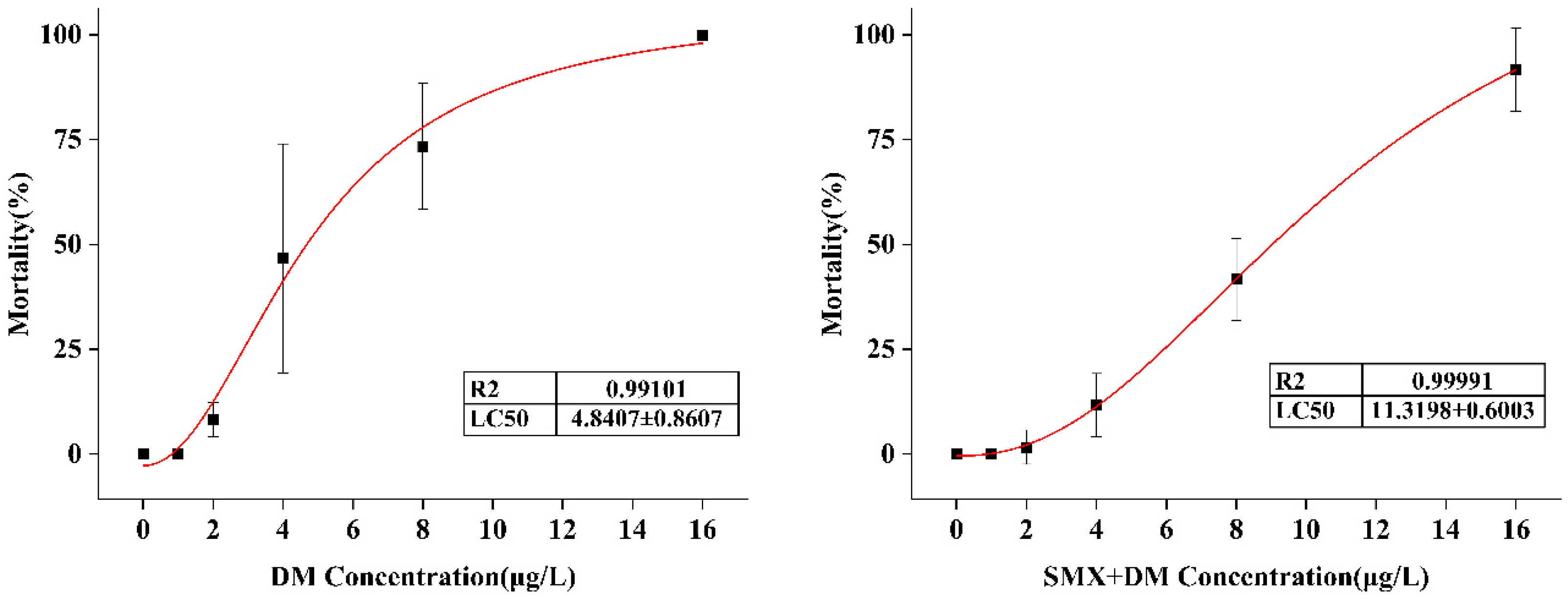
3.1. Acute Toxicity of DM and SMX in Adult Zebrafish
3.2. Neurotransmitter Alterations in the Zebrafish Brain Following Chemical Exposure
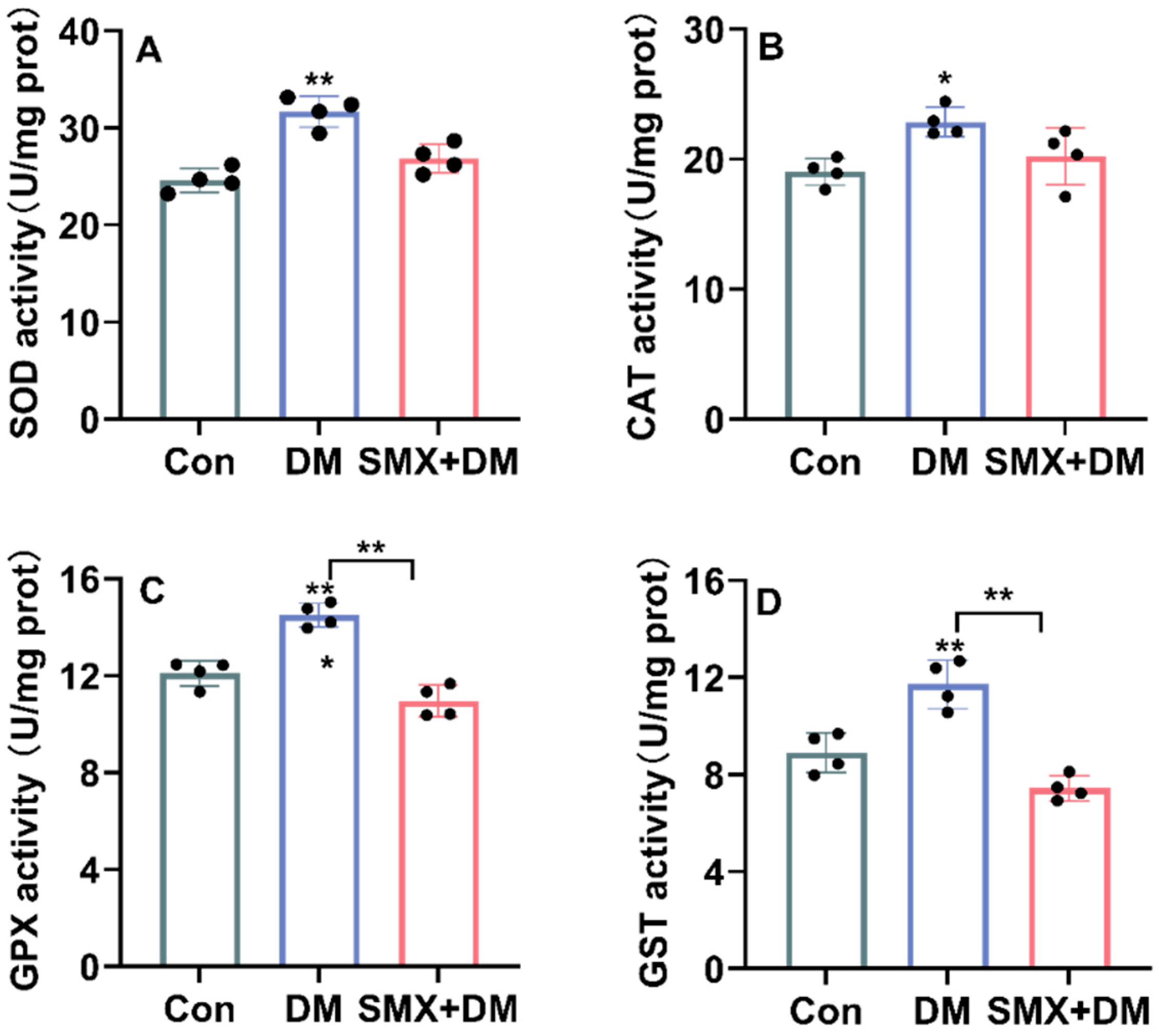
3.3. Oxidative Stress Response in the Zebrafish Brain After Chemical Exposure
3.4. Changes in Immune-Related Gene Expression in the Zebrafish Brain
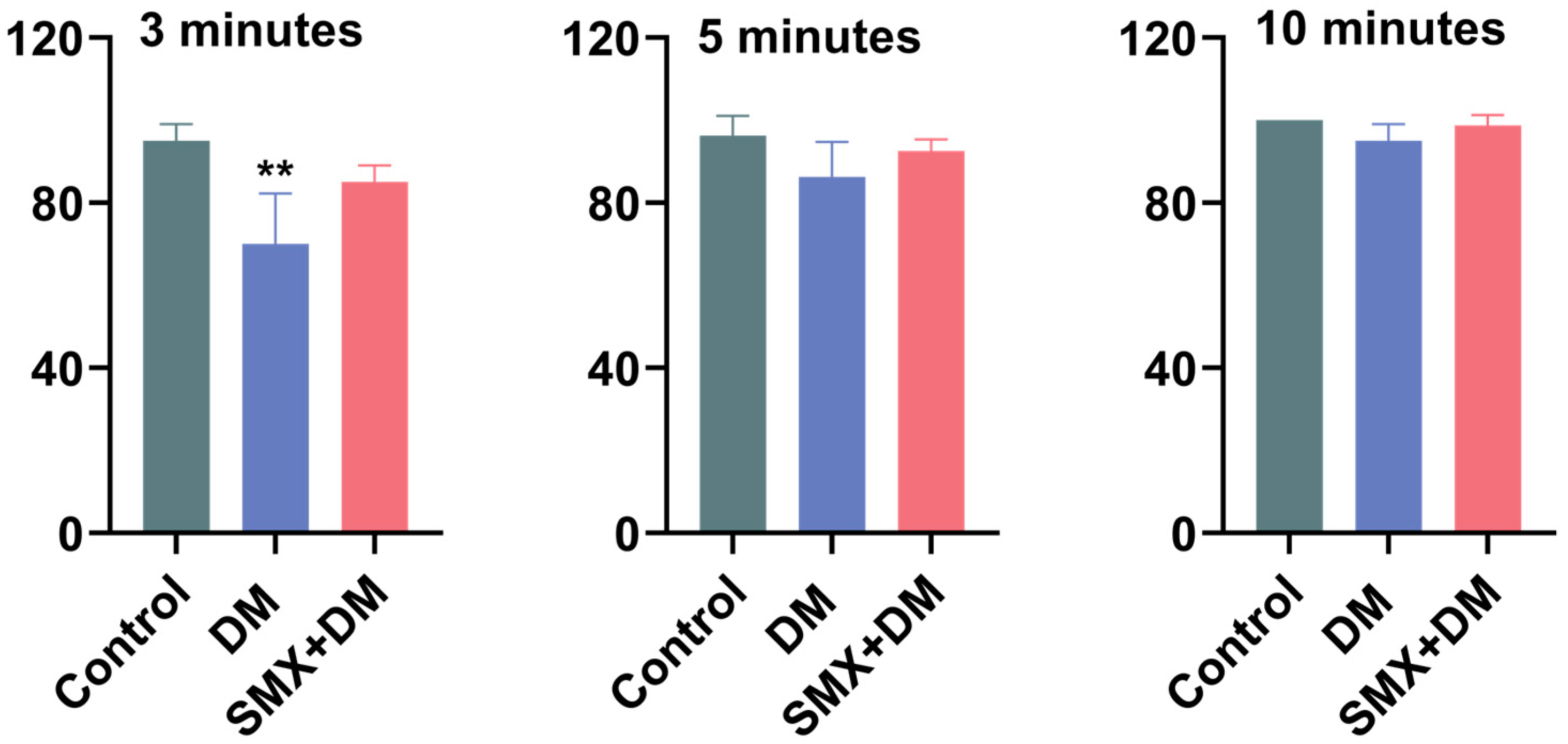
3.5. Effects of Deltamethrin and Sulfamethoxazole on Feeding Behavior of Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitzer, E.M.; Williams, M.T.; Vorhees, C.V. Effects of pyrethroids on brain development and behavior: Deltamethrin. Neurotoxicol. Teratol. 2021, 87, 106983. [Google Scholar] [CrossRef]
- Kumar, A.; Jasrotia, S.; Dutta, J.; Kyzas, G.Z. Pyrethroids toxicity in vertebrates and invertebrates and amelioration by bioactive compounds: A review. Pestic. Biochem. Physiol. 2023, 196, 105615. [Google Scholar] [CrossRef] [PubMed]
- Wolansky, M.J.; Harrill, J.A. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: A critical review. Neurotoxicol. Teratol. 2008, 30, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Feo, M.L.; Ginebreda, A.; Eljarrat, E.; Barceló, D. Presence of pyrethroid pesticides in water and sediments of Ebro River Delta. J. Hydrol. 2010, 393, 156–162. [Google Scholar] [CrossRef]
- Lao, W.; Tsukada, D.; Greenstein, D.J.; Bay, S.M.; Maruya, K.A. Analysis, occurrence, and toxic potential of pyrethroids, and fipronil in sediments from an urban estuary. Environ. Toxicol. Chem. 2010, 29, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Pérez, M.D.; Torres-Dosal, A.; Batres, L.E.; López-Guzmán, O.D.; Grimaldo, M.; Carranza, C.; Pérez-Maldonado, I.N.; Martínez, F.; Pérez-Urizar, J.; Díaz-Barriga, F. Environmental health assessment of deltamethrin in a malarious area of Mexico: Environmental persistence, toxicokinetics, and genotoxicity in exposed children. Environ. Health Perspect. 2005, 113, 782–786. [Google Scholar] [CrossRef]
- Fang, W.; Peng, Y.; Muir, D.; Lin, J.; Zhang, X. A critical review of synthetic chemicals in surface waters of the US, the EU and China. Environ. Int. 2019, 131, 104994. [Google Scholar] [CrossRef]
- Peris, A.; Barbieri, M.V.; Postigo, C.; Rambla-Alegre, M.; López de Alda, M.; Eljarrat, E. Pesticides in sediments of the Ebro River Delta cultivated area (NE Spain): Occurrence and risk assessment for aquatic organisms. Environ. Pollut. 2022, 305, 119239. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Lima, G.D.A.; Destro, A.L.F.; Condessa, S.; Zuanon, J.A.S.; Freitas, M.B.; Oliveira, L.L. Short-term intake of deltamethrin-contaminated fruit, even at low concentrations, induces testicular damage in fruit-eating bats (Artibeus lituratus). Chemosphere 2021, 278, 130423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pei, J.; Zhang, R.; Wang, S.; Zeng, W.; Huang, D.; Wang, Y.; Zhang, Y.; Wang, Y.; Yu, K. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood. Ecotoxicol. Environ. Saf. 2018, 154, 27–35. [Google Scholar] [CrossRef]
- Radke, M.; Lauwigi, C.; Heinkele, G.; Mürdter, T.E.; Letzel, M. Fate of the antibiotic sulfamethoxazole and its two major human metabolites in a water sediment test. Environ. Sci. Technol. 2009, 43, 3135–3141. [Google Scholar] [CrossRef]
- Mo, W.Y.; Chen, Z.; Leung, H.M.; Leung, A.O. Application of veterinary antibiotics in China’s aquaculture industry and their potential human health risks. Environ. Sci. Pollut. Res. Int. 2017, 24, 8978–8989. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Straub, J.O. Aquatic environmental risk assessment for human use of the old antibiotic sulfamethoxazole in Europe. Environ. Toxicol. Chem. 2016, 35, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Jijie, R.; Solcan, G.; Nicoara, M.; Micu, D.; Strungaru, S.A. Antagonistic effects in zebrafish (Danio rerio) behavior and oxidative stress induced by toxic metals and deltamethrin acute exposure. Sci. Total Environ. 2020, 698, 134299. [Google Scholar] [CrossRef] [PubMed]
- Trush, M.A.; Thompson, D.C. Enhancement of Chemical Activation via Radical-Dependent Mechanisms: An Emerging Concept in Chemical-Chemical Interactions. In Oxygen Radicals in Biology and Medicine; Simic, M.G., Taylor, K.A., Ward, J.F., von Sonntag, C., Eds.; Springer: Boston, MA, USA, 1988; pp. 739–744. [Google Scholar] [CrossRef]
- Desalegn, A.; Bopp, S.; Asturiol, D.; Lamon, L.; Worth, A.; Paini, A. Role of Physiologically Based Kinetic modelling in addressing environmental chemical mixtures—A review. Comput. Toxicol. 2019, 10, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Sipes, N.S.; Padilla, S.; Knudsen, T.B. Zebrafish: As an integrative model for twenty-first century toxicity testing. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 256–267. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Gao, J.M.; Huang, C.J.; Li, C.Q. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol. Teratol. 2014, 42, 35–42. [Google Scholar] [CrossRef]
- Blanco, A.M.; Bertucci, J.I.; Hatef, A.; Unniappan, S. Feeding and food availability modulate brain-derived neurotrophic factor, an orexigen with metabolic roles in zebrafish. Sci. Rep. 2020, 10, 10727. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.P.; Rosemberg, D.B.; Seibt, K.J.; Capiotti, K.M.; Da Silva, R.S.; Bonan, C.D. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol. Teratol. 2011, 33, 608–617. [Google Scholar] [CrossRef]
- Parng, C.; Roy, N.M.; Ton, C.; Lin, Y.; McGrath, P. Neurotoxicity assessment using zebrafish. J. Pharmacol. Toxicol. Methods 2007, 55, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bally-Cuif, L.; Vernier, P. 2-Organization and physiology of the zebrafish nervous system. In Fish Physiology; Perry, S.F., Ekker, M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 29, pp. 25–80. [Google Scholar]
- Balmus, I.M.; Ciobica, A.; Antioch, I.; Dobrin, R.; Timofte, D. Oxidative Stress Implications in the Affective Disorders: Main Biomarkers, Animal Models Relevance, Genetic Perspectives, and Antioxidant Approaches. Oxidative Med. Cell. Longev. 2016, 2016, 3975101. [Google Scholar] [CrossRef]
- Huh, J.R.; Veiga-Fernandes, H. Neuroimmune circuits in inter-organ communication. Nat. Rev. Immunol. 2020, 20, 217–228. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature reviews. Neuroscience 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Parlak, V. Evaluation of apoptosis, oxidative stress responses, AChE activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere 2018, 207, 397–403. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Wang, C.; Li, T.; Zheng, G.; Sun, W.; An, L.; Bai, Y.; Wu, F. Sex-Specific Effects of Environmental Exposure to the Antimicrobial Agents Benzalkonium Chloride and Triclosan on the Gut Microbiota and Health of Zebrafish (Danio rerio). Environ. Sci. Technol. 2024, 58, 15450–15462. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Zhang, T.; Li, S.; Ren, Z.; Yang, M.; Pan, H.; Xu, S.; Qi, L.; Chon, T.S. Integrative Characterization of Toxic Response of Zebra Fish (Danio rerio) to Deltamethrin Based on AChE Activity and Behavior Strength. BioMed Res. Int. 2016, 2016, 7309184. [Google Scholar] [CrossRef] [PubMed]
- Görge, G.; Nagel, R. Toxicity of lindane, atrazine, and deltamethrin to early life stages of zebrafish (Brachydanio rerio). Ecotoxicol. Environ. Saf. 1990, 20, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Lummis, S.C.R.; Chow, S.C.; Holan, G.; Johnston, G.A.R. γ-Aminobutyric Acid Receptor Ionophore Complexes: Differential Effects of Deltamethrin, Dichlorodiphenyltrichloroethane, and Some Novel Insecticides in a Rat Brain Membrane Preparation. J. Neurochem. 1987, 48, 689–694. [Google Scholar] [CrossRef]
- Alasmari, F. Caffeine induces neurobehavioral effects through modulating neurotransmitters. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2020, 28, 445–451. [Google Scholar] [CrossRef]
- Lee, J.G.; Cho, H.J.; Jeong, Y.M.; Lee, J.S. Genetic Approaches Using Zebrafish to Study the Microbiota-Gut-Brain Axis in Neurological Disorders. Cells 2021, 10, 566. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Maharajan, K.; Habibi, H.R.; Kadirvelu, K.; Venkataramana, M. Zearalenone induced embryo and neurotoxicity in zebrafish model (Danio rerio): Role of oxidative stress revealed by a multi biomarker study. Chemosphere 2018, 198, 111–121. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Deisseroth, A.; Dounce, A.L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol. Rev. 1970, 50, 319–375. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Song, G. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2020, 234, 108758. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Mashoof, S.; Criscitiello, M.F. Fish Immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Kemp, D.E.; Soczynska, J.K.; McIntyre, R.S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: A systematic review of the literature. J. Clin. Psychiatry 2009, 70, 1078–1090. [Google Scholar] [CrossRef]
- Medina-Gali, R.M.; Ortega-Villaizan, M.D.M.; Mercado, L.; Novoa, B.; Coll, J.; Perez, L. Beta-glucan enhances the response to SVCV infection in zebrafish. Dev. Comp. Immunol. 2018, 84, 307–314. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, F.; Wang, C. Acute Toxicity, Neurotoxic, Immunotoxic, and Behavioral Effects of Deltamethrin and Sulfamethoxazole in Adult Zebrafish: Insights into Chemical Interactions and Environmental Implications. Toxics 2025, 13, 128. https://doi.org/10.3390/toxics13020128
Liu Y, Liu F, Wang C. Acute Toxicity, Neurotoxic, Immunotoxic, and Behavioral Effects of Deltamethrin and Sulfamethoxazole in Adult Zebrafish: Insights into Chemical Interactions and Environmental Implications. Toxics. 2025; 13(2):128. https://doi.org/10.3390/toxics13020128
Chicago/Turabian StyleLiu, Yueyue, Fengyu Liu, and Chen Wang. 2025. "Acute Toxicity, Neurotoxic, Immunotoxic, and Behavioral Effects of Deltamethrin and Sulfamethoxazole in Adult Zebrafish: Insights into Chemical Interactions and Environmental Implications" Toxics 13, no. 2: 128. https://doi.org/10.3390/toxics13020128
APA StyleLiu, Y., Liu, F., & Wang, C. (2025). Acute Toxicity, Neurotoxic, Immunotoxic, and Behavioral Effects of Deltamethrin and Sulfamethoxazole in Adult Zebrafish: Insights into Chemical Interactions and Environmental Implications. Toxics, 13(2), 128. https://doi.org/10.3390/toxics13020128





